Abstract
Multipotent mesenchymal stem/stromal cells (MSCs) offer great promise for future regenerative and anti-inflammatory therapies. Panels of functional and phenotypical markers are currently used in characterization of different therapeutic stem cell populations from various sources. The i antigen (linear poly-N-acetyllactosamine) from the Ii blood group system has been suggested as a marker for MSCs derived from umbilical cord blood (UCB). However, there are currently no commercially available antibodies recognizing the i antigen. In the present study, we describe the use of antibody phage display technology to produce recombinant antibodies recognizing a structure from the surface of mesenchymal stem cells. We constructed IgM phage display libraries from the lymphocytes of a donor with an elevated serum anti-i titer. Antibody phage display technology is not dependent on immunization and thus allows the generation of antibodies against poorly immunogenic molecules, such as carbohydrates. Agglutination assays utilizing i antigen–positive red blood cells (RBCs) from UCB revealed six promising single-chain variable fragment (scFv) antibodies, three of which recognized epitopes from the surface of UCB-MSCs in flow cytometric assays. The amino acid sequence of the VH gene segment of B12.2 scFv was highly similar to the VH4.21 gene segment required to encode anti-i specificities. Further characterization of binding properties revealed that the binding of B12.2 hyperphage was inhibited by soluble linear lactosamine oligosaccharide. Based on these findings, we suggest that the B12.2 scFv we have generated is a prominent anti-i antibody that recognizes i antigen on the surface of both UCB-MSCs and RBCs. This binder can thus be utilized in UCB-MSC detection and isolation as well as in blood group serology.
Key words: i blood group antigen, MSC, phage display, recombinant antibody development
Introduction
Mesenchymal stem or stromal cells (MSCs) are multipotent self-renewing progenitor cells, originally isolated from the bone marrow and subsequently also identified in various fetal and postnatal organs and tissues.1 Because of the lack of a specific marker, there are difficulties in defining human MSCs, and these cells are now characterized using minimal criteria suggested by the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy.2 MSCs are currently under intense clinical and scientific investigation. Stem cells from nonembryonic sources provide a less controversial and technically more feasible alternative for embryonic stem cells in future cell therapy applications.3,4 Due to the unique multilineage differentiation capacity5–7 MSCs are a promising cell type for various applications in the field of tissue engineering.8 MSCs have also been shown to be capable of improving engraftment of hematopoietic stem cells after allogeneic transplantation9 and they evoke immunomodulatory effects, which makes these cells an attractive candidate for therapy in several immune-mediated disorders.10–13 In order to fulfill the expectations raised by MCSs for stem cell therapy, several biological questions need to be addressed. A specific molecular marker for MSCs to be used in the preparation of pure stem cell populations aimed at therapy has not been defined.
Cell surface glycans are the first cellular components encountered by approaching cells, pathogens, signaling molecules, and other binders. As the cell surface glycans form cell type specific signatures, they are ideal for identifying and isolating specific cell types from heterogeneous populations.14 We have previously shown the linear poly-N-acetyllactosamine carbohydrate structure (i antigen) to be a marker for MSCs from umbilical cord blood (UCB).15
The Ii blood group system consists of two structurally related poly-N-acetyllactosamine carbohydrate antigens I and i. The corresponding phenotypes are I or i (I-). Poly-N-acetyllactosamines (polylactosamines, poly-LacNAc) consist of repeating N-acetyllactosamine disaccharide units (LacNAc; Galβ1-4GlcNAc, where Gal is D-galactose and GlcNAc is N-acetyl-D-glucosamine). The LacNAc units can form linear (i type) or branched (I type) chains. The linear i antigen is abundantly expressed on the surface of embryonic red blood cells (RBCs) and on RBCs during times of altered erythropoiesis. During the first 18 months of life the amount of i antigens on RBC surfaces decreases to very low levels and the erythrocytes start to express I antigen.16 This developmental regulation is presumed to be controlled by the regulated expression of β1-6 N-acetylglucosaminyltransferases, enzymes responsible for the formation of β1-6 branch to the poly-N-acetyllactosamine chain and thus formation of the I antigen.17 The adult i phenotype is rare, although there are some individuals and families, whose RBCs never start to express I antigen but express i antigen lifelong.18,19 There is currently no antibody commercially available against the i antigen, and the serological testing needs to be performed using human antisera.
The expression of the I and i antigens is not restricted to erythrocytes, and they are present on the surfaces of many other human cell types as well.16 Anti-I antibody is a common cold reactive antibody, which reacts strongly with adult RBCs but not with UCB-derived RBCs. It can be often found as an autoantibody in patients with cold hemagglutinin disease or Mycoplasma pneumoniae infection and as an alloantibody in i phenotypic adult sera. Anti-i antibody is relatively uncommon antibody in healthy individuals, but is often found in patients with infectious mononucleosis.16 Anti-I and anti-i antibodies can cause problems in blood group typing, antibody screening and compatibility testing, as well as in blood transfusions if they have high titer and/or high thermal amplitude.
Carbohydrates are generally considered to be poor immunogens. Many existing monoclonal antiglycan antibodies have been raised against intact cells, often from cancer, and later on defined as having glycan-binding specificity.20 Monoclonal antibodies to specific glycan structures have been generated by using glycoconjugates, such as glycans coupled to carrier proteins. Further, some glycoproteins and glycolipids have been successfully used as immunogens.20 Antibodies resulting from carbohydrate immunization are typically of the IgM class, and therefore they are not well applicable for in vivo diagnostics or therapy.21 Some of the challenges involved in using these conventional approaches for generating antibodies to carbohydrate moieties can be overcome by using phage display technology, which is not dependent on immunization and thus allows the generation of antibodies against poorly immunogenic molecules or even self-antigens. Antibody phage display technology has been successfully used to generate several anticarbohydrate antibodies, such as antibodies against Lewis x and sialyl Lewis x,22 Thomsen-Friedenreich antigen,21 ganglioside GM3,23 mannotriose carbohydrate antigens,24–26 as well as glycosaminoglycan fragments.27,28 One blood group antibody, blood group B, has also been produced using recombinant technology, where RBCs were used in panning.29 Using this technology, it has been possible to produce completely human monoclonal antibodies from both immune and nonimmune sources, rendering recombinant antibodies a promising source of antibodies for immunotherapy.22
In this article we describe the isolation and characterization of a novel binder that specifically recognizes a structure on the surface of MSCs, known to express the i blood group antigen.15 We used antibody phage display technology and constructed IgM single-chain variable fragment (scFv) phage display libraries from the lymphocytes of a donor with elevated anti-i antibody level. The selection of the libraries was performed utilizing RBC antigens. Characterization of potential binders resulted in the selection of one prominent antibody specific for i antigen.
Materials and Methods
Cells
Red blood cells
RBCs were obtained either from healthy voluntary adult donors or from UCB units. Adult blood was obtained from the Finnish Red Cross Blood Service and UCB units via the Finnish Cord Blood Bank (Finnish Red Cross Blood Service). UCB was collected after normal vaginal delivery from voluntary donors, who gave informed consent for the blood to be used for research. The study protocol was accepted by the ethical review boards of the Helsinki University Central Hospital (Helsinki, Finland) and the Finnish Red Cross Blood Service. The ABO and RhD blood groups were determined from the blood samples. The red blood cells from each cell source were isolated using density gradient centrifugation.30 The remaining RBC fraction was washed three times and stored at 4°C for maximum of 7 days, if not used immediately.
UCB-derived mesenchymal stem cells
UCB units were obtained as already mentioned. UCB was processed within 24 h of collection as previously described.31 The UCB-MSC lines used in this study were prepared from the mononuclear cell fraction as previously described.32 Briefly, the mononuclear cell fraction was plated on fibronectin-coated (Sigma Aldrich, St. Louis, MO) six-well plates at 106 cells/well in minimum essential alpha-medium supplemented with 10% fetal bovine serum, 50 nM dexamethasone, 10 ng/mL epidermal growth factor, 10 ng/mL recombinant human platelet–derived growth factor (BB), 100 U/mL penicillin, and 1% streptomycin. Most of the nonadherent cells were removed as the medium was replaced the next day. The cells were subcultured by plating the cells at a density of 1000–3000 cells/cm2 in the same media and replacing the medium two or three times a week. UCB-MSCs were characterized by flow cytometry to be negative for CD14, CD34, CD45, and HLA-DR and positive for CD13, CD29, CD44, CD90, CD105, and HLA-ABC.2 The cells were shown to be able to differentiate along osteogenic, adipogenic, and chondrogenic lineages as previously described.33
Materials
Enzymes used to modify the cell surface, neuraminidase from Vibrio cholera (N6514), and papain from papaya latex (P4762), were both obtained from Sigma Aldrich. Papain was used in 5 mM cysteine hydrochloride buffer (pH 7.2), L-cysteine hydrochloride (C-7880) from Sigma Aldrich, prepared immediately before use. Anti-myc antibody, clone 9E10 (05-419), which recognizes scFvs containing the myc tag, was from Millipore (Billerica, MA). Anti-M13 monoclonal antibody (27942001) recognizing hyperphages was obtained from GE Healthcare (Little Chalfont, United Kingdom) and Alexa Fluor488 goat anti-mouse IgG (H+L) (A11001) recognizing anti-M13 antibody from Invitrogen (Molecular Probes, Invitrogen by Life Technologies, Carlsbad, CA). Oligosaccharides used in oligosaccharide competition assay were branched lacto-N-hexaose (LNH) from Dextra Laboratories (Reading, United Kingdom) (L604), linear para-lacto-N-neohexaose (LNnH) from Elicityl (Crolles, France) (GLY022), and N-acetyl-D-lactosamine (LacNAc) from Sigma Aldrich (A7791). The assay was repeated with branched (Gal-β1-4GlcNAcβ1-3(Galβ1-4GlcNAcβ1-6)Galβ1-4Glc) and linear (Gal-β1-4GlcNAcβ1-3Galβ1-4GlcNAcβ1-3Galβ1-4Glc) lactosamine oligosaccharides synthesized by Glykos Finland Ltd.
Construction of blood donor specific scFv phage display library
Whole blood sample having anti-i antibodies was obtained from a serologically selected blood donor after approval of the Ethics Committee of the Helsinki and Uusimaa Hospital District. The blood donor had been earlier characterized to have anti-i antibodies by using a panel of I negative and I positive red RBCs. Lymphocytes were isolated according to the Ig-Prime kit protocol (Novagen, EMD Biosciences Inc., Merk KGaA, Darmstadt, Germany). Briefly, the blood sample (90 mL) was mixed with 270 mL of the lysis buffer (155 mM NH4Cl, 10 mM NH4HCO3, 0.1 mM EDTA, pH 7.4), incubated for 15 min on ice with gentle shaking and centrifuged at 450 g for 10 min at room temperature. The pellet containing white blood cells was washed twice with lysis buffer and resuspended into 5 mL of sterile phosphate-buffered saline (PBS). RNA was isolated using Qiagen's (Austin, TX) RNeasy midi Total RNA isolation kit according to manufacturer's protocol. First strand cDNA synthesis was carried out using Transcriptor first strand cDNA synthesis kit (Roche Applied Science, Mannheim, Germany) and primers specific to the human constant regions of human IgM heavy chain as well as human kappa and lambda light chain. For the construction of single-chain antibody (scFv) phage display libraries the variable regions of the heavy and light chains cDNA fragments were amplified by polymerase chain reaction (PCR). Each subgroup of heavy and light chains was amplified separately: 14 PCR amplifications for the VH region, 13 for Vκ, and 15 for Vλ. The amplified VH and VL regions were pooled and cloned separately into the scFv phagemid vector. The size of the resulting scFv libraries was analyzed and the their diversity was studied by sequencing the VH and VL regions by using an ABI 3100 Genetic Analyzer (Applied Biosystems, Invitrogen). For the selection, the scFv libraries were converted to a multivalent scFv display format using hyperphage (Progen Biotechnik GmbH, Heidelberg, Germany).
Selection and screening of recombinant antibodies
The depletion of the libraries was performed using I-positive AB blood group adult RBCs to remove phages that bind to structures other than i blood group antigen. The selection of the libraries was carried out using i-positive O blood group UCB-RBCs. Four affinity selection rounds were performed, and scFv phages enriched were further characterized. Soluble scFv fragments containing myc tag were produced in Escherichia coli HB2151 strain. Production of the soluble scFv to the culture supernatant was confirmed by dot blot analysis using anti-myc antibody detection. Finally RBC agglutination assay was used to determine which scFvs bind to UCB-derived O blood group RBCs having i antigen on their surface [see Agglutination assay (scFv)]. After the agglutination tests the DNA sequences of the selected scFv clones were determined.
Preparation of individual phage clones
Escherichia coli XL-1 Blue clones containing phagemid vector desired scFvs expression units were grown in SB medium with 10 μg/mL ampicillin, 20 μg/mL tetracyclin, and 1% (v/v) glucose with shaking at 37°C for 18 h. The bacterial cultures were diluted 1:50 into 10 mL of SB medium containing ampicillin and tetracyclin as just described, and the cultivation was carried on until OD600 ∼ 1 and infected with the hyperphage (Progen Biotechnik GmbH) for 30 min at 37°C. The infected cultivations were diluted with 90 mL of SB medium supplemented with ampicillin and tetracyclin and grown with shaking at 37°C for 2 h. Finally kanamycin was added for final concentration of 70 μg/mL and cultivations were further grown with shaking at 34°C for 18 h. The multivalent scFv phage stocks were prepared from overnight cultivations by PEG precipitation as follows: after centrifugation at 5700 g at 4°C for 15 min the supernatant containing the phages was precipitated twice with 20% PEG-6000, 2.5 M NaCl. The titers of resulting multivalent scFv phage stocks were determined by infecting E. coli XL-1 Blue cells with diluted phage stocks and counting the colonies growing on LB plates containing 100 μg/mL ampicillin. The titers of the resulting scFv-phage pools were analyzed (Tables 1 and 2).
Table 1.
Characteristics of Red Blood Cells Used in the Selection of scFv Clones from Antibody Phage Display Library
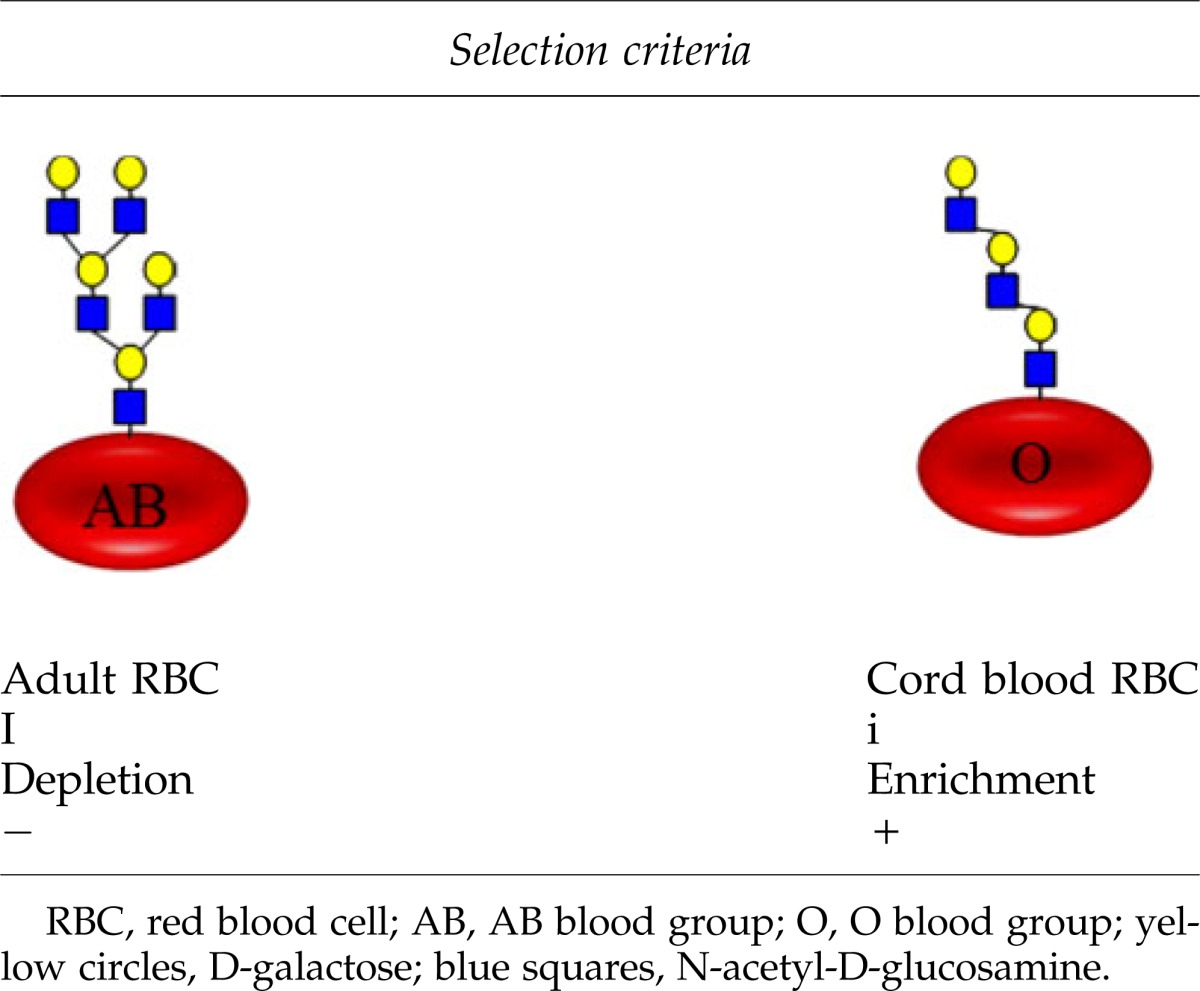 |
Table 2.
Steps Used to Select the i Blood Group–Specific Binders from the Phage-Display Library
| Step | No. of clones tested | Positive clones | |
|---|---|---|---|
| 1 | RBC agglutination assay, HTS | ∼100 | 17 |
| 2 | RBC agglutination assay, 3×i/I RBCs | 17 | 10 |
| 3 | DNA sequencing | 10 | 5 |
| 4 | FACS analysis with MSCs | 5 | 3 |
In step 1, a high-throughput-screening (HTS) method with only one technical replicate was made. In step 2, a more thorough approach with three different cord blood RBCs and three different adult RBCs, with three technical replicates, was performed.
HTS, high-throughput-screening; FACS, fluorescence associated cell sorting.
Enzymatic cell surface treatments
RBCs, 12.5% suspension in 2 mL of PBS, were treated with 50 mU neuraminidase for 2 h at 37°C. Papain treatment was performed by adding 1 mL of 25% RBCs in PBS to an equal volume of 5 mM cysteine hydrochloride buffer (pH 7.2) containing 0.35 mg/mL (3.5 U/mL) papain and incubating resulting 12.5% RBC suspension for 2 h at 37°C. UCB-MSCs were grown to 80% confluency in a 10-cm culture dish and treated first with 50 mU neuraminidase in 2 mL alphaMEM Glutamax (Gibco, Invitrogen) containing 0.5% bovine serum albumin (BSA; Sigma Aldrich) for 30 min at 37°C. UCB-MSCs were then detached from the culture dish with freshly prepared papain (15 min, 37°C) using 3 mg/1.5 mL (20 U/mL) papain in 5 mM cysteine hydrochloride buffer (pH 7.2).
Agglutination assay (scFv)
UCB-derived (blood group O) and adult-derived (blood group AB) RBCs treated with either sialidase, papain, both, or neither were added as a 2% suspension in PBS to a U-bottom 96-well plate (Costar, Corning Incorporated, Corning, NY) with an equal volume (50 μL) of scFv supernatants. The assay was performed with two or three technical replicates. Cell and scFv supernatant cocktails were gently stirred and allowed to agglutinate at 4°C for 1 h. Results were visually observed, and anti-myc Ab (20 μg/mL) was then added, and the solution was gently stirred and again allowed to agglutinate at 4°C for 1 h. Agglutination was interpreted manually and photographed. The data were then converted into table format using −, +, and ++ symbols indicating agglutination strength (Table 3).
Table 3.
Agglutination Assay With Umbilical Cord Blood–Derived Red Blood Cells With and Without Enzyme Treatment
| scFv | UCB-RBC no enzyme | UCB-RBC+sialidase+papain | UCB-RBC+sialidase | UCB-RBC+papain |
|---|---|---|---|---|
| B11.1 | − | ++ | − | − |
| D10.1/D10.2 | − | ++ | − | ++ |
| F9 | − | ++ | − | + |
| G8 | − | ++ | − | ++ |
| C3 | − | ++ | − | ++ |
| C11 | − | ++ | − | ++ |
| E8.2 | − | ++ | − | ++ |
| E11 | − | ++ | − | ++ |
| B12.1/B12.2 | − | + | − | ++ |
| G2 | − | ++ | − | ++ |
UCB-RBC, umbilical cord blood–derived red blood cell.
Flow cytometry
In cell stainings for flow cytometry, the UCB-MSCs (50,000 MSCs in 100 μL) were incubated with scFv hyperphages (each with titer 1.32×1010 plaque-forming units [pfu]/mL) for 30 min at 4°C followed by secondary antibody labeling with anti-M13 monoclonal Ab (10 μg/mL for 50,000 MSCs/100 μL) for 30 min at 4 °C. Fluorescently labeled tertiary antibody, Alexa Fluor 488 goat anti-mouse IgG (H+L) (4 μg/mL for 50,000 MSCs/100 μL), was added and incubated in the dark for 30 min at 4°C. As a negative control, cells were incubated without the primary binder or with irrelevant phage, but otherwise treated similar to labeled cells. The binding properties of the scFv hyperphages were analyzed by flow cytometry (FACSAria, Becton Dickinson, San Jose, CA), and a minimum of 10,000 events were measured. Data analysis was carried out with BD FACSDiva™ Flow Cytometry Software Version 5.0.2.
Oligosaccharide competition assay
In the competition assay the oligosaccharides were first dissolved in 0.3% BSA-PBS and then allowed to interact with scFv hyperphages on ice for 30 min. UCB-MSCs were added to each reaction, incubated on ice for 30 min, and stained with anti-M13 antibody and Alexa Fluor488 goat anti-mouse IgG (H+L) as already described. The final concentration of oligosaccharides was 3 mM, scFv hyperphages 1.32×1010 pfu/mL, and cells 50,000 UCB-MSCs in 50-μL reaction volume.
Results
Approximately 100 potential i-specific scFvs were enriched from a phage display library constructed from the blood of an anti-i positive donor
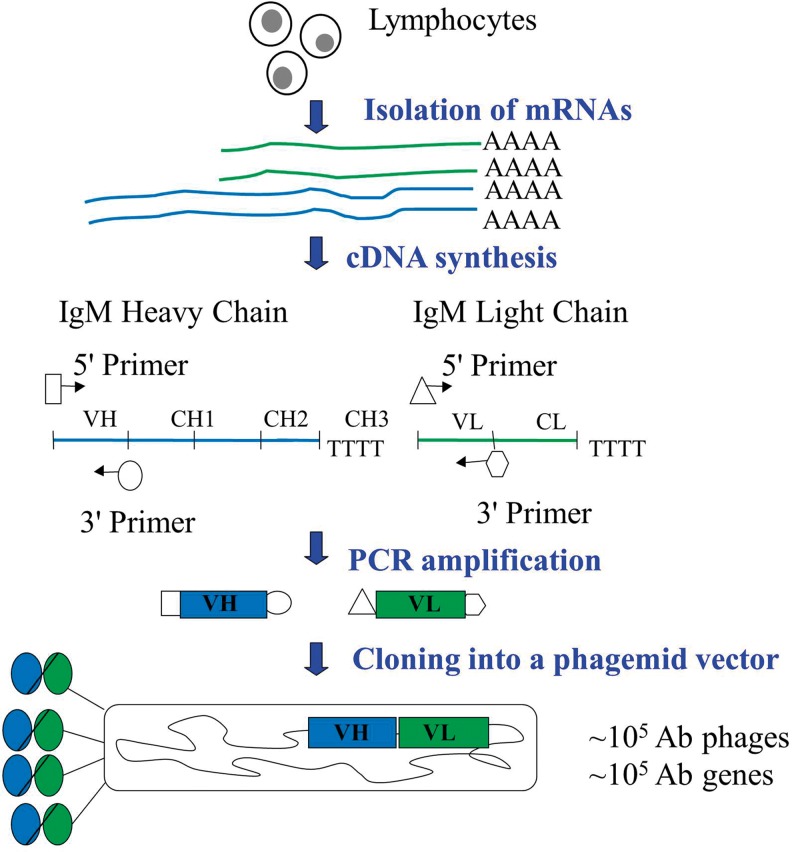
Very few people have anti-i antibodies in their blood,34 so it would have been unlikely to find an anti-i antibody from a naïve pooled antibody library. A blood donor having circulating anti-i antibody was identified in the Finnish Red Cross Blood Service by using a panel of I-negative and I-positive red blood cells. The anti-i antibody directly agglutinated I-negative adult red blood cells at 4°C but did not react with I-positive cells. To develop antibodies recognizing i antigen, but not I antigen, an IgM scFv library was constructed from the lymphocytes of the particular donor as summarized in Figure 1. Both scFv-lambda and scFv-kappa libraries contained 105 clones.
FIG. 1.
A workflow of the construction of the scFv phage display library. scFv, single-chain variable fragment; Ab, antibody; VH, variable region of immunoglobulin heavy chain; CH, constant region of immunoglobulin heavy chain; VL, variable region of immunoglobulin light chain; CL constant region of immunoglobulin light chain.
RBCs from UCB have naturally i antigen on their surface.35 This gives an ideal opportunity to use these cells for the affinity selection of anti-i antibodies from the phage display library. The depletion of the libraries was performed using I-positive adult RBCs that expressed blood group antigens A and B (AB blood group) to remove hyperphages that bind to heterogeneous blood group structures other than i antigen. The selection of the libraries was carried out using i-positive O blood group UCB-RBCs (Table 1). The other 30 known blood group systems on the RBC surface were not taken into account to keep the experimental setting manageable. Four affinity selection rounds were performed and ∼100 scFv phages enriched to i-positive cells. These were further characterized with RBC agglutination assay. To enhance the agglutination efficiency and improve carbohydrate accessibility, RBCs, both UCB and adult derived, were enzymatically treated with sialidase and papain. After two separate high-throughput screening agglutination assays with ∼100 scFvs, 17 clones were selected for further studies based on their ability to agglutinate linear poly-LacNAc (i antigen) expressing UCB-derived red blood cells (UCB-RBCs) compared to branched poly-LacNAc (I antigen) containing adult RBCs (Table 2).
Ten scFvs agglutinated UCB-RBCs better than RBCs from adult donor
The 17 scFvs selected for further studies were tested with more thorough agglutination assays. Agglutinations were repeated three times with three replicates, each time with adult RBCs from different donors and UCB-RBCs from different units. Out of 17 scFvs tested, 10 were able to agglutinate UCB-RBCs (i+/I−) better than adult-derived RBCs (i−/I+) repeatedly using RBCs from different individuals. The effect of RBC surface proteins and carbohydrate sialic acids on agglutination was studied with resulting 10 scFv binders (Table 3). Enzymatic treatment was performed either with sialidase and papain simultaneously or with only one or the other. Sialidase was selected to remove terminal sialic acid and make repeated lactosamine structures more accessible. This treatment was observed unnecessary for the induction of agglutination, however. Papain, in contrast, was shown to increase agglutination efficiency. Therefore, removal of bulk cell surface proteins was shown to be critical for the binding of scFvs to RBCs. The results showed a similar type of agglutination pattern for each of the repeated experiments. Small differences were most likely due to the variable scFv concentrations in different production batches. This was verified with dilution series experiment showing some of the undiluted scFv samples agglutinating both RBC types, while 1:3 dilution of the same sample agglutinated only UCB-RBCs (data not shown).
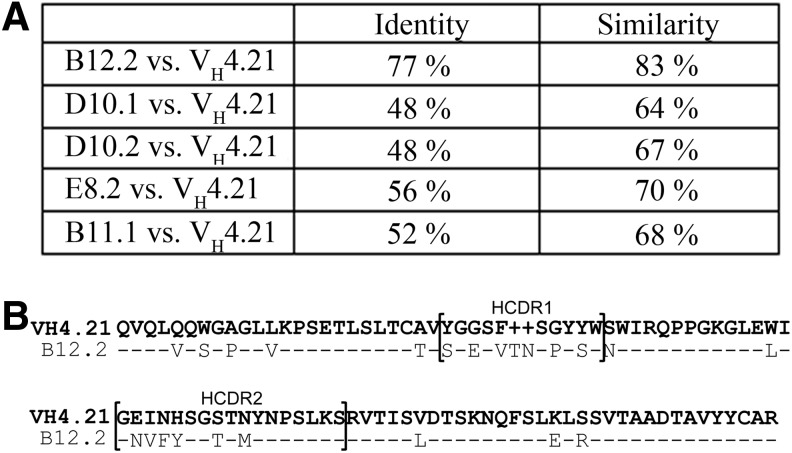
The DNA sequences of 10 individual scFv clones were determined and five clones, B12.2, D10.1, D10.2, E8.2, and B11.1, contained the cDNAs encoding the whole length scFvs. These five sequences were compared to immunoglobulin VH4.21 gene segment encoding anti-I and anti-i specificities of cold agglutinins.36–38 Figure 2A shows the amount of identical and similar amino acids of each scFv compared to Ig VH4.21 gene segment. The B12.2 scFv was highly similar (83%) to VH4.21 gene segment. The amino acid sequences of these two VH sequences are compared in Figure 2B. All five of the promising scFvs were produced as hyperphages, each containing approximately three to five antigen binding sites of a certain scFv.
FIG. 2.
Amino acid sequences of five scFvs were compared to the VH4.21 gene sequence known to be involved in i blood group binding. (A) The amount of identical and similar amino acids. (B) The amino acid sequences of B12.2 scFv and VH4.21 are highly similar. HCDR, heavy chain complementarity determining region. HCDR1 and HCDR2 are denoted by brackets; –, identical amino acid.
Three hyperphages showed characteristic MSC binding
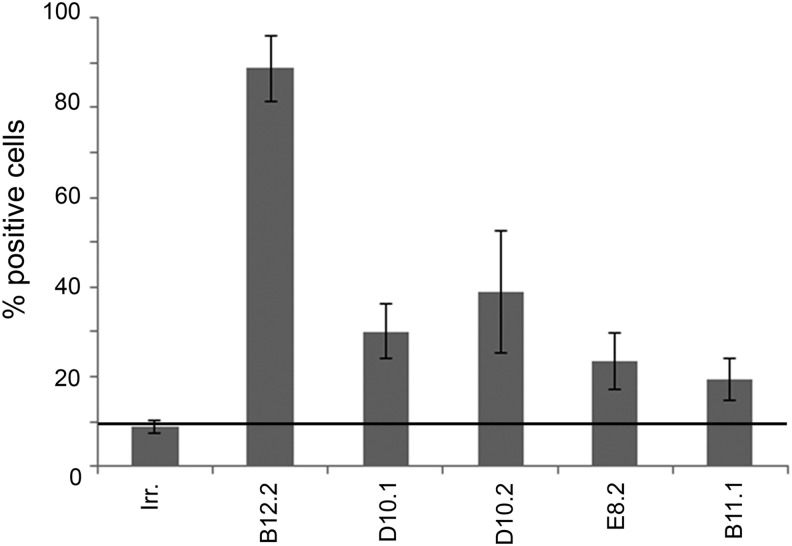
We have previously shown that linear polylactosamines are markers for UCB-MSCs.15 The capability of the five scFv hyperphages to recognize i antigen on the UCB-MSC surface was analyzed by flow cytometry. Three scFv hyperphages, B12.2, D10.1, and D10.2, bound to UCB-MSCs significantly better than the irrelevant hyperphage (Fig. 3). The B12.2 hyperphage recognized 81%–96% of the cells in three repeated experiments. Similarly, 25%–37% of the cells were recognized by D10.1 and 23%–48% with D10.2 hyperphage. The binding of E8.2 and B11.1 hyperphages to UCB-MSCs in two repeated experiments was considered to be low level, and these scFv hyperphages were excluded from further characterization.
FIG. 3.
The percentage of each hyperphage recognizing UCB-MSCs in flow cytometric analysis. The results are shown as averages of three independent experiments for B12.2, D10.1, and D10.2 and two for E8.2 and B11.1. The threshold line represents the amount of irrelevant phage binding to UCB-MSCs. Irr., irrelevant hyperphage; MSC, mesenchymal stem cell.
The binding properties of B12.2, D10.1, and D10.2 hyperphages were also studied with bone marrow (BM)-derived MSCs and fibroblasts, one of the end products of mesenchymal differentiation. All three scFv hyperphages bound more strongly to UCB-MSCs than to BM-MSCs (data not shown). This is consistent with our previous unpublished results showing that the polyclonal anti-i serum binds to UCB-MSCs better than to BM-MSCs. Fibroblasts have previously been shown to have i and I antigens on their surface.39 The B12.2 and D10.2 hyperphages bound to fibroblasts with the same binding level as to UCB-MSCs, but the binding of D10.1 hyperphage to fibroblasts was at the same level as the binding of irrelevant hyperphage (data not shown).
One of the developed binders exhibit clear characteristics of an i blood group antibody
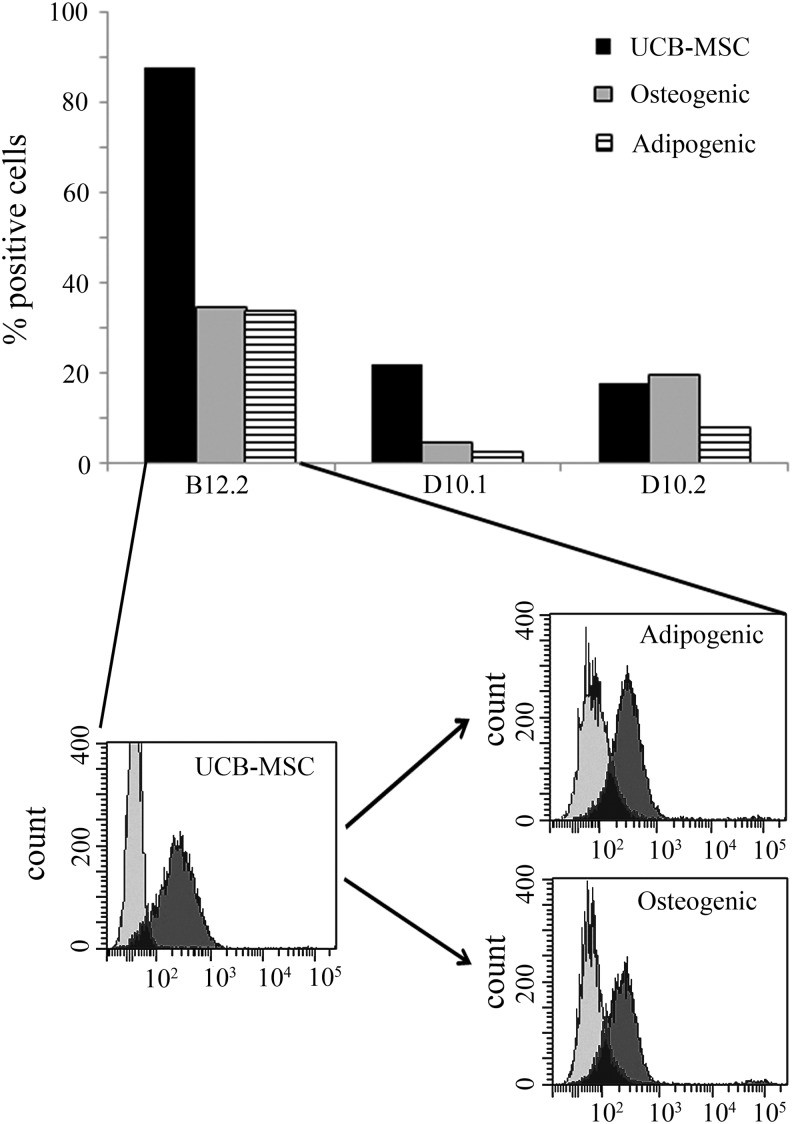
We have shown earlier, by using patient serum containing anti-i IgM, that the i antigen expression diminishes when UCB-MSCs are differentiated to osteoblasts or adipocytes.15 The same phenomenon was also seen with the three selected scFv hyperphages (Fig. 4). The B12.2 hyperphage recognized 88% of the UCB-MSCs, but only 35% and 34% of the osteogenic and adipogenic cells, respectively. D10.1 hyperphage recognized 22% of the UCB-MSCs and only 5% osteoblasts and 3% adipocytes. D10.2 recognized 18% of UCB-MSCs, and the binding level was approximately the same with osteoblasts (20%) but only 8% with adipocytes. Notably, however, the amount of epitopes for these binders transiently increases at the early stage of the differentiation, before it drastically diminishes (data not shown). This transition stage expression profile has not been previously analyzed with polyclonal anti-i serum.
FIG. 4.
Flow cytometric analysis showing that all three hyperphages bind to UCB-MSCs, but the amount of binding activity decreases when the cells are differentiated to osteoblasts or adipocytes. A histogram of the B12.2 hyperphage binding is also shown. Light gray curve, control cells stained only with secondary Ab; dark gray curve, cells stained with B12.2 hyperphage.
We tried to find out if the UCB-MSC recognition by scFv hyperphages is carbohydrate epitope dependent by using periodate treatment known to oxidize hydroxyl groups of carbohydrates.40 Periodate treatment did not have any effect to the binding of these hyperphages (data not shown). However, all carbohydrate antigenic determinants are not sensitive to periodate treatment. Determinants, like linear poly-LacNAc (i antigen), consisting of linear carbohydrate chains with linkages at carbon 3 are considered not to be periodate sensitive.40,41
Detailed epitope characterization of glycan binding proteins (GBPs) is challenging. Although array formats have been developed to test antibodies and other GBPs,42–45 hyperphages seem to behave differently from antibodies in these arrays. We did try to screen the carbohydrate specificity of our scFv hyperphages in the Consortium for Functional Glycomics glycan array (www.functionalglycomics.org) but weren't able to get any specificities. However, currently it is unclear if glycan array is a suitable method for assaying glycan-binding specificity of hyperphages since there is no previous experience in successfully using this technology for hyperphages (personal communication, David F. Smith and Jamie Heimburg-Molinaro, Consortium for Functional Glycomics).
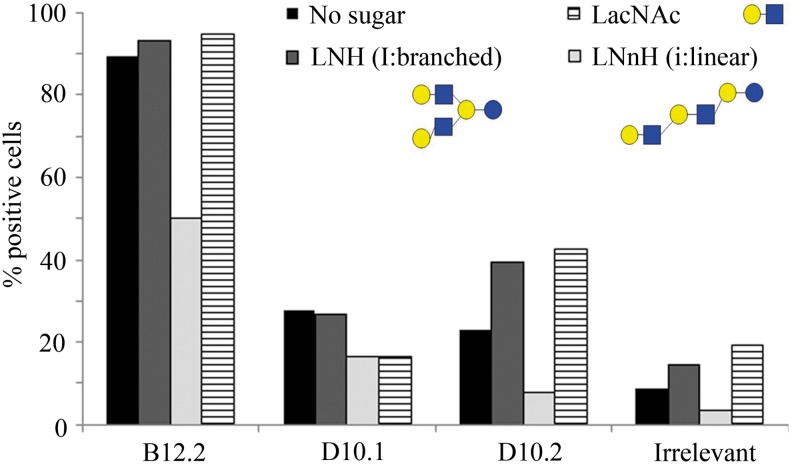
To find out if the epitope recognized by these scFv hyperphages was a linear polylactosamine, a competition binding assay was carried out. Three scFv hyperphages, B12.2, D10.1, and D10.2, were allowed to interact with three different lactosamine oligosaccharides and the formed hyperphage–glycan complexes were then introduced to UCB-MSCs. A flow cytometric analysis showed that the linear lactosamine carbohydrate, containing two LacNAc units and one lactose unit inhibited the cellular binding of all the hyperphages (Fig. 5). The result was verified using lactosamine oligosaccharides from a different provider (data not shown). These results indicate that these scFv hyperphages bind to linear poly-LacNAc structures (i.e., i antigens), with the binding of B12.2 hyperphage being the strongest.
FIG. 5.
A competition binding assay showing that a linear lactosamine structure inhibits the binding of all three scFv hyperphages to UCB-MSCs. The data are representative of two individual flow cytometric experiments. LNH, lacto-N-hexaose; LNnH, para-lacto-N-neohexaose; LacNAc, N-acetyl-D-lactosamine. In glycan structures: blue square symbols, N-acetyl-D-glucosamine (GlcNAc); yellow circle symbols, D-galactose (Gal).
Discussion
The i antigen (linear poly-LacNAc) is known to be expressed on RBCs of fetal and UCB origin. However, the expression of i antigens is not restricted to erythrocytes, and polylactosamine epitopes are present on the surfaces of various human cell types.16 We have previously reported that MSCs derived from UCB express linear poly-LacNAc structures on their surface and this structure can be used as an UCB-MSC marker.15 An anti-i antibody could thus be useful both in the identification of MSCs aimed at therapy and in blood group serology. Such antibodies, however, are not currently commercially available. Glyco Epitope database (www.glyco.is.ritsumei.ac.jp/epitope) lists five antibodies for i antigen (epitope EP0139): I- and i-antigen recognizing A5,46 antiserum anti-i Den,47 GL-2,48 MH21-134,49 and NCC-1004.50 We personally requested to obtain these antibodies, but none of them were available anymore. In blood group serology the typing of i antigen on RBCs is typically performed using polyclonal human antisera expressing the antibody with high enough titer.
Antibody phage display technology has been successfully used to isolate recombinant human antibodies specific to cell surface markers that distinguish otherwise similar cell types.51 These include many human carbohydrate structures, such as the blood group B antigen.29 Unlike hybridoma technology, antibody phage display technology enables producing recombinant human antibodies against poorly immunogenic carbohydrates.22 In this study we present a novel way to use antibody phage display technology to produce a specific antibody for linear poly-LacNAc structure (i antigen). We utilized UCB-derived RBCs naturally expressing i antigen and adult RBCs, which lack the expression of i antigen but express a branched poly-LacNAc structure, I antigen, instead.
We constructed an IgM phage display library from the white blood cells of a voluntary blood donor with elevated anti-i level and were able to isolate promising scFvs, one in particular. We used agglutination assay to identify the scFvs able to bind and agglutinate the UCB-RBCs but not adult-derived RBCs. Papain treatment was shown to increase agglutination efficiency, indicating that the removal of bulk cell surface proteins is critical for the binding of scFvs to RBCs, which could reflect expression of linear poly-LacNAc in glycolipids. Papain is a cysteine protease enzyme that is commonly used in blood group serology to modify interfacial properties of RBCs so that the agglutination is possible under conditions in which untreated cells would not have been able to agglutinate.52,53 It was already reported in 1958–1971 that the increase in I and i antigen reactivity after proteolytic enzyme treatment is a significant phenomenon. However, the reactivities of blood group antigens bound to the glycoprotein moiety of the RBC membrane like M, N, or Pr antigens are reduced and disappear after proteolytic enzyme treatment.54
Our results show that three of the five UCB-RBC agglutinating scFv hyperphages recognized an epitope from the surface of UCB-MSCs. The binding profile was shown to be similar to previously reported anti-i serum binding to UCB-MSCs, recognizing undifferentiated MSCs but decreasing drastically when cells are differentiated.15 This indicates potential use for anti-i antibody as a UCB-MSC marker. Other markers for the “stemness” of cells, mainly for embryonal stem cells, are known. These markers are thought to be associated with pluripotency and are widely used to characterize embryonal stem cells. They are displayed on the surface of undifferentiated pluripotent cells and disappear rapidly when cells differentiate. These markers include the protein antigens CD9, Thy-1 (CD90), tissue nonspecific alkaline phosphatase (Tra-2-49 and Tra-2-54), class-1 human leukocyte antigen, and podocalyxin (GCTM2), the globoseries glycosphingolipid antigens stage-specific embryonic antigen (SSEA)-3 and SSEA-4, and the carbohydrate epitopes recognized by the monoclonal antibodies Tra-1-60 and Tra-1-81.55 We have previously shown that hematopoietic cells, embryonic cells, and MSCs display a characteristic glycosylation profile that distinguishes them from differentiated cell types.56–58 Many of the stem cell marker antibodies in embryonic stem cells, such as SSEA-3, SSEA-4,33 Tra-1-60, and Tra-1-80,59 as well as the binder we generated for i antigen in UCB-MSCs, recognize carbohydrate epitopes. The Tra-1-60 and Tra-1-80 antibodies recognize type-1 linear poly-LacNAc structures closely related to i antigen.59 As stem cell therapies become more common, cell surface markers to identify and extract different stem cell populations will become important for efficient and safe cell therapy applications.
It has been reported that the variable domain of heavy (VH) chain regions of all human cold agglutinin anti-i/I autoantibodies is encoded by a single Ig VH gene segment VH4.21.37,38 The VH4.21 gene segment is essential for cold agglutinin activity and restriction at the VH gene segment level is absolute.36 It has been reported that VH4.21-encoded heavy chains strongly predispose for anti-i binding capacity, regardless of the variable domain of light (VL) regions or heavy chain complementarity-determining region 3 (CDR3) structure.37 The amino acid sequence of the VH region of the B12.2 binder we developed was found to be highly similar (83%) to the VH4.21 gene segment. The most prominent similarity is found on the gene segments outside CDR regions, substantially contributing to the cold agglutinin anti-i structure. Most of the differences in amino acid constitution are found in the two CDRs, as is expected since these regions are responsible for the fine-tuning of the specificity and affinity of the binding of a specific binder.
Evaluation and characterization of GBPs (antibodies and lectins) is challenging. Most lectins are known to have broad specificity, so they have been primarily used to monitor general changes of carbohydrate expression.45 In contrast, anticarbohydrate antibodies typically have more limited specificity and are therefore used to monitor the expression of individual carbohydrate antigens. However, in a broad high-throughput profiling study, more than half of the antibodies studied displayed inappropriate binding relative to the listed specificity. More than half of the antibodies also had measurable cross-reactivity with at least one other glycan on the array.42 There are many methods for analyzing carbohydrate–protein interactions. Traditional methods, such as X-ray crystallography, nuclear magnetic resonance, affinity chromatography, enzyme-linked immunosorbent assay, and mono- or oligosaccharide inhibition studies are usually time consuming and require large amounts of material. Therefore, they are not optimal for the analysis of thousands of potential carbohydrate–protein interactions. Carbohydrate microarrays are an emerging technology that provide a high-throughput means of detecting the interactions of proteins with diverse oligosaccharide sequences of glycoproteins, glycolipids, and polysaccharides. However, glycan-based microarrays vary due to variations in ligand presentation, the origin of glycans (isolated from natural sources or chemically synthesized), assay conditions, detection method, and immobilization of the carbohydrates on flat surfaces. All these features contribute to the affinity and selectivity of binding, and thus the assay may not reflect the actual conditions on the cell surface.42
Although we did not get any specificity for our hyperphages in the glycan array, we were able to show using competition binding assay that the binding of B12.2 hyperphage was inhibited by soluble linear oligolactosamine structure. This indicates that the B12.2 hyperphage recognizes and binds to i antigen on the UCB-MSC surface.
In this study we have identified a blood donor with elevated anti-i level in serum, constructed an antibody library, and isolated an antibody that was able to agglutinate UCB-derived RBCs and bind to UCB-MSCs. Both of these cell types are known to naturally express i antigen. The VH gene segment of B12.2 scFv is also highly similar to the VH4.21 gene segment previously shown to encode anti-i specificities. Finally, the binding of B12.2 hyperphage was inhibited by soluble linear lactosamine structure. Hereby we conclude that the B12.2 antibody is a prominent anti-i binder and thus it can be utilized and further engineered for both UCB-MSC detection and blood group serology.
Abbreviations
- Ab
antibody
- BB
recombinant human platelet–derived growth factor
- BM
bone marrow
- BSA
bovine serum albumin
- CDR
complementarity-determining region
- FACS
fluorescence-associated cell sorting
- Gal
D-galactose
- GBP
glycan binding protein
- GlcNAc
N-acetyl-D-glucosamine
- HTS
high-throughput screen
- i antigen
the linear poly-N-acetyllactosamine carbohydrate structure
- LacNAc
N-acetyllactosamine disaccharide
- LNH
lacto-N-hexaose
- LNnH
para-lacto-N-neohexaose
- MSC
mesenchymal stem/stromal cell
- PBS
phosphate-buffered saline
- Poly-LacNAc
poly-N-acetyllactosamine (polylactosamine)
- RBC
red blood cell
- scFv
a single-chain variable fragment (a single chain antibody)
- UCB
umbilical cord blood
- UCB-RBC
UCB-derived red blood cell
- VH
immunoglobulin variable domain of heavy chain region
- VL
immunoglobulin variable domain of light chain region.
Acknowledgments
The authors would like to thank the Finnish Red Cross Blood Service Cord Blood Bank and acknowledge Birgitta Rantala, Iris Tallus, and Sirkka Hirschovits-Gerz for excellent technical assistance. We also wish to acknowledge the Consortium for Functional Glycomics grant number GM62116 and GM098791 for glycan array analysis of the scFv hyperphages and thank especially David F. Smith and Jamie Heimburg-Molinaro of the Consortium for Functional Glycomics for performing the array screening experiments. This work was supported by the Finnish Funding Agency for Technology and Innovation (TEKES), and the Finnish Glycoscience Graduate School.
Author Disclosure Statement
The authors declare potentially competing interests: Tero Satomaa is a shareholder of Glykos Finland Ltd. and a co-owner in a related patent application.
References
- 1.da Silva Meirelles L. Chagastelles PC. Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 2.Dominici M. Le Blanc K. Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 3.Moore KE. Mills JF. Thornton MM. Alternative sources of adult stem cells: a possible solution to the embryonic stem cell debate. Gend Med. 2006;3:161–168. doi: 10.1016/s1550-8579(06)80204-4. [DOI] [PubMed] [Google Scholar]
- 4.Pessina A. Gribaldo L. The key role of adult stem cells: therapeutic perspectives. Curr Med Res Opin. 2006;22:2287–2300. doi: 10.1185/030079906X148517. [DOI] [PubMed] [Google Scholar]
- 5.Pittenger MF. Mackay AM. Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 6.Kopen GC. Prockop DJ. Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci USA. 1999;96:10711–10716. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato Y. Araki H. Kato J, et al. Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood. 2005;106:756–763. doi: 10.1182/blood-2005-02-0572. [DOI] [PubMed] [Google Scholar]
- 8.Pittenger MF. Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 9.Dazzi F. Ramasamy R. Glennie S, et al. The role of mesenchymal stem cells in haemopoiesis. Blood Rev. 2006;20:161–171. doi: 10.1016/j.blre.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Siegel G. Schafer R. Dazzi F. The immunosuppressive properties of mesenchymal stem cells. Transplantation. 2009;87:S45–9. doi: 10.1097/TP.0b013e3181a285b0. [DOI] [PubMed] [Google Scholar]
- 11.Uccelli A. Moretta L. Pistoia V. Immunoregulatory function of mesenchymal stem cells. Eur J Immunol. 2006;36:2566–2573. doi: 10.1002/eji.200636416. [DOI] [PubMed] [Google Scholar]
- 12.Krampera M. Glennie S. Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 13.Le Blanc K. Rasmusson I. Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 14.Lanctot PM. Gage FH. Varki AP. The glycans of stem cells. Curr Opin Chem Biol. 2007;11:373–380. doi: 10.1016/j.cbpa.2007.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirvonen T. Suila H. Kotovuori A, et al. The i blood group antigen as a marker for umbilical cord blood-derived mesenchymal stem cells. Stem Cells Dev. 2012;21:455–464. doi: 10.1089/scd.2011.0405. [DOI] [PubMed] [Google Scholar]
- 16.Cooling L. Polylactosamines, there's more than meets the “Ii”: a review of the I system. Immunohematology. 2010;26:133–155. [PubMed] [Google Scholar]
- 17.Reid ME. The gene encoding the I blood group antigen: review of an I for an eye. Immunohematology. 2004;20:249–252. [PubMed] [Google Scholar]
- 18.Cooper AG. Brown MC. Serum i antigen: a new human blood-group glycoprotein. Biochem Biophys Res Commun. 1973;55:297–304. doi: 10.1016/0006-291x(73)91087-5. [DOI] [PubMed] [Google Scholar]
- 19.Feizi T. The blood group Ii system: a carbohydrate antigen system defined by naturally monoclonal or oligoclonal autoantibodies of man. Immunol Commun. 1981;10:127–156. doi: 10.3109/08820138109050693. [DOI] [PubMed] [Google Scholar]
- 20.Kannagi R. Hakomori S. A guide to monoclonal antibodies directed to glycotopes. Adv Exp Med Biol. 2001;491:587–630. doi: 10.1007/978-1-4615-1267-7_38. [DOI] [PubMed] [Google Scholar]
- 21.Ravn P. Danielczyk A. Jensen KB, et al. Multivalent scFv display of phagemid repertoires for the selection of carbohydrate-specific antibodies and its application to the Thomsen-Friedenreich antigen. J Mol Biol. 2004;343:985–996. doi: 10.1016/j.jmb.2004.08.052. [DOI] [PubMed] [Google Scholar]
- 22.Mao S. Gao C. Lo CH, et al. Phage-display library selection of high-affinity human single-chain antibodies to tumor-associated carbohydrate antigens sialyl Lewisx and Lewisx. Proc Natl Acad Sci USA. 1999;96:6953–6958. doi: 10.1073/pnas.96.12.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee KJ. Mao S. Sun C, et al. Phage-display selection of a human single-chain fv antibody highly specific for melanoma and breast cancer cells using a chemoenzymatically synthesized G(M3)-carbohydrate antigen. J Am Chem Soc. 2002;124:12439–12446. doi: 10.1021/ja020737j. [DOI] [PubMed] [Google Scholar]
- 24.Sakai K. Shimizu Y. Chiba T, et al. Isolation and characterization of phage-displayed single chain antibodies recognizing nonreducing terminal mannose residues. 1. A new strategy for generation of anti-carbohydrate antibodies. Biochemistry. 2007;46:253–262. doi: 10.1021/bi061875e. [DOI] [PubMed] [Google Scholar]
- 25.Zhang W. Matsumoto-Takasaki A. Kusada Y, et al. Isolation and characterization of phage-displayed single chain antibodies recognizing nonreducing terminal mannose residues. 2. Expression, purification, and characterization of recombinant single chain antibodies. Biochemistry. 2007;46:263–270. doi: 10.1021/bi0618767. [DOI] [PubMed] [Google Scholar]
- 26.Yuasa N. Zhang W. Goto T, et al. Production of anti-carbohydrate antibodies by phage display technologies: potential impairment of cell growth as a result of endogenous expression. J Biol Chem. 2010;285:30587–30597. doi: 10.1074/jbc.M110.107284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Kuppevelt TH. Dennissen MA. van Venrooij WJ, et al. Generation and application of type-specific anti-heparan sulfate antibodies using phage display technology. Further evidence for heparan sulfate heterogeneity in the kidney. J Biol Chem. 1998;273:12960–12966. doi: 10.1074/jbc.273.21.12960. [DOI] [PubMed] [Google Scholar]
- 28.Smits NC. Lensen JF. Wijnhoven TJ, et al. Phage display-derived human antibodies against specific glycosaminoglycan epitopes. Methods Enzymol. 2006;416:61–87. doi: 10.1016/S0076-6879(06)16005-X. [DOI] [PubMed] [Google Scholar]
- 29.Chang TY. Siegel DL. Isolation of an IgG anti-B from a human Fab-phage display library. Transfusion. 2001;41:6–12. doi: 10.1046/j.1537-2995.2001.41010006.x. [DOI] [PubMed] [Google Scholar]
- 30.Jaatinen T. Laine J. Isolation of mononuclear cells from human cord blood by Ficoll-Paque density gradient. Curr Protoc Stem Cell Biol. 2007;Chapter. 2(Unit 2A.1) doi: 10.1002/9780470151808.sc02a01s1. [DOI] [PubMed] [Google Scholar]
- 31.Kekarainen T. Mannelin S. Laine J, et al. Optimization of immunomagnetic separation for cord blood-derived hematopoietic stem cells. BMC Cell Biol. 2006;7:30. doi: 10.1186/1471-2121-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laitinen A. Nystedt J. Laitinen S. The isolation and culture of human cord blood-derived mesenchymal stem cells under low oxygen conditions. Methods Mol Biol. 2011;698:63–73. doi: 10.1007/978-1-60761-999-4_6. [DOI] [PubMed] [Google Scholar]
- 33.Suila H. Pitkanen V. Hirvonen T, et al. Are globoseries glycosphingolipids SSEA-3 and - 4 markers for stem cells derived from human umbilical cord blood? J Mol Cell Biol. 2011;3:99–107. doi: 10.1093/jmcb/mjq041. [DOI] [PubMed] [Google Scholar]
- 34.Huflejt ME. Vuskovic M. Vasiliu D, et al. Anti-carbohydrate antibodies of normal sera: findings, surprises and challenges. Mol Immunol. 2009;46:3037–3049. doi: 10.1016/j.molimm.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 35.Marsh WL. Anti-i: a cold antibody defining the Ii relationship in human red cells. Br J Haematol. 1961;7:200–209. doi: 10.1111/j.1365-2141.1961.tb00329.x. [DOI] [PubMed] [Google Scholar]
- 36.Pascual V. Victor K. Spellerberg M, et al. VH restriction among human cold agglutinins. The VH4-21 gene segment is required to encode anti-I and anti-i specificities. J Immunol. 1992;149:2337–2344. [PubMed] [Google Scholar]
- 37.Schutte ME. van Es JH. Silberstein LE, et al. VH4.21-encoded natural autoantibodies with anti-i specificity mirror those associated with cold hemagglutinin disease. J Immunol. 1993;151:6569–6576. [PubMed] [Google Scholar]
- 38.Smith G. Spellerberg M. Boulton F, et al. The immunoglobulin VH gene, VH4-21, specifically encodes autoanti-red cell antibodies against the I or i antigens. Vox Sang. 1995;68:231–235. doi: 10.1111/j.1423-0410.1995.tb02578.x. [DOI] [PubMed] [Google Scholar]
- 39.Toh BH. Diggle TA. Koh SH. Ii blood group antigens on fibroblast cell surfaces. Clin Immunol Immunopathol. 1979;12:177–182. doi: 10.1016/0090-1229(79)90006-0. [DOI] [PubMed] [Google Scholar]
- 40.Woodward MP. Young WW., Jr Bloodgood RA. Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J Immunol Methods. 1985;78:143–153. doi: 10.1016/0022-1759(85)90337-0. [DOI] [PubMed] [Google Scholar]
- 41.Niemann H. Watanabe K. Hakomori S. Blood group i and I activities of “lacto-N-norhexaosylceramide” and its analogues: the structural requirements for i-specificities. Biochem Biophys Res Commun. 1978;81:1286–1293. doi: 10.1016/0006-291x(78)91275-5. [DOI] [PubMed] [Google Scholar]
- 42.Manimala JC. Roach TA. Li Z, et al. High-throughput carbohydrate microarray profiling of 27 antibodies demonstrates widespread specificity problems. Glycobiology. 2007;17:17C–23C. doi: 10.1093/glycob/cwm047. [DOI] [PubMed] [Google Scholar]
- 43.Pochechueva T. Jacob F. Goldstein DR, et al. Comparison of printed glycan array, suspension array and ELISA in the detection of human anti-glycan antibodies. Glycoconj J. 2011;28:507–517. doi: 10.1007/s10719-011-9349-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Partyka K. Maupin KA. Brand RE, et al. Diverse monoclonal antibodies against the CA 19-9 antigen show variation in binding specificity with consequences for clinical interpretation. Proteomics. 2012;12:2212–2220. doi: 10.1002/pmic.201100676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manimala JC. Roach TA. Li Z, et al. High-throughput carbohydrate microarray analysis of 24 lectins. Angew Chem Int Ed Engl. 2006;45:3607–3610. doi: 10.1002/anie.200600591. [DOI] [PubMed] [Google Scholar]
- 46.Fenderson BA. Nichols EJ. Clausen H, et al. A monoclonal antibody defining a binary N-acetyllactosaminyl structure in lactoisooctaosylceramide (IV6Gal beta 1—4GlcNAcnLc6): a useful probe for determining differential glycosylation patterns between normal and transformed human fibroblasts. Mol Immunol. 1986;23:747–754. doi: 10.1016/0161-5890(86)90086-6. [DOI] [PubMed] [Google Scholar]
- 47.Feizi T. Kapadia A. Yount WJ. I and i antigens of human peripheral blood lymphocytes cocap with receptors for concanavalin A. Proc Natl Acad Sci USA. 1980;77:376–380. doi: 10.1073/pnas.77.1.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nagatsuka Y. Watarai S. Yasuda T, et al. Production of human monoclonal antibodies to i blood group by EBV-induced transformation: possible presence of a new glycolipid in cord red cell membranes and human hematopoietic cell lines. Immunol Lett. 1995;46:93–100. doi: 10.1016/0165-2478(95)00028-4. [DOI] [PubMed] [Google Scholar]
- 49.Miyake M. Kohno N. Nudelman ED, et al. Human IgG3 monoclonal antibody directed to an unbranched repeating type 2 chain (Gal beta 1-4GlcNAc beta 1-3Gal beta 1-4GlcNAc beta 1-3Gal beta 1-R) which is highly expressed in colonic and hepatocellular carcinoma. Cancer Res. 1989;49:5689–5695. [PubMed] [Google Scholar]
- 50.Hirohashi S. Clausen H. Nudelman E, et al. A human monoclonal antibody directed to blood group i antigen: heterohybridoma between human lymphocytes from regional lymph nodes of a lung cancer patient and mouse myeloma. J Immunol. 1986;136:4163–4168. [PubMed] [Google Scholar]
- 51.Marks JD. Ouwehand WH. Bye JM, et al. Human antibody fragments specific for human blood group antigens from a phage display library. Biotechnology (NY) 1993;11:1145–1149. doi: 10.1038/nbt1093-1145. [DOI] [PubMed] [Google Scholar]
- 52.Aho K. Christian CL. Studies of incomplete antibodies. 1. Effect of papain on red cells. Blood. 1966;27:662–669. [PubMed] [Google Scholar]
- 53.Hyono A. Gaboriaud F. Mazda T, et al. Impacts of papain and neuraminidase enzyme treatment on electrohydrodynamics and IgG-mediated agglutination of type A red blood cells. Langmuir. 2009;25:10873–10885. doi: 10.1021/la900087c. [DOI] [PubMed] [Google Scholar]
- 54.Doinel C. Ropars C. Salmon C. Effects of proteolytic enzymes and neuraminidase on the I and i erythrocyte antigen sites. Quantitative and thermodynamic studies. Immunology. 1978;34:653–662. [PMC free article] [PubMed] [Google Scholar]
- 55.International Stem Cell Initiative. Adewumi O. Aflatoonian B. Ahrlund-Richter L, et al. Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nat Biotechnol. 2007;25:803–816. doi: 10.1038/nbt1318. [DOI] [PubMed] [Google Scholar]
- 56.Satomaa T. Heiskanen A. Mikkola M, et al. The N-glycome of human embryonic stem cells. BMC Cell Biol. 2009;10:42-2121-10–42. doi: 10.1186/1471-2121-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heiskanen A. Hirvonen T. Salo H, et al. Glycomics of bone marrow-derived mesenchymal stem cells can be used to evaluate their cellular differentiation stage. Glycoconj J. 2009;26:367–384. doi: 10.1007/s10719-008-9217-6. [DOI] [PubMed] [Google Scholar]
- 58.Hemmoranta H. Satomaa T. Blomqvist M, et al. N-glycan structures and associated gene expression reflect the characteristic N-glycosylation pattern of human hematopoietic stem and progenitor cells. Exp Hematol. 2007;35:1279–1292. doi: 10.1016/j.exphem.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 59.Natunen S. Satomaa T. Pitkanen V, et al. The binding specificity of the marker antibodies Tra-1-60 and Tra-1-81 reveals a novel pluripotency-associated type 1 lactosamine epitope. Glycobiology. 2011;21:1125–1130. doi: 10.1093/glycob/cwq209. [DOI] [PMC free article] [PubMed] [Google Scholar]