Abstract
OBJECTIVE
The purpose of this research was to report results on long-term administration of dichloroacetate in 36 children with congenital lactic acidosis who participated previously in a controlled trial of this drug.
PATIENTS AND METHODS
We conducted a randomized control trial, followed by an open-label study. Data were analyzed for each patient from the time they began treatment through May 2005.
RESULTS
Subject exposure to dichloroacetate totaled 110.42 years. Median height and weight increased over time, but the standardized values declined slightly and remained below the first percentile. There were no significant changes in biochemical metabolic indices, except for a 2% rise in total protein and a 22% increase in 24-hour urinary oxalate. Both the basal and carbohydrate meal-induced rises in lactate were blunted by dichloroacetate. The median cerebrospinal fluid lactate also decreased over time. Conduction velocity decreased and distal latency increased in peroneal nerves. Mean 3-year survival for all of the subjects was 79%.
CONCLUSIONS
Oral dichloroacetate is generally well tolerated in young children with congenital lactic acidosis. Although continued dichloroacetate exposure is associated with evidence of peripheral neuropathy, it cannot be determined whether this is attributable mainly to the drug or to progression of underlying disease.
Keywords: dichloroacetate, lactic acidosis, mitochondria, pyruvate dehydrogenase, respiratory chain
Dichloroacetate has been administered for several years to children and adults with genetic mitochondrial diseases. The primary rationale for its use is its ability to stimulate the activity of the pyruvate dehydrogenase complex, thereby facilitating aerobic glucose and lactate oxidation and energetics.1 Most published information on the safety and efficacy of dichloroacetate in this patient population is derived from open-label studies in 1 or a few individuals. Recently, a randomized, controlled trial evaluated oral dichloroacetate at a dose of 12.5 mg/kg twice daily for the chronic treatment of older adolescents and adults (mean age at entry: 30 years) with the common A3243G mutation in mitochondrial DNA that gives rise to the syndrome of mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes.2 This study was terminated prematurely because of an undue incidence of worsening or new onset of peripheral neuropathy associated with dichloroacetate administration compared with placebo administration.
Another recently completed randomized, controlled trial investigated the same dichloroacetate dose in 43 young children (mean age at entry: 5.6 years) with congenital lactic acidosis (CLA) because of defects in pyruvate dehydrogenase or in ≥1 complex of the respiratory chain or a mitochondrial DNA mutation.3 In contrast to the findings in older subjects with mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes, this study demonstrated good tolerability of dichloroacetate, and there were no significant differences in the severity or frequency of adverse events between the drug and placebo groups after 6 months of treatment.
Here we report our experience with long-term administration of dichloroacetate in the 36 children on the trial who received dichloroacetate, from their starting times on the drug. Some of these patients have received treatment continuously for ~10 years.
PATIENTS AND METHODS
This study was approved by the University of Florida Institutional Review Board. The DCA/CLA Clinical Trial was a single-center, double-blind, randomized, controlled trial of oral dichloroacetate that was conducted in the General Clinical Research Center (GCRC) at the University of Florida between December 1994 and October 2001.3 The primary outcome results were measures of neuromuscular and behavioral function, quality of life, linear growth, blood lactate concentration, frequency and severity of intercurrent illness and hospitalizations, and safety, including tests of liver and peripheral nerve function.
Participants
Of 43 children randomized to the DCA/CLA Clinical Trial, 36 (17 boys and 19 girls) received dichloroacetate during the study, and these patients are the subject of this report. The median age at random assignment was 5.3 years (range: 1.3–20.3 years). Inclusion criteria included biochemical or molecular genetic proof of a deficiency in pyruvate dehydrogenase or in ≥1 complexes of the respiratory chain or a pathologic mutation in mitochondrial DNA and a persistent or episodic elevation of the concentration of lactate in blood, cerebrospinal fluid, or both.
Study Design
Patients were admitted quarterly to the GCRC for clinical and biochemical evaluation throughout the 2-year double-blind period. All of the patients received placebo for 6 months, and then were randomly assigned to receive placebo or dichloroacetate (12.5 mg/kg every 12 hours by mouth) for an additional 6 months, so that month 12 was the point of comparison between the 2 treatments. Subsequently, patients receiving placebo were switched to dichloroacetate, and all of the subjects were continued on the double-blind study for an additional 12 months.
At the end of the double-blind phase, patients were given the opportunity to continue receiving 25 mg/kg per day of dichloroacetate, providing that they would return every 6 months for evaluation and that the data safety monitoring board that oversaw the clinical trial determined that there was no clinical or biochemical evidence of significant drug-related toxicity. Based on the analysis of the trial,3 all of the patients were considered eligible for continued treatment with dichloroacetate, and 36 subjects elected to participate in the open-label study.
Statistical Considerations
We analyzed data accumulated for each patient from the time they began dichloroacetate treatment in the DCA/CLA Clinical Trial through May 2005 or last clinical contact, whichever was first. The statistical question for long-term trends was whether the value of a given parameter tended to increase versus decrease with time. To address this in a distribution-free way we proceeded as follows. For each patient contributing ≥2 observations on dichloroacetate for a given variable, we computed a personal estimated slope in terms of unit change per year. The Wilcoxon signed rank test was applied to these slopes to test the null hypothesis that the distribution of slopes was symmetric at ~0 (ie, not associated with time).
To put the slopes into perspective, a summary statistic for the value at the initiation of dichloroacetate was also provided. For quantitative variables, this was the median (50th percentile). For normal or abnormal variables, this was the percentage abnormal. A P value of ≤.05 was considered statistically significant.
RESULTS
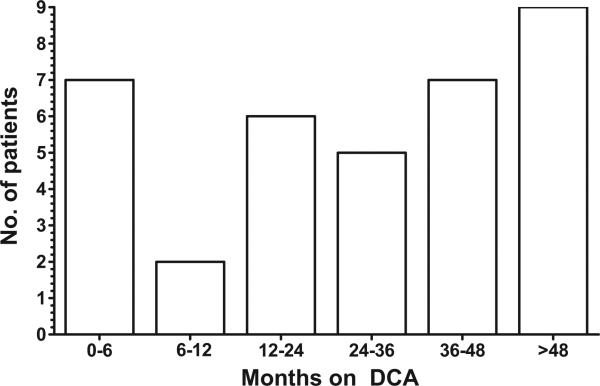
Table 1 lists the clinical characteristics of the 36 patients who participated in this investigation. The most common diagnosis was pyruvate dehydrogenase deficiency (10 patients), whereas 26 subjects had ≥1 defects in respiratory chain complexes or had a pathologic mutation in mitochondrial DNA. Over the course of evaluation, subject exposure to dichloroacetate totaled 110.42 years, 76.4 years of which were not reported in the earlier study.3 The median exposure per subject was 2.38 years (range: 0.00–9.67 years). The distribution of subjects who attained various treatment exposure durations was fairly stable over time (Fig 1). For example, 19% (7 of 36 patients) received dichloroacetate for a period between 0 (<30 days) and 6 months, and 25% (9 subjects) received dichloroacetate for >48 months.
TABLE 1.
Patient Diagnosis and Demographics
| Patient | Diagnosis | Gender | Age at Entry | Age Randomized to Dichloroacetate |
|---|---|---|---|---|
| 1 | PDH | Female | 10 mo | 1 year 4 mo |
| 2 | PDH | Female | 1 y 8 mo | 2 y 8 mo |
| 3 | PDH | Male | 6 y 7mo | 7 y 1 mo |
| 4 | PDH | Female | 2 y 4 mo | 2 y 10 mo |
| 5 | PDH | Female | 3 y 9 mo | 4 y 9 mo |
| 6 | PDH | Female | 3 y 9 mo | 4 y 9 mo |
| 7 | PDH | Male | 2 y 9 mo | 3 y 9 mo |
| 8 | PDH | Female | 5 y 9 mo | 6 y 3 mo |
| 9 | PDH | Female | 2 y 11 mo | 3 y 11 mo |
| 10 | PDH | Male | 4 y 9 mo | 5 y 3 mo |
| 11 | Complex I | Male | 1 y 5 mo | 1 y 11 mo |
| 12 | Complex I | Female | 1 y 9 mo | 2 y 9 mo |
| 13 | Complex I | Male | 19 y 3 mo | 20 y 3 mo |
| 14 | Complex I | Female | 9 y 8 mo | 10 y 2 mo |
| 15 | Complex I | Female | 4 y 11 mo | 5 y 11 mo |
| 16 | Complex II | Male | 1 y 6 mo | 2 y 6 mo |
| 17 | Complex III | Female | 1 y 6 mo | 2 y |
| 18 | Complex III | Female | 2 y 4 mo | 2 y 10 mo |
| 19 | Complex IV | Female | 5 y 5 mo | 6 y 5 mo |
| 20 | Complex IV | Male | 7 y 1 mo | 8 y 1 mo |
| 21 | Complex IV | Female | 9 y 8 mo | 10 y 8 mo |
| 22 | Complex I and IV | Female | 4 y 7 mo | 5 y 7 mo |
| 23 | Complex I and IV | Male | 1 y 6 mo | 2 y |
| 24 | Complex I and IV | Male | 6 y 11 mo | 7 y 5 mo |
| 25 | Complex I and IV | Male | 5 y 10 mo | 6 y 10 mo |
| 26 | Complex I and IV | Male | 7 y | 8 y |
| 27 | Complex I and IV | Male | 4 y 3 mo | 5 y 3 mo |
| 28 | Complex I, II and IV | Male | 4 y 4 mo | 5 y 4 mo |
| 29 | Complex I, III, and IV | Female | 1 y 10 mo | 2 y 10 mo |
| 30 | Complex II, III, and IV | Male | 2 y 3 mo | 3 y 3 mo |
| 31 | MELAS | Male | 6 y | 7 y |
| 32 | MELAS | Female | 19 y 2 mo | 19 y 8 mo |
| 33 | MELAS | Female | 13 y 9 mo | 14 y 3 mo |
| 34 | MELAS | Female | 11 y 7 mo | 12 y 1 mo |
| 35 | MELAS | Male | 13 y | 13 y 6 mo |
| 36 | OXPHOS | Male | 1 y 2 mo | 2 y 2 mo |
PDH indicates pyruvate dehydrogenase; MELAS, mitochondrial encephalomyelopathy, lactic acidosis, and stroke-like episodes; OXPHOS, generalized deficiency of oxidative phosphorylation pathway. For further description of biochemical diagnostic criteria, see Reference3.
FIGURE 1.
Duration of DCA administration among 36 patients.
Table 2 summarizes changes in anthropomorphic variables and vital sign measurements in the patients. At least 2 individual recordings of each index were obtained in most of the 36 children who participated in this long-term evaluation. The median height and weight of the patients increased over time, but their standardized values declined slightly and remained below the first percentile (z =–2.33).
TABLE 2.
Individual Trends in Anthropomorphic and Physiological Measurements of Patients
| Variable | n | Starting Median | Percentile for Annual Slopes |
P | ||
|---|---|---|---|---|---|---|
| 25th | Median | 75th | ||||
| Height, cm | 33 | 108.00 | 2.7100 | 4.2200 | 7.8400 | <.001 |
| Height, z score | 33 | –2.54 | –0.0012 | –0.0006 | 0.0001 | .047 |
| Weight, kg | 33 | 17.90 | 0.9000 | 1.3400 | 1.8000 | <.001 |
| Weight, z score | 33 | –2.83 | –0.0010 | –0.0004 | 0.0003 | .053 |
| BMI | 33 | 14.95 | –0.0800 | 0.0700 | 0.6800 | .14 |
| Pulse, beats per min | 31 | 104.00 | –6.1900 | –0.1300 | 5.9300 | .83 |
| Systolic blood pressure | 32 | 109.00 | –3.5000 | 0.2100 | 4.7800 | .53 |
| Diastolic blood pressure | 32 | 61.00 | –2.5300 | 0.2500 | 3.9700 | .99 |
| Respiration, breaths per min | 31 | 22.00 | –0.6400 | –0.2000 | 0.8200 | .86 |
There were no significant changes over time in the serum concentrations of glucose, β-hydroxybutyrate, electrolytes, and in tests of renal or hepatic function (including serum transaminases; data not shown), except for a 2% median annual increase in total protein concentration (n = 33; P = .042). Venous pH, white blood cell count, and platelet count also did not change significantly, but the median 24-hour urinary oxalate excretion (normal, ≤30 mg/24 hours) increased at a median annual rate of 3.0 mg/24 hours or 22% per year, from a median baseline of 13.6 mg/24 hours to 16.6 mg/24 hours (n = 27; P = .0033).
We measured venous whole-blood lactate concentrations (Glucose/Lactate Analyzer, YSI, Yellow Springs, OH), obtained ≥4 hours after a meal (“basal” state) and after a provocative carbohydrate challenge test (compare with 3). Table 3 shows that basal lactate levels were relatively normal and increased transiently after the meal challenge. Continued exposure of the subjects to dichloroacetate was associated with further decline in both basal and meal-stimulated lactate concentrations. The median cerebrospinal lactate at the start of this investigation was 3.8 mmol/L in 20 subjects and decreased by a median of 0.62 mmol/L (P = .044) per year. The median initial cerebrospinal glucose concentration was 51.5 mg/dL and did not change significantly (P = .74).
TABLE 3.
Individual Trends in Blood Lactate Response to Carbohydrate Meal Challenge
| Condition | n | Starting Median Lactate, mmol/L | Percentiles for Annual Slopes |
P | ||
|---|---|---|---|---|---|---|
| 25th | Median | 75th | ||||
| Basal | 33 | 1.28 | –0.70 | – 0.33 | –0.06 | <.001 |
| Meal + 60 min | 33 | 1.80 | –0.61 | –0.33 | 0.07 | .0052 |
| Meal + 120 min | 32 | 1.50 | –0.55 | –0.27 | –0.02 | .0039 |
Upper (median nerve) and lower (peroneal nerve and sural nerve) sensory and motor electrical activities were evaluated every 6 months as measures of peripheral nerve function, according to previously published methods (Table 4).4 As described in the “Methods,” we determined several changes in both the proportion of cases that demonstrated an abnormality in specific nerve function over time and the median values for conduction velocity and amplitude of a particular nerve group over time. Median and sural nerve functions were largely unaffected during the course of dichloroacetate exposure, except for a modestly increased proportion of patients who demonstrated an abnormality of median sensory measurements (P = .048). In contrast, the proportion of subjects who showed ≥1 abnormality of peroneal nerve function increased (P = .0053), and this was associated with a decrease in both the conduction velocity (from a mean ± SD baseline of 4.1 ± 3.6 mV at the start of dichloroacetate administration; P < .001) and the amplitude of the compound muscle action potential (from a baseline of 46.6 ± 8.3 m per second; P = .0019) of the nerve.
TABLE 4.
Individual Trends in Nerve Conduction Measurements
| Variable | n | Starting Median or Fraction Abnormal | Percentiles for Annual Slopes |
P | ||
|---|---|---|---|---|---|---|
| 25th | Median | 75th | ||||
| Median nerve | ||||||
| Motor: abnormal | 30 | 0.20 | –0.00 | 0.00 | 0.039 | .42 |
| Motor: conduction velocity | 31 | 50.00 | –2.96 | –0.43 | 1.101 | .24 |
| Motor: CMAP amp | 31 | 8.20 | –1.11 | –0.10 | 0.436 | .40 |
| Sensory: abnormality | 30 | 0.20 | 0.00 | 0.000 | 0.048 | .048 |
| Sensory: conduction velocity | 28 | 47.00 | –1.09 | 0.079 | 1.001 | .86 |
| Sensory: amplitude | 33 | 25.10 | –0.23 | 0.430 | 0.890 | .061 |
| Peroneal nerve | ||||||
| Abnormal | 30 | 0.20 | 0.00 | 0.010 | 0.234 | .0053 |
| Conduction velocity | 30 | 44.00 | –4.97 | –1.050 | 0.076 | <.001 |
| CMAP amplitude | 30 | 2.70 | –1.34 | –0.410 | –0.010 | .0019 |
| Sural nerve | ||||||
| Abnormal | 30 | 0.23 | 0.00 | 0.00 | 0.022 | .27 |
| Conduction velocity | 27 | 44.00 | –1.97 | –0.390 | 1.464 | .59 |
| Amplitude | 27 | 20.90 | –4.98 | –0.950 | 1.542 | .20 |
CMAP indicates compound muscle action potential.
We monitored overall quality of life by administering 2 questionnaires: a parent form and a nurse form that were developed for the DCA/CLA Clinical Trial.3 Both forms consisted of items designed to track physical and behavioral changes over time while the subject was at home or in the GCRC. There were no significant changes in median quality of life reported by parents or nurses during long-term administration of dichloroacetate (data not shown).
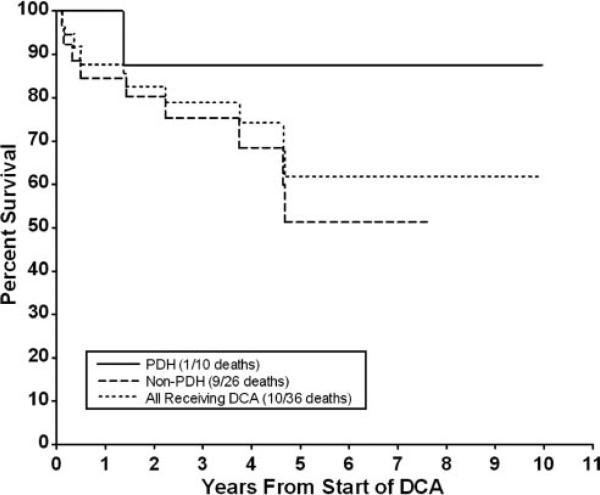
Finally, we conducted a survival analysis among all of the patients receiving dichloroacetate and further determined survival among those who had pyruvate dehydrogenase deficiency and those who did not (Fig 2). This latter group, therefore, included patients with a respiratory chain defect (N = 22) or a pathologic mitochondrial DNA mutation (N = 5). The estimated mean ± SE 3 year survival for all 36 subjects was 78.9% ± 8.3%. Mortality was accounted for primarily by deaths among children who did not have pyruvate dehydrogenase deficiency.
FIGURE 2.
Kaplan-Meier plot of percentage of survival of 36 subjects.
DISCUSSION
These data indicate that long-term oral administration of dichloroacetate, at a daily dose of 25 mg/kg, is generally well-tolerated in young children with genetic mitochondrial diseases, many of whom have been exposed to the drug for ≥4 years. Consistent with findings from the DCA/CLA Clinical Trial,3 long-term treatment with dichloroacetate continued to demonstrate a significant lactate-lowering effect that occurs independent of genotype.
However, in contrast to our previous observations in these and other children who received dichloroacetate for ≤6 months,3 it seems that continued drug administration is associated with an increased frequency of peripheral neuropathy, involving primarily the peroneal nerve. None of the nerve conduction results in these patients met the criteria for the diagnosis of a primarily demyelinating neuropathy, but a demyelinating component of pathogenesis is not excluded by these data. In older individuals harboring the A4243G mt DNA point mutation studied by Kaufmann et al,2 dichloroacetate exposure was associated with significant decreases in the amplitude of both the sural nerve action potential and peroneal nerve compound muscle action potential, particularly during the first 6 months of treatment. The results from both trials must be interpreted with caution, however, because both sensory and motor peripheral neuropathy are very common manifestations of genetic mitochondrial diseases in children4 and adults5 and may show signs consistent with a primarily demyelinating or axonal condition or a mixture of both. Indeed, among the 43 children who participated in the DCA/CLA Clinical Trial (including the 36 subjects of this study) the amplitude of the peroneal nerve and the conduction velocities of the median motor and peroneal nerves were uniformly lower than in healthy age-matched children reported in the literature.4 The neuropathy of the patients was independent of age, gender, or congenital mitochondrial disorder. Thus, the progression of peripheral neuropathy found in patients who composed this study may be due, in part, to the natural course of disease. Unfortunately, because of the generally impaired neuromuscular condition of the patients, it could not be determined with certainty whether any child experienced clinical signs or symptoms of worsening peripheral neuropathy, despite careful neurologic examination of the patients and interviews with parents at each 6-month evaluation. This further emphasizes the importance of formal electrical testing when characterizing the effects of mitochondrial genetic diseases on the peripheral nervous system.
Nevertheless, dichloroacetate is clearly a potential neurotoxin. Recent studies using cultured rat Schwann cells and dorsal root ganglia cells demonstrated that in vitro exposure to dichloroacetate caused a dose- and duration-dependent, but reversible, decrease of myelination, associated with a reduction in the expression of several myelin proteins.6
In the DCA/CLA Clinical Trial, patients exposed to dichloroacetate had higher urinary concentrations of maleylacetone and δ-aminolevulinate than patients who received placebo.3 These effects are because of the inhibitory action of the drug on maleylacetoacetate isomerase, the penultimate enzyme in the catabolism of tyrosine, which also dechlorinates dichloroacetate to glyoxylate.7 Increases in maleylacetone and δ-aminolevulinate have been considered responsible, respectively, for the hepatocellular and neurologic complications of hereditary tyrosinemia type 1, caused by loss-of-function mutations in the terminal enzyme of tyrosine catabolism, fumarylacetoacetate hydrolase.8 Although neither maleyl-acetone nor δ-aminolevulinate was measured during the long-term administration of dichloroacetate in the patients studied here, it is noteworthy that in vitro exposure of cultured rat Schwann cells and dorsal root ganglia cells to δ-aminolevulinate9 leads to change in the expression of myelin proteins similar to those observed in dichloroacetate-treated cells.6
Preliminary investigations in rats administered oral dichloroacetate at concentrations as high or higher than those administered to humans suggest that the drug also induces a sensory-motor peripheral neuropathy that is associated with axonal atrophy and is also directly dependent on dose and animal age, with older rats demonstrating both increased susceptibility to peripheral neuropathy and a decrease in clearance of dichloroacetate from plasma.10 These findings are consistent with the apparent age-dependent susceptibility of humans to peripheral neuropathy after treatment with dichloroacetate.2,3 Based on these findings, it is reasonable to assume that the peripheral neuropathy observed in the patients composing this report is the result of the chronicity of both their primary mitochondrial disease and their exposure to dichloroacetate, although the relative impact of each on peripheral nerve conduction cannot be quantified.
Urinary oxalate levels increased during dichloroacetate administration but remained within the reference range. This effect is expected, based on previous clinical investigations,11,12 because oxalate is an end product of dichloroacetate biotransformation.7 To our knowledge, there is no evidence that this consequence of dichloroacetate exposure has caused toxicity to humans or animals.
It is noteworthy that no other adverse events in our patients were associated with long-term administration of dichloroacetate. This is particularly important in assessing the effect of dichloroacetate on liver function. Dichloroacetate is a hepatic carcinogen in certain inbred strains of rodents when administered chronically at doses generally higher than those used clinically, although the mechanism accounting for tumorigenesis is unknown.13 Despite anecdotal reports of asymptomatic and reversible increases in circulating levels of aspartate transaminase and alanine transaminase in a few patients, there were no significant differences in the indices or in other measures of liver function between the 2 treatment groups in the DCA/CLA Clinical Trial. Furthermore, a recent report indicates that dichloroacetate may exert a tumoricidal effect in vitro and in vivo in human tumor cell lines or in human tumors implanted in immunocompromised rodents.14 These effects seem to be mediated by the drug's ability to stimulate pyruvate dehydrogenase activity and thus oxidative metabolism in cancer cells. This, in turn, is thought to increase respiratory chain activity, and the resulting increase in mitochondrial free radical production induces selective apoptosis in malignant, but not in benign, cells. If this scenario is accurate, then the same underlying mechanism invoked to rationalize the usefulness of dichloro-acetate in patients with mitochondrial energy failure1 explains its potential as an antitumor agent.
The current study provides only exploratory information on the efficacy of dichloroacetate in genetic mitochondrial diseases. Unfortunately, we know too little of the natural course of disease progression in patients with pyruvate dehydrogenase or respiratory chain complex deficiency to determine whether dichloroacetate significantly influences their clinical outcome. Ten of 36 patients who received long-term dichloroacetate expired, for an actuarial rate of 79% at 3 years. Nine deaths occurred among the 26 patients who had a respiratory chain defect and/or a mitochondrial DNA mutation, whereas only 1 death occurred among the 10 patients who had pyruvate dehydrogenase deficiency. It is interesting to speculate that children with pyruvate dehydrogenase deficiency may be particularly responsive to chronic dichloroacetate treatment. This notion is consistent with our recent analysis of 46 patients described in the literature who had pyruvate dehydrogenase deficiency who also received dichloroacetate.15 Dichloroacetate decreased blood and cerebrospinal fluid actate concentrations and was generally well tolerated when administered over a 20- to 135-mg/kg daily dose range and for a duration that ranged from 10 days to 9 years 9 months (median duration: 16 months). Four of these patients (3 girls) who enrolled in the DCA/CLA Clinical Trial and who are included in the present analysis received dichloroacetate for 7 years 6 months to 9 years 9 months, and this was temporally associated with biochemical and clinical improvement of their disease.
CONCLUSIONS
In conclusion, long-term oral dichloroacetate administration, at a daily dose of 25 mg/kg, to young children with various genetic mitochondrial diseases is generally well tolerated but may precipitate or worsen underlying peripheral neuropathy in some cases. Other drug-associated adverse effects are uncommon or rare. Whether dichloroacetate actually benefits patients with pyruvate dehydrogenase deficiency or other genetic subgroups with mitochondrial diseases remains to be determined.
What's Known on This Subject
Dichloroacetate is an investigational drug that has been widely used for the treatment of genetic mitochondrial diseases, such as those that lead to congenital forms of lactic acidosis. Recently, a phase 3 clinical trial was completed with dichloroacetate.
What This Study Adds
This study extends the recently completed phase 3 clinical trial of dichloroacetate to provide new information about the long-term tolerability of this compound.
ACKNOWLEDGMENTS
This study was supported by grants from the Muscular Dystrophy Association (MDA/92–95), the Orphan Products Division of the Food and Drug Administration (FDR-001500), and the National Institutes of Health (R01 ES07355, P42 ES 07375, and M01 RR00082).
We thank the patients and their families for their participation, the dedicated staff of the General Clinical Research Center, the pilots and staff of Mercy Medical Airlift and Angel Flight of Florida for facilitating patient transport, the members of the Data Safety Monitoring Board (Stephen Cederbaum, Chairman, Elizabeth Wright, John McReynolds, and Patricia Huff), and Candace Caputo for administrative assistance.
Abbreviations
- CLA
congenital lactic acidosis
- GCRC
General Clinical Research Center
Footnotes
The authors have indicated they have no financial relationships relevant to this article to disclose.
REFERENCES
- 1.Stacpoole PW, Barnes CL, Hurbanis MD, Cannon SL, Kerr DS. Treatment of congenital lactic acidosis with dichloroacetate. Arch Dis Child. 1997;77(6):535–541. doi: 10.1136/adc.77.6.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaufmann P, Engelstad K, Wei Y, et al. Dichloroacetate as a treatment for MELAS: a randomized, controlled clinical trial. Neurology. 2006;66(3):324–330. doi: 10.1212/01.wnl.0000196641.05913.27. [DOI] [PubMed] [Google Scholar]
- 3.Stacpoole PW, Kerr DS, Barnes C, et al. A controlled clinical trial of dichloroacetate for treatment of congenital lactic acidosis in children. Pediatrics. 2006;117(5):1519–1521. doi: 10.1542/peds.2005-1226. [DOI] [PubMed] [Google Scholar]
- 4.Stickler D, Valenstein E, Neiberger RE, et al. Peripheral neuropathy in genetic mitochondrial diseases. Pediatr Neurology. 2006;34(2):127–131. doi: 10.1016/j.pediatrneurol.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 5.Kaufmann P, Pascual JM, Anziska Y, et al. Nerve conduction abnormalities in patients with MELAS and the A3243G mutation. Arch Neurol. 2006;63(5):746–748. doi: 10.1001/archneur.63.5.746. [DOI] [PubMed] [Google Scholar]
- 6.Felitsyn N, Stacpoole PW, Notterpek L. Dichloroacetate causes reversible demyelination in vitro: Potential mechanism for its neuropathic effect. J Neurochem. 100(2):429–436. doi: 10.1111/j.1471-4159.2006.04248.x. 3007. [DOI] [PubMed] [Google Scholar]
- 7.Cornett R, James MO, Henderson GN, Cheung J, Shroads AL, Stacpoole PW. Inhibition of glutathione S-transferase zeta and tyrosine metabolism by dichloroaceate: a potential unifying mechanism for its altered biotransformation and toxicity. Biochem Biophys Res Commun. 1999;262(3):752–756. doi: 10.1006/bbrc.1999.1287. [DOI] [PubMed] [Google Scholar]
- 8.Langlois C, Jorquera R, Finegold M, Shroads AL, Stacpoole PW, Tanguay RM. Evaluation of dichloroacetate treatment in a murine model of hereditary tyrosinemia type 1. Biochem Pharmacol. 2006;71(11):1648–1661. doi: 10.1016/j.bcp.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 9.Felitsyn N, Stacpoole PW, Notterpek L. Delta-aminolevulinic acid inhibits myelination by Schwann cells.. Presented at Neuroscience; San Diego, CA. November 3–7, 2007.2007. [Google Scholar]
- 10.Stacpoole PW, Shroads AL, Felitsyn NM, Notterpek L, Calcutt NA. Mechanism of Age Dependence of Dichloroacetate-Induced Peripheral Neuropathy. United Mitochondrial Disease Foundation; Atlanta, GA: 2006. pp. 14–18. [Google Scholar]
- 11.Curry SH, Chu PI, Baumgartner TG, Stacpoole PW. Plasma concentrations and metabolic effects of intravenous sodium dichloroacetate. Clin Pharmacol Ther. 1985;37(1):89–93. doi: 10.1038/clpt.1985.17. [DOI] [PubMed] [Google Scholar]
- 12.Stacpoole PW, Henderson GN, Yan Z, Cornett R, James MO. Pharmacokinetics, metabolism and toxicology of dichloroacetate. Drug Metab Rev. 1998;30(3):499–539. doi: 10.3109/03602539808996323. [DOI] [PubMed] [Google Scholar]
- 13.Ammini CV, Stacpoole PW. Biotransformation, toxicology and pharmacogenomics of dichloroacetate. In: Gribble GW, editor. Natural Production of Organohalogen Compounds. Vol. 3/P in the series The Handbook of Environmental Chemistry. Springer-Verlag; Berlin: 2003. pp. 215–234. [Google Scholar]
- 14.Bonnet S, Archer SL, Allalunis-Turner J, et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11(1):37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 15.Berendzen K, Theriaque D, Shuster J, Stacpoole PW. Therapeutic potential of dichloroacetate for pyruvate dehydrogenase complex deficiency. Mitochondrion. 2006;6(3):126–135. doi: 10.1016/j.mito.2006.04.001. [DOI] [PubMed] [Google Scholar]