Abstract
Objective
To evaluate the efficacy of ovarian stimulation with higher doses of gonadotropins in fertility preservation (FP) cycles with the intention to maximize the likelihood of future pregnancies.
Design
Retrospective (secondary analysis).
Setting
Academic medical centers.
Patient(s)
Low-dose (LD, 150 IU; n = 34) versus high-dose (HD, >150 IU; n = 117) FSH start in 151 patients with breast cancer (BCa) undergoing ovarian stimulation for embryo cryopreservation with letrozole (LE) before cancer treatment.
Intervention(s)
None.
Main Outcome Measure(s)
FP cycle outcomes.
Result(s)
Mean total FSH dose (2,037 ± 679 IU vs. 1,128 ± 381 IU) and FSH level on trigger day (21.1 ± 8.9 vs. 10.6 ± 4.5 mIU/mL) were higher in the HD group, confirming the receipt of higher-dose FSH. There was no difference in other patient characteristics. Despite the larger number of follicles >17 mm in diameter in the HD group (5.0 ± 2.0 vs. 3.4 ± 1.4), neither peak E2 (498.0 ± 377.5 vs. 397.9 ± 320.3), number of oocytes (13.3 ± 8.7 vs. 12.3 ± 8.0), nor number of embryos (6.3 ± 4.7 vs. 5.4 ± 3.8) were significantly different from the LD group. Of those undergoing frozen embryo transfer (ET), live birth rate (LBR)/ET trended higher in the LD (9/15) compared with HD (2/11) group, with 2.1 ± 0.8 vs. 1.9 ± 0.3 embryos transferred, respectively.
Conclusion(s)
Higher-dose FSH stimulation in LE cycles does not improve outcomes and may be associated with lower LBR. Our findings may support minimal stimulation in young noninfertile women with BCa.
Keywords: Follicle-stimulating hormone, letrozole, starting dose, fertility preservation, breast cancer
Breast cancer is the most common malignancy in women during reproductive age (1). The number of breast cancer survivors is increasing thanks to early diagnosis and new therapeutic strategies. Because of the dramatic rise in survival rates, quality of life issues have gained more significance. A large number of breast cancer survivors face infertility after chemotherapy and/or radiotherapy, and fertility preservation (FP) is a key part of maintaining quality of life after breast cancer (2, 3).
Three main options for FP are embryo, oocyte, and ovarian tissue freezing. Both embryo and oocyte freezing require ovarian stimulation with gonadotropins, which results in excessive levels of estrogen production during ovarian stimulation. We previously developed a novel ovarian stimulant protocol using an aromatase inhibitor, letrozole, to reduce the estrogen exposure in women with breast cancer (4).
In FP cycles, because there is limited time available before the onset of chemotherapy in breast cancer patients (5), ovarian stimulation is often performed with higher doses of gonadotropins to maximize the number of embryos or oocytes cryopreserved to increase the likelihood of future pregnancies. The purpose of the present study was to determine whether administration of higher doses of gonadotropins confer an advantage on the outcomes in letrozole cycles.
MATERIALS AND METHODS
The data were generated by secondary analysis of a previously collected and deidentified database (6). The study protocol was approved by the Institutional Review Board. After the first introduction of the letrozole-gonadotropin protocol, we used low-dose FSH (LD) in a majority of the patients. Once the safety profile was established, we then gradually transitioned to using higher doses of FSH (HD). Therefore, this was a comparison of the initial low-dose FSH protocol (150 IU) with the later higher-dose FSH protocol (>150 IU) (4). The inclusion criteria were age <45 years, breast cancer stage ≤3, no prior chemotherapy, no prior history of ovarian surgery or infertility. Of the 170 patients in the database, 151 met the inclusion criteria for secondary analysis. All patients received 5 mg/d letrozole starting on cycle day 2 (CD2) until the day of hCG administration, and recombinant FSH (range 150–375 IU) was added from CD4. Serum FSH levels were monitored throughout the cycle.
Statistical analysis was performed with the SPSS 17 for Windows package. Continuous data (presented as mean ± SD) were analyzed by t test. Nonparametric data were analyzed by Mann-Whitney U test. Levene test of homogeneity of variances (P<.01) and Kolmogorov-Smirnov test of normality (P<.01) were performed to choose the appropriate statistical test. Chi-square and Fisher exact tests were performed to analyze the relation between two categoric variables. A receiver operating characteristic (ROC) curve analysis was performed to identify the cutoff point for gonadotropin dose. When the P value was <.05, the difference was considered to be statistically significant in all statistical tests.
RESULTS
There were 34 women in the LD and 117 in the HD group. There was no difference in the rate of cancellation between the two groups (8.8% vs. 9.4%; P=.92). Mean age was 36.2 ± 4.1 years in the LD group and 34.9 ± 4.5 years in the HD group. Mean total FSH dose (2,037 ± 679 vs. 1,128 ± 381; P<.001) and FSH level on trigger day (21.1 ± 8.9 vs. 10.6 ± 4.5 mIU/mL; P<.001) were higher in the HD group than in the LD group, confirming the receipt of higher-dose FSH in the former. There was no difference in other clinical characteristics between the two groups (Table 1).
TABLE 1.
Patient clinical characteristics and IVF outcomes (mean ± SD).
| FSH 150 IU | FSH >150 IU | P value | |
|---|---|---|---|
| Total FSH dose, IU | 1,128.6 ± 381.1 | 2,037.4 ± 678.8 | <.001 |
| Total letrozole dose, mg | 47.6 ± 13.3 | 46.3 ± 12.5 | NS |
| Age, y | 36.2 ± 4.1 | 34.9 ± 4.5 | NS |
| Body mass index, kg/m2 | 23.2 ± 3.6 | 22.6 ± 3.7 | NS |
| Antral follicle count | 14.8 ± 13.4 | 12.8 ± 8.6 | NS |
| No. of follicles >17 mm | 3.4 ± 1.4 | 5.0 ± 2.0 | <.001 |
| FSH level on day 2, mIU/mL | 7.3 ± 3.3 | 8.0 ± 3.7 | NS |
| LH level on day 2, mIU/mL | 5.0 ± 2.6 | 4.6 ± 2.4 | NS |
| E2 level on day 2, pg/ml | 40.1 ± 26.0 | 45.8 ± 23.9 | NS |
| E2 on trigger day, pg/ml | 397.9 ± 320.3 | 498.0 ± 377.5 | NS |
| FSH on trigger day, mIU/mL | 10.6 ± 4.5 | 21.1 ± 8.9 | <.001 |
| No. of oocytes | 12.3 ± 8.0 | 13.3 ± 8.7 | NS |
| No. of mature oocytes | 8.0 ± 4.4 | 8.5 ± 6.0 | NS |
| Maturation rate, % | 74.1 ± 21.5 | 66.6 ± 27.9 | NS |
| No. of embryos | 5.4 ± 3.8 | 6.3 ± 4.7 | NS |
Lee. FSH dose and fertility preservation. Fertil Steril 2012.
Despite the larger number of follicles developing to >17 mm in the HD group (5.0 ± 2.0 vs. 3.4 ± 1.4), neither the peak E2 (498.0 ± 377.5 vs. 397.9 ± 320.3), number of oocytes (13.3 ± 8.7 vs. 12.3 ± 8.0), nor number of embryos (6.3 ± 4.7 vs. 5.4 ± 3.8) were significantly different from the LD group.
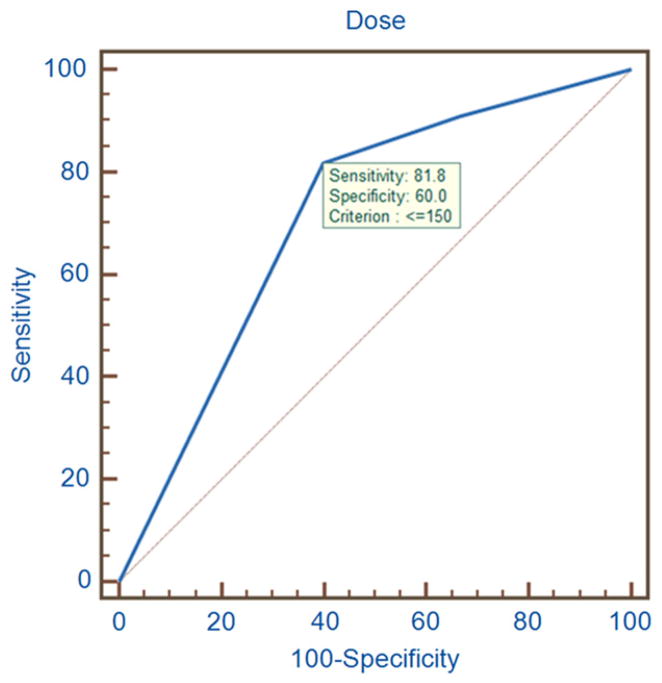
A total of 26 frozen embryo transfers (FETs) were performed from 21 patients. Of those undergoing FET, live birth rate (LBR) per ET trended higher in the LD group (9/15) compared with the HD group (2/11; P=.051) with a mean of 2.1 ± 0.8 vs. 1.9 ± 0.3 (P=.496) embryos transferred. A ROC curve analysis with the starting FSH dose validated 150 IU as the optimal cutoff value based on LBR (Fig. 1). There was no difference in abortion or clinical pregnancy rates (Table 2). The clinical characteristics of patients who had live births and those who did not were similar except for the total FSH dose (1,224 ± 487 vs. 1,734 ± 746, respectively; P=.049). Mean age was similar between the HD and LD groups among those who conceived (34.2 ± 3.3 vs. 35.9 ± 4.5, respectively; P=.29). To rule out the possibility that changes in the embryology laboratory quality over time affected success rate, the pregnancy rates were analyzed based on the year the embryos were frozen. We did not find any relationship among the year, the mean age, and pregnancy rates (Supplemental Table 1).
FIGURE 1.
A receiver operating characteristic (ROC) curve analysis identified 150 IU starting FSH dose as the cutoff point with 81.8% sensitivity and 60.0% specificity when live birth rate is used as the outcome measure.
Lee. FSH dose and fertility preservation. Fertil Steril 2012.
TABLE 2.
Comparison of pregnancy outcomes between the low- and high-dose FSH regimens, % (ratio).
| FSH 150 IU (n = 15) | FSH 150 IU (n = 11) | P value | |
|---|---|---|---|
| Clinical pregnancy rate | 80 (12/15) | 54 (6/11) | .218 |
| Abortion rate | 20 (3/15) | 36 (4/11) | .407 |
| Live birth rate | 60 (9/15) | 18 (2/11) | .051 |
Lee. FSH dose and fertility preservation. Fertil Steril 2012.
DISCUSSION
In this study, we found that the higher-dose FSH stimulation in letrozole cycles did not improve pregnancy outcomes, and may be associated with a lower live birth rate. Our findings may support minimal stimulation in young noninfertile women with breast cancer.
Although our study is the first to test the impact of starting FSH dose on FP outcomes in women with breast cancer receiving letrozole, the benefit of high-dose gonadotropins has been questioned in earlier studies in noncancer infertility patients. In one, increased gonadotropin use for ovarian stimulation was associated with lower rates of clinical pregnancy and live birth as well as a suggestion of higher likelihood for spontaneous miscarriages (7). One of the plausible theories is that the high doses of FSH may stimulate the recruitment of incompetent or chromosomally abnormal oocytes, which may result in a higher abortion rate or lower LBR (8, 9). In another study, there was no significant improvement in oocyte and embryo yield or pregnancy rates after an upward adjustment of FSH starting dose (10). Another intriguing possibility is estrogen-induced DNA damage. Recent research has indicated that estrogen exposure induces DNA double-strand breaks (DSBs) (11). We have shown that oocytes incur DNA DSBs in response to genotoxic agents and have an active DNA repair mechanism triggered in response to this damage (12). Therefore, one may speculate that higher estrogen levels associated with high-dose FSH use may be associated with increased incidence of DSBs and impaired oocyte health in IVF cycles. In the present study, although the difference in peak E2 levels did not reach statistical significance, owing to letrozole suppression, the HD group had a larger number of follicles >17 mm. It is possible that this created higher intraovarian estrogen levels with potential consequences on DNA integrity.
We compared low-dose (150 IU) FSH start with high-dose (>150 IU) in women with breast cancer undergoing FP with letrozole. Consistent with our findings, a recent meta-analysis reported that the optimal daily FSH stimulation dose was 150 IU/d in presumed normal responders <39 years old undergoing IVF, because this dose was associated with a slightly lower oocyte yield but similar pregnancy and embryo freezing rates compared with higher doses (13).
Physicians should also consider the potential side effects of higher-dose FSH treatment when dealing with women with breast cancer. Even though not statistically significant, the mean peak E2 levels trended higher in the HD group, which can be a disadvantage in breast cancer. Overall, we showed that breast cancer recurrence rates are not increased after ovarian stimulation with the letrozole-gonadotropin protocol that we previously described (4). Future studies are planned to compare the recurrence rates between low-dose and high-dose FSH groups, which will require larger numbers of patients.
Ideally, ovarian stimulation should be started within the first 4 days of the menstrual cycle to be most effective and requires ~2 weeks for completion (4). Depending on whether the referral is made before or after breast surgery, no more than usually one or two cycles of ovarian stimulation can be performed without delaying chemotherapy in women with breast cancer (5, 14). Because of this limited time period, reproductive specialists tend to use higher starting dose of gonadotropins to maximize the likelihood for future pregnancies. However, one should also consider the cost of gonadotropins, which represents a significant proportion, up to 30%, of the total expenditure for an IVF treatment cycle (15). FP procedures are generally excluded under infertility coverage, because the patients do not meet the classic definition of infertility. Therefore, cost reduction may be of higher significance for women undergoing FP, and using a lower dose protocol may make embryo or oocyte cryopreservation more affordable.
Although our study indicates that minimal stimulation may improve reproductive outcomes in women with breast cancer who are undergoing ovarian stimulation with letrozole and gonadotropins, it has certain limitations. It was a secondary analysis of a prospective database, the number of patients in the LD group was relatively low compared with the HD group, and antimüllerian hormone levels were not available in all patients owing to its recent use as a routine marker.
After the safety profile with low-dose FSH was established, we gradually transitioned to using higher doses of FSH. One may ask that it is not really possible to adequately compare pregnancy and live birth rates in the two groups because these may have changed in the time period. However, we analyzed the pregnancy rates based on the year the embryos were frozen and did not find any relationship among year, mean age, and pregnancy rates.
It could be questioned whether after the initial publication of successful FP with the use of letrozole plus FSH for ovarian stimulation, a broader cross-section of women agreed to have this treatment, and it could be that subtle differences occurred in the make-up of the later HD group. However, we compared all available characteristics, including parity, and did not find any differences between the two groups. In addition, because breast cancer patients are referred as a result of an unexpected occurrence of malignancy by their medical oncologists, publicity is unlikely to affect patient behavior as it would for elective IVF. It must be clarified that our protocol is not a minimal-stimulation protocol in a strict sense, because letrozole is also providing ovarian stimulation. Mitwally and Casper originally demonstrated that combination of gonadotropins with aromatase inhibitors reduce gonadotropin requirements in ovulation induction cycles (16). Our previous work with IVF cycles also showed that similar outcomes can be obtained between this protocol and standard protocols used for male factor infertility patients with 40% lower amount of gonadotropins (17). Based on the present work, we estimate that 150 IU FSH plus letrozole is similar to a short-protocol FSH dose of 210 IU. This estimation provides another rationale for not using a high start dose of FSH along with letrozole.
In conclusion, higher-dose FSH stimulation with letrozole in women with breast cancer undergoing FP treatment does not improve reproductive outcomes and may be associated with lower LBR. In addition, it may increase estrogen exposure as well as the costs of FP. Infertility specialists should consider lowest possible dose in young women with breast cancer undergoing ovarian stimulation for FP.
Supplementary Material
Acknowledgments
Supported in part by National Institutes of Health grant HD 053112.
Footnotes
S.L. has nothing to disclose. K.O. has nothing to disclose.
Presented in preliminary form at the American Society for Reproductive Medicine 66th annual meeting, 2010.
References
- 1.American Cancer Society. [Last accessed July 2, 2012];Cancer facts & figures. 2009 Available at: http://www.cancer.org/downloads/STT/500809web.pdf.
- 2.Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, Oktay K. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–31. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 3.Oktay K. Fertility preservation: an emerging discipline in the care of young patients with cancer. Lancet Oncol. 2005;6:192–3. doi: 10.1016/S1470-2045(05)70070-X. [DOI] [PubMed] [Google Scholar]
- 4.Azim AA, Costantini-Ferrando M, Oktay K. Safety of fertility preservation by ovarian stimulation with letrozole and gonadotropins in patients with breast cancer: a prospective controlled study. J Clin Oncol. 2008;26:2630–5. doi: 10.1200/JCO.2007.14.8700. [DOI] [PubMed] [Google Scholar]
- 5.Lee S, Ozkavukcu S, Heytens E, Moy F, Oktay K. Value of early referral to fertility preservation in women with breast cancer. J Clin Oncol. 2010;28:4683–6. doi: 10.1200/JCO.2010.30.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oktay K, Kim JY, Barad D, Babayev SN. Association of BRCA1 mutations with occult primary ovarian insufficiency: a possible explanation for the link between infertility and breast/ovarian cancer risks. J Clin Oncol. 2010;28:240–4. doi: 10.1200/JCO.2009.24.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pal L, Jindal S, Witt BR, Santoro N. Less is more: increased gonadotropin use for ovarian stimulation adversely influences clinical pregnancy and live birth after in vitro fertilization. Fertil Steril. 2008;89:1694–701. doi: 10.1016/j.fertnstert.2007.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devroey P, Fauser BC, Diedrich K Evian Annual Reproduction (EVAR) Workshop Group 2008. Approaches to improve the diagnosis and management of infertility. Hum Reprod Update. 2009;15:391–408. doi: 10.1093/humupd/dmp012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baart EB, Martini E, Eijkemans MJ, van Opstal D, Beckers NG, Verhoeff A, et al. Milder ovarian stimulation for in-vitro fertilization reduces aneuploidy in the human preimplantation embryo: a randomized controlled trial. Hum Reprod. 2007;22:980–8. doi: 10.1093/humrep/del484. [DOI] [PubMed] [Google Scholar]
- 10.Lekamge DN, Lane M, Gilchrist RB, Tremellen KP. Increased gonadotrophin stimulation does not improve IVF outcomes in patients with predicted poor ovarian reserve. J Assist Reprod Genet. 2008;25:515–21. doi: 10.1007/s10815-008-9266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williamson LM, Lees-Miller SP. Estrogen receptor alpha–mediated transcription induces cell cycle-dependent DNA double-strand breaks. Carcinogenesis. 2011;32:279–85. doi: 10.1093/carcin/bgq255. [DOI] [PubMed] [Google Scholar]
- 12.Soleimani R, Heytens E, Darzynkiewicz Z, Oktay K. Mechanisms of chemotherapy-induced human ovarian aging: double strand DNA breaks and microvascular compromise. Aging. 2011;3:782–93. doi: 10.18632/aging.100363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sterrenburg MD, Veltman-Verhulst SM, Eijkenams MJC, Hughes EG, Macklon NS, Broekmans FJ, et al. Clinical outcomes in relation to the daily dose of recombinant follicle-stimulating hormone for ovarian stimulation in in vitro fertilization in presumed normal responders younger than 39 years: a meta-analysis. Hum Reprod Update. 2011;17:184–96. doi: 10.1093/humupd/dmq041. [DOI] [PubMed] [Google Scholar]
- 14.Cold S, During M, Ewertz M, Knoop A, Moller S. Does timing of adjuvant chemotherapy influence the prognosis after early breast cancer? Results of the Danish Breast Cancer Cooperative Group (DBCG) Br J Cancer. 2005;93:627–32. doi: 10.1038/sj.bjc.6602734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wechowski J, Connolly M, Schneider D, McEwan P, Kennedy R. Cost-saving treatment strategies in in vitro fertilization: a combined economic evaluation of two large randomized clinical trials comparing highly purified human menopausal gonadotropin and recombinant follicle-stimulating hormone alpha. Fertil Steril. 2009;91:1067–76. doi: 10.1016/j.fertnstert.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 16.Mitwally MF, Casper RF. Aromatase inhibition reduces gonadotrophin dose required for controlled ovarian stimulation in women with unexplained infertility. Hum Reprod. 2003;18:1588–97. doi: 10.1093/humrep/deg311. [DOI] [PubMed] [Google Scholar]
- 17.Oktay K, Hourvitz A, Sahin G, Oktem O, Safro B, Cil A, et al. Letrozole reduces estrogen and gonadotropin exposure in women with breast cancer undergoing ovarian stimulation before chemotherapy. J Clin Endocrinol Metab. 2006;91:3885–90. doi: 10.1210/jc.2006-0962. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.