Abstract
Does breastfeeding alter early brain development? The prevailing consensus from large epidemiological studies posits that early exclusive breastfeeding is associated with improved measures of IQ and cognitive functioning in later childhood and adolescence. Prior morphometric brain imaging studies support these findings, revealing increased white matter and sub-cortical gray matter volume, and parietal lobe cortical thickness, associated with IQ, in adolescents who were breastfed as infants compared to those who were exclusively formula-fed. Yet it remains unknown when these structural differences first manifest and when developmental differences that predict later performance improvements can be detected. In this study, we used quiet magnetic resonance imaging (MRI) scans to compare measures of white matter microstructure (mcDESPOT measures of myelin water fraction) in 133 healthy children from 10 months through 4 years of age, who were either exclusively breastfed a minimum of 3 months; exclusively formula-fed; or received a mixture of breast milk and formula. We also examined the relationship between breastfeeding duration and white matter microstructure. Breastfed children exhibited increased white matter development in later maturing frontal and association brain regions. Positive relationships between white matter microstructure and breastfeeding duration are also exhibited in several brain regions, that are anatomically consistent with observed improvements in cognitive and behavioral performance measures. While the mechanisms underlying these structural differences remains unclear, our findings provide new insight into the earliest developmental advantages associated with breastfeeding, and support the hypothesis that breast milk constituents promote healthy neural growth and white matter development.
Abbreviations: MCR, Multicomponent Relaxometry; MRI, Magnetic Resonance Imaging; MWF, Myelin Water Fraction; VFM, mcDESPOT Derived Myelin Water Fraction; T1, Longitudinal Relaxation Time; T2, Transverse Relation Time
Keywords: Brain development, Breastfeeding, Myelin maturation, White matter development, Infant imaging, Myelin, Myelin water fraction, Magnetic resonance imaging
Highlights
-
•
First investigation of breast-feeding and early infant brain myelination.
-
•
Breastfed infants shown improved brain development by 2 years of age.
-
•
Duration of breastfeeding is positively associated with behavioral performance.
Introduction
The decision to breastfeed is an early parental decision that may have important consequences for a child's later cognitive and behavioral functioning. The prevailing consensus from large-scale epidemiological studies is that children who were breastfed perform, on average, higher on tests of IQ and cognitive functioning than do children who were exclusively formula-fed, even when factors such as birth-weight, gestation duration, and maternal education and socioeconomic status (SES) are accounted for (Anderson et al., 1999; McCrory and Murray, 2012). These neuropsychological findings are complemented by morphometric brain imaging studies in adolescents, showing volumetric increases in total white matter, sub-cortical gray matter and parietal lobe cortical thickness in those who were breastfed as infants, as well as a relationship linking duration of breastfeeding and IQ (Hallowell and Spatz, 2012; Isaacs et al., 2010; Kafouri et al., 2012). Non-imaging assessments of early neural pathway maturation using evoked potentials have further supported the developmental benefits of breastfeeding, with formula-fed infants found to have greater wave latencies in their visual and auditory pathways at 1 year of age (Khedr et al., 2004), suggestive of delayed or immature myelination of these pathways, compared to breastfed infants.
The primary hypothesized substrate for these developmental advantages is the rich compliment of long-chain fatty acids found in breast milk, specifically docosahexaenoic (DHA) and arachidonic (AA) acids (McCann and Ames, 2005). Together, DHA and AA comprise approximately 20% of the fatty acid content of the brain and are involved in early neurodevelopment by promoting healthy neuronal growth, repair, and myelination (Guesnet and Alessandri, 2011). Recent studies have also highlighted differing gut microbial profiles in breastfed versus formula-fed infants (Azad et al., 2013), which may also promote brain and myelin development (Heijtz et al., 2011), resulting in improved brain function. Cumulatively, these studies intimate a preferential association between breastfeeding and structural neurodevelopment that ultimately gives rise to improved reasoning, cognition, and behavior.
Of particular interest to functional development is the role of the myelinated white matter, which forms the backbone of the brain's eloquent neural systems. As white matter facilitates the rapid and synchronized brain messaging required for higher-order cognitive functions, the spatio-temporal establishment of the myelin sheath (Yakovlev and Lecours, 1967) closely mirrors the development of co-ordinated movement, social and emotional processing, and other behaviors (Wozniak and Lim, 2006). Aberrations in myelination, or deficiencies in myelin content or integrity, can have profound deleterious effects on brain function (Ahima et al., 1999; Fields, 2008), as evidenced by various leukodystrophies, and demyelinating disorders such as multiple sclerosis. Converging neuropsychology, brain imaging and electrical activity evidence suggests that breastfed infants may display preferential myelination and white matter development. The involvement of major constituents of breast milk in myelin promotion and development (Guesnet and Alessandri, 2011), evidence of increased white matter volume in adolescents breastfed as infants (Isaacs et al., 2010; Kafouri et al., 2012), and delayed brain messaging in formula-fed infants (Khedr et al., 2004; Makrides et al., 1993), suggest a structural foundation for observed improved functional performance.
Prior brain imaging studies, however, have exclusively examined adolescent-aged children using measures of gross morphometry. As a result, early developmental differences that may be more closely related to neural growth or myelination remain to be identified. Studies specifically examining white matter microstructure in early childhood, coincident with evolving cognition and behavior, are needed to clarify the developmental influence of breast milk in healthy and typically developing children; how this influence may change through early neurodevelopment; and how they may be associated with early behavioral differences.
In this work, we sought to investigate the influence of breastfeeding and breast milk on early white matter and myelin development using a multicomponent relaxation (MCR) magnetic resonance imaging (MRI) approach. MCR provides novel information on tissue microstructure that may be preferentially sensitive to myelin content or change (MacKay et al., 2006). Specifically, MCR decomposes the MRI signal into contribution from distinct micro-environments. In CNS tissue, MCR analysis has consistently revealed the presence of at least two relaxation species: a fast relaxing water pool commonly attributed to water trapped between the lipid bilayers of the myelin sheath; and a slower relaxing pool associated with the intra and extra-axonal water. MCR analysis estimates not only the relaxation properties of these water pools, but also their relative volume fraction. The myelin-associated water fraction, termed the MWF, derived from established techniques, has been shown to correlate strongly with histological assessments of myelin content (Gareau et al., 2000; Laule et al., 2006, 2008; Webb et al., 2003). In this work, a recently developed MRC technique, termed mcDESPOT (Deoni et al., 2008), was used to acquire data of 133 healthy, typically developing toddlers and young children, 10 months to 4 years of age. These children were divided into one of three groups: exclusively breastfed; exclusively formula-fed; and those who received a mixture of breast milk and formula. mcDESPOT also provides a measure of the myelin-associated water pool, termed VFM, which although different from conventional MCR measures, have been qualitatively compared with histological myelin content measures in a Shaking Pup model of dysmyelination (Hurley et al., 2010), and have been used to investigate structure–function impairment in MS (Kitzler et al., 2012; Kolind et al., 2012), and healthy infant neurodevelopment (Deoni et al., 2011, 2012b).
In this work, we examined differences in the cross-sectional VFM development profiles in exclusively breastfed, exclusively formula-fed, and combined formula plus breast milk-fed children 10 months through 4 years of age, as well as mean group VFM values and behavioral functioning in children 2.2 to 4 years of age. We also investigated potential associations between breastfeeding duration and VFM measures of white matter microstructure and behavioral functioning.
Methods
Infant participants
Brain white matter maturation was investigated in 133 healthy male and female toddlers, 305 to 1541 days of age (corrected to a 40 week gestation), or approximately 10 months to 4 years of age. Complete subject demographics are provided in Table 1.
Table 1.
Child demographic information.
| Group #1: exclusively breastfed | Group #2: exclusively formula-fed | Group #3: breast + formula-fed | p-Values (#1 vs. #2); (#1 vs. #3); (#2 vs. #3) |
|
|---|---|---|---|---|
| Participants (n) | 85 | 38 | 51 | |
| Age (days) | 775 ± 350 | 807.5 ± 369 | 772.7 ± 347 | (0.65); (0.97); (0.63) |
| Male:female | 56:29 | 24:14 | 30:21 | (0.63); (0.2); (0.98) |
| Gestation (days) | 272 ± 34 | 272 ± 12 | 271 ± 40 | (0.97); (0.89); (0.87) |
| Birth weight (lbs) | 7.59 ± 1.02 | 7.07 ± 1.56 | 7.32 ± 0.95 | (0.16); (0.25); (0.49) |
| Maternal age (years) | 31 ± 3.2 | 29.9 ± 6.5 | 30.1 ± 4.8 | (0.5); (0.48); (0.87) |
| Maternal SES | 5.8 ± 1.04 | 5.38 ± 1.02 | 5.75 ± 1.0 | (0.12); (0.77); (0.23) |
| Mullen gross motor raw scorea | 22.3 ± 5.02 | 21.4 ± 5.03 | 22.4 ± 5.6 | (0.43); (0.98); (0.49) |
| Mullen fine motor raw score | 24.7 ± 8.5 | 24.6 ± 8.3 | 24.7 ± 8.0 | (0.94); (0.99); (0.95) |
| Mullen receptive language raw score | 24.9 ± 11 | 24.4 ± 9.8 | 24.0 ± 10.1 | (0.82); (0.63); (0.83) |
| Mullen expressive language raw score | 23.1 ± 11.2 | 23.2 ± 11.7 | 23.3 ± 10.6 | (0.97); (0.94); (0.98) |
| Mullen visual reception raw score | 27.8 ± 12 | 28.0 ± 1 | 27.2 ± 10.2 | (0.92); (0.73); (0.71) |
Scored only for children under 2 years, 8 months.
From a detailed medical history and parental interview, participants were divided into one of three groups: exclusively breastfed for a minimum of 90 days (Group 1); exclusively formula-fed (Group 2); or received a mixture of breast milk and formula (Group 3). Final group sizes and mean ages were: Group #1: N = 85, mean age = 775 ± 350 days; Group #2: N = 38, mean age = 807 ± 369 days; and Group #3: N = 51, mean age = 773 ± 347 days; with no significant group differences in mean age, male:female ratio; birth weight; gestation duration; or maternal age, education or SES (Table 1). Maternal SES was tabulated using the Hollingshead Social Status (Hollingshead, 1975). While maternal IQ was not specifically measured, the combination of education and SES was believed to provide an adequate alternative. Duration of breastfeeding was also assessed, and ranged from 90 to 900 days (mean = 413 ± 186 days) in the exclusively breastfed group, and from 14 to 610 days (mean = 149 ± 136 days) in the breast milk plus formula-fed group.
To help mitigate potential confounds beyond infant feeding choice, selection criteria included the following: 1. Healthy singleton birth between 37 and 42 weeks gestation; 2. APGAR score of at least 8; 3. No abnormalities on fetal ultrasound; 4. No reported history of neurological events or disorders in the infant (i.e., head trauma, ischemia, epilepsy, etc.); 5. No admissions to the neonatal intensive care unit; 6. No family history of a psychiatric or neurological disorder in the infants' parents or siblings; 7. No complications (i.e. preeclampsia) during pregnancy; and 8. No reports of illicit drug or alcohol use during pregnancy.
Alongside the parental interview, all children were assessed using the Mullen Scales of Early Learning (Mullen, 1992) within 7 days of scanning. This provided a broad assessment of behavioral development in the domains of fine and gross motor control, receptive and expressive language, and visual reception. No significant group raw score differences in any of these domains were found between the groups (Table 1).
Imaging methods & analysis
Each infant was scanned using the mcDESPOT (multicomponent Driven Equilibrium Single Pulse Observation of T1 and T2) white matter imaging technique (Deoni et al., 2012a), which provides a quantitative measure of the myelin water fraction (VFM) at each imaging point throughout the brain. All infants were scanned during natural (i.e. non-sedated) sleep using acoustically-muffled mcDESPOT imaging protocols described previously (Deoni et al., 2012b) and summarized in Table 2. Each protocol comprises 8 T1-weighted spoiled gradient recalled echo (SPGR) images; 2 inversion (IR-) prepared SPGR images; and 15 T1/T2 weighted steady-state free precession (SSFP, FIESTA or TrueFISP) images. Total imaging times ranged from 19 min for the youngest toddlers to 24 min for the older 4 year-old children.
Table 2.
mcDESPOT acquisition parameters by age.
| Parameter | 9–16 months | 16–28 months | 28–48 months | > 48 months |
|---|---|---|---|---|
| Orientation | Sagittal | Sagittal | Sagittal | Sagittal |
| Field of view | (17 × 17) cm2 | (18 × 18) cm2 | (20 × 20) cm2 | (20 × 20) cm2 |
| Matrix | 96 × 96 | 100 × 100 | 112 × 112 | 112 × 112 |
| Slice thickness | 1.8 mm | 1.8 mm | 1.8 mm | 1.8 mm |
| Number of slices | 80 | 88 | 88 | 92 |
| SPGR TE/TR | 5.9/12 ms | 5.4/12 ms | 5.2/11 ms | 4.8/10 ms |
| SPGR flip angles | (2,3,4,5,7,9,11,14)° | (2,3,4,5,7,9,11,14)° | (2,3,4,5,7,9,12,16)° | (3,4,5,6,7,13,18)° |
| SPGR bandwidth | 350 Hz/voxel | 350 Hz/voxel | 350 Hz/voxel | 350 Hz/voxel |
| SPGR partial k-space | 5/8 | 5/8 | 5/8 | 5/8 |
| IR-SPGR TE/TR/TI | 5.9/12/ 600, 900 ms | 5.4/12/ 500, 850 ms | 5.2/11/ 500, 800 ms | 4.8/10/ 450 750 ms |
| IR-SPGR flip angle | 5° | 5° | 5° | 5° |
| SSFP TE/TR | 5.1/10.2 ms | 5/10 ms | 4.4/9.8 | 5/10 ms |
| SSFP flip angles | (9,14,20,27,34,41,56,70)° | (9,14,20,27,34,41,56,70)° | (9,14,20,27,34,41,56,70)° | (9,14,20,27,34,41,56,70)° |
| SSFP bandwidth | 350 Hz/voxel | 350 Hz/voxel | 350 Hz/voxel | 350 Hz/voxel |
| SSFP partial k-space | 6/8 | 6/8 | 6/8 | 6/8 |
| SSFP phase-cycling | 0° and 180° | 0° and 180° | 0° and 180° | 0° and 180° |
All data were acquired on a Siemens 3 T Tim Trio scanner equipped with a 12 channel head RF array. To minimize intra-scan motion, children were swaddled with a pediatric MedVac vacuum immobilization bag (CFI Medical Solutions, USA) and foam cushions. Scanner noise was reduced by lessening the peak gradient amplitudes and slew-rates, and using a noise-insulating scanner bore insert (Quiet Barrier HD Composite, UltraBarrier, USA). MiniMuff pediatric ear covers and electrodynamic headphones (MR Confon, Germany) were also used. Children were continuously monitored with a pediatric pulse-oximetry system and infrared camera. All children remained asleep for the duration of the MRI scan and no motion-artifacts were present in the analyzed data.
Following image alignment, non-brain signal removal, and correction for main and transmit magnetic field (B0 and B1) inhomogeneities (Deoni, 2011), a three-pool signal model (comprising the myelin-associated water; intra-extra axonal water; and a non-exchanging free-water pool) was fit to the mcDESPOT data to derive voxel-wise VFM maps (Deoni et al., 2012a). The three-pool signal model was fit using a stochastic region contraction approach (Deoni et al., 2012b), with bounds for the search region defined as: 250 ms ≤ T1,M ≤ 650 ms; 750 ms ≤ T1,IE ≤ 2500 ms; 2500 ms ≤ T1,F ≤ 7500 ms; 0 ms ≤ T2,M ≤ 50 ms; 75 ms ≤ T2,IE ≤ 250 ms; 350 ms ≤ T2,F ≤ 1000 ms; 0.00 ≤ VFM ≤ 0.49; 0.00 ≤ VFF ≤ 0.9; and 50 ms ≤ τM ≤ 1500 ms. Two additional constraints, IEF = 1.00 − (VFM + VFF); and VFM + VFF ≤ 0.95, were also imposed. Subscripts M, IE and F correspond to the myelin, intra/extra-cellular, and free water pools, respectively.
Each child's map was then non-linearly aligned to a study specific template (Deoni et al., 2012b) using the Advanced Normalization Tools software package (Avants et al., 2008). A white matter mask was derived from the mean template image using the FAST package, part of the FMRIB FSL tool library (Zhang et al., 2001). All subsequent analyses were restricted to voxels within this masked region (Fig. 1).
Fig. 1.
White matter mask. All analyses were restricted to this masked region to avoid gray matter.
Developmental differences
To examine developmental differences logarithmic trajectories (Deoni et al., 2012b), defined as
| (1) |
where α is the development rate and β is the initial VFM value, were fit voxel-wise to each group's cross-sectional VFM data.
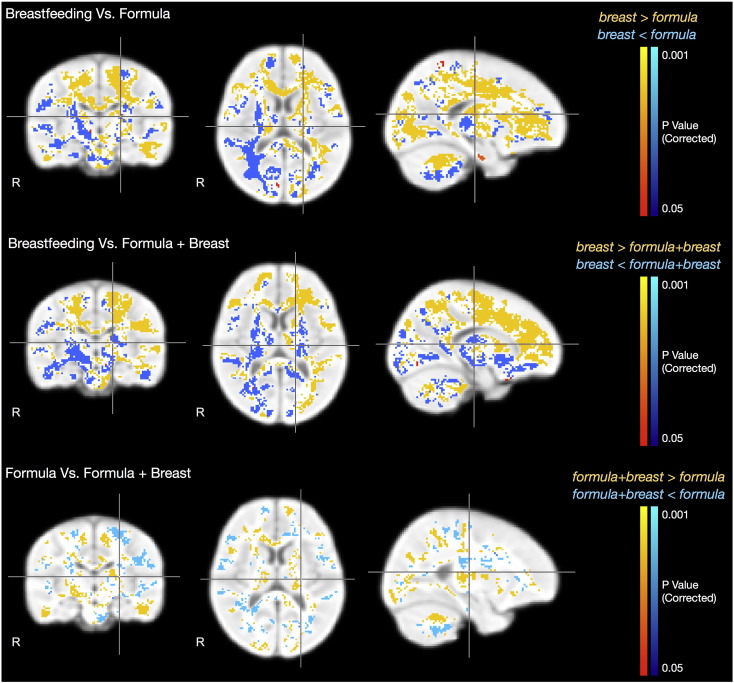
All groups were matched for age; male:female ratio; gestation duration; birth weight; maternal age, education and SES. A bootstrap resampling approach (Efron, 1979) with 5000 resamples was used to estimate the distribution of α and β for each group, at each voxel, and an unpaired t-test was used (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Randomise) to compare α between each combination of the three groups. Significance was defined as p < 0.05 corrected for multiple comparisons using a cluster-based technique, a commonly used multiple testing method for determining corrected significances while accounting for the high level of spatial dependencies between adjacent voxels (Worsley et al., 1999). Contiguous clusters were first identified using a threshold of p < 0.005 (t-stat > 3.1).
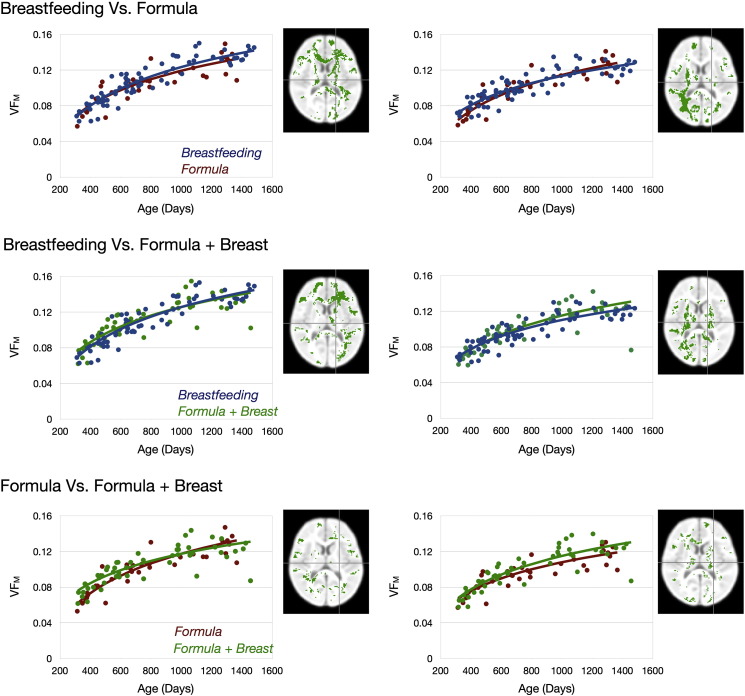
To visually compare the developmental trajectories, areas with significant differences were identified and divided into areas where breastfed > formula-fed, breastfed < formula-fed; breastfed > breast milk and formula-fed, breastfed < breast milk and formula-fed; and formula-fed > breast milk and formula-fed and formula-fed < breast milk and formula-fed. Mean VFM developmental trajectories were calculated for these regions.
Based on the results of this developmental trajectory analysis (please see Results, Fig. 3), we investigated group differences in an subset of older children, approx. 2.2 to 4 years (800 to 1541 days) of age, matched for age, male:female ratio; birth weight; gestation duration; or maternal age, education or SES. This older age range was used given the similarity in each group's developmental profiles below 800 days. Mean VFM values were compared between the: 1. Breastfed and formula-fed children; and 2. Breastfed and breast milk plus formula children. Prior to comparisons, all data were smoothed with a conservative 2.5 mm full-width-at-half-maximum (FWHM) Gaussian kernel and unpaired t-tests performed at each voxel to identify significant differences. Significance was defined as p < 0.05 corrected for multiple comparisons using a cluster-based technique as above. In addition to VFM comparisons, we also compared motor and behavioral functioning in this older subset of children. Raw Mullen scores for fine motor, expressive and receptive language, and visual reception were compared between the breastfed and formula-fed children; and breastfed and breast milk plus formula children. Gross motor scores were not examined since all participants obtained the maximum score in this domain. Significance group difference was defined as p < 0.05 corrected for type 1 errors using the Holm–Bonferroni method (Holm, 1979).
Fig. 3.
Mean VFM development trajectories calculated from identified areas in Fig. 2. In the Top Row, the left panel corresponds to the mean VFM trajectories from areas where breastfed children showed greater developmental rate than formula-fed children and the right panel shows the mean VFM trajectories from areas where breastfed children showed slower developmental rate than formula-fed children. In the Middle Row, the left panel corresponds to the mean VFM trajectories from areas where breastfed children showed greater developmental rate than breast milk and formula-fed children and the right panel shows the mean VFM trajectories from areas where breastfed children showed slower developmental rate than breast milk and formula-fed children. In the Bottom Row, the left panel corresponds to the mean VFM trajectories from areas where formula-fed children showed greater developmental rate than breast milk and formula-fed children and the right panel shows the mean VFM trajectories from areas where formula-fed children showed slower developmental rate than breast milk and formula-fed children.
Relationships between VFM and nursing duration
To investigate potential relationships between nursing duration and VFM, correlation analysis was applied to data from the exclusively breastfed children. To account for age in this analysis, logarithmic VFM development curves (Eq. [1]) were first fit voxel-wise to the data. This model was then subtracted from the data and the regression analysis was performed with respect to these residuals. As a precautionary second step, age was also included as a variable of no interest in the regression analysis. Prior to model fitting and analysis, all data were smoothed was a 2.5 mm FWHM Gaussian kernel. Areas of significant difference were defined as p < 0.05 cluster corrected for multiple comparisons as above.
As a follow-up to correlation analysis, we also investigated mean VFM differences in children who were breastfed for extended durations (longer than 15 months) and less than 12 months (in line with current recommendations from the American Academy of Pediatrics and the World Health Organization (More, 2003)). Groups were matched for age, male:female ratio; birth weight; gestation duration; or maternal age, education or SES. Data were smoothed with a 2.5 mm FWHM Gaussian kernel and unpaired t-tests performed at each voxel to identify significant differences. Significance was defined as p < 0.05 corrected for multiple comparisons using a cluster-based technique as above. Motor and behavioral function differences were also examined between the short and extended breastfeeding groups across all Mullen domains: fine and gross motor, expressive and receptive language, and visual reception. Children who had maximal score in the gross motor domain (n = 3) were excluded from the gross motor comparison. Significance group difference was defined as p < 0.05 corrected for type 1 errors using the Holm–Bonferroni method (Holm, 1979).
Results
Statistical results contrasting the VFM developmental rate in the breastfed and formula-fed; breastfed and breast milk plus formula-fed; and formula-fed and breast plus formula-fed groups are shown superimposed on the T1-weighted study template image in Fig. 2. Mean VFM developmental trajectories for the identified areas of significance are shown in Fig. 3. Compared with the formula-fed and breast milk plus formula-fed infants, breastfed infants showed increased VFM development rate throughout many later maturing white matter regions, including frontal brain and left temporal lobe, as well as early maturing regions such as corpus callous, internal capsule and corticospinal tract, cerebellum, and left optic radiation. In contrast, formula-fed and breast milk plus formula-fed infants showed increased VFM development rate in the right optic radiation and occipital lobe and right internal capsule compared to breastfed infants.
Fig. 2.
Regions of statistical difference (p < 0.05, corrected) in VFM development rate (α) between each comparison set. For each image row, cross-hairs correspond to the same point.
Logarithmic curves were chosen to model the developmental trajectory as they provided improved goodness of fit compared to linear curves. This is illustrated in Table 3, where correlation coefficients for linear and logarithmic fits are shown for each of the curves in Fig. 3.
Table 3.
Comparison of linear and logarithmic fits to the developmental data presented in Fig. 3.
| Developmental trajectory | Linear r2 | Logarithmic r2 | |
|---|---|---|---|
| Breastfed vs. formula-fed breast > formula |
Breastfed | 0.80 | 0.86 |
| Formula-fed | 0.71 | 0.77 | |
| Breastfed vs. breast + formula breast > breast + formula |
Breastfed | 0.8 | 0.85 |
| Breast + formula | 0.63 | 0.73 | |
| Formula-fed vs. breast + formula formula > breast + formula |
Formula-fed | 0.76 | 0.83 |
| Breast + formula | 0.59 | 0.69 | |
| Breastfed vs. formula-fed breast < formula |
Breastfed | 0.77 | 0.84 |
| Formula-fed | 0.71 | 0.76 | |
| Breastfed vs. breast + formula breast < breast + formula |
Breastfed | 0.72 | 0.77 |
| Breast + formula | 0.63 | 0.72 | |
| Formula-fed vs. breast + formula formula < breast + formula |
Formula-fed | 0.71 | 0.77 |
| Breast + formula | 0.67 | 0.76 | |
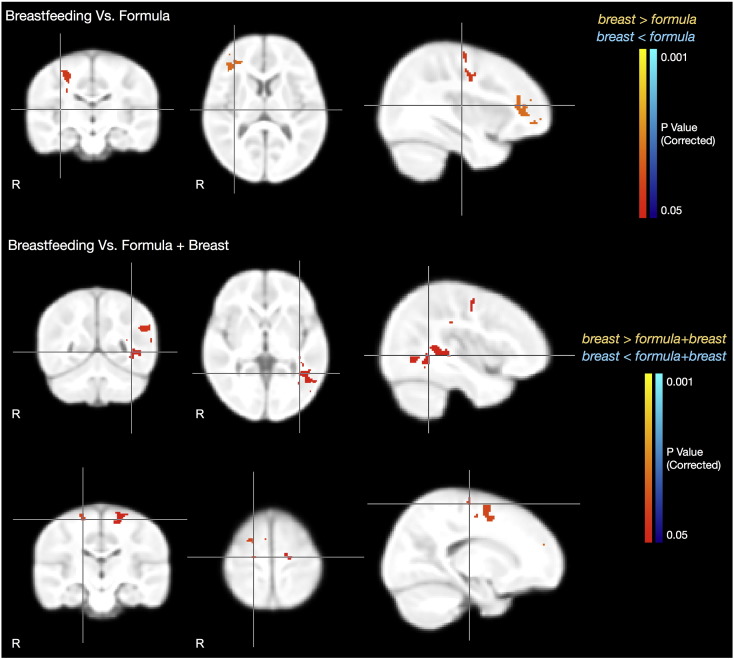
Since early (less than 800 days) VFM development was found to be similar for each of the 3 groups (visual inspection of Fig. 3), we examined VFM and behavioral differences in children 800 days of age and older, approximately 2.2 to 4 years of age. Results of this comparison are shown in Fig. 4 and Table 4. Compared to formula-fed children, breastfed children had greater VFM values (p < 0.05, FER corrected) in areas including right hemisphere inferior frontal white matter, near Broca's area (Brodmann area 44) and right corticospinal tract and premotor cortex (Brodmann area 6). Behaviorally (Table 5), breastfed children showed improved receptive language scores compared to formula-fed children (p < 0.05, FER corrected). The linkage between these structure and functional findings, however, is not immediately clear. Broca's area is more commonly associated with word generation than comprehension and is functionally lateralized to left hemisphere in adults (Josse and Tzourio-Mazoyer, 2004). It may be that this functional lateralization is incomplete at this age (Berl et al., 2012) or that decreased lateralization yields improved performance.
Fig. 4.
Statistical images of group mean VFM difference in an older subset of children (2.2–4 years of age). The Top Row displays regions of significant (p < 0.05, corrected) VFM differences between the breastfed and formula-fed children, and the Bottom Rows display regions of significant difference between the breastfed and breast plus formula-fed children. No areas were found where the exclusively formula-fed or breast plus formula fed children had greater VFM than the exclusively breastfed children. For each image row, cross-hairs correspond to the same point.
Table 4.
Mean VFM differences between 2.2 and 4 year old breastfed, formula-fed and breast plus formula-fed children.
| Breastfed vs. formula-fed | |||||
|---|---|---|---|---|---|
| Atlas coordinatea |
Brain region | Mean % change (breastfed > formula-fed) |
Cluster size (voxels) | ||
| X | Y | Z | |||
| 33.0 | 30.0 | 13.5 | Right inferior frontal white matter/superior longitudinal fasciculus. | 34.4 | 1399 |
| 34.5 | − 10.5 | 40.5 | Right corticospinal tract/Brodmann area 44 | 30.5 | 951 |
| Breastfed vs. formula + breast milk | |||||
| Atlas coordinatea |
Brain region | Mean % change (breastfed > formula + breast) |
|||
| X | Y | Z | |||
| 12 | − 1.5 | 49.5 | Right corticospinal tract | 29.3 | 403 |
| − 16.5 | − 19.5 | 60 | Left corticospinal tract | 34.4 | 637 |
| − 42 | − 51 | 6 | Left superior longitudinal fasciculus | 15.1 | 808 |
| − 43.5 | − 27 | 33 | Left superior longitudinal fasciculus/Brodmann area 40 | 24.6 | 555 |
Coordinates from Talairach's brain atlas, such that x is the distance in millimeters to the right (+) or left (−) of midline, y is the distance anterior (+) or posterior (−) to the anterior commissure, and z is the distance above (+) or below (−) a horizontal plane through the anterior and posterior commissures.
Table 5.
Comparison of behavioral test scores for participants older than 2.2 years of age. Bold p-values indicate those identified as statistically significant, corrected for type 1 error using the method of Holm–Bonferroni.
| Subset comparison of older members of Group #1 and Group #2 | |||
|---|---|---|---|
| Group #1 (breastfed) |
Group #2 (formula-fed) |
p-Value | |
| Participants (n) | 21 | 12 | |
| Age (days) | 1287 ± 153 | 1281 ± 118 | 0.91 |
| Fine motor | 36.8 ± 5.3 | 34.3 ± 4.8 | 0.19 |
| Receptive language | 41.1 ± 3.3 | 34.5 ± 5.6 | 0.0019 |
| Expressive language | 39.1 ± 3.9 | 37 ± 5.8 | 0.28 |
| Visual reception | 44.4 ± 4.6 | 41.6 ± 4.5 | 0.09 |
| Subset comparison of older members of Group #1 and Group #3 | |||
| Group #1 (breastfed) |
Group #3 (breast + formula-fed) |
p-Value | |
| Participants (n) | 21 | 15 | |
| Age (days) | 1287 ± 153 | 1219 ± 150 | 0.19 |
| Fine motor | 36.8 ± 5.3 | 32.9 ± 6.4 | 0.067 |
| Receptive language | 41.1 ± 3.3 | 34.7 ± 5.8 | 0.0011 |
| Expressive language | 39.1 ± 3.9 | 35.2 ± 6.4 | 0.05 |
| Visual reception | 44.4 ± 4.6 | 38.8 ± 6.1 | 0.0056 |
| Subset comparison of older members of Group #2 and Group #3 | |||
| Group #2 (formula-fed) |
Group #3 (breast + formula-fed) |
p-Value | |
| Participants (n) | 12 | 15 | |
| Age (days) | 1281 ± 118 | 1219 ± 150 | 0.23 |
| Fine motor | 34.3 ± 4.8 | 32.9 ± 6.4 | 0.52 |
| Receptive language | 34.5 ± 5.6 | 34.7 ± 5.8 | 0.91 |
| Expressive language | 37 ± 5.8 | 35.2 ± 6.4 | 0.45 |
| Visual reception | 41.6 ± 4.5 | 38.8 ± 6.1 | 0.18 |
In contrast with children who received both breast milk and formula, exclusively breastfed children had significantly greater VFM (p < 0.05, FER corrected) in brain regions including: left optic radiation adjacent to the angular gyrus (Brodmann area 39); right inferior parietal lobe, near the somatosensory cortex (Brodmann area 7); bilateral premotor cortex (Brodmann area 4); and right prefrontal cortex (Brodmann area 8). These regional VFM differences may underlie the improved visual reception performance (specifically related to Brodmann area 7) and receptive language scores (Brodmann area 39) of the breastfed children (Table 5). No significant behavioral score or MWF differences were found between the exclusively formula-fed and breast milk plus formula-fed children.
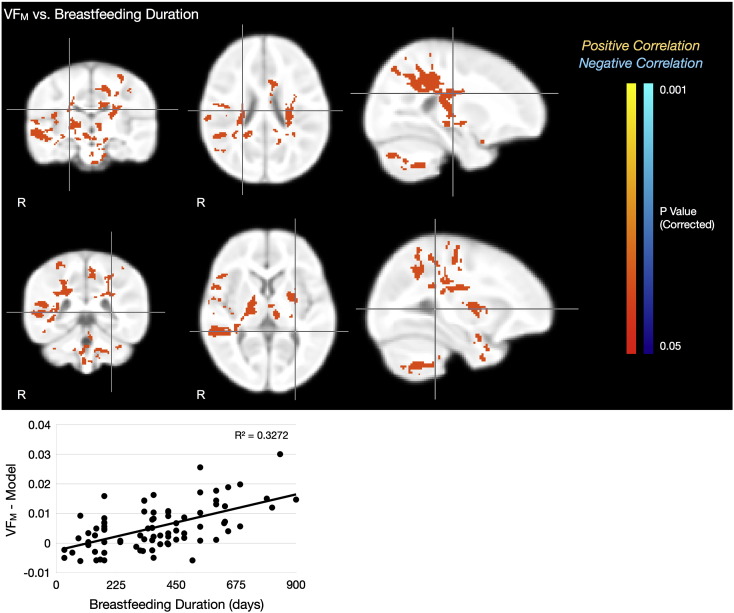
Regions exhibiting a significant correlation between breastfeeding duration and VFM, as well as significant group VFM differences between short and extended breastfeeding, are shown in Figs. 5 and 6, Tables 6 and 7. Brain areas exhibiting a significant correlation between breastfeeding duration and VFM included bilateral internal capsule; corticospinal tract; and superior occipito-frontal fasiculus; left cerebellar white matter and superior parietal lobe; and right auditory and somatosensory cortices.
Fig. 5.
Top Rows: Statistical images showing regions exhibiting a significant (p < 0.05, corrected) positive association between VFM and breastfeeding duration, accounting for the effects of age. Mean VFM residuals (i.e., raw VFM values with the logarithmic age model subtracted) from these regions vs. breastfeeding duration are shown in the Bottom Row. For each image row, cross-hairs correspond to the same anatomical point.
Fig. 6.
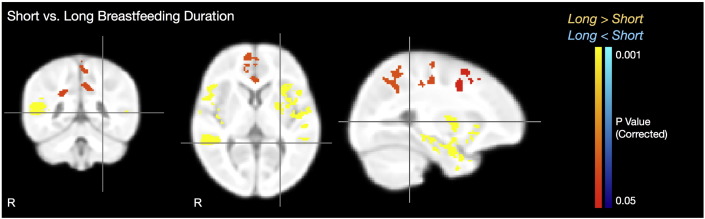
Statistical image showing regions of significant VFM difference between children breastfed for prolonged durations (greater than 15 months) and children breastfed for less than 12 months. For each image row, cross-hairs correspond to the same point.
Table 6.
Mean VFM differences between short and extended breastfed children.
| Short vs. extended breastfeeding duration | |||||
|---|---|---|---|---|---|
| Atlas coordinatea |
Brain region | Mean % change (extended > short duration) |
Cluster size (voxels) | ||
| X | Y | Z | |||
| 10.5 | 33 | 28.5 | Right Brodmann area 9 | 25.1 | 1001 |
| 42 | − 36 | 13.5 | Right Brodmann area 41 | 30.2 | 2783 |
| − 43.5 | − 1.5 | − 3 | Left Brodmann area 13 | 28.3 | 3567 |
| − 24 | − 49.5 | 51 | Left Brodmann area 7 | 23.9 | 996 |
| − 24 | − 21 | 58.5 | Left Brodmann area 4 | 21.2 | 912 |
| 42 | − 57 | 33 | Left Brodmann area 39 | 23.3 | 692 |
| − 4.5 | − 36 | 57 | Left Brodmann area 5 | 21.8 | 500 |
| − 24 | 0 | 49.5 | Left Brodmann area 6 | 22.7 | 604 |
| 37.5 | 3 | 42 | Right Brodmann area 6 | 28.8 | 270 |
Coordinates from Talairach's brain atlas, such that x is the distance in millimeters to the right (+) or left (−) of midline, y is the distance anterior (+) or posterior (−) to the anterior commissure, and z is the distance above (+) or below (−) a horizontal plane through the anterior and posterior commissures.
Table 7.
Comparison of behavioral test scores for breast-fed children divided into short and long feeding durations. Bold values indicate statistically different scores corrected for type 1 error using Holm–Bonferroni correction.
| Short breast feeding duration | Long breast feeding duration | p-Value | |
|---|---|---|---|
| Participants (n) | 22 | 25 | |
| Age (days) | 691 ± 324 | 807 ± 341 | 0.24 |
| Breast feeding duration | 220 ± 81 | 600 ± 124 | |
| Gross motor | 20.41 ± 4.7 | 23 ± 5 | 0.046 |
| Fine motor | 20.4 ± 5.5 | 25.3 ± 8.6 | 0.028 |
| Receptive language | 19.2 ± 8.9 | 26.7 ± 11.2 | 0.015 |
| Expressive language | 16.9 ± 7.9 | 25.6 ± 10.7 | 0.0036 |
| Visual reception | 20.9 ± 9.2 | 30 ± 11.1 | 0.0042 |
Significant (p < 0.05 FER corrected) group VFM differences (Fig. 6, Table 6) were found in bilateral Broca's area (Brodmann areas 44 and 45); parietal lobes; and secondary somatosensory cortices; right auditory cortex and frontal lobe; left optic radiation, and premotor and primary somatosenory cortices (Brodmann areas 3 and 6). These group differences provide a structural link to the functional differences found in all Mullen domains (Table 7), for example, Brodmann areas 44 and 45, associated with language; and secondary somatosensory cortices and Brodmann areas 3 and 6 associated with motor control.
Discussion
This study describes some of the earliest changes in human white matter development associated with breastfeeding. While prior imaging studies have shown increased brain volume and cortical thickness in adolescents who were breastfed as infants (Hallowell and Spatz, 2012; Isaacs et al., 2010; Kafouri et al., 2012), none have examined the early developmental trajectories that may ultimately give rise to this later structural differentiation. Cumulatively, our results associate early exclusive breastfeeding with increased development in late maturing white matter regions, including the frontal and temporal white matter, peripheral aspects of the internal capsule and corticospinal tracts, superior longitudinal fasciculus and superior occipital–frontal fasciculus. These regions and pathways are commonly associated with higher-order cognition, such as executive functioning, planning, social–emotional functioning, and language (Grossmann and Johnson, 2007; Johnson, 2003), domains in which breastfed infants were also found to have improved performance. We have also shown extended breastfeeding is positively associated with increased VFM in somatosensory, auditory and language areas and, in turn, with increased language performance, visual reception and motor control performance.
While the exact mechanism(s) that underlie these observed VFM differences remain unclear, our results lend support to the hypothesis that the docosahexaenoic and arachidonic acids present in breast milk promote preferential neural growth and white matter development. For example, early weaning in Wistar rats resulted in reduced myelin basic protein expression (Kodama et al., 2008). Other constituents of breast milk, however, may also play a significant role (Rey, 2003). For example, the high cholesterol content of breast milk may provide a ready supply from which oligodendrocytes can develop myelin membranes. Limited cholesterol availability to oligodendrocytes has been shown to inhibit brain maturation in mice (Saher et al., 2005). In contrast to breast milk, formula milk contains only the precursors to DHA and AA, and thus must be synthesized by the infant, and has limited cholesterol content (Reynolds, 2001).
While myelin water fraction imaging has a long history in the field of known demyelinating disorders, such as multiple sclerosis (MacKay et al., 2009), it's use in examining structural and functional development is new. Further, mcDESPOT differs from the conventional and established techniques that derive the MWF from analysis of the transverse magnetization decay curve and have been verified histologically (Laule et al., 2006; Webb et al., 2003). Similar verification of mcDESPOT has been limited to qualitative histological comparisons in the Shaking Pup model of dysmyelination (Hurley et al., 2010) and indirectly through comparison with the known histological time-course of myelination in human infants (Deoni et al., 2011, 2012a,b), and demyelination studies in MS (Kolind et al., 2012; Kitzler et al., 2012). Thus, the specificity of mcDESPOT VFM measures as a reflection solely of myelin may be questioned. Additional effects, such as magnetization transfer may also influence mcDESPOT values (Bieri et al., 2008). However, animal model and in vivo results garnered so far give confidence that if not specific to myelin, mcDESPOT provides novel information regarding white matter microstructure, and offers differing, perhaps enhanced, sensitivity to myelin changes relative to T1 and T2 relaxation times (Deoni et al., 2012b). Nevertheless, though tempting, we cannot confidently attribute the observed VFM differences specifically to increased myelin, though prior work suggests it may be closely related.
While the cross-sectional nature of our study precludes us from performing the predictive analysis to more conclusively demonstrate a causal link between breastfeeding, structural development, and cognitive outcome, results do show consistent trends across our study data, and with prior studies. The cross-sectional developmental trajectories predicted the increased VFM identified in frontal and association brain regions of the breastfed infants, which are also brain regions related to observed cognitive improvements. White matter pathways connecting somatosensory regions were also found to exhibit a strong relationship between VFM and duration of breastfeeding. This corresponds well to prior studies that have shown that the percentage of breast milk in an infant's diet positively predicts cortical thickness of the parietal lobe as well as verbal IQ (Isaacs et al., 2010). However, we did not quantify the percentage of breast milk in our infants' diet and, hence, a similar dose effect could not be directly established herein.
Though outside the scope of this work, an important public health concern is the personal, familial, societal or work pressures that lead some mothers to chose to not breastfeed or stop prior to 3 months, well before the 6 month guidelines of the American Academy of Pediatrics and the World Health Organization (WHO) (More, 2003). Indeed, the results herein lend further support to the WHO recommendation that breastfeeding be continued up to 2 years of age and beyond. Thus, this work may have significant implications for our understanding of the earliest neurodevelopmental advantages provided by breastfeeding.
Conclusions
While the exact mechanisms that underlie the well documented cognitive advantages associated with breastfeeding remain unclear, potentially related to the long-chain polyunsaturated or other essential fatty acids prevalent in breast milk, our results show infant breastfeeding is associated with improved developmental growth in late maturing white matter association regions, and extended breastfeeding duration is associated with improved white matter structure and cognitive performance. Our findings are consistent with prior cognitive and IQ performance studies from older children, as well as imaging studies, and add to the consensus that breastfeeding has a positive impact of brain development.
Acknowledgments
This work was supported by the National Institutes of Mental Health (R01 MH087510). JOM is supported by a Sir Henry Wellcome Postdoctoral Fellowship awarded by the Wellcome Trust (No. 096195).
Conflict of interestThe authors report no financial conflict of interest with regards to the content of this paper.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Ahima R.S., Bjorbaek C., Osei S., Flier J.S. Regulation of neuronal and glial proteins by leptin: implications for brain development. Neuroendocrinology. 1999;140:2755. doi: 10.1210/endo.140.6.6774. [DOI] [PubMed] [Google Scholar]
- Anderson J.W., Johnstone B.M., Remley D.T. Breast-feeding and cognitive development: a meta-analysis. Am. J. Clin. Nutr. 1999;70:525–535. doi: 10.1093/ajcn/70.4.525. [DOI] [PubMed] [Google Scholar]
- Avants B.B., Epstein C.L., Grossman M., Gee J.C. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 2008;12:26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad M.B., Konya T., Maughan H., Guttman D.S., Field C.J. Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ. 2013;9:15. doi: 10.1503/cmaj.121189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berl M.M., Mayo J., Parks E.N., Rosenberger L.R., Vanmeter J. Regional differences in the developmental trajectory of lateralization of the language network. Hum. Brain Mapp. 2012 doi: 10.1002/hbm.22179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieri O., Mamisch T.C., Trattnig S., Scheffler K. Steady-state free precession magnetization transfer imaging. Magn. Reson. Med. 2008;60:1261–1266. doi: 10.1002/mrm.21781. [DOI] [PubMed] [Google Scholar]
- Deoni S.C. Correction of main and transmit magnetic field (B0 and B1) inhomogeneity effects in multicomponent driven equilibrium single pulse observation of T1 and T2. Magn. Reson. Med. 2011;65:1021–1035. doi: 10.1002/mrm.22685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni S.C.L., Rutt B.K., Arun T., Puerpaoli C., Jones D.K. Gleaning multicomponent T1 and T2 information from steady-state imaging data. Magn. Reson. Med. 2008;60:1372–1387. doi: 10.1002/mrm.21704. [DOI] [PubMed] [Google Scholar]
- Deoni S.C.L. Mapping infant brain myelination with magnetic resonance imaging. J. Neurosci. 2011;31(2):784–791. doi: 10.1523/JNEUROSCI.2106-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni S.C., Dean D.C., III, O'Muircheartaigh J., Driks H., Jerskey B.A. Investigating white matter development in infancy and childhood using myelin water fraction and relaxation time mapping. NeuroImage. 2012;63:1038–1053. doi: 10.1016/j.neuroimage.2012.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni S.C., Matthews L., Kolind S.H. One component? Two components? Three? The effect of including a non-exchanging “free” water component in multicomponent driven equilibrium single pulse observation of T1 and T2. Magn. Reson. Med. 2012 doi: 10.1002/mrm.24429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B. Bootstrap methods: another look at the jackknife. Ann. Stat. 1979;7:1–26. [Google Scholar]
- Fields R.D. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008;31:361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareau P.J., Rutt B.K., Karlik S.J., Mitchell J.R. Magnetization transfer and multicomponent T2 relaxation measurements with histopathologic correlation in an experimental Model of MS. J. Magn. Reson. Imaging. 2000;11:586–595. doi: 10.1002/1522-2586(200006)11:6<586::aid-jmri3>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Grossmann T., Johnson M.H. The development of the social brain in human infants. Eur. J. Neurosci. 2007;25:809–819. doi: 10.1111/j.1460-9568.2007.05379.x. [DOI] [PubMed] [Google Scholar]
- Guesnet P., Alessandri J.M. Docosahexaenoic acid (DHA) and the developing nervous system (CNS) — implications for dietary recommendations. Biochimie. 2011;93:7–12. doi: 10.1016/j.biochi.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Hallowell S.G., Spatz D.L. The relationship of brain development and breastfeeding in the late-preterm infant. J. Pediatr. Nurs. 2012;27:154–162. doi: 10.1016/j.pedn.2010.12.018. [DOI] [PubMed] [Google Scholar]
- Heijtz R.D., Wang S., Anuar S., Qian Y., Bjorkholm B. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead, A.A., 1975. Four-Factor Index of Social Status. Unpublished Manuscript, Yale University, New Haven CT.
- Holm S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979;6:65–70. [Google Scholar]
- Hurley S.A., Mossahebi P.M., Samsonov A.A., Alexander A.L., Deoni S.C., Fisher R., Ducan I.D., Field A.S. Proc. 18th Annual Meeting of the ISMRM. Stockholm, SWE. 2010. Multicomponent relaxometry (mcDESPOT) in the Shaking Pup Model of dysmyelination; p. 4516. [Google Scholar]
- Isaacs E.B., Fischl B.R., Quinn B.T., Chong W.K., Gadian D.G., Lucas A. Impact of breast milk on intelligence quotient, brain size, and white matter development. Pediatr. Res. 2010;67:357–362. doi: 10.1203/PDR.0b013e3181d026da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M.H. Development of human brain functions. Biol. Psychiatry. 2003;54:1312–1316. doi: 10.1016/s0006-3223(03)00426-8. [DOI] [PubMed] [Google Scholar]
- Josse G., Tzourio-Mazoyer N. Hemispheric specialization for language. Brain Res. Rev. 2004;44:1–12. doi: 10.1016/j.brainresrev.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Kafouri S., Kramer M., Leonard G., Perron M., Pike B. 2012. Breastfeeding and Brain Structure in Adolescence. [DOI] [PubMed] [Google Scholar]
- Khedr E.M.H., Farghaly W.M.A., Amry S.E.D., Osman A.A.A. Neural maturation of breastfed and formula-fed infants. Acta Paediatr. 2004;93:734–738. doi: 10.1111/j.1651-2227.2004.tb03011.x. [DOI] [PubMed] [Google Scholar]
- Kitzler H.H., Su J., Zeineh M., Harper-Little C., Leung A., Kremenchutzky M., Deoni S., Rutt B.K. Deficient MWF mapping in multiple sclerosis using 3D whole-brain multi-component relaxation MRI. NeuroImage. 2012;59:2670–2677. doi: 10.1016/j.neuroimage.2011.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama Y., Kikusui T., Takeuchi Y., Mori Y. Effects of early weaning on anxiety and prefrontal cortical and hippocampal myelination in male and female Wistar rats. Dev. Psychobiol. 2008;50:332–342. doi: 10.1002/dev.20289. [DOI] [PubMed] [Google Scholar]
- Kolind S., Matthews L., Johansen-Berg H., Leite M.I., Williams S.C., Deoni S., Palace J. Myelin water imaging reflects clinical variability in multiple sclerosis. NeuroImage. 2012;60:263–270. doi: 10.1016/j.neuroimage.2011.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laule C., Leung E., Lis D.K.B., Traboulsee A.L., Paty D.W., MacKay A.L., Moore G.R.W. Myelin water imaging in multiple sclerosis: quantitative correlations with histopathology. Mult. Scler. 2006;12:747–753. doi: 10.1177/1352458506070928. [DOI] [PubMed] [Google Scholar]
- Laule C., Kozlowski P., Leung E., Li D.K.B., MacKay A.L., Moore G.R.W. Myelin water imaging of multiple sclerosis at 7 T: correlations with histopathology. NeuroImage. 2008;40:1575–1580. doi: 10.1016/j.neuroimage.2007.12.008. [DOI] [PubMed] [Google Scholar]
- MacKay A., Laule C., Vavsour I., Bjarnason T., Kolling S., Madler B. Insights into brain microstructure from the T2 distribution. Magn. Reson. Imaging. 2006;24:515–525. doi: 10.1016/j.mri.2005.12.037. [DOI] [PubMed] [Google Scholar]
- MacKay A.L., Vavasour I.M., Rauscher A., Kolind S.H., Madler B., Moore G.R., Traboulsee A.L., Li D.K., Laule C. MR relaxation in multiple sclerosis. Neuroimaging Clin. N. Am. 2009;19:1–26. doi: 10.1016/j.nic.2008.09.007. [DOI] [PubMed] [Google Scholar]
- McCann J.C., Ames B.N. Is docosahexaenoic acid, an N − 3 long-chain polyunsaturated fatty acid, required for development of normal brain function? An overview of evidence for cognitive and behavioral tests in humans and animals. Am. J. Clin. Nutr. 2005;83:281–295. doi: 10.1093/ajcn.82.2.281. [DOI] [PubMed] [Google Scholar]
- McCrory C., Murray A. The effect of breast-feeding on neuro-development in infancy. Matern. Child Health J. 2012 doi: 10.1007/s10995-012-1182-9. [DOI] [PubMed] [Google Scholar]
- More J. New guidelines on infant feeding in the first 12 months of life. J. Fam. Health Care. 2003;13:89–90. [PubMed] [Google Scholar]
- Mullen E.M. TOTAL Child; Cranston, RI: 1992. Mullen Scales of Early Learning. [Google Scholar]
- Rey J. Breastfeeding and cognitive development. Acta Paediatr. 2003;92:11–18. doi: 10.1111/j.1651-2227.2003.tb00659.x. [DOI] [PubMed] [Google Scholar]
- Reynolds A. Breastfeeding and brain development. Pediatr. Clin. N. Am. 2001;48:159–171. doi: 10.1016/s0031-3955(05)70291-1. [DOI] [PubMed] [Google Scholar]
- Saher G., Brugger B., lappe-Siefke C., Mobius W., Tozawa R. High cholesterol level is essential for myelin membrane growth. Nat. Neurosci. 2005;6:468–475. doi: 10.1038/nn1426. [DOI] [PubMed] [Google Scholar]
- Webb S., Munro C.A., Midha R., Stanisz G.J. Is multicomponent T2 a good measure of myelin content in peripheral nerve? Magn. Reson. Med. 2003;49:638–645. doi: 10.1002/mrm.10411. [DOI] [PubMed] [Google Scholar]
- Worsley K.J., Andermann M., Koulis T., MacDonald D., Evans A.C. Detecting changes in nonisotropic images. Hum. Brain Mapp. 1999;8:98–101. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<98::AID-HBM5>3.0.CO;2-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak J.R., Lim K.O. Advances in white matter imaging: a review of in vivo magnetic resonance methodologies and their applicability to the study of development and aging. Neurosci. Biobehav. Rev. 2006;30:762–774. doi: 10.1016/j.neubiorev.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovlev P.I., Lecours A.R. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A., editor. Regional Development of the Brain in Early Life. Blackwell Scientific Publications; Oxford, UK: 1967. pp. 3–65. [Google Scholar]
- Zhang Y., Brady M., Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation–maximization algorithm. IEEE Trans. Med. Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]