Abstract
Leukocytes can “gnaw away” the plasma membrane of other cells. This phenomenon, called trogocytosis, occurs subsequent to cell-to-cell adhesion. Currently, two mechanisms of trogocytosis, adhesion molecule-mediated trogocytosis and Fcγ receptor-(FcγR-) mediated trogocytosis, have been identified. In our earlier study, we established an in vitro model of FcγR-mediated trogocytosis, namely, CD8 translocation model from T cells to neutrophils. By using this model, we demonstrated that the molecules transferred to neutrophils via FcγR-mediated trogocytosis were taken into the cytoplasm immediately. This result suggests that the chance of molecules transferred via FcγR-mediated trogocytosis to play a role on the cell surface could be time-limited. Thus, we consider the physiological role of FcγR-mediated trogocytosis as a means to remove antibodies (Abs) that bind with self-molecules rather than to extract molecules from other cells. This concept means that FcγR-mediated trogocytosis can be a defense mechanism to Ab-mediated autoimmune response. Moreover, the activity of FcγR-mediated trogocytosis was revealed to be parallel to the endocytotic activity of neutrophils, which was critically related to the susceptibility to systemic autoimmune diseases. The collective findings suggest that FcγR-mediated trogocytosis could physiologically play a role in removal of Abs bound to self-antigens and prevent autoimmune diseases.
1. Introduction
Trogocytosis is the exchange of plasma membrane fragments between immune cells which form a conjugate [1, 2]. The phenomenon was first described on CD8+ T cells [3]. The CD8+ T cells can take plasma membrane fragments of antigen-presenting cells (APCs) via T-cell receptor (TCR) and antigen (Ag)/class I major histocompatibility complex (MHC), when these cells form an immunological synapse. To date, it has been shown that not only CD8+ T cells but also other immune cells, including CD4+ T cells [4], γδT cells [5], B cells [6], natural killer cells [7], dendritic cells [8], monocytes [9], and macrophages [10], have potential for trogocytosis. These cells are able to accept plasma membrane fragments of other cells after recognition of cell surface Ags by the specific receptors. This mechanism is called adhesion molecule-mediated trogocytosis.
Recently, another mechanism of trogocytosis mediated by Ag/antibody (Ab) immune complex and Fcγ receptor (FcγR), namely, FcγR-mediated trogocytosis, is advocated [11–13]. The major difference of FcγR-mediated trogocytosis from adhesion molecule-mediated trogocytosis is the intervention of Ab. This phenomenon is well characterized, wherein CD20 molecules on malignant B cells are lost after infusion of the humanized anti-CD20 monoclonal Ab, rituximab [14–16]. In this situation, FcγR+ immune cells capture the plasma membrane fragments of B cells via the CD20/anti-CD20 immune complexes and FcγRs. Similar phenomenon is seen when CD22 [17] and CD25 [18] are targeted by therapeutic Abs. Although the best known cells that can perform FcγR-mediated trogocytosis are monocytes and macrophages [12, 19–21], the most abundant FcγR+ cells in peripheral blood, neutrophils, also have potential for FcγR-mediated trogocytosis [22, 23].
The physiological roles of trogocytosis have been discussed concerning T cells that acquired the Ag/MHC from the APCs in particular [24, 25]. The acceptor CD8+ T cells for the Ag/MHC are prone to be killed by the fratricide mechanism. In another way, the acquisition of HLA-G1 from APCs via trogocytosis could change the T-cell phenotype from effector to regulatory phenotype [26, 27]. It has been also demonstrated that CD4 CD8 double negative regulatory T cells, which accept the Ag/MHC from APCs via trogocytosis, can function as cytotoxic cells toward the Ag-specific CD8+ T cells [28]. These could be related to the convergence or suppression of the immune response. On the contrary, it is indicated that the T cells with the Ag/MHC can possibly function as new APCs resulting in the progression of the immune response. These controversial properties of T cells through adhesion molecule-mediated trogocytosis could be displayed in time and environment-dependent manners. In addition, recent studies have demonstrated that adhesion molecule-mediated trogocytosis could be related to the exercise of naturally occurring FOXP3+ regulatory T cells [29]. However, the physiological role of FcγR-mediated trogocytosis remains unrevealed. In this perspective, the role of FcγR-mediated trogocytosis in the physiological immune system is discussed according to the current data.
2. Establishment of In Vitro Model of FcγR-Mediated Trogocytosis
Daubeuf et al. had established a simple method to detect adhesion molecule-mediated trogocytosis by flow cytometry (FCM) [30]. On the other hand, we earlier established an in vitro model of FcγR-mediated trogocytosis [23]. The protocol was simple and easy as follows: (1) heparinized peripheral blood samples were incubated with anti-CD8 and anti-CD15 Abs: (2) after removal of erythrocytes by treatment with ammonium chloride, cells were subjected to FCM. Results demonstrated the presence of CD8+ cells in CD15+ neutrophils. Since neutrophils do not express CD8 innately, the CD8 molecules detected on neutrophils seem to be derived from other cells. Our studies revealed that CD8 molecules on T cells were transferred to neutrophils via the anti-CD8 Ab and FcγRs (FcγRII and FcγRIII) on neutrophils. The usage of FcγRIII appears to be a specific characteristic of neutrophils because FcγRII, especially FcγRIIB, among the FcγRs mediates trogocytosis in monocytes and macrophages [19, 31]. Moreover, bystander molecules, such as TCR and CD3, were also transferred from T cells to neutrophils accompanied by CD8 molecules. Thus, this phenomenon was considered as FcγR-mediated trogocytosis (Figure 1). The polymerization of actin was involved in the process of neutrophil FcγR-mediated trogocytosis similar to T cells [32]. By using this model, we demonstrated that human anti-mouse IgG Abs in serum accelerated FcγR-mediated trogocytosis. This is consistent with the finding that FcγR-mediated trogocytosis is prone to occur in arthritic patients positive for rheumatoid factor, which is an anti-IgG Ab [33].
Figure 1.
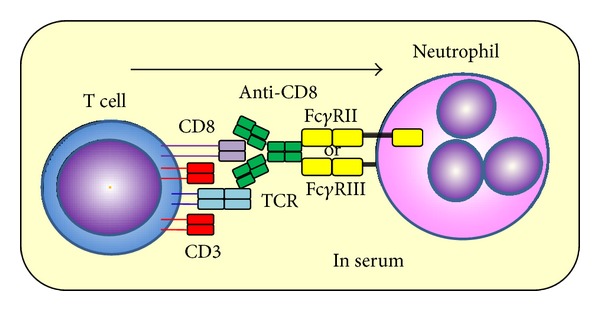
Schemas of CD8 translocation model from T cells to neutrophils CD8 molecules on T cells were transferred to neutrophils when these cells were incubated with anti-CD8 Ab in the serum. TCR and CD3 molecules on T cells were also transferred to neutrophils. FcγRs (FcγRII and FcγRIII) were involved in the mechanism.
3. Immediate Intake of Molecules Deprived via FcγR-Mediated Trogocytosis
By using the in vitro model of FcγR-mediated trogocytosis, namely, CD8 translocation model from T cells to neutrophils, the dynamism of the molecules deprived by FcγR+ cells via FcγR-mediated trogocytosis was determined. For this purpose, two anti-CD8 monoclonal Abs that could recognize diverse epitopes were applied. First, CD8 molecules on T cells were made to transfer to neutrophils via FcγR-mediated trogocytosis using the PE-labeled anti-CD8 Ab. After incubation for 0–8 hours, CD8 molecules that remained on the cell surface of neutrophils were detected by the other PECy5-labeled anti-CD8 Ab. Although the number of neutrophils labeled by PE (neutrophils that performed FcγR-mediated trogocytosis) was substantially stable during the incubation period, the number of PECy5-labeled CD8 molecules on the cell surface of neutrophils diminished and reached low level by 2 hours (Figure 2). These findings indicated that the molecules transferred to neutrophils via FcγR-mediated trogocytosis were taken into the cytoplasm immediately.
Figure 2.
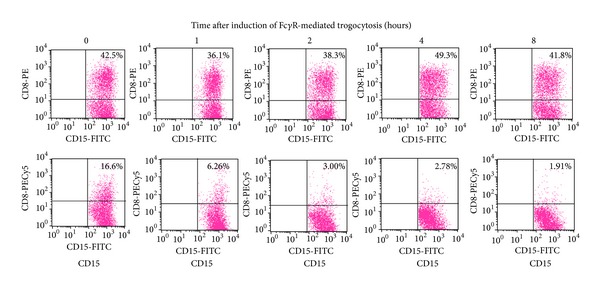
Dynamism of molecules transferred to neutrophils via FcγR-mediated trogocytosis. Heparinized peripheral blood samples (100 μL) were made to react with 0.1 μg/mL of PE-labeled anti-CD8 Ab for 30 min at room temperature. PE-labeled mouse IgG1 was used as an isotype-matched control. After depletion of erythrocytes, the cells were incubated in RPMI-1640 medium with 10% FBS for 0–8 hours at 37°C. Subsequently, the cells were resuspended in 100 μL of PBS and then made to react with 0.1 μg of PECy5-labeled anti-CD8 Ab for 20 min at room temperature. After removal of unbounded Ab, the cells were resuspended in 100 μL of PBS followed by reaction with 0.1 μg of FITC-labeled anti-CD15 Ab for 20 min at room temperature and then served for FCM. The PECy5-labeled anti-CD8 Ab used in this experiment could recognize a different epitope from that recognized by the PE-labeled anti-CD8 Ab. Experiments were repeated at least 3 times. Similar results were reproduced.
4. Association between FcγR-Mediated Trogocytosis and Endocytosis
Next, the association between FcγR-mediated trogocytosis and endocytosis was examined using the CD8 translocation model from T cells to neutrophils. Endocytosis is a fundamental function of neutrophils. Two diverse mechanisms of endocytosis include phagocytosis and pinocytosis, wherein the difference between the two is the size of target molecules. Pinocytosis is used for the absorption of extracellular fluids, and in contrast to phagocytosis, the target molecules are very small. To determine the association between FcγR-mediated trogocytosis and endocytosis, experiments were performed using yellow-green carboxylate-modified latex beads and FITC-labeled ovalbumin (OVA). The process of intake of latex bead and OVA represents phagocytosis and pinocytosis, respectively. After enhancing FcγR-mediated trogocytosis by the CD8 translocation model from T cells to neutrophils, fluorescence-labeled latex beads and OVA were added to the cells. Subsequently, the engulfment of these molecules by CD8+ neutrophils that underwent FcγR-mediated trogocytosis was examined, and the data were compared with those by CD8− neutrophils that did not perform FcγR-mediated trogocytosis. The activities of both phagocytosis and pinocytosis were higher in CD8+ neutrophils than those in CD8− neutrophils (Figure 3). These findings suggest that the activity of FcγR-mediated trogocytosis is parallel to the phagocytic and pinocytic activities of neutrophils.
Figure 3.
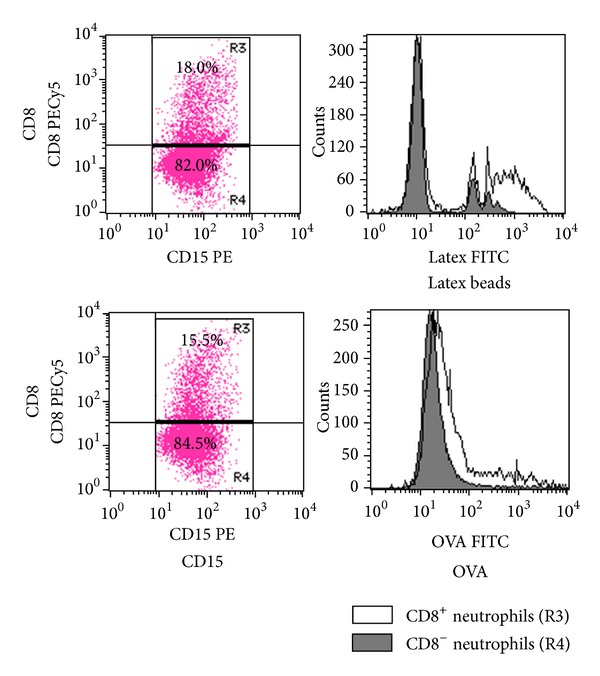
Comparison of endocytotic activities between neutrophils with and without display of FcγR-medicated trogocytosis. Peripheral blood polymorphonuclear cells (0.5 × 106) and mononuclear cells (0.5 × 106) were mixed and preincubated in 100 μL of the autologous serum for 20 min at 37°C. The cells were made to react with 0.1 μg of PECy5-labeled anti-CD8 Ab for 1 hour at room temperature. After washing with PBS, the cells were resuspended in 1 mL of RPMI-1640 medium with 10% FBS. Subsequently, the cells were made to react with 2 μL of yellow-green carboxylate-modified latex beads for 90 min at 37°C or with 100 μg of FITC-labeled OVA for 30 min at 37°C. After washing 3 times with PBS, the cells were re-suspended in 100 μL of PBS and then made to react with 0.1 μg of PE-labeled anti-CD15 Ab, followed by serving for FCM. Experiments were repeated at least 3 times. Similar results were reproduced.
5. Physiological Role of FcγR-Mediated Trogocytosis
It was revealed that the CD8 molecules transferred to neutrophils via FcγR-mediated trogocytosis were taken into the cytoplasm immediately. This evidence suggests that the chance of molecules transferred via FcγR-mediated trogocytosis to play a role on the cell surface could be time-limited. Thus, we consider the physiological role of FcγR-mediated trogocytosis as a means to remove Abs that bind with self-molecules rather than to extract molecules from other cells. Essentially, a living body is always exposed to invading pathogens. The immune system produces Abs to compete with the pathogens; however, these Abs sometimes cross-react with self-Ags. In such situation, the presence of mechanism that can prevent Ab-mediated autoimmune response is beneficial. FcγR-mediated trogocytosis can be a defense mechanism to remove Abs, which unexpectedly bind to self-Ags (Figure 4).
Figure 4.
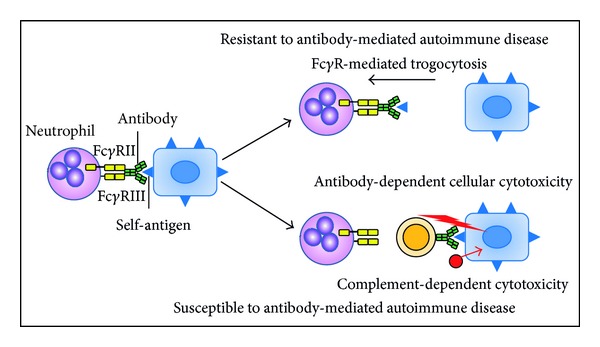
Relation between FcγR-mediated trogocytosis and susceptibility to Ab-mediated autoimmune disease. FcγR-mediated trogocytosis can play a role in the removal of Abs that bind to self-Ags on the cell surface and prevent Ab-mediated autoimmune diseases.
In this study, the relationship between FcγR-mediated trogocytosis and endocytosis of neutrophils was examined using the established CD8 translocation model from T cells to neutrophils. Results clearly indicated that the activity of FcγR-mediated trogocytosis was closely linked to the endocytosis activity of neutrophils. The low activity of endocytosis of neutrophils is critically related to the high susceptibility to systemic autoimmune diseases including systemic lupus erythematosus [34]. Therefore, it is reasonable to consider that the low ability of FcγR-mediated trogocytosis could be also linked to the high susceptibility to systemic autoimmune diseases. This is compatible with our concept that FcγR-mediated trogocytosis can play a role in the removal of autoantibodies and prevent autoimmune diseases. However, in autoimmune hemolytic anemia, trogocytosis mediated by anti-red blood cell (RBC) polymeric IgA Abs and Fcα receptors is involved in the destruction of RBCs [35]. Thus, further prospective studies are needed to clarify if the ability of FcγR-mediated trogocytosis would be actually related to the susceptibility to systemic autoimmune diseases.
Conflict of Interests
The authors have no financial conflict of interests.
Acknowledgments
This work was supported by the Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan and by the grant from Hokkaido University, Faculty of Health Sciences.
References
- 1.Joly E, Hudrisier D. What is trogocytosis and what is its purpose? Nature Immunology. 2003;4(9):p. 815. doi: 10.1038/ni0903-815. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed KA, Xiang J. Mechanisms of cellular communication through intercellular protein transfer. Journal of Cellular and Molecular Medicine. 2011;15(7):1458–1473. doi: 10.1111/j.1582-4934.2010.01008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold PY, Davidian DK, Mannie MD. Antigen presentation by T cells: T cell receptor ligation promotes antigen acquisition from professional antigen-presenting cells. European Journal of Immunology. 1997;27(12):3198–3205. doi: 10.1002/eji.1830271217. [DOI] [PubMed] [Google Scholar]
- 4.Puaux AL, Campanaud J, Salles A, et al. A very rapid and simple assay based on trogocytosis to detect and measure specific T and B cell reactivity by flow cytometry. European Journal of Immunology. 2006;36(3):779–788. doi: 10.1002/eji.200535407. [DOI] [PubMed] [Google Scholar]
- 5.Espinosa E, Tabiasco J, Hudrisier D, Fournié J-J. Synaptic transfer by human γδ T cells stimulated with soluble or cellular antigens. The Journal of Immunology. 2002;168(12):6336–6343. doi: 10.4049/jimmunol.168.12.6336. [DOI] [PubMed] [Google Scholar]
- 6.Batista FD, Iber D, Neuberger MS. B cells acquire antigen from target cells after synapse formation. Nature. 2001;411(6836):489–494. doi: 10.1038/35078099. [DOI] [PubMed] [Google Scholar]
- 7.Carlin LM, Eleme K, McCann FE, Davis DM. Intercellular transfer and supramolecular organization of human leukocyte antigen C at inhibitory natural killer cell immune synapses. Journal of Experimental Medicine. 2001;194(10):1507–1517. doi: 10.1084/jem.194.10.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herrera OB, Golshayan D, Tibbott R, et al. A novel pathway of alloantigen presentation by dendritic cells. The Journal of Immunology. 2004;173(8):4828–4837. doi: 10.4049/jimmunol.173.8.4828. [DOI] [PubMed] [Google Scholar]
- 9.Xu H, Dhanireddy KK, Kirk AD. Human monocytes as intermediaries between allogeneic endothelial cells and allospecific T cells: a role for direct scavenger receptor-mediated endothelial membrane uptake in the initiation of alloimmunity. The Journal of Immunology. 2006;176(2):750–761. doi: 10.4049/jimmunol.176.2.750. [DOI] [PubMed] [Google Scholar]
- 10.Harvey BP, Quan TE, Rudenga BJ, Roman RM, Craft J, Mamula MJ. Editing antigen presentation: antigen transfer between human B lymphocytes and macrophages mediated by class A scavenger receptors. The Journal of Immunology. 2008;181(6):4043–4051. doi: 10.4049/jimmunol.181.6.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hudrisier D, Aucher A, Puaux A-L, Bordier C, Joly E. Capture of target cell membrane components via trogocytosis is triggered by a selected set of surface molecules on T or B cells. The Journal of Immunology. 2007;178(6):3637–3647. doi: 10.4049/jimmunol.178.6.3637. [DOI] [PubMed] [Google Scholar]
- 12.Daubeuf S, Lindorfer MA, Taylor RP, Joly E, Hudrisier D. The direction of plasma membrane exchange between lymphocytes and accessory cells by trogocytosis is influenced by the nature of the accessory cell. The Journal of Immunology. 2010;184(4):1897–1908. doi: 10.4049/jimmunol.0901570. [DOI] [PubMed] [Google Scholar]
- 13.Daubeuf S, Aucher A, Bordier C, et al. Preferential transfer of certain plasma membrane proteins onto T and B cells by trogocytosis. PLoS ONE. 2010;5(1) doi: 10.1371/journal.pone.0008716.e8716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beum PV, Kennedy AD, Williams ME, Lindorfer MA, Taylor RP. The shaving reaction: rituximab/CD20 complexes are removed from mantle cell lymphoma and chronic lymphocytic leukemia cells by THP-1 monocytes. The Journal of Immunology. 2006;176(4):2600–2609. doi: 10.4049/jimmunol.176.4.2600. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Williams ME, Cousar JB, Pawluczkowycz AW, Lindorfer MA, Taylor RP. Rituximab-CD20 complexes are shaved from Z138 mantle cell lymphoma cells in intravenous and subcutaneous SCID mouse models. The Journal of Immunology. 2007;179(6):4263–4271. doi: 10.4049/jimmunol.179.6.4263. [DOI] [PubMed] [Google Scholar]
- 16.Beum PV, Mack DA, Pawluczkowycz AW, Lindorfer MA, Taylor RP. Binding of rituximab, trastuzumab, cetuximab, or mAb T101 to cancer cells promotes trogocytosis mediated by THP-1 cells and monocytes. The Journal of Immunology. 2008;181(11):8120–8132. doi: 10.4049/jimmunol.181.11.8120. [DOI] [PubMed] [Google Scholar]
- 17.Rossi EA, Goldenberg DM, Michel R, Rossi DL, Wallace DJ, Chang CH. Trogocytosis of multiple B-cell surface markers by CD22-targeting with epratuzumab. Blood. 2013 doi: 10.1182/blood-2012-12-473744. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, McClellan M, Efros L, et al. Daclizumab reduces CD25 levels on T cells through monocyte-mediated trogocytosis. Multiple Sclerosis Journal. 2013 doi: 10.1177/1352458513494488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwasaki S, Masuda S, Baba T, et al. Plasma-dependent, antibody- and Fcγ receptor-mediated translocation of CD8 molecules from T cells to monocytes. Cytometry A. 2011;79(1):46–56. doi: 10.1002/cyto.a.20984. [DOI] [PubMed] [Google Scholar]
- 20.Pham T, Mero P, Booth JW. Dynamics of macrophage trogocytosis of rituximab-coated B cells. PLoS ONE. 2011;6(1) doi: 10.1371/journal.pone.0014498.e14498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beum PV, Peek EM, Lindorfer MA, et al. Loss of CD20 and bound CD20 antibody from opsonized B cells occurs more rapidly because of trogocytosis mediated by Fc receptor-expressing effector cells than direct internalization by the B cells. The Journal of Immunology. 2011;187(6):3438–3447. doi: 10.4049/jimmunol.1101189. [DOI] [PubMed] [Google Scholar]
- 22.Horner H, Frank C, Dechant C, et al. Intimate cell conjugate formation and exchange of membrane lipids precede apoptosis induction in target cells during antibody-dependent, granulocyte-mediated cytotoxicity. The Journal of Immunology. 2007;179(1):337–345. doi: 10.4049/jimmunol.179.1.337. [DOI] [PubMed] [Google Scholar]
- 23.Masuda S, Iwasaki S, Tomaru U, et al. Mechanism of Fcγ receptor-mediated trogocytosis-based false-positive results in flow cytometry. PLoS ONE. 2012;7(12) doi: 10.1371/journal.pone.0052918.e52918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis DM. Intercellular transfer of cell-surface proteins is common and can affect many stages of an immune response. Nature Reviews Immunology. 2007;7(3):238–243. doi: 10.1038/nri2020. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed KA, Munegowda MA, Xie Y, Xiang J. Intercellular trogocytosis plays an important role in modulation of immune responses. Cellular and Molecular Immunology. 2008;5(4):261–269. doi: 10.1038/cmi.2008.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LeMaoult J, Caumartin J, Daouya M, et al. Immune regulation by pretenders: cell-to-cell transfers of HLA-G make effector T cells act as regulatory cells. Blood. 2007;109(5):2040–2048. doi: 10.1182/blood-2006-05-024547. [DOI] [PubMed] [Google Scholar]
- 27.Brown R, Suen H, Favaloro J, et al. Trogocytosis generates acquired regulatory T cells adding further complexity to the dysfunctional immune response in multiple myeloma. Oncoimmunology. 2012;1(9):1658–1660. doi: 10.4161/onci.22032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ford McIntyre MS, Young KJ, Gao J, Joe B, Zhang L. Cutting edge: in vivo trogocytosis as a mechanism of double negative regulatory T cell-mediated antigen-specific suppression. The Journal of Immunology. 2008;181(4):2271–2275. doi: 10.4049/jimmunol.181.4.2271. [DOI] [PubMed] [Google Scholar]
- 29.Bahcheli D, Hay V, Nadeau JL, Piccirillo CA. Transfer of cell membrane components via trogocytosis occurs in CD4+Foxp3+CD25+ regulatory T-cell contact-dependent suppression. Autoimmunity. 2011;44(8):607–615. doi: 10.3109/08916934.2011.571730. [DOI] [PubMed] [Google Scholar]
- 30.Daubeuf S, Puaux AL, Joly E, Hudrisier D. A simple trogocytosis-based method to detect, quantify, characterize and purify antigen-specific live lymphocytes by flow cytometry, via their capture of membrane fragments from antigen-presenting cells. Nature Protocols. 2007;1(6):2536–2542. doi: 10.1038/nprot.2006.400. [DOI] [PubMed] [Google Scholar]
- 31.Boross P, Jansen JHM, Pastula A, van der Poel CE, Leusen JHW. Both activating and inhibitory Fcγ receptors mediate rituximab-induced trogocytosis of CD20 in mice. Immunology Letters. 2012;143(1):44–52. doi: 10.1016/j.imlet.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Aucher A, Magdeleine E, Joly E, Hudrisier D. Capture of plasma membrane fragments from target cells by trogocytosis requires signaling in T cells but not in B cells. Blood. 2008;111(12):5621–5628. doi: 10.1182/blood-2008-01-134155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones JD, Shyu I, Newkirk MM, Rigby WF. A rheumatoid factor paradox: inhibition of rituximab effector function. Arthritis Research and Therapy. 2013;15(1, article R20) doi: 10.1186/ar4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de la Fuente H, Richaud-Patin Y, Jakez-Ocampo J, González-Amaro R, Llorente L. Innate immune mechanisms in the pathogenesis of systemic lupus erythematosus (SLE) Immunology Letters. 2001;77(3):175–180. doi: 10.1016/s0165-2478(01)00220-6. [DOI] [PubMed] [Google Scholar]
- 35.Chadebech P, Michel M, Janvier D, et al. IgA-mediated human autoimmune hemolytic anemia as a result of hemagglutination in the spleen, but independent of complement activation and FcαRI. Blood. 2010;116(20):4141–4147. doi: 10.1182/blood-2010-03-276162. [DOI] [PubMed] [Google Scholar]


