Abstract
Improvements in survival among central nervous system (CNS) tumor patients has made the risk of developing a subsequent cancer an important survivorship issue. Such a risk is likely influenced by histological and treatment differences between CNS tumors. De-identified data for 41,159 patients with a primary CNS tumor diagnosis from 9 Surveillance, Epidemiology and End Results (SEER) registries were used to calculate potential risk for subsequent cancer development. Relative risk (RR) and 95 % confidence interval (CI) of subsequent cancer was calculated using SEER*Stat 7.0.9, comparing observed number of subsequent cancers versus expected in the general United States population. For all CNS tumors studied, there were 830 subsequent cancers with a RR of 1.26 (95 % CI, 1.18–1.35). Subsequent cancers were observed in the CNS, digestive system, bones/joints, soft tissue, thyroid and leukemia. Radiotherapy was associated with an elevated risk, particularly in patients diagnosed with a medulloblastoma/primitive neuroectodermal tumor (MPNET). MPNET patients who received radiotherapy were at a significant risk for development of cancers of the digestive system, leukemia, bone/joint and cranial nerves. Glioblastoma multiforme patients who received radiotherapy were at lower risks for female breast and prostate cancers, though at an elevated risk for cancers of the thyroid and brain. Radiotherapy is associated with subsequent cancer development, particularly for sites within the field of radiation, though host susceptibility and post-treatment status underlie this risk. Variation in subsequent cancer risk among different CNS tumor histological subtypes indicate a complex interplay between risk factors in subsequent cancer development.
Keywords: Central nervous system cancer; Subsequent cancer; Radiotherapy; Surveillance, Epidemiology and End Results (SEER) Program
Introduction
Central nervous system (CNS) tumors are a substantial cause of morbidity and mortality in the United States, and will likely result in more than 13,000 estimate deaths in 2012 alone [1]. Increases in survival after a CNS tumor diagnosis have raised awareness of treatment related morbidity, the most concerning of these is the risk of developing a subsequent cancer [2]. Published data have indicated an elevated risk in patients with a primary CNS tumor for development of a variety of tumors, including bone, brain, soft tissue, thyroid, and salivary gland cancers as well as melanoma, leukemia and lymphoma [3–5], with one such study associating subsequent cancer risk with radiotherapy [6]. However, the aforementioned studies included data until 1998, before mandated reporting of benign brain tumors in 2004, and therefore did not stratify subsequent cancer risk by histological subtype of CNS tumor [7]. Our study focuses on utilizing only the most recently available data as collected by the Surveillance, Epidemiology, and End Results (SEER) Program, with data updated through 2009, which captures benign brain tumors, to estimate risk of subsequent cancer after primary CNS tumor diagnosis, stratifying by histological subtype of tumor and radiation status.
Materials and methods
Publically available, de-identified data from the original SEER 9 registries was used to identify a cohort of individuals diagnosed with a primary CNS tumor between 1973 and 2009. The 9 SEER registries included Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, and Utah [8]. Radiation status was limited to any type of radiotherapy versus no/refused radiotherapy. Using the International Classification of Diseases for Oncology, Third Edition (ICD-O-3) [9], CNS tumors were categorized into pilocytic astrocytoma (ICD-O-3 histological code 9421), astrocytoma grade II/III (AII/III) (9383, 9384, 9401, 9410, 9411, 9420, 9424), astrocytoma-not otherwise specified (NOS) (9400), glioblastoma multiforme (GBM) (9440–9442), oligodendroglioma grade II/III (OII/III) (9450, 9451, 9460), ependymoma (9391–9394), mixed glioma (9382), glioma NOS (9380), medulloblastoma/primitive neuroectodermal tumor (MPNET) (8901, 8921, 8963, 9363–9364, 9470–9474, 9501–9503, 9508) and meningioma (9530–9534, 9537–9539). The primary site of the CNS tumor for each histological type except meningioma was categorized into the following areas: cerebrum (ICD-O-3 site code C71.0), frontal lobe (C71.1), temporal lobe (C71.2), parietal lobe (C71.3), occipital lobe (C71.4), ventricle—NOS (C71.5), cerebellum—NOS (C71.6), brain stem (C71.7), overlapping lesion of brain (C71.8) and brain—NOS (C71.9). Meningiomas were limited to cerebral meninges (C70.0), spinal meninges (C70.1) and meninges—NOS (C70.9). Excluded from study were patients with diagnoses made at autopsy or on death certificate, non-surgically confirmed CNS tumors (i.e., patients who had no biopsy or no surgical resection), CNS tumors of borderline/uncertain malignant potential, or patients with an unknown age at diagnosis or race/ethnicity.
This study was reviewed and approved by the Internal Review Board at University Hospitals Case Medical Center in Cleveland, OH.
SEER*Stat 7.0.9 was used to compare the observed number of subsequent cancers versus the expected number in the general United States population in order to calculate the relative risk (RR) including the 95 % confidence interval (CI), of subsequent cancers following a diagnosis of a primary CNS tumor, calculated as a standardized incidence ratio, in which the comparison cohort was matched by age, sex, race and year of diagnosis [8]. The expected numbers of subsequent cancers were based on the assumption that incidence rates for new primary tumors equaled corresponding SEER rates for invasive primary cancers of that type. Person-years at risk for developing subsequent cancers included the time beginning 2 months after primary CNS tumor diagnosis to the date of death, the date of last known follow-up, or the end of the currently available data (i.e. 2009), whichever occurred first.
Results
Study sample
A total of 41,159 patients were diagnosed with a primary CNS tumor between 1973 and 2009. Tumor characteristics and demographics are presented in Online Resource 1. The most common histological diagnosis was GBM (47.1 %), particularly among adult patients age 20+ (53.4 %). The most common histological diagnosis in patients 0–19 years of age was MPNET (24.5 %), followed by pilocytic astrocytoma (21.4 %). Radiation status varied by histological subtype of tumor.
Risk of subsequent cancer
There were 830 subsequent new tumors with an overall RR of 1.26 (95 % CI, 1.18–1.35) (Table 1). The risk of developing a subsequent cancer was significantly elevated in all patients diagnosed with a glioma, with the exception of GBM, and highest in MPNET patients (RR 4.31; 95 % CI 3.33–5.49). Risk of a subsequent CNS tumor and cranial nerve/other central nervous system (CN/Other CNS) tumor was significantly elevated for those individuals diagnosed with a primary pilocytic astrocytoma, AII/III, astrocytoma NOS, ependymoma, mixed glioma, and MPNET. Other subsequent cancers with significantly elevated RRs include those of the digestive system, bone/joint, soft tissue, thyroid and leukemia. Significantly decreased risks were observed in GBM patients for subsequent female breast and male prostate cancers.
Table 1.
Relative risk (RR) of subsequent cancer development following diagnosis of a primary CNS tumor according to histological diagnosis (SEER 9 Registries, 1973–2009)
| Second cancer primary site | PA |
AII/III |
ANOS |
GBM |
OII/III |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| O | RR (95 % CI) | O | RR (95 % CI) | O | RR (95 % CI) | O | RR (95 % CI) | O | RR (95 % CI) | |
| All sites | 29 | 1.32 (0.88–1.89) | 88 | 1.35# (1.08–1.66) | 188 | 1.21# (1.04–1.4) | 131 | 0.86 (0.72–1.02) | 144 | 1.43# (1.21–1.69) |
| Salivary gland | 0 | 0 (0–15.94) | 1 | 5.39 (0.14–30.01) | 4 | 9.09# (2.48–23.28) | 0 | 0 (0–9.86) | 3 | 10.74# (2.22–31.4) |
| Digestive systema | 2 | 0.67 (0.08–2.41) | 15 | 1.43 (0.8–2.37) | 22 | 0.83 (0.52–1.26) | 31 | 1.09 (0.74–1.55) | 12 | 0.73 (0.38–1.27) |
| Bone/joint | 1 | 5.83 (0.15–32.5) | 2 | 12.88# (1.56–46.54) | 4 | 9.82# (2.68–25.15) | 1 | 5.38 (0.14–30) | 1 | 5.19 (0.13–28.92) |
| Soft tissue including heart | 3 | 10.98# (2.27–32.1) | 1 | 2.24 (0.06–12.47) | 4 | 3.76# (1.03–9.64) | 1 | 1.33 (0.03–7.39) | 5 | 7.77# (2.52–18.13) |
| Female breast | 3 | 0.91 (0.19–2.65) | 6 | 0.53 (0.2–1.16) | 15 | 0.65 (0.37–1.08) | 5 | 0.28# (0.09–0.66) | 13 | 0.88 (0.47–1.51) |
| Prostate | 3 | 1.16 (0.24–3.4) | 3 | 0.34 (0.07–1) | 14 | 0.64 (0.35–1.08) | 13 | 0.44# (0.23–0.75) | 21 | 1.25 (0.77–1.91) |
| Brain | 5 | 7.99# (2.6–18.66) | 21 | 20.10# (12.45–30.73) | 34 | 13.19# (9.13–18.43) | 11 | 5.95# (2.97–10.64) | 28 | 18.86# (12.53–27.25) |
| Cranial nerves other nervous system | 2 | 39.81# (4.82–143.8) | 2 | 29.53# (3.58–106.66) | 6 | 36.73# (13.48–79.94) | 0 | 0 (0–39.73) | 1 | 11.11 (0.28–61.92) |
| Thyroid | 2 | 1.95 (0.24–7.04) | 2 | 1.13 (0.14–4.07) | 9 | 2.42# (1.11–1.6) | 6 | 3.04# (1.12–6.62) | 4 | 1.59 (0.43–1.07) |
| Leukemia | 3 | 3.59 (0.74–10.49) | 4 | 2.48 (0.68–6.34) | 4 | 0.98 (0.27–2.51) | 5 | 1.33 (0.43–3.11) | 10 | 4.12# (1.98–7.57) |
| Otherb | 5 | 0.5 (0.16–1.16) | 31 | 1.05 (0.72–1.49) | 72 | 1 (0.78–1.26) | 58 | 0.85 (0.65–1.1) | 46 | 1.03 (0.75–1.37) |
|
| ||||||||||
| Second cancer primary site | E |
Mixed |
Glioma |
MPNET |
Meningioma |
|||||
| O | RR (95 % CI) | O | RR (95 % CI) | O | RR (95 % CI) | O | RR (95 % CI) | O | RR (95 % CI) | |
|
| ||||||||||
| All sites | 50 | 1.69# (1.26–2.23) | 31 | 1.14 (0.77–1.61) | 42 | 1.79# (1.29–2.42) | 65 | 4.31# (3.33–5.49) | 62 | 0.95 (0.73–1.21) |
| Salivary gland | 0 | 0 (0–46.51) | 0 | 0 (0–17.41) | 0 | 0 (0–59) | 1 | 16.99 (0.43–94.66) | 0 | 0 (0–24.65) |
| Digestive systema | 5 | 1.04 (0.34–2.42) | 7 | 1.62 (0.65–3.34) | 5 | 1.23 (0.4–2.86) | 9 | 4.72# (2.16–8.95) | 15 | 1.09 (0.61–1.8) |
| Bone/joint | 1 | 13.09 (0.33–72.93) | 1 | 15.61 (0.4–86.98) | 0 | 0 (0–66.25) | 4 | 29.12# (7.93–74.56) | 0 | 0 (0–60.85) |
| Soft tissue including heart | 0 | 0 (0–18.5) | 0 | 0 (0–19.52) | 1 | 6.55 (0.17–36.52) | 3 | 14.34# (2.96–41.92) | 0 | 0 (0–12.49) |
| Female breast | 8 | 1.82 (0.78–3.58) | 2 | 0.39 (0.05–1.42) | 2 | 0.59 (0.07–2.12) | 4 | 1.72 (0.47–1.41) | 6 | 0.62 (0.23–1.35) |
| Prostate | 5 | 1.04 (0.34–2.43) | 2 | 0.64 (0.08–2.31) | 6 | 1.64 (0.6–3.56) | 3 | 1.97 (0.41–5.77) | 9 | 0.91 (0.41–1.72) |
| Brain | 10 | 21.40# (10.26–39.36) | 9 | 20.34# (9.3–38.62) | 15 | 42.71# (23.91–70.45) | 14 | 28.53# (15.6–17.87) | 0 | 0 (0–5.88) |
| Cranial nerves other nervous system | 2 | 64.63# (7.83–233.45) | 0 | 0 (0–128.23) | 2 | 86.58# (10.49–312.77) | 2 | 48.77# (5.91–176.16) | 2 | 59.47# (7.2–214.83) |
| Thyroid | 1 | 1.5 (0.04–8.38) | 0 | 0 (0–1.46) | 0 | 0 (0–7.17) | 10 | 13.71# (6.57–25.21) | 2 | 3.2 (0.39–11.54) |
| Leukemia | 3 | 3.7 (0.76–10.83) | 0 | 0 (0–5.54) | 3 | 4.88# (1.01–14.26) | 10 | 15.89# (7.62–29.23) | 1 | 0.59 (0.02–3.31) |
| Otherb | 15 | 1.14 (0.64–1.88) | 10 | 0.8 (0.39–1.48) | 8 | 0.76 (0.33–1.49) | 5 | 0.71 (0.23–1.66) | 27 | 0.94 (0.62–1.37) |
O observed, O/E observed/expected, SEER Surveillance, Epidemiology and End Results, PA pilocytic astrocytoma, A NOS astrocytoma not otherwise specified, AII/III astrocytoma grade II and III, GBM glioblastoma multiforme, OII/III oligodendroglioma grade II and III, MPNET medulloblastoma/primitive neuroectodermal tumor
Statistically significant (p < 0.05)
Digestive system includes esophagus, stomach, small intestine, colon, rectum, anus, liver, gallbladder and pancreas
Other includes pharynx, respiratory system, female genital system, testis, skin, urinary system, thymus, lymphoma, myeloma and mesothelioma
Risk stratified by radiation status
To account for a potential influence of radiotherapy, results were stratified by radiation status and histological subtype of the CNS tumor (Table 2). Patients who received radiation, compared to those that did not, were at a significantly elevated risk for developing a subsequent cancer, particularly patients diagnosed with a primary astrocytoma NOS, OII/III, ependymoma and MPNET. Among those patients diagnosed with a MPNET who underwent radiotherapy, risks were significantly elevated for cancers of the thyroid, CNS/other CNS, and the digestive system. Interestingly, GBM patients who received radiotherapy had a significantly decreased risk of subsequent cancer development overall, compared to the same patients who did not received radiotherapy. The risk of subsequent cancer in the brain was significantly elevated in patients who received radiotherapy for those diagnosed with a primary GBM or MPNET. Similar significant risks were found in patients who received radiotherapy, compared to those that did not, for cancers of the CN/Other CNS, salivary gland, digestive system, thyroid, bone/joint, soft tissues and heart, though individual risks for these cancers varied by the primary CNS tumor histology (Table 2). Interestingly, the risk of female breast cancer in patients with astrocytoma NOS and GBM treated with radiotherapy decreased, and patients with GBM treated with radiotherapy had a lower rate of prostate cancer.
Table 2.
Relative risk (RR) of subsequent cancer development among primary CNS tumor patients according to histological diagnosis and radiation status (SEER 9 Registries, 1973–2011)
| Second cancer primary site |
First primary cancer histological type |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PA |
AII/III |
A NOS |
GBM |
|||||||||||||
| Radiation |
No radiation |
Radiation |
No radiation |
Radiation |
No radiation |
Radiation |
No radiation |
|||||||||
| O | RR O/E (95 % CI) |
O | RR O/E (95 % CI) |
O | RR O/E (95 % CI) |
O | RR O/E (95 % CI) |
O | RR O/E (95 % CI) |
O | RR O/E (95 % CI) |
O | RR O/E (95 % CI) |
O | RR O/E (95 % CI) |
|
| All sites | 8 | 1.43 (0.62–2.82) |
21 | 1.29 (0.8–1.97) | 66 | 1.29 (1–1.64) | 17 | 1.32 (0.77–2.12) | 120 | 1.23#(1.02–1.48) | 66 | 1.21 (0.94–1.54) |
109 | 0.80# (0.66–0.97) |
21 | 1.43 (0.89–2.19) |
| Salivary gland | 0 | 0 (0–176.25) | 0 | 0 (0–62.85) | 1 | 6.9 (0.17–38.42) | 0 | 0 (0–98.51) | 3 | 11.10# (2.29–32.43) |
1 | 6.27 (0.16–34.94) |
0 | 0 (0–11.06) | 0 | 0 (0–105.04) |
| Digestive systema | 1 | 1.3 (0.03–7.24) |
1 | 0.45 (0.01–2.52) |
10 | 1.21 (0.58–2.23) | 5 | 2.44 (0.79–5.69) | 16 | 0.96 (0.55–1.57) | 6 | 0.66 (0.24–1.43) |
22 | 0.88 (0.55–1.33) |
8 | 2.71# (1.17–5.35) |
| Bone/joint | 0 | 0 (0–96.78) | 1 | 7.62 (0.19–42.47) |
2 | 18.19# (2.2–65.7) |
0 | 0 (0–85.69) | 4 | 17.67# (4.81–15.24) |
0 | 0 (0–21.55) | 1 | 6.02 (0.15–33.55) |
0 | 0 (0–215.46) |
| Soft tissue including heart |
2 | 30.45# (3.69–110) |
1 | 4.88 (0.12–27.21) |
1 | 2.94 (0.07–16.41) |
0 | 0 (0–36.9) | 2 | 3.18 (0.38–11.48) | 2 | 4.9 (0.59–17.7) | 1 | 1.49 (0.04–8.29) |
0 | 0 (0–51.57) |
| Female breast | 0 | 0 (0–5.34) | 3 | 1.17 (0.24–3.41) |
5 | 0.6 (0.19–1.39) | 1 | 0.39 (0.01–2.19) | 7 | 0.47#(0.19–0.97) | 8 | 1.09 (0.47–2.14) |
2 | 0.13# (0.02–0.46) |
3 | 1.68 (0.35–.92) |
| Prostate | 1 | 1.34 (0.03–7.48) |
2 | 1.09 (0.13–3.94) |
3 | 0.41 (0.08–1.19) | 0 | 0 (0–2.89) | 10 | 0.77 (0.37–1.41) | 4 | 0.47 (0.13–1.21) |
11 | 0.41# (0.2–0.73) |
2 | 0.77 (0.09–2.79) |
| Brain | 2 | 13.24# (1.6–47.84) |
3 | 6.40# (1.32–18.71) |
16 | 19.92# (11.39–32.35) |
4 | 17.81# (4.85–45.6) |
18 | 11.47# (6.8–18.12) |
15 | 15.79# (8.84–26.05) |
11 | 6.65# (3.32–11.9) |
0 | 0 (0–22.09) |
| Cranial nerves/other nervous system |
0 | 0 (0–327.03) | 2 | 52.03# (6.3–187.95) |
1 | 19.82 (0.5–110.43) |
1 | 61.67# (1.56–343.59) |
5 | 51.97# (16.87–121.27) |
1 | 15.8 (0.4–88.01) |
0 | 0 (0–44.54) | 0 | 0 (0–428.39) |
| Thyroid | 1 | 3.85 (0.1–21.45) |
1 | 1.33 (0.03–7.39) |
1 | 0.76 (0.02–4.22) | 1 | 2.37 (0.06–13.23) |
4 | 1.86 (0.51–.77) | 5 | 3.36# (1.09–7.85) |
5 | 2.8 (0.91–6.53) |
1 | 6.11 (0.15–34.06) |
| Leukemia | 1 | 5.23 (0.13–29.15) |
2 | 3.14 (0.38–11.33) |
3 | 2.42 (0.5–7.07) | 1 | 2.88 (0.07–16.07) |
3 | 1.22 (0.25–3.56) | 1 | 0.66 (0.02–3.65) |
4 | 1.2 (0.33–3.08) |
1 | 2.69 (0.07–14.96) |
| Otherb | 0 | 0 (0–1.39) | 5 | 0.68 (0.22–1.58) |
23 | 1 (0.63–1.49) | 4 | 0.69 (0.19–1.77) | 48 | 1.06 (0.78–1.41) | 23 | 0.93 (0.59–1.39) |
52 | 0.86 (0.64–1.12) |
6 | 0.92 (0.34–2.01) |
| Second cancer primary site | First Primary Cancer Histological Type |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OII/III |
Ependymoma |
Mixed G |
||||||||||
| Radiation |
No radiation |
Radiation |
No radiation |
Radiation |
No radiation |
|||||||
| O | RR O/E (95 % CI) | O | RR O/E (95 % CI) | O | RR O/E (95 % CI) | O | RR O/E (95 % CI) | O | RR O/E (95 % CI) | O | RR O/E (95 % CI) | |
| All sites | 88 | 1.52# (1.22–1.87) | 51 | 1.28 (0.95–1.68) | 28 | 1.61# (1.07–2.33) | 19 | 1.63 (0.98–2.55) | 22 | 1.16(0.73–1.76) | 9 | 1.13 (0.51–2.14) |
| Salivary gland | 3 | 18.86# (3.89–55.1) | 0 | 0 (0–32.4) | 0 | 0 (0–76.5) | 0 | 0 (0–125.08) | 0 | 0 (0–68.7) | 0 | 0 (0–162.28) |
| Digestive systema | 7 | 0.72 (0.29–1.48) | 4 | 0.63 (0.17–1.6) | 2 | 0.71 (0.09–2.55) | 2 | 1.05 (0.13–3.78) | 6 | 1.97 (0.72–4.28) | 1 | 0.83 (0.02–4.62) |
| Bone/joint | 1 | 10.11 (0.26–56.33) | 0 | 0 (0–41) | 1 | 19.79 (0.5–110.25) | 0 | 0 (0–154.65) | 1 | 24.84 (0.63–138.42) | 0 | 0 (0–161.77) |
| Soft tissue including heart | 1 | 2.82 (0.07–15.74) | 4 | 14.50# (3.95–37.13) | 0 | 0 (0–30.19) | 0 | 0 (0–50.91) | 0 | 0 (0–29.23) | 0 | 0(0–62) |
| Female breast | 7 | 0.86 (0.35–1.77) | 5 | 0.81 (0.26–1.89) | 4 | 1.41 (0.38–3.61) | 4 | 2.7 (0.74–6.92) | 2 | 0.58 (0.07–2.11) | 0 | 0 (0–2.29) |
| Prostate | 13 | 1.29 (0.69–2.21) | 7 | 1.12 (0.45–2.3) | 3 | 1.22 (0.25–3.56) | 2 | 0.87 (0.1–3.13) | 2 | 0.89(0.11–3.21) | 0 | 0 (0–4.45) |
| Brain | 17 | 20.29# (11.82–32.48) | 10 | 16.24# (7.79–29.86) | 6 | 20.78# (7.63–15.23) | 2 | 12.01# (1.45–43.38) | 4 | 13.26# (3.61–33.94) | 5 | 37.50# (12.18–87.5) |
| Cranial nerves/other nervous system | 1 | 20.21 (0.51–112.59) | 0 | 0 (0–95.72) | 2 | 103.26# (12.5–372.99) | 0 | 0 (0–345.03) | 0 | 0 (0–194.54) | 0 | 0 (0–396.09) |
| Thyroid | 3 | 2.27 (0.47–6.64) | 1 | 0.88 (0.02–4.91) | 1 | 2.35 (0.06–13.07) | 0 | 0 (0–16.68) | 0 | 0 (0–6.68) | 0 | 0 (0–14.19) |
| Leukemia | 6 | 4.36# (1.6–9.48) | 4 | 4.00# (1.09–10.23) | 0 | 0 (0–7.67) | 3 | 9.59# (1.98–28.01) | 0 | 0 (0–8.17) | 0 | 0 (0–18.08) |
| Otherb | 29 | 1.12 (0.75–1.61) | 16 | 0.9 (0.51–1.46) | 9 | 1.15 (0.53–2.18) | 6 | 1.17 (0.43–2.55) | 7 | 0.81 (0.33–1.67) | 3 | 0.82 (0.17–2.41) |
|
| ||||||||||||
| Second cancer primary site | First Primary Cancer Histological Type |
|||||||||||
| Glioma NOS |
MPNET |
Meningioma |
||||||||||
| Radiation |
No radiation |
Radiation |
No radiation |
Radiation |
No radiation |
|||||||
| O | RR O/E (95 % CI) | O | RR O/E (95 % CI) | O | RR O/E (95 % CI) | O | RR O/E (95 % CI) | O | RR O/E (95 % CI) | O | RR O/E (95 % CI) | |
|
| ||||||||||||
| All sites | 20 | 1.62 (0.99–2.5) | 21 | 1.92# (1.19–2.93) | 60 | 4.59# (3.5–5.91) | 5 | 2.96 (0.96–6.9) | 20 | 1.04 (0.63–1.61) | 42 | 0.92 (0.66–1.24) |
| Salivary gland | 0 | 0 (0–111.86) | 0 | 0(0–127) | 1 | 19.2 (0.49–106.99) | 0 | 0 (0–671.19) | 0 | 0 (0–83.57) | 0 | 0 (0–35.27) |
| Digestive systema | 3 | 1.38 (0.28–.03) | 1 | 0.54 (0.01–2.98) | 9 | 5.49# (2.51–10.41) | 0 | 0 (0–15.38) | 3 | 0.79 (0.16–2.3) | 12 | 1.22 (0.63–2.13) |
| Bone/joint | 0 | 0 (0–130.54) | 0 | 0 (0–136.51) | 3 | 24.64# (5.08–72.02) | 1 | 79.21# (2.01–141.31) | 0 | 0 (0–189.66) | 0 | 0 (0–90.32) |
| Soft tissue including heart | 1 | 12.75 (0.32–71.04) | 0 | 0 (0–50.53) | 2 | 11.02# (1.33–39.81) | 1 | 43.99# (1.11–245.09) | 0 | 0 (0–41.44) | 0 | 0 (0–18.05) |
| Female breast | 1 | 0.58 (0.01–3.25) | 1 | 0.61 (0.02–3.39) | 4 | 2.07 (0.57–5.31) | 0 | 0 (0–11.7) | 0 | 0 (0–1.33) | 6 | 0.88 (0.32–1.91) |
| Prostate | 3 | 1.57 (0.32–4.58) | 3 | 1.73 (0.36–5.06) | 3 | 2.16 (0.44–6.3) | 0 | 0 (0–29.26) | 3 | 0.95 (0.2–2.77) | 6 | 0.9 (0.33–1.97) |
| Brain | 8 | 43.15# (18.63–85.02) | 7 | 42.92# (17.26–88.43) | 14 | 32.93# (18–55.25) | 0 | 0 (0–68.3) | 0 | 0 (0–19.14) | 0 | 0 (0–8.56) |
| Cranial nerves/other nervous system | 2 | 169.55# (20.53–612.48) | 0 | 0 (0–332.49) | 2 | 57.39# (6.95–207.3) | 0 | 0 (0–721.59) | 0 | 0 (0–341.53) | 2 | 88.50# (10.72–319.69) |
| Thyroid | 0 | 0 (0–13.85) | 0 | 0 (0–15.3) | 10 | 15.62# (7.49–28.73) | 0 | 0 (0–57.38) | 2 | 9.40# (1.14–33.97) | 0 | 0 (0–8.99) |
| Leukemia | 1 | 3.18 (0.08–17.7) | 2 | 6.75 (0.82–24.39) | 7 | 13.12# (5.28–27.04) | 3 | 36.42# (7.51–106.44) | 1 | 2.1 (0.05–11.7) | 0 | 0 (0–3.09) |
| Otherb | 1 | 0.18# (0–0.99) | 7 | 1.44 (0.58–2.97) | 5 | 0.82 (0.26–1.9) | 0 | 0 (0–4.83) | 11 | 1.3 (0.65–2.33) | 16 | 0.8 (0.46–1.3) |
PA pilocytic astrocytoma, AII/IH astrocytoma grade II and III, A NOS astrocytoma not otherwise specified, GBM glioblastoma multiforme, OII/III oligodendroglioma grade II and III, Mixed G mixed glioma, MPNET medulloblastoma/primitive neuroectodermal tumor, O observed, O/E observed/expected, SEER surveillance, epidemiology and end results
Statistically significant (p < 0.05)
Digestive system includes esophagus, stomach, small intestine, colon, rectum, anus, liver, and gallbladder
Other includes pharynx, respiratory system, female genital system, testis, skin, urinary system, thymus, lymphoma, myeloma and mesothelioma
Risk of subsequent cancer in MPNET and GBM patients
With the incidence of MPNET peaking before the age of 19 years and GBM peaking later in life (Fig. 1), further investigation of these two tumor types was performed by latency period to further assess the development of subsequent cancers. Overall risk of developing a subsequent cancer was significantly elevated in MPNET patients ages 0–19 years, who had received radiotherapy, in all latency periods (Table 3). In these treated patients there was a significantly elevated risk of developing cancers of the digestive system, urinary system, brain and thyroid and leukemia within the first 10 years. Risk of subsequent brain, CN/other CNS and thyroid tumors was significantly elevated 10 years post-diagnosis. However, significant risks for developing leukemia, soft tissue and bone/joint cancers among children diagnosed with a MPNET were observed independent of radiation status.
Fig 1.
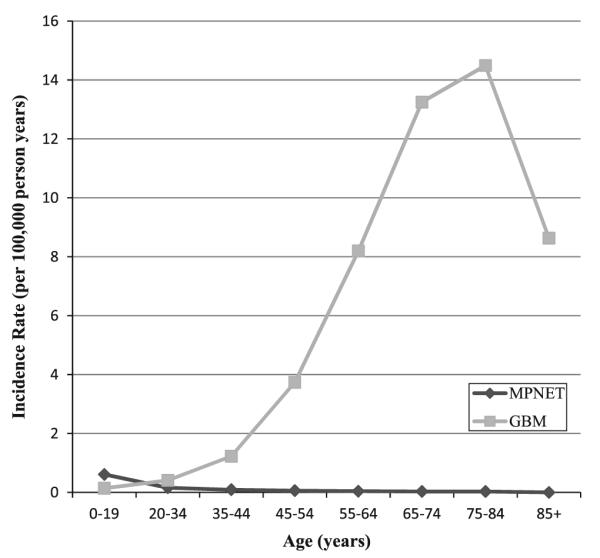
United States incidence rates of medulloblastoma/primitive neuroectodermal tumor (MPNET) and glioblastoma multiforme (GBM) tumors [50]
Table 3.
Relative risk (RR) of subsequent cancer development among primary medulloblastoma/primitive neuroectodermal tumor (MPNET) patients age 0–19 years according to Radiation Status and Latency Period (SEER 9 Registries, 1973–2011)
| Second cancer primary site |
Latency period |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2–11 months |
12–59 months |
60–119 months |
120 months+ |
|||||||||||||
| Radiation |
No radiation |
Radiation |
No radiation |
Radiation |
No radiation |
Radiation |
No radiation |
|||||||||
| O | RR (95 % CI) | O | RR (95 % CI) | O | RR (95 % CI) | O | RR (95 % CI) | O | RR (95 % CI) | O | RR (95 % CI) | O | RR (95 % CI) | O | RR (95 % CI) | |
| All sites | 2 | 16.75# (2.03–60.51) |
0 | 0 (0–130.61) | 5 | 12.00# (3.9–28.01) |
1 | 15.55 (0.39–86.62) |
13 | 27.63# (14.71–7.25) |
3 | 64.98# (13.4–189.89) |
18 | 7.77# (4.6–12.28) |
1 | 6.59 (0.17–36.73) |
| Digestive systema | 1 | 257.52# (6.52–1434.82) |
0 | 0 (0–2848.5) | 1 | 90.48# (2.29–504.11) |
0 | 0 (0–1531.45) | 1 | 69.76# (1.77–388.65) |
0 | 0 (0–3152.81) | 2 | 11.21# (1.36–40.5) |
0 | 0 (0–359.21) |
| Bone/joint | 0 | 0 (0–644.44) | 0 | 0 (0–6904.28) | 0 | 0 (0–144.62) | 0 | 0 (0–1752.24) | 1 | 34.46 (0.87–192.01) |
0 | 0 (0–1304.33) | 2 | 55.57# (6.73–200.74) |
1 | 246.32# (6.24–1372.43) |
| Soft tissue including heart |
0 | 0 (0–527.76) | 0 | 0 (0–1934.51) | 0 | 0 (0–166.08) | 0 | 0 (0–925.46) | 1 | 45.56# (1.15–253.85) |
1 | 433.84# (10.98–2417.18) |
0 | 0 (0–70.52) | 0 | 0 (0–879.97) |
| Urinary system | 0 | 0 (0–591.48) | 0 | 0 (0–1590.73) | 0 | 0 (0–263.76) | 0 | 0(0–907) | 1 | 113.38# (2.87–631.7) |
0 | 0 (0–2989.47) | 0 | 0 (0–53.28) | 0 | 0 (0–754.92) |
| Brain | 0 | 0 (0–165.68) | 0 | 0 (0–746.66) | 1 | 13.91 (0.35–77.48) |
0 | 0 (0–315.85) | 3 | 54.17# (11.17–158.3) |
0 | 0 (0–456.67) | 6 | 59.59# (21.87–129.7) |
0 | 0 (0–397.61) |
| Cranial nerves/other nervous system |
0 | 0 (0–1489.75) | 0 | 0 (0–5201.92) | 0 | 0 (0–520.81) | 0 | 0 (0–2385.85) | 0 | 0 (0–734.85) | 0 | 0 (0–5061.06) | 2 | 238.15# (28.84–860.29) |
0 | 0 (0–5025.92) |
| Thyroid | 0 | 0 (0–1494.63) | 0 | 0 (0–13270.38) | 0 | 0 (0–225.41) | 0 | 0 (0–2366.85) | 2 | 56.66# (6.86–204.67) |
0 | 0 (0–1548.35) | 5 | 20.75# (6.74–18.43) |
0 | 0 (0–256.03) |
| Leukemia | 1 | 30.19 (0.76–168.21) |
0 | 0 (0–392.24) | 2 | 21.70# (2.63–78.39) |
1 | 52.55# (1.33–292.81) |
4 | 63.07# (17.18–161.47) |
2 | 216.96# (26.28–783.75) |
0 | 0 (0–35.13) | 0 | 0 (0–375.8) |
| Otherb | 0 | 0 (0–101.84) | 0 | 0 (0–538.07) | 1 | 6.4 (0.16–35.64) |
0 | 0 (0–205.62) | 0 | 0 (0–15.55) | 0 | 0 (0–202.4) | 1 | 0.66 (0.02–3.65) | 0 | 0 (0–39.23) |
O observed, O/E, observed/expected, SEER surveillance, epidemiology and end results
Statistically significant (p < 0.05)
Digestive system includes esophagus, stomach, small intestine, colon, rectum, anus, liver, and gallbladder
Other includes oral cavity, pharynx, respiratory system, female genital system, breast, prostate, testis, skin, thymus, lymphoma, myeloma and mesothelioma
Patients over age 20, diagnosed with a GBM, who received radiotherapy were at a significantly decreased overall risk for subsequent cancer development during the first year after diagnosis, particularly for female breast cancer and prostate cancer (Table 4). There were significantly elevated risks in patients who had received radiotherapy for developing cancers of the colon and thyroid during the first 5 years and brain tumors from 5 to 10 years after diagnosis. Interestingly, there was a significantly increased overall risk of cancer in patients not treated with radiotherapy 5–10 years after diagnosis.
Table 4.
Relative risk (RR) of subsequent cancer development among primary GBM patients age 20+ years according to Radiation Status and Latency Period (SEER 9 Registries, 1973–2011)
| Second cancer primary site |
Latency period |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2–1 months |
12–59 months |
60–119 months |
120 months+ |
|||||||||||||
| Radiation |
No radiation |
Radiation |
No radiation |
Radiation |
No radiation |
Radiation |
No radiation |
|||||||||
| O | RR (95 % CI) | O | RR 95 % CI) | O | RR 95 % CI) | O | RR (95 % CI) | O | RR (95 % CI) | O | RR (95 % CI) | O | RR (95 % CI) | O | RR (95 % CI) |
|
| All sites | 50 | 0.60# (0.45–0.79) | 11 | 1.19 (0.59–2.12) | 39 | 1 (0.71–1.37) | 6 | 1.7 (0.62–3.69) | 10 | 1.56 (0.75–2.86) | 4 | 3.83# (1.04–9.8) | 6 | 0.86 (0.32–1.88) | 0 | 0 (0–5.51) |
| Colon | 3 | 0.46 (0.09–1.33) | 2 | 2.37 (0.29–8.57) | 7 | 2.61# (1.05–5.37) | 2 | 7.31 (0.89–26.41) | 0 | 0 (0–9.4) | 0 | 0 (0–42.16) | 1 | 2.27 (0.06–12.64) | 0 | 0 (0–101.76) |
| Female breast |
2 | 0.23# (0.03–0.83) | 0 | 0 (0–3.58) | 0 | 0.00# (0–0.78) | 2 | 4.37 (0.53–15.78) | 0 | 0 (0–3.98) | 1 | 5.08 (0.13–28.33) | 0 | 0 (0–3.25) | 0 | 0 (0–38.43) |
| Prostate | 5 | 0.30# (0.1–0.69) | 1 | 0.58 (0.01–3.26) | 4 | 0.54 (0.15–1.37) | 0 | 0 (0–5.9) | 1 | 0.87 (0.02–4.83) | 1 | 11.66 (0.3–64.95) | 1 | 0.79 (0.02–4.38) | 0 | 0 (0–23.92) |
| Brain | 2 | 2.09 (0.25–7.54) | 0 | 0 (0–37.76) | 2 | 4.05 (0.49–14.64) | 0 | 0 (0–87.57) | 4 | 44.43# (12.11–113.77) | 0 | 0 (0–280.06) | 1 | 12.33 (0.31–68.7) | 0 | 0 (0–485.26) |
| Thyroid | 1 | 1.19 (0.03–6.61) | 1 | 13.36 (0.34–74.42) | 3 | 4.90# (1.01–14.31) | 0 | 0 (0–74.26) | 1 | 7.17 (0.18–39.93) | 0 | 0 (0–185.49) | 0 | 0 (0–25.64) | 0 | 0 (0–248.17) |
| Othera | 37 | 0.75 (0.53–1.04) | 7 | 1.27 (0.51–2.62) | 23 | 1.01 (0.64–1.51) | 2 | 0.96 (0.12–3.46) | 4 | 1.07 (0.29–2.75) | 2 | 3.12 (0.38–11.25) | 3 | 0.77 (0.16–2.26) | 0 | 0 (0-10.24) |
O observed, O/E observed/expected, SEER surveillance, epidemiology and end results
Statistically significant (p < 0.05)
Other includes oral cavity, pharynx, digestive system excluding colon, bones/joints, soft tissue, urinary system, respiratory system, female genital system, cranial nerves/other nervous system, testis, skin, thymus, leukemia, lymphoma, myeloma and mesothelioma
Discussion
Primary CNS tumor patients have been associated with a risk for subsequent cancers [3, 4, 10]. However, we provide the first known large-scale study stratifying the risk by the histological subtype of the CNS tumor, with attention to such potential risk factors as radiation status, age and latency period. Our study showed there is a risk for subsequent cancer in patients diagnosed with a MPNET, AII/III, astrocytoma NOS, OII/III, ependymoma and glioma NOS. The subsequent cancers that develop in such patients include those of the brain, CN/other CNS, digestive system, bone/joint, soft tissue, thyroid and leukemia. Though previous reports found significant risks for cancers of the bone, soft tissue, brain/CNS, thyroid, and leukemia, this study found that subsequent cancer risk varied by the histological subtype of the CNS tumor, which the aforementioned studies did not account for (Table 1) [3].
Though reports have implicated radiation therapy as a risk factor for development of a subsequent cancer [3, 4, 6,11–16], our study provided the first known large-scale comparison of subsequent cancer risk in patients who had radiation therapy for their primary CNS tumor to those that did not, separated by histological subtypes. Significantly elevated risks were found for patients diagnosed with an astrocytoma NOS, OII/III, ependymoma and MPNET and who had received radiotherapy (Table 2). These risks were not found for patients with the same histological subtype of primary CNS tumor who did not receive radiotherapy, therefore supporting the hypothesis that radiotherapy is associated with development of a subsequent cancer. For patients who received radiotherapy, they were at risk not only for subsequent cancer likely related to the field of radiation (such as brain, CNS/other CNS and thyroid), but also cancer of the bone/joint, heart, and soft tissue. Past studies with a primary CNS tumor cohort found similar subsequent cancer sites, particularly in the CNS [3, 12, 15], though our study is the first to distinguish between histological subtypes of primary CNS tumors.
There still exists some uncertainty, however, in the link between the primary CNS tumor, radiotherapy and the development of digestive system, bone/joint and soft tissue cancers [3], given such radiotherapy is generally limited to the CNS. Cancer of the upper GI tract, such as esophageal tumors, may have originated in the radiotherapy field, though such sites were not at a significant risk for developing cancer when studied alone. An alternative explanation is that in patients treated with radiation and chemotherapy, severe immunosuppression may result in diminished immune response [17]. Furthermore, genetic/epigenetic propensities related to various primary CNS tumor histologies, such as mutations in p53, and damage induced by ionizing radiation, may underlie subsequent cancer risk in these patients [18, 19]. Additionally, improved survival due to the beneficial effects of radiotherapy on the tumor may also contribute by improving survival, thereby increasing the opportunities to develop and be diagnosed with subsequent cancers.
Since MPNET patients had the highest risk of subsequent cancer, particularly those who received radiotherapy, focus was placed on MPNET tumors in patients age 0–19. Significant risk was found for development of a digestive system cancer and leukemia within the first 5 years after a MPNET diagnosis and cancers of the digestive system, soft tissue, urinary system, bone/joint, brain, CN/other CNS and thyroid, as well as leukemia 5–10 years post-diagnosis (Table 3). Risks for cancer of the digestive system, urinary system, brain, CN/other CNS and thyroid were found in patients who had received radiotherapy for the MPNET, and comparatively not found in those that did not receive radiotherapy, indicating that radiotherapy appears to be a risk factor in the development of such subsequent cancers. This is particularly important for patients diagnosed with a MPNET, in which current standard of care involves combination radiotherapy and chemotherapy, though newer approaches avoid radiotherapy [20].
Patients treated with radiotherapy likely have a measureable dose of radiation to nearby structures, potentially putting these areas at risk for development of a malignancy, while chemotherapy may be related to development of hematologic malignancies such as leukemia. The implications of radiotherapy in pediatric patients have been well documented [5, 21–46]. The Childhood Cancer Survivor Study (CCSS) is a population-based cancer registry utilized in numerous reports to assess the subsequent cancer risk in patients diagnosed with a childhood cancer [21,22, 25, 28, 35, 37, 42–44]. The results of these studies have shown that pediatric patients diagnosed with a cancer, particularly those who received radiation therapy, are at a significant risk for development of subsequent cancer, particularly of the thyroid, soft tissue, CNS and lymphoma, similar to the findings presented here [21, 28, 35]. Another study reported increased incidence of leukemia, gliomas, fibrosarcoma, thyroid, and bone cancers in pediatric patients diagnosed with a MPNET [5]. However, the aforementioned studies were limited by their inability to stratify by both histological subtype of CNS tumor and radiation status. Our results confirm that MPNET survivors are at an elevated risk for development of subsequent cancers, particularly patients treated with CNS radiation.
Due to the short survival of GBM patients, the median survival time of approximately 12–15 months is likely not enough time to develop a subsequent cancer. In fact, patients diagnosed with a GBM who received radiation therapy were at a significantly lower risk for development of female breast and prostate cancers (Table 2). Interestingly, the risk for development of a subsequent cancer following diagnosis of a GBM was insignificant for most sites but increased for brain and thyroid cancer a year post-diagnosis (Table 4). Though these areas would be exposed to radiation, development of such cancers a year post-diagnosis more likely suggests potential host susceptibility for cancer. Prior studies on adult GBM patients and risk of subsequent cancer proved to be very limited, though one study, utilizing the SEER database, found insignificant risks for development of a subsequent cancer after a GBM diagnosis [4], though radiation status and latency were not accounted for.
The large sample size of the SEER data makes for one of the greatest strengths of this study. SEER’s ability to limit the cohort for only those who had surgical confirmation of the histological diagnosis allowed us to perform analysis stratified by histological subtype. Additionally, the data presented here included changes in CNS tumor collection, with the beginning of collection of benign brain tumors in 2004 [7]. However, the SEER 9 registries, though allowing for the greatest range of time for follow up and subsequent cancer development, only represent approximately 10 % of the United States population. SEER does not have its own central pathology review board, allowing for potential inconsistencies in diagnosis of the primary CNS tumors over time, though studies have shown that the diagnostic criteria for GBM is uniform among neuropathologists [47, 48]. Though spinal meningiomas were included in the analysis, they represented a small portion of all meningiomas studied (2.8 %), with all other primary CNS tumors located in the brain. Additionally, benign and malignant meningiomas were included, though in a sensitivity analysis zero cases of subsequent cancer were found among benign meningioma patients (results not presented). Grade II and III gliomas, particularly oligodendroglioma and astrocytoma, were grouped together due to limitations within the SEER data. A majority of oligodendrogliomas were NOS (89.5 %), which limits our data to analyze differences between grade II and III tumors. Similar issues plagued astrocytoma grade II and III analysis, though stratification of the tumors by their respective ICD-O-3 codes found subsequent cancer risk for grade III astrocytomas, and not for grade II. Our data also includes tumors of unclear pathology/location, designated Glioma NOS; however these patients represented only a small portion of our cohort (3 %), hence their potential bias effect would be limited. Within the SEER data, only first course of treatment data is available on radiotherapy, hence if patients received second course radiotherapy, that information is not available. Hence the relationship between subsequent treatment and risk of subsequent cancer cannot be examined. There is also the possibility of confusion between subsequent cancer development and CNS tumor recurrence. Furthermore, changes in WHO classification of CNS tumors, most recently in 2007 [49], do not result in retroactive reclassification in SEER to reflect current WHO classification. Though SEER limits these issues by recording only newly diagnosed primary tumors, the possibility of over or under-estimation of tumor incidence is possible.
The variations found in subsequent cancer risk between histological subtypes of CNS tumors are particularly important given that this is the first known large-scale study to stratify such data by histological subtype. The data indicates that many of these results can be explained by receipt of radiotherapy, though not for all histological subtypes. The finding that MPNET tumors in children increase the risk of multiple subsequent secondary cancers at various latencies indicates that particular attention must be placed on continuing monitoring and survivorship in such patients, particularly those that receive radiotherapy. Given the complexity of these results, especially with differences across histological subtypes of CNS tumors, further study is warranted to understand the underlying biological reasons towards progression to a subsequent cancer with the goal of decreasing the risk, or finding alternative treatment modalities.
Supplementary Material
Acknowledgment
This project was funded by the Case Comprehensive Cancer Center Support Grant (P30 CA043703).
Footnotes
Conflict of interest None of the authors had any conflict of interest to disclose.
Electronic supplementary material The online version of this article (doi:10.1007/s11060-013-1063-0) contains supplementary material, which is available to authorized users.
Contributor Information
Kyle Strodtbeck, Case Western Reserve University School of Medicine, 10900 Euclid Avenue, Cleveland, OH 44106, USA Kxs213@case.edu.
Andrew Sloan, Department of Neurological Surgery, University Hospitals Neurological Institute, 11100 Euclid Avenue, Hanna House 524, Cleveland, OH 44106, USA Andrew.sloan@uhhospitals.org; Seidman Cancer Center, 11100 Euclid Avenue, Hanna House 524, Cleveland, OH 44106, USA; Case Comprehensive Cancer Center, 11100 Euclid Avenue, Hanna House 524, Cleveland, OH 44106, USA.
Lisa Rogers, Department of Neurology, University Hospitals Neurological Institute, 11100 Euclid Avenue, Hanna House Rm. 506, Cleveland, OH 44106, USA Lisa.rogers1@uhhospitals.org; Seidman Cancer Center, 11100 Euclid Avenue, Hanna House 524, Cleveland, OH 44106, USA; Case Comprehensive Cancer Center, 11100 Euclid Avenue, Hanna House 524, Cleveland, OH 44106, USA.
Paul Graham Fisher, Department of Pediatrics, Lucile Packard Children’s Hospital, Stanford University, 750 Welch Road, Suite 317, Palo Alto, CA 94304-1510, USA pfisher@stanford.edu; Department of Child Neurology, Lucile Packard Children’s Hospital, Stanford University, 750 Welch Road, Suite 317, Palo Alto, CA 94304-1510, USA.
Duncan Stearns, Division of Pediatric Hematology/Oncology, Rainbow Babies and Children’s Hospital, 11100 Euclid Avenue, Suite 340 Mailstop: RBC6054, Cleveland, OH 44106, USA Duncan.stearns@case.edu; Seidman Cancer Center, 11100 Euclid Avenue, Hanna House 524, Cleveland, OH 44106, USA; Case Comprehensive Cancer Center, 11100 Euclid Avenue, Hanna House 524, Cleveland, OH 44106, USA.
Laura Campbell, Case Western Reserve University School of Medicine, 10900 Euclid Avenue, Cleveland, OH 44106, USA Lbc24@case.edu.
Jill Barnholtz-Sloan, Case Western Reserve University School of Medicine, 10900 Euclid Avenue, Cleveland, OH 44106, USA; Case Comprehensive Cancer Center, 11100 Euclid Avenue, Hanna House 524, Cleveland, OH 44106, USA jsb42@case.edu.
References
- 1.American Cancer Society . Cancer facts & figures. The Society; Atlanta: 2012. [Google Scholar]
- 2.Kohler BA, et al. Annual report to the nation on the status of cancer, 1975–2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst. 2011;103(9):714–736. doi: 10.1093/jnci/djr077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inskip PD. Multiple primary tumors involving cancer of the brain and central nervous system as the first or subsequent cancer. Cancer. 2003;98(3):562–570. doi: 10.1002/cncr.11554. [DOI] [PubMed] [Google Scholar]
- 4.Salminen E, Pukkala E, Teppo L. Second cancers in patients with brain tumours—impact of treatment. Eur J Cancer. 1999;35(1):102–105. doi: 10.1016/s0959-8049(98)00341-4. [DOI] [PubMed] [Google Scholar]
- 5.Peterson KM, et al. An analysis of SEER data of increasing risk of secondary malignant neoplasms among long-term survivors of childhood brain tumors. Pediatr Blood Cancer. 2006;47(1):83–88. doi: 10.1002/pbc.20690. [DOI] [PubMed] [Google Scholar]
- 6.Berrington de Gonzalez A, et al. Proportion of second cancers attributable to radiotherapy treatment in adults: a cohort study in the US SEER cancer registries. Lancet Oncol. 2011;12(4):353–360. doi: 10.1016/S1470-2045(11)70061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Control CfD. [cited 2012];Benign Brain Tumor Cancer Registries Amendment Act. 2004 www.cdc.gov/cancer/npcr/pdf/btr/Amendment_Act.pdf.
- 8.Surveillance EaERSP [cited 2012];SEER*Stat Database. 2012 http://www.seer.cancer.gov/
- 9.Fritz AG. International classification of diseases for oncology: ICD-O. 3rd edn World Health Organization; Geneva: 2000. p. 240. [Google Scholar]
- 10.Dong C, Hemminki K. Second primary neoplasms in 633,964 cancer patients in Sweden, 1958–1996. Int J Cancer. 2001;93(2):155–161. doi: 10.1002/ijc.1317. [DOI] [PubMed] [Google Scholar]
- 11.Berrington de Gonzalez A, et al. Second solid cancers after radiotherapy for breast cancer in SEER cancer registries. Br J Cancer. 2010;102(1):220–226. doi: 10.1038/sj.bjc.6605435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gessi M, et al. Radiation-induced glioblastoma in a medulloblastoma patient: a case report with molecular features. Neuropathology. 2008;28(6):633–639. doi: 10.1111/j.1440-1789.2008.00900.x. [DOI] [PubMed] [Google Scholar]
- 13.Maddams J, Parkin DM, Darby SC. The cancer burden in the United Kingdom in 2007 due to radiotherapy. Int J Cancer. 2011;129(12):2885–2893. doi: 10.1002/ijc.26240. [DOI] [PubMed] [Google Scholar]
- 14.Travis LB, et al. Second malignant neoplasms and cardiovascular disease following radiotherapy. J Natl Cancer Inst. 2012;104(5):357–370. doi: 10.1093/jnci/djr533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang SY, et al. Radiation-induced cerebellar glioblastoma at the site of a treated medulloblastoma: case report. J Neurosurg. 2005;102(4 Suppl):417–422. doi: 10.3171/ped.2005.102.4.0417. [DOI] [PubMed] [Google Scholar]
- 16.Zhu G, et al. Risk of second primary cancer after treatment for esophageal cancer: a pooled analysis of nine cancer registries. Dis Esophagus. 2012;25(6):505–511. doi: 10.1111/j.1442-2050.2011.01273.x. [DOI] [PubMed] [Google Scholar]
- 17.Grossman SA, et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17(16):5473–5480. doi: 10.1158/1078-0432.CCR-11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Louis DN. Molecular pathology of malignant gliomas. Annu Rev Pathol. 2006;1:97–117. doi: 10.1146/annurev.pathol.1.110304.100043. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe K, et al. Overexpression of the EGF receptor and p53 mutations are mutually exclusive in the evolution of primary and secondary glioblastomas. Brain Pathol. 1996;6(3):217–223. doi: 10.1111/j.1750-3639.1996.tb00848.x. discussion 23–24. [DOI] [PubMed] [Google Scholar]
- 20.Rutkowski S, et al. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med. 2005;352(10):978–986. doi: 10.1056/NEJMoa042176. [DOI] [PubMed] [Google Scholar]
- 21.Armstrong GT. Long-term survivors of childhood central nervous system malignancies: the experience of the Childhood Cancer Survivor Study. Eur J Paediatr Neurol. 2010;14(4):298–303. doi: 10.1016/j.ejpn.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armstrong GT, Stovall M, Robison LL. Long-term effects of radiation exposure among adult survivors of childhood cancer: results from the childhood cancer survivor study. Radiat Res. 2010;174(6):840–850. doi: 10.1667/RR1903.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bassal M, et al. Risk of selected subsequent carcinomas in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2006;24(3):476–483. doi: 10.1200/JCO.2005.02.7235. [DOI] [PubMed] [Google Scholar]
- 24.Berger C, et al. Second malignant neoplasms following childhood cancer: a study of a recent cohort (1987–2004) from the childhood cancer registry of the Rhone-Alpes region (ARC-ERRA) in France. Pediatr Hematol Oncol. 2011;28(5):364–379. doi: 10.3109/08880018.2011.562601. [DOI] [PubMed] [Google Scholar]
- 25.Davies SM. Subsequent malignant neoplasms in survivors of childhood cancer: Childhood Cancer Survivor Study (CCSS) studies. Pediatr Blood Cancer. 2007;48(7):727–730. doi: 10.1002/pbc.21113. [DOI] [PubMed] [Google Scholar]
- 26.Devarahally SR, et al. Second malignant neoplasms after primary central nervous system malignancies of childhood and adolescence. Pediatr Hematol Oncol. 2003;20(8):617–625. [PubMed] [Google Scholar]
- 27.Farwell J, Flannery JT. Second primaries in children with central nervous system tumors. J Neurooncol. 1984;2(4):371–375. doi: 10.1007/BF00178120. [DOI] [PubMed] [Google Scholar]
- 28.Friedman DL, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2010;102(14):1083–1095. doi: 10.1093/jnci/djq238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inskip PD, Curtis RE. New malignancies following childhood cancer in the United States, 1973–2002. Int J Cancer. 2007;121(10):2233–2240. doi: 10.1002/ijc.22827. [DOI] [PubMed] [Google Scholar]
- 30.Jazbec J, Ecimovic P, Jereb B. Second neoplasms after treatment of childhood cancer in Slovenia. Pediatr Blood Cancer. 2004;42(7):574–581. doi: 10.1002/pbc.20025. [DOI] [PubMed] [Google Scholar]
- 31.Kaatsch P, Michaelis J. Second neoplasms after malignant diseases in childhood. Klin Padiatr. 1995;207(4):158–163. doi: 10.1055/s-2008-1046533. [DOI] [PubMed] [Google Scholar]
- 32.Little MP, et al. Risks of brain tumour following treatment for cancer in childhood: modification by genetic factors, radiotherapy and chemotherapy. Int J Cancer. 1998;78(3):269–275. doi: 10.1002/(SICI)1097-0215(19981029)78:3<269::AID-IJC1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 33.Maule M, et al. Second malignancies after childhood noncentral nervous system solid cancer: results from 13 cancer registries. Int J Cancer. 2011;129(8):1940–1952. doi: 10.1002/ijc.26135. [DOI] [PubMed] [Google Scholar]
- 34.Maule M, et al. Risk of second malignant neoplasms after childhood central nervous system malignant tumours: an international study. Eur J Cancer. 2008;44(6):830–839. doi: 10.1016/j.ejca.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 35.Meadows AT, et al. Second neoplasms in survivors of childhood cancer: findings from the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27(14):2356–2362. doi: 10.1200/JCO.2008.21.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muller HL, et al. Meningioma as second malignant neoplasm after oncological treatment during childhood. Strahlenther Onkol. 2012;188(5):438–441. doi: 10.1007/s00066-012-0082-7. [DOI] [PubMed] [Google Scholar]
- 37.Nathan PC, et al. Screening and surveillance for second malignant neoplasms in adult survivors of childhood cancer: a report from the childhood cancer survivor study. Ann Intern Med. 2010;153(7):442–451. doi: 10.1059/0003-4819-153-7-201010050-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neglia JP, et al. Second malignant neoplasms in five-year survivors of childhood cancer: childhood cancer survivor study. J Natl Cancer Inst. 2001;93(8):618–629. doi: 10.1093/jnci/93.8.618. [DOI] [PubMed] [Google Scholar]
- 39.Neglia JP, et al. New primary neoplasms of the central nervous system in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2006;98(21):1528–1537. doi: 10.1093/jnci/djj411. [DOI] [PubMed] [Google Scholar]
- 40.Pang D, et al. Cancer incidence and mortality among the parents of a population-based series of 2604 children with cancer. Cancer Epidemiol Biomarkers Prev. 2003;12(6):538–544. [PubMed] [Google Scholar]
- 41.Pearce MS, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380:499–505. doi: 10.1016/S0140-6736(12)60815-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robison LL. The Childhood Cancer Survivor Study: a resource for research of long-term outcomes among adult survivors of childhood cancer. Minn Med. 2005;88(4):45–49. [PubMed] [Google Scholar]
- 43.Robison LL. Treatment-associated subsequent neoplasms among long-term survivors of childhood cancer: the experience of the Childhood Cancer Survivor Study. Pediatr Radiol. 2009;39(Suppl 1):S32–S37. doi: 10.1007/s00247-008-1066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robison LL, et al. Long-term outcomes of adult survivors of childhood cancer. Cancer. 2005;104(11 Suppl):2557–2564. doi: 10.1002/cncr.21249. [DOI] [PubMed] [Google Scholar]
- 45.Tukenova M, et al. Second malignant neoplasms in digestive organs after childhood cancer: a cohort-nested case-control study. Int J Radiat Oncol Biol Phys. 2012;82(3):e383–e390. doi: 10.1016/j.ijrobp.2011.05.069. [DOI] [PubMed] [Google Scholar]
- 46.Woodward E, et al. Late effects in survivors of teenage and young adult cancer: does age matter? Ann Oncol. 2011;22(12):2561–2568. doi: 10.1093/annonc/mdr044. [DOI] [PubMed] [Google Scholar]
- 47.Davis FG, et al. Issues of diagnostic review in brain tumor studies: from the Brain Tumor Epidemiology Consortium. Cancer Epidemiol Biomarkers Prev. 2008;17(3):484–489. doi: 10.1158/1055-9965.EPI-07-0725. [DOI] [PubMed] [Google Scholar]
- 48.Aldape K, et al. Discrepancies in diagnoses of neuroepithelial neoplasms: the San Francisco Bay Area Adult Glioma Study. Cancer. 2000;88(10):2342–2349. [PubMed] [Google Scholar]
- 49.Louis DN, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.CBTRUS [cited 2012];CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2004–2008. 2012 revised March 23, 2012. www.cbtrus.org/reports/reports.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


