Abstract
The identification and diagnosis of diabetic foot ulcer (DFU) infections remains a complex problem. Because inflammatory responses to microbial invasion may be diminished in persons with diabetes, clinical signs of infection are often absent in persons with DFUs when infection is limited to localized tissue. In the absence of these clinical signs, microbial load is believed to be the best indicator of infection. Some researchers, however, believe microbial load to be insignificant and type of organism growing in the ulcer to be most important. Previous studies on the microbiology of DFUs have not provided enough evidence to determine the microbiological parameters of importance.
Infection-related complications of DFUs include wound deterioration, osteomyelitis, and amputation. Risk factors for amputation include age, peripheral vascular disease, low transcutaneous oxygen, smoking, and poor glycemic control. These risk factors are best measured directly with physiological measures of arterial perfusion, glycemic control, sensory neuropathy, plantar pressures, and activity level and by controlling off-loading. DFU bioburden has not been examined as a risk factor for infection-related complications. To address the relationship between wound bioburden and the development of infection-related complications in DFUs, tightly controlled prospective studies based on clearly defined, valid measures of wound bioburden and wound outcomes are needed. This article reviews the literature and proposes a model of hypothesized relationships between wound bioburden—including microbial load, microbial diversity, and pathogenicity of organisms—and the development of infection-related complications.
Keywords: wound infection, diabetic foot ulcers, amputation, osteomyelitis
Diabetic foot ulcers (DFUs) are a common type of chronic wound. The prevalence of diabetes is 6.3% in the general population, 8.7% among persons 20 years of age and older (Centers for Disease Control and Prevention, 2004), and 17% among veterans enrolled in the Veterans' Health Administration (VHA; Reiber et al., 2001). Approximately 15–25% of persons with diabetes will develop a DFU in their lifetime (American Diabetes Association, 1999; Boulton, Meneses, & Ennis, 1999). Persons with diabetes are at higher risk for developing infections than persons without diabetes (Grossi et al., 1991; Josephs, Cordts, DiEdwardo, LaMorte, & Menzoian, 1993; Reiber et al., 2001; Wymenga, van Horn, Theeuwes, Muytjens, & Slooff, 1992). Outcomes associated with infection-related complications among persons with DFUs are striking. For example, 14–24% of persons with DFUs will have an amputation (American Diabetes Association, 1999), and complications associated with foot ulcers account for 20–25% of all hospital days among persons with diabetes (Reiber, 1995).
Nonetheless, the identification and diagnosis of DFU infection, as with chronic wound infection in general, remains a complex problem (Bowler, 2003). During the past 10 years, 50 articles in the nursing and medical literature were devoted specifically to identifying and diagnosing infection in the chronic wound. Although a few of these have provided data to advance the knowledge base in this area, most address the problems associated with the uncertainty regarding the definition of infection, the issues surrounding wound cultures, rationales supporting best culturing techniques, and the role of clinical signs of infection. The reiteration of these issues underscores the continued frustration felt by clinicians in screening chronic wounds for localized infection. The problem is that studies on chronic wound infection have used variable definitions to determine wound infection, methods of wound sampling, and laboratory procedures for processing wound cultures (Bowler, 1998).
Despite the continued attention given to this problem, there is a critical gap in the knowledge base that centers on the value of wound culture findings in predicting clinical outcomes. To address this limitation in wound healing science, tightly controlled prospective studies based on clearly defined, valid measures of wound bioburden and wound outcomes are needed. Below, we will review the literature and describe a model of proposed relationships between wound bioburden and infection-related complications from which a prospective study could be designed. We conducted a search of the literature using Medline and CINAHL databases as well as a hand search of the bibliographies of published articles. Keywords that we used to search the literature included “diabetic foot ulcer” and “wound infection.”
DFU
Foot ulcers in persons with diabetes are most commonly caused by peripheral neuropathy (Boulton, 1997; Pecoraro, Reiber, & Burgess, 1990). The prevalence of neuropathy increases with both age and duration of diabetes (Boulton, 1997). Neuropathy leads to foot ulcers through two related pathways. In motor neuropathy alterations in muscle tension and tone cause foot deformities and increased plantar pressures. The resulting foot deformities are often referred to as charcot deformities, claw toes, and hammerhead toes. In claw toes, the toes pull up and the metatarsal heads become prominent, reducing the amount of weight bearing performed by the toes. The pad of skin below the metatarsal heads is exposed to increased shear pressures and is a common site for ulceration (Steed, 2001). Other foot deformities, such as charcot midfoot collapse, occur as a result of muscle weakness, muscle wasting, and abnormal foot postures. These deformities result in a “rocker” bottom shape of the foot and midfoot ulcers over areas of maximal weight bearing. High plantar foot pressures underlie the primary treatment of DFUs, which is to “off-load” the area of ulceration and protect it from repeated trauma during ambulation. Off-loading is accomplished through the use of specially designed shoes, sandals, or removable boots, or the application of total contact casts. These devices are designed to prevent pressure on the ulcer during ambulation. In sensory neuropathy protective sensation is lost, allowing minor trauma from poorly fitting shoes (Fotieo, Reiber, Carter, & Smith, 1999), walking on foreign objects, or exposure to heat to lead to foot ulceration. The nature of these two pathways is consistent with neuropathic ulcers being frequently located on the plantar surface of the foot, especially over metatarsal heads (Laing, 1994). The presence of motor neuropathy is determined by the presence of foot deformities and measures of maximal plantar pressures. The presence of sensory neuropathy is determined by assessing the threshold to light touch with Semmes-Weinstein monofilaments. Inability to feel a 5.07 monofilament applied to standardized locations on the plantar surface of the foot has been associated with risk of ulceration (Birke & Sims, 1986).
Once a DFU occurs, peripheral vascular disease may contribute to poor outcomes (Singh, Armstrong, & Lipsky, 2007). Persons with diabetes have more atherosclerosis than persons without diabetes. Peripheral vascular disease of the infrapopliteal vessels causes occlusive ischemia in a variety of locations on the foot, heel, and ankle (Fotieo et al., 1999), resulting in soft tissue necrosis. The presence of peripheral vascular disease can be determined by assessing arterial blood supply to the foot. Arterial toe pressures are most valid for measuring the macrovascular status of the diabetic foot (Boulton et al., 1999) because ankle-brachial indexes (ABI), a common measure of arterial blood supply, are unreliable in persons with diabetes because of calcification of the arterial walls. Transcutaneous oxygen measures on the dorsum of the foot are a measure of microvascular status, with TCPO2 values greater than 30 mmHg indicative of adequate perfusion for healing of chronic wounds, including DFUs (Boulton et al., 1999).
DFU Infections
Definition of Wound Infection
Wound infection occurs when the virulence factors of one or more wound organisms overwhelm host resistance resulting in invasion and replication of the organism and local tissue damage (Bowler, Duerden, & Armstrong, 2001). Invasion and multiplication of organisms occur in the wound tissue in contrast to both wound contamination and colonization. Wound contamination is defined as the presence of bacteria on the wound surface with no multiplication of bacteria (Baxter & Mertz, 1992; Gilchrist, 1994; Hutchinson & McGuckin, 1990), while wound colonization is the replication of organisms on the wound surface without invasion of wound tissue and with no host immune response (Mertz & Ovington, 1993). Contamination and colonization are conditions common to all wounds healing by secondary intention (Mertz & Ovington, 1993; Stotts & Hunt, 1997). Similarly, the mere presence of organisms in nonviable tissue, without invasion of viable tissue, does not constitute wound infection. Nonviable wound tissues, such as necrotic tissue, are known to support bacterial growth (Barnett, Dave, & Ksander, 1986). Another essential element of wound infection is that microorganisms must replicate in large enough numbers to cause injury and/or impair healing.
Clinical Signs of Infection
The final element of wound infection is host response and/or tissue injury. Host response and pathophysiological tissue injury are related in that they present clinical signs and symptoms of infection and infection-related complications. Although inflammatory responses are the first line of defense against microbial invasion and the first indication of infection, many chronic wounds do not express these signs of clinical infection despite high microbial load and/or the presence of pathogenic organisms (Gardner, Frantz, & Doebbeling, 2001). This is especially true in persons with diabetes (Gardner, Frantz, & Saltzman, 2005). The manifestation of inflammation may be altered in persons with diabetes because of population-specific factors. Age (Gilchrist, 1997), hyperglycemia, tissue perfusion and oxygenation, and other aspects of immunocompetence and anti-inflammatory drug use influence inflammatory responses to microbial invasion. These factors are of particular relevance in persons with diabetes because they have a high prevalence of peripheral vascular disease, microvascular disease, and elevated blood sugars. Therefore, clinical signs of infection among persons with DFUs are often absent when infection is limited to localized soft tissue and are expressed robustly only when the infection has advanced to underlying tissue and/or bone. Invasive, limb-threatening DFU infections are more obvious because the systemic inflammatory response or acute-phase response is triggered, amplifying local inflammatory responses so they become clinically apparent. The resulting extensive erythema and elevated body temperature, white blood cell count, and blood sugars are more easily recognized than the signs of localized infections.
Consistent with these observations, the Infectious Diseases Society of America developed a grading system that characterizes DFU infections as mild, moderate, or severe (Lipsky et al., 2004). Infections deemed to be mild are those without systemic signs of toxicity, with less than 2 cm of surrounding cellulitis, and without deep abscesses, but with two or more signs of local inflammation (purulence, erythema, pain, tenderness, warmth, or induration). Moderate infections are also without systemic or metabolic instability but also have at least one of the following symptoms: cellulitis extending greater than 2 cm from the ulcer margin, lymphangitic streaking spread beneath the superficial fascia, deep-tissue abscess, gangrene, or involvement of muscle, tendon, joint, or bone. Severe infections are characterized by systemic toxicity or metabolic instability (fever, chills, tachycardia, hypotension, confusion, vomiting, leukocytosis, acidosis, severe hypoglycemia, or azotemia).
Wound Bioburden
Wound bioburden refers to all of the dimensions of wound microbiology believed to be important in the development of wound infection. These dimensions include microbial load, microbial diversity, and the presence of pathogenic organisms. In the absence of clinical signs of infection, the quantity of organisms, or microbial load, is believed to be the best indicator of wound infection (Dow, 2001; U.S. Department of Health and Human Services, 1994). The gold standard method for examining microbial load is quantitative culture of viable wound tissue. Wound tissue is viewed as the most valid specimen for culture because tissue cultures reflect organisms invading the wound rather than those contaminating the surface (Stotts & Whitney, 1999). However, wound tissue biopsy is impractical for routine diagnostic applications because it is invasive, expensive, and not widely available given the specialized equipment and skills needed to obtain and process tissue specimens. Quantitative swab cultures, obtained using Levine's technique (Levine, Lindberg, Mason, & Pruitt, 1976), which expresses wound fluid from deep tissue layers, are valid measures of wound bioburden compared to cultures of wound tissue (Gardner et al., 2006).
Although numerous studies (Bendy et al., 1964; Daltrey, Rhodes, & Chattwood, 1981; Krizek & Robson, 1975; Lookingbill, Miller, & Knowles, 1978; Robson & Heggers, 1969) have demonstrated a microbial load greater than 105 organisms per gram of tissue to be the critical threshold for diagnosing infection, the critical threshold pertinent to DFUs and other chronic wounds may be higher. Some have questioned the 105 guideline, asserting that the interactions among specific types of pathogens may be more important than microbial load in promoting infection (Bowler, 2003; Bowler et al., 2001). Supporting this assertion is microbiological evidence that chronic wounds contain multiple species of microbial organisms (Bendy et al., 1964; Bryan, Dew, & Reynolds, 1983; Chow, Galpin, & Guze, 1977; Daltrey et al., 1981; Gilchrist & Reed, 1989; Peromet, Labbe, Yourassowsky, & Schoutens, 1973; Trengove, Stacey, McGechie, Stingemore, & Mata, 1996) and that those wounds with four or more different species have poor healing outcomes (Trengove et al., 1996). Nonetheless, it is unclear which organisms represent a definitive threat to the wound or which synergistically interact with others (Bowler et al., 2001).
Of the numerous organisms that colonize chronic wounds, experts believe Staphylococcus aureus, Pseudomonas aeruginosa, β-hemolytic streptococcus, and anaerobes are the primary causes of delayed healing and infection (Bowler et al., 2001). Of these organisms, S. aureus is most commonly isolated from chronic wounds (Bowler, 1998; Gardner et al., 2001), with the others occurring at relatively low frequencies. S. aureus is a known pathogen with an extensive array of virulence factors including proteases and toxins. As with most bacteria, these factors are primarily expressed at higher densities to enable the organism to further colonize and subsequently invade surrounding tissue. Such factors are rarely expressed at lower densities, where adherence and survival are paramount. For this reason, the significance of S. aureus in a chronic wound—pathogen versus colonizer—is difficult to ascertain. In a previous study, we found that the presence of S. aureus in the wound was associated with high microbial load in a sample of chronic wounds (Gardner, Frantz, Saltzman, & Dodgson, 2004). Although methicillin-resistant S. aureus (MRSA) is of particular concern for infection control purposes, whether methicillin resistance increases the pathogenicity of S. aureus in chronic wounds is unclear (Roghmann, Siddiqui, Plaisance, & Standiford, 2001). In addition, Panton-Valentine Leukocidin (PVL) is a cytotoxin produced by less than 5% of S. aureus strains but is implicated in severe necrotic skin infections (Lina et al., 1999). Its role in DFU complications has not been specifically explored. A positive relationship between nasal colonization with MRSA and wound MRSA has been described (Stanaway, Johnson, Moulik, & Gill, 2007).
Others believe wound anaerobes pose a greater, though unrecognized, threat because they are difficult to isolate using traditional specimen collection and transport techniques. An integrated synthesis of 62 studies on the aerobic and anaerobic microbiology of wounds (Bowler, 1998) concluded that anaerobes were more consistently isolated from infected than from noninfected chronic wounds.
Microbiological Studies of DFUs
The microbiology of clinically infected DFUs has been examined in several studies over the past 30 years (Armstrong, Liswood, & Todd, 1995; Diamantopoulos et al., 1998; El-Tahawy, 2000; Foster, McColgan, & Edmonds, 1998; Goldstein, Citron, & Nesbit, 1996; Johnson, Lebahn, Peterson, & Gerding, 1995; Lipsky, Pecoraro, Larson, Hanley, & Ahroni, 1990; Louie, Bartlett, Tally, & Gorbach, 1976; Prabhakar, Rao, & Hira, 1981; Sapico et al., 1980; Sapico, Witte, Canawati, Montgomerie, & Bessman, 1984; Wheat et al.,1986). Table 1 presents these studies along with pertinent study information. The criteria to define clinical infection among the various studies ranged from explicit clinical markers, such as purulence plus two or more signs of inflammation, to less explicit criteria, such as the presence of the diagnosis in the medical record, to unstated definitions. The level of infection severity ranged from mild, or uncomplicated, infections to severe, or limb-threatening, infections; only one study examined the microbiology of noninfected ulcers to compare differences with infected ulcers (Louie et al., 1976). Another compared the microbiology of gangrenous to nongangrenous ulcers (Prabhakar et al., 1981). The specific type of wound specimens obtained for culture also varied from study to study and included swab, exudates, aspirate, and wound tissue. Two studies used necrotic wound tissue specimens (Sapico et al., 1980; Sapico et al., 1984). Finally, the majority of studies described study ulcers using qualitative culture findings, with only two describing study ulcers using quantitative culture procedures (Sapico et al., 1980; Sapico et al., 1984).
Table 1. Microbiological Studies of Diabetic Foot Ulcers.
| Study | Definition of Clinical Infection | Severity of Infection | Wound Specimen | Culture Procedures | Number of Isolates | Organisms |
|---|---|---|---|---|---|---|
| Armstrong et al. (1995) (N = 112) | Dx of infection in medical record | Unclear | Wound tissue or bone | Qualitative | 1.5 | S. aureus 51%, anaerobes 7% |
| Diamantopoulos et al. (1998) (N = 84) | Explicit criteria: Extensive cellulitis, necrotizing fasciitis, and osteomyelitis | Limb-threatening | Wound tissue | Qualitative | 2.8 | S. aureus 51%, anaerobes 21 % |
| El-Tahawy (2000) (N = 111) | Unstated | Unclear | Exudates, aspirate, or wound tissue | Qualitative | Not reported | S. aureus 28%, anaerobes 11 % |
| Foster et al. (1998) (N = 64) | Unstated | Unclear | Swab | Unclear | Not reported | Not reported |
| Goldstein et al. (1996) (N = 25) | Vague criteria | Mild to moderate | Swab or exudates | Qualitative | 3.0 | S. aureus 76%, anaerobes 40% |
| Johnson et al. (1995) (N = 52) | Unstated | Limb-threatening | Swab (anaerobic) and aspirate | Qualitative (anaerobes only) | Not reported | Anaerobes 74% |
| Lipsky et al. (1990) (N = 50) | Explicit criteria: Pus plus two signs of inflammation or drainage | Mild | Swab and exudates or aspirate | Qualitative | 2.09 | S. aureus 54%, anaerobes 13% |
| Louie et al. (1976) (N = 20) | Cellulitis | Noninfected and infected with range of severity | Wound tissue | Qualitative | 5.8 | Peptococcus 80%, S. aureus 35% |
| Prabhakar et al. (1981) (N = 61) | Unstated | Gangrenous and infected | Wound tissue or swab | Qualitative | Not reported | Gangrenous: Proteus 72%, Nongangrene: S. pyogenes 46% |
| Sapico et al. (1980) (N =13) | Unstated | Scheduled for amputation | Swab, aspirate, and necrotic wound tissue | Quantitative | 4.7 | Proteus 31 %, S. aureus 23%, anaerobes 23% |
| Sapico et al. (1984) (N = 32) | Unstated | Scheduled for amputation | Swab, aspirate, and necrotic wound base | Quantitative | 4.81 | Group D strep 41%, anaerobes 28%, S. aureus 25% |
| Wheat et al. (1986) (N = 28 with reliable specimens) | Explicit criteria: Cellulitis, osteomyelitis, necrotizing fasciitis, and abscess | Mild to limb-threatening | Exudates or wound tissue or bone | Qualitative | Not reported | S. aureus 37%, anaerobes 22% |
Note: Dx = diagnosis; S. aureus = Staphylococcus aureus; S. pyogenes = Streptococcus pyogenes; Group D strep = Group D streptococci.
Similar to the findings of microbiological studies of other chronic wounds, S. aureus was the most commonly isolated organism regardless of infection severity (see Table 1), with isolation rates ranging from 23% to 76% of the ulcers. Among studies that examined more severe levels of infection, S. aureus was the most common organism isolated in some (Diamantopoulos et al., 1998; Wheat et al., 1986) but not all (Louie et al., 1976; Sapico et al., 1980; Sapico et al., 1984). The prevalence of anaerobes among study ulcers ranged from 7% to 80%. Louie and colleagues (1976) found that anaerobes were more common than S. aureus when precise sampling, transporting, and processing techniques were employed. Finally, the number of different microbial species isolated per wound ranged from 1.5 to 5.8. Louie and colleagues found that the mean number of species in stable, noninfected ulcers was 4.9 compared to 7.3 in those with extensive cellulitis.
Methodological limitations preclude comparisons among studies and the ability to draw definitive conclusions on proper interpretation/significance of wound culture findings. First, the criteria used to diagnose clinical infection varied or were not made explicit. The interobserver reliability among clinicians when judging the infection status of wounds without a criterion standard is not high (Greenwald et al., 2002). Therefore, the misclassification of ulcers as infected may have introduced substantial sampling bias. Second, only one study (Foster et al., 1998) compared microbiological findings between infected and noninfected ulcers to explore which microbiological indicators are associated with clinical infection. Yet, though the authors reported that clinically infected ulcers had significantly more positive swab cultures than noninfected ulcers, they neither defined a positive culture nor reported the organisms present. Third, the techniques used to obtain swab specimens were not described, and variation in technique can alter microbiological data. Fourth, some wound tissue samples were of necrotic tissue (Sapico et al., 1980; Sapico et al., 1984). Necrotic tissue is known to harbor more organisms than viable tissue. However, infection by definition is a phenomenon of viable wound tissue, not of necrotic tissue (Gardner & Frantz, 2003). Fifth, although one study prospectively examined the relationship between antibiotic treatment and DFU outcomes (Foster et al., 1998), none of the studies prospectively examined the predictive relationship between wound microbiology and ultimate wound outcomes. Prospective studies on wound bioburden and DFU outcomes are needed to provide a more definitive foundation on which to build prospective studies on effective treatment. The essential need is to determine the significance of specific microbiological indicators for clinical prediction and screening so that this information can then be used to guide treatment.
As noted by Gerding (1995), the science of DFU infections has not advanced significantly since the early work of Louie et al. (1976). Perplexing issues remain with respect to the significance of microbial load and diversity and the role of specific microbes, including anaerobes.
Infection-Related Complications
Wound infection is believed to underlie up to 90% of the lower extremity amputations in persons with diabetes (Boulton, Vileikyte, Ragnarson-Tennvall, & Apelqvist, 2005; Lavery, Armstrong, Wunderlich, Tredwell, & Boulton, 2003). High bioburden may lead to wound deterioration, osteomyelitis, and eventually amputation (Bamberger, Daus, & Gerding, 1987). Although risk factors for wound deterioration and/or osteomyelitis have not been explicitly examined, multiple risk factors for diabetes-related amputations have been examined among persons with DFUs, including social, behavioral, demographic, environmental, and pathophysiological variables (Reiber, Pecoraro, & Koepsell, 1992). Documented risk factors include age (Reiber, 1996), peripheral vascular disease (Adler, Boyko, Ahroni, & Smith, 1999; Reiber et al., 1992), sensory neuropathy (Adler et al., 1999), low transcutaneous oxygen (Adler et al., 1999; Reiber et al., 1992), smoking (Reiber et al., 1992), and poor glycemic control (Reiber et al., 1992). Peripheral vascular disease and smoking are linked to amputation because they ultimately decrease arterial perfusion and microcirculation to the lower extremities. Arterial perfusion can be more directly measured via toe pressures and ABI, and microcirculation can be more directly measured via transcutaneous oxygen measures of the foot than by either the presence of peripheral vascular disease or smoking history. Similarly, diabetes type and duration are linked to amputation via their impact on arterial perfusion and microcirculation, glycemic control, and degree of neuropathy. Glycemic control can be more directly measured via hemoglobin A1c levels, and degree of neuropathy can be more directly measured via Semmes-Weinstein monofilament assessment (sensory neuropathy) and magnitude of plantar pressures (motor neuropathy). Because neuropathy leads to increased plantar pressures, measures of activity combined with off-loading therapy are undoubtedly important in the pathway to foot ulcer complications. Activity level can be more directly measured through electronic activity monitors than through self-report levels of activity, and off-loading can be implemented for most patients. All of these factors (i.e., age, arterial perfusion, microcirculation, glycemic control, sensory and motor neuropathy, off-loading, and activity) are potential physiological contributors to DFU complications. Finally, Reiber and colleagues (1992) found a positive association between self-reported propensity for infection and amputation incidence. However, ulcer bioburden has not been examined as a predictive factor in studies of DFU complications.
Conceptual Framework of the Relationship Between Wound Bioburden and Infection-related Complications
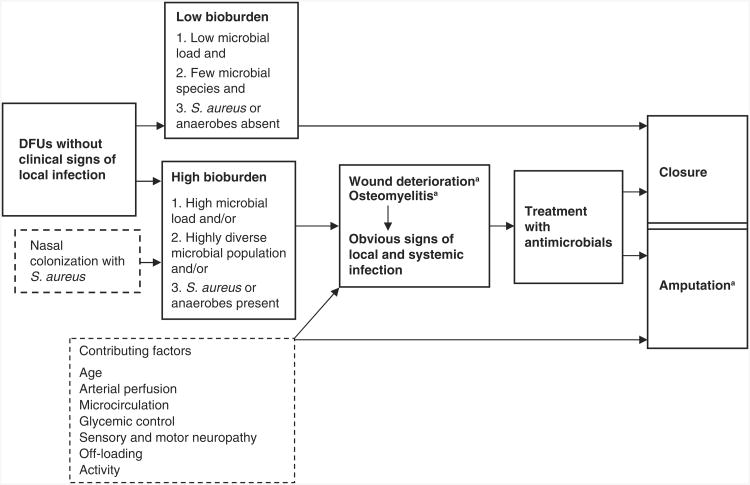
To address the issues surrounding DFU infection, we developed a model (see Figure 1) to depict the time-dependent relationship between wound bioburden and wound outcomes. In this model, DFUs without clinical signs of local infection may have low or high wound bioburden. DFUs with low bioburden progress to closure. DFUs with high bioburden are not treated because they lack clinically robust signs of infection. If we had effective screening tests to identify these DFUs, they could be targeted for preventive treatment before high bioburden led to the infection-related complications of wound deterioration and/or osteomyelitis. Wound deterioration and osteomyelitis are depicted together because these two complications, although technically different, often occur simultaneously. The rationale for distinguishing between the two rather than aggregating them is that osteomyelitis can develop without discernable clinical evidence of wound deterioration, and wound deterioration can lead to amputation in the absence of osteomyelitis. Therefore, these two complications are identified separately for descriptive purposes.
Figure 1.
Model of diabetic foot ulcer bioburden and outcomes. S. aureus = Staphylococcus aureus; DFUs = diabetic foot ulcers. Note: Bold text and solid-line boxes indicate primary variables; dotted-line boxes indicate confounding variables. a. Infection-related complication.
Wound deterioration and/or osteomyelitis, which may be accompanied by clinical signs of infection, trigger treatment with systemic antibiotics. If effective, these treatments contribute to wound closure. If treatment is not effective or is administered too late in the natural progression of the infection, amputation may result as the ultimate infection-related complication. Based on this model, DFUs that result in amputation would be assumed to be accompanied by wound deterioration and possibly osteomyelitis prior to amputation.
The sequential nature and partial dependence among the three complications provides the rationale for including each of them in the model because the focus is on infection-related complications prior to wound closure. If amputations were the only complication included in the model, other pertinent negative outcomes would be missed. We believe these less severe complications are important to include because they result in additional monitoring, treatment, and cost and may lead to the most severe outcome, amputation. Their inclusion provides the opportunity to more fully examine the prognostic efficacy of wound bioburden measures and to more fully capture the progression of infection-related complications.
Three microbiological dimensions of wound bioburden (denoted as 1, 2, and 3 in the bioburden-related boxes in Figure 1) are proposed to be associated either alone or in combination with subsequent infection-related complications prior to wound closure. These dimensions include high microbial load, a highly diverse microbial population, and/or the presence of S. aureus or anaerobes. The validity of each as a screening criterion can be determined by examining the probabilities associated with its value and wound deterioration and/or osteomyelitis and by examining the probabilities associated with its value and the ultimate complication, amputation (Greenberg, Daniels, Flanders, Eley, & Boring, 1996).
Also included in the model are other clinical variables that may contribute to DFU complications based on previous research of risk factors for amputation (these are listed in the dashed box). The variables included in this model are the more direct clinical and/or physiological measures of documented risk factors. Finally, the model includes the variable of nasal colonization with S. aureus as a factor that may contribute to high bioburden among patients with DFUs. Nasal colonization with S. aureus may thus contribute to infection-related complications.
Summary
The proposed model provides a means to prospectively examine the role of wound bioburden in the development of infection-related complications in conjunction with clinical signs of infection. It uniquely includes the role of wound bioburden using multiple microbiological dimensions or indicators. Studies that examine the prognostic efficacy of each specific dimension (i.e., microbial load, diversity of microbial population, and presence of pathogenic organisms) would positively impact the science of wound healing because they would address a central controversy surrounding appropriate dimensions to include in definitions of infection and the optimal thresholds to associate with these dimensions.
Acknowledgments
This work was supported by funding from National Institutes of Health, National Institute for Nursing Research (1RO1NR009448-01A2).
References
- Adler AI, Boyko EJ, Ahroni JH, Smith DG. Lower-extremity amputation in diabetes. The independent effects of peripheral vascular disease, sensory neuropathy, and foot ulcers. Diabetes Care. 1999;22(7):1029–1035. doi: 10.2337/diacare.22.7.1029. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. Consensus development conference on diabetic foot wound care. Diabetes Care. 1999;22(8):1354–1360. doi: 10.2337/diacare.22.8.1354. [DOI] [PubMed] [Google Scholar]
- Armstrong DG, Liswood PJ, Todd WF. 1995 William J. Stickel Bronze Award. Prevalence of mixed infections in the diabetic pedal wound. A retrospective review of 112 infections. Journal of the American Podiatric Medical Association. 1995;85(10):533–537. doi: 10.7547/87507315-85-10-533. [DOI] [PubMed] [Google Scholar]
- Bamberger D, Daus GP, Gerding DN. Osteomyelitis in the feet of diabetic patients. The American Journal of Medicine. 1987;83:653–660. doi: 10.1016/0002-9343(87)90894-1. [DOI] [PubMed] [Google Scholar]
- Barnett A, Dave B, Ksander GA. A concentration gradient of bacteria within wound tissue and scab. Journal of Surgical Research. 1986;41(3):326–332. doi: 10.1016/0022-4804(86)90044-2. [DOI] [PubMed] [Google Scholar]
- Baxter CR, Mertz PM. Local factors that affect wound healing. Nursing RSA Verpleging. 1992;7(2):16–23. [PubMed] [Google Scholar]
- Bendy RH, Nuccio PA, Wolfe E, Collins B, Tamburro C, Glass W, et al. Relationship of quantitative wound bacterial counts to healing of decubiti: Effect of topical gentamicin. Antimicrobial Agents and Chemotherapy. 1964;4:147–155. [PubMed] [Google Scholar]
- Birke JA, Sims DS. Plantar sensory threshold in the ulcerative foot. Leprosy Review. 1986;57(3):261–267. doi: 10.5935/0305-7518.19860028. [DOI] [PubMed] [Google Scholar]
- Boulton AJM, editor. Diabetic neuropathy. Carnforth, Lancashire, UK: Marius Press; 1997. Late sequelae of diabetic neuropathy; pp. 63–75. [Google Scholar]
- Boulton AJM, Meneses P, Ennis WJ. Diabetic foot ulcers: A framework for prevention and care. Wound Repair and Regeneration. 1999;7(1):7–16. doi: 10.1046/j.1524-475x.1999.00007.x. [DOI] [PubMed] [Google Scholar]
- Boulton AJM, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. The Lancet. 2005;366:1719–1724. doi: 10.1016/S0140-6736(05)67698-2. [DOI] [PubMed] [Google Scholar]
- Bowler PG. The anaerobic and aerobic microbiology of wounds: A review. Wounds. 1998;10(6):170–178. [Google Scholar]
- Bowler PG. The 10(5) bacterial growth guideline: Reassessing its clinical relevance in wound healing. Ostomy/Wound Management. 2003;49(1):44–53. [PubMed] [Google Scholar]
- Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clinical Microbiology Reviews. 2001;14(2):244–269. doi: 10.1128/CMR.14.2.244-269.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan CS, Dew CE, Reynolds KL. Bacteremia associated with decubitus ulcers. Archives of Internal Medicine. 1983;143:2093–2095. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. National diabetes fact sheet: General information and national estimates on diabetes in the United States, 2003 (Rev ed) Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2004. Retrieved February, 27 2008, from http://www.cdc.gov/diabetes/pubs/factsheet.htm. [Google Scholar]
- Chow AW, Galpin JE, Guze LB. Clindamycin for treatment of sepsis by decubitus ulcers. Journal of Infectious Diseases. 1977;135:S65–S68. doi: 10.1093/infdis/135.supplement.s65. [DOI] [PubMed] [Google Scholar]
- Daltrey DC, Rhodes B, Chattwood JG. Investigation into the microbial flora of healing and non-healing decubitus ulcers. Journal of Clinical Pathology. 1981;34:701–705. doi: 10.1136/jcp.34.7.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamantopoulos EJ, Haritos D, Yfandi G, Grigoriadou M, Margariti G, Paniara O, et al. Management and outcome of severe diabetic foot infections. Experimental and Clinical Endocrinology and Diabetes. 1998;106(4):346–352. doi: 10.1055/s-0029-1211996. [DOI] [PubMed] [Google Scholar]
- Dow G. Infection in chronic wounds. In: Krasner D, Rodeheaver G, Sibbald RG, editors. Chronic wound care: A clinical source book for healthcare professionals. Wayne, PA: HMP Communications; 2001. pp. 343–356. [Google Scholar]
- El-Tahawy AT. Bacteriology of diabetic foot. Saudi Medical Journal. 2000;21(4):344–347. [PubMed] [Google Scholar]
- Foster AVM, McColgan M, Edmonds ME. Should oral antibiotics be given to clean″ foot ulcers with no cellulitis? [Abstract 27] Diabetes Medicine. 1998;15(Suppl. 2):S10. [Google Scholar]
- Fotieo GG, Reiber GE, Carter JS, Smith DG. Diabetic amputations in the VA: Are there opportunities for interventions? Journal of Rehabilitation Research and Development. 36(1):55–59. [PubMed] [Google Scholar]
- Gardner SE, Frantz RA. Wound bioburden. In: Baronoski S, Ayello EA, editors. Wound care essentials: Practice principles. Philadelphia: Lippincott, Williams & Wilkins; 2003. pp. 91–116. [Google Scholar]
- Gardner SE, Frantz RA, Doebbeling BN. The validity of the clinical signs and symptoms used to identify localized chronic wound infection. Wound Repair and Regeneration. 2001;9(3):178–186. doi: 10.1046/j.1524-475x.2001.00178.x. [DOI] [PubMed] [Google Scholar]
- Gardner SE, Frantz RA, Saltzman CL. Diabetes and inflammation in infected chronic wounds. Wounds. 2005;17(8):203–205. [Google Scholar]
- Gardner SE, Frantz RA, Saltzman CL, Dodgson KJ. Staphylococcus aureus is associated with high microbial load in chronic wounds. Wounds. 2004;16(8):251–257. [Google Scholar]
- Gardner SE, Frantz RA, Saltzman CL, Hillis SL, Park H, Scherubel M. Diagnostic validity of three swab techniques in identifying chronic wound infection. Wound Repair & Regeneration. 2006;14(5):548–557. doi: 10.1111/j.1743-6109.2006.00162.x. [DOI] [PubMed] [Google Scholar]
- Gerding DN. Foot infections in diabetic patients: The role of anaerobes. Clinical Infectious Diseases. 1995;20(Suppl. 2):S283–S288. doi: 10.1093/clinids/20.supplement_2.s283. [DOI] [PubMed] [Google Scholar]
- Gilchrist B. Treating bacterial wound infection. Nursing Times. 1994;90(50):55–58. [PubMed] [Google Scholar]
- Gilchrist B. Infection and culturing. In: Krasner D, Kane D, editors. Chronic wound care: A clinical sourcebook for healthcare professionals. Wayne, PA: Health Management Publications; 1997. pp. 109–114. [Google Scholar]
- Goldstein EJ, Citron DM, Nesbit CA. Diabetic foot infections. Bacteriology and activity of 10 oral antimicrobial agents against bacteria isolated from consecutive cases. Diabetes Care. 1996;19(6):638–641. doi: 10.2337/diacare.19.6.638. [DOI] [PubMed] [Google Scholar]
- Greenberg R, Daniels S, Flanders W, Eley J, Boring J, editors. Medical epidemiology. Norwalk: Appleton & Lange; 1996. Diagnostic testing; pp. 75–83. [Google Scholar]
- Greenwald PW, Schaible DD, Ruzich JV, Prince SJ, Birnbaum AJ, Bijur PE. Is single observer identification of wound infection a reliable endpoint? Journal of Emergency Medicine. 23(4):333–335. doi: 10.1016/s0736-4679(02)00564-4. [DOI] [PubMed] [Google Scholar]
- Grossi EA, Esposito R, Harris LJ, Crooke GA, Galloway AC, Colvin SB, et al. Sternal wound infections and use of internal mammary artery grafts. Journal of Thoracic and Cardiovascular Surgery. 1991;102(3):342–346. discussion 346-347. [PubMed] [Google Scholar]
- Hutchinson JJ, McGuckin M. Occlusive dressings: A microbiologic and clinical review. American Journal of Infection Control. 1990;18(4):257–268. doi: 10.1016/0196-6553(90)90167-q. [DOI] [PubMed] [Google Scholar]
- Johnson S, Lebahn F, Peterson LR, Gerding DN. Use of an anaerobic collection and transport swab device to recover anaerobic bacteria from infected foot ulcers in diabetics. Clinical Infectious Diseases. 1995;20(Suppl. 2):S289–S290. doi: 10.1093/clinids/20.supplement_2.s289. [DOI] [PubMed] [Google Scholar]
- Josephs LG, Cordts PR, DiEdwardo CL, LaMorte WW, Menzoian JO. Do infected inguinal lymph nodes increase the incidence of postoperative groin wound infection? Journal of Vascular Surgery. 17(6):1077–1080. discussion 1080-1082. [PubMed] [Google Scholar]
- Krizek TJ, Robson MC. Evolution of quantitative bacteriology in wound management. American Journal of Surgery. 1975;130:579–584. doi: 10.1016/0002-9610(75)90516-4. [DOI] [PubMed] [Google Scholar]
- Laing P. Diabetic foot ulcers. American Journal of Surgery. 1994;167(Suppl. 1A):31S–36S. doi: 10.1016/0002-9610(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Lavery LA, Armstrong DG, Wunderlich RP, Tredwell J, Boulton AJM. Diabetic foot syndrome: Evaluating the prevalence of foot pathology in Mexican Americans and non-Hispanic whites from a disease management cohort. Diabetes Care. 2003;26:1435–1438. doi: 10.2337/diacare.26.5.1435. [DOI] [PubMed] [Google Scholar]
- Levine NS, Lindberg RB, Mason AD, Pruitt BA. The quantitative swab culture and smear: A quick, simple method for determining the number of viable aerobic bacteria on open wounds. Journal of Trauma. 1976;16(2):89–94. [PubMed] [Google Scholar]
- Lina G, Piemont Y, Godail-Gamot F, Bes M, Peter M, Gauduchon V, et al. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clinical Infectious Diseases. 1999;29:1128–1132. doi: 10.1086/313461. [DOI] [PubMed] [Google Scholar]
- Lipsky BA, Berendt AR, Deery HG, Embil JM, Joseph WS, Karchmer AW, et al. IDSA Guidelines: Diagnosis and treatment of diabetic foot infections. Clinical Infectious Diseases. 2004;39:885–910. doi: 10.1086/424846. [DOI] [PubMed] [Google Scholar]
- Lipsky BA, Pecoraro RE, Larson SA, Hanley ME, Ahroni JH. Outpatient management of uncomplicated lower extremity infections in diabetic patients. Archives of Internal Medicine. 1990;150:790–797. [PubMed] [Google Scholar]
- Lookingbill DP, Miller SH, Knowles RC. Bacteriology of chronic leg ulcers. Archives of Dermatology. 1978;114(12):1765–1768. [PubMed] [Google Scholar]
- Louie TJ, Bartlett JG, Tally FP, Gorbach SL. Aerobic and anaerobic bacteria in diabetic foot ulcers. Annals of Internal Medicine. 1976;85(4):461–463. doi: 10.7326/0003-4819-85-4-461. [DOI] [PubMed] [Google Scholar]
- Mertz PM, Ovington LG. Wound healing microbiology. Dermatology Clinics. 1993;11:739–747. [PubMed] [Google Scholar]
- Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care. 1990;13(5):513–521. doi: 10.2337/diacare.13.5.513. [DOI] [PubMed] [Google Scholar]
- Peromet M, Labbe M, Yourassowsky E, Schoutens E. Anaerobic bacteria isolated from decubitus ulcers. Infection. 1973;1(4):205–207. doi: 10.1007/BF01639650. [DOI] [PubMed] [Google Scholar]
- Prabhakar P, Rao AB, Hira J. Bacteriological study of diabetic foot ulcers. Tropical and Geographical Medicine. 1981;33(3):249–252. [PubMed] [Google Scholar]
- Reiber GE. Lower extremity foot ulcers and amputations in diabetes. In: Mi H, editor. Diabetes in America. Washington, DC: National Institutes of Health; 1995. [Google Scholar]
- Reiber GE. The epidemiology of diabetic foot problems. Diabetic Medicine. 1996;13(Suppl. 1):S6–S11. [PubMed] [Google Scholar]
- Reiber GE, Pecoraro RE, Koepsell TD. Risk factors for amputation in patients with diabetes mellitus. A case-control study. Annals of Internal Medicine. 1992;117(2):97–105. doi: 10.7326/0003-4819-117-2-97. [DOI] [PubMed] [Google Scholar]
- Reiber GE, Smith DG, Carter J, Fotieo G, Deery HG, II, Sangeorzan JA, et al. A comparison of diabetic foot ulcer patients managed in VHA and non-VHA settings. Journal of Rehabilitation Research and Development. 2001;38(3):309–317. [PubMed] [Google Scholar]
- Robson MC, Heggers JP. Bacterial quantification of open wounds. Military Medicine. 1969;134:19–24. [PubMed] [Google Scholar]
- Roghmann MC, Siddiqui A, Plaisance K, Standiford H. MRSA colonization and the risk of MRSA bacteraemia in hospitalized patients with chronic ulcers. Journal of Hospital Infection. 2001;47:98–103. doi: 10.1053/jhin.2000.0903. [DOI] [PubMed] [Google Scholar]
- Sapico FL, Canawati HN, Witte JL, Montgomerie JZ, Wagner FW, Bessman AN. Quantitative aerobic and anaerobic bacteriology of infected diabetic feet. Journal of Clinical Microbiology. 1980;12(3):413–420. doi: 10.1128/jcm.12.3.413-420.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapico FL, Witte JL, Canawati HN, Montgomerie JZ, Bessman AN. The infected foot of the diabetic patient: Quantitative microbiology and analysis of clinical features. Reviews of Infectious Diseases. 1984;6(Suppl. 1):S171–S176. doi: 10.1093/clinids/6.supplement_1.s171. [DOI] [PubMed] [Google Scholar]
- Singh N, Armstrong DG, Lipsky BA. Preventing foot ulcers in patients with diabetes. Journal of the American Medical Association. 2007;293:217–228. doi: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- Stanaway S, Johnson D, Moulik P, Gill G. Methicillin-resistant Staphylococcus aureus (MRSA) isolation from diabetic foot ulcers correlates with nasal MRSA carriage. Diabetes Research and Clinical Practice. 2007;75:47–50. doi: 10.1016/j.diabres.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Steed DL. Diabetic wounds, Assessment, classification, and management. In: Krasner D, Rodeheaver G, Sibbald RG, editors. Chronic wound care: A clinical source book for healthcare professionals. Wayne, PA: Health Management Publications; 2001. pp. 575–581. [Google Scholar]
- Stotts NA, Hunt TK. Managing bacterial colonization and infection. Clinics in Geriatric Medicine. 1997;13(3):565–573. [PubMed] [Google Scholar]
- Stotts NA, Whitney JD. Identifying and evaluating wound infection. Home Healthcare Nurse. 1999;17(3):159–165. doi: 10.1097/00004045-199903000-00008. [DOI] [PubMed] [Google Scholar]
- Trengove NJ, Stacey MC, McGechie DF, Stingemore NF, Mata S. Qualitative bacteriology and leg ulcer healing. Journal of Wound Care. 1996;5(6):277–280. doi: 10.12968/jowc.1996.5.6.277. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Treatment of pressure ulcers. Rockville, MD: Author; 1994. (AHCPR Publication No. 95-0652 Agency for Health Care Policy and Research, Public Health Service) [Google Scholar]
- Wheat LJ, Allen SD, Henry M, Kernek CB, Siders JA, Kuebler T, et al. Diabetic foot infections: Bacteriologic analysis. Archives of Internal Medicine. 1986;146:1935–1940. [PubMed] [Google Scholar]
- Wymenga AB, van Horn JR, Theeuwes A, Muytjens HL, Slooff TJ. Perioperative factors associated with septic arthritis after arthroplasty. Prospective multicenter study of 362 knee and 2,651 hip operations. Acta Orthopaedica Scandinavica. 1992;63(6):665–671. doi: 10.1080/17453679209169732. [DOI] [PubMed] [Google Scholar]