Abstract
Diet-derived luminal factors have a major influence on zinc available for uptake across the apical membrane of enterocytes. Malabsorption and possibly intestinal microbiota limit this zinc availability. The transporter ZIP4 is expressed along the entire gastrointestinal tract and acts as a major processor of dietary zinc for loading into enterocytes from the apical membrane. Zip4 and other Zip family genes expressed in the gastrointestinal tract are up-regulated in periods of dietary zinc restriction. This provides for powerful homeostatic control. The transporter ZIP14 is up-regulated along the entire gastrointestinal tract by proinflammatory conditions. Intracellular transporters such as ZnT7, influence the transcellular movement of zinc across the enterocyte. Metallothionein, an intracellular metal buffer, and the transporter ZnT1 at the basolateral membrane, regulate the amount of zinc released to the portal circulation for systemic distribution. Pancreatic release of zinc by acinar cells is through the secretory process and apical membrane and involves transporters ZnT2 and ZnT1, respectively. Expression of both transporters is zinc-responsive. Enterocytes and acinar cells constitutively express Zip5 at the basolateral membrane, where it may serve as a monitor of zinc status.
Keywords: zinc absorption, zinc transporters, zinc homeostasis
Introduction
The absorption of trace elements is generally favored by conditions that increase solubility and uptake by the apical surface of absorptive cells of the gastrointestinal tract. It is generally accepted that the bulk of zinc absorption occurs in the small intestine. Localization and expression studies of zinc transporters have established that some of these proteins are produced in the epithelium lining the entire gastrointestinal tract. Hence it is likely that some zinc absorption occurs in the entire tract. Well before the first zinc transporter was cloned and characterized [1] , zinc transport by the intestinal cells was shown to exhibit the kinetic properties of saturable, carrier-mediated transport [2]. The Km of ~30μM and Vmax of ~2 mmol Zn/g mucosa/minute in conditions of normal zinc status and increased Vmax with low zinc status were consistent with this characterization of zinc transport. These estimates also tend to agree with estimates of intralumenal zinc concentrations following a meal.
Zinc transporters fall into two families: ZnT with 10 members and ZIP with 14 members [3]. The ZnT protein family lowers the cellular zinc concentration while the ZIP proteins act to increase cellular zinc. Well over half of these proteins are expressed in enterocytes or enterocyte-like cell lines; e. g. HT-29 and Caco-2. A view is emerging that integrates the locations and apparent functions of these transporters with recognized events related to the absorption process. For the purpose of this report, the movement of dietary zinc into the systemic circulation will be divided into uptake, transcellular, and efflux steps with attention also given to zinc functions within intestinal cells.
Apical Zinc Transport by Enterocytes
Since the zinc malabsorption disorder, acrodermatitis enteropathica, is produced by a variety of mutations in hZip4, this transporter has received most of the attention [4]. As the defect in Zip4 produces a ZIP4 protein of reduced transport efficiency, it is relevant that the condition can be remediated by supplemental zinc. Considering that hZip4 has multiple polymorphisms, it is likely that these produce altered ZIP4 transport activity. Such differences could alter the structure and hence function of ZIP4 as a zinc transporter molecule. Altered affinity of the transporter could influence the effect of bioavailability on zinc uptake. These polymorphisms have yet to be investigated. Immunofluorescence studies have documented that ZIP4 is located at the apical membrane of enterocytes [5, 6]. Abundance of the protein increases with zinc deficiency [5 – 7]. The capacity of ZIP4 to transport zinc has been demonstrated in transfected cells but not directly in intestinal epithelial cells. In these model systems, ZIP4-mediated transport produces saturable zinc uptake [5, 8]. Zip4 is expressed in the stomach, small intestine, and colon [5 – 7] and has recently been found to be expressed in the cecum (Maki and Cousins, unpublished results). The critical role that ZIP4 plays in zinc delivery is demonstrated through the embryonic lethality of the Zip4 knockout mouse model [7].
The mechanism to explain Zip4 up-regulation upon zinc restriction of mice or cultured cells may involve multiple processes. It has been shown that zinc restriction influences Zip4 mRNA stability [8]. Subsequently, the enhanced Zip4 transcription associated with zinc restriction has been related to the up-regulation of the transcription factor (TF) Kruppel-like factor 4 (KLF4) [9]. This TF is highly expressed in the gastrointestinal tract and is known to regulate expression of important intestinal enzymes and proteins; e. g., intestinal alkaline phosphatase (IAP), which has a protective function for the intestinal mucosa [10]. The Zip4 promoter has multiple KLF4 binding sites. When KLF4 expression is inhibited by siRNA or the KLF4 sites of Zip4 are mutated, Zip4 promoter activity is not responsive to zinc restriction and 65Zn transport by mouse intestinal cells is decreased [9]. In support of the role for KLF4, in intestinal cells (HT-29) of colonic origin, zinc chelation produced by an anticancer molecule also up-regulates KLF4 [11]. As has been recently shown with iron absorption, TFs play a role in regulating the proteins involved in iron homeostasis [12]. Most likely Zip4 expression at the mRNA level is regulated by transcription and degradation which act together for this adaptive response to intestinal zinc availability. ZIP4 protein is similarly responsive to cellular zinc with changes in endocytosis at the plasma membrane and ubiquitination and degradation [13, 14].
ZnT5 (variant B) is also been localized to the apical plasma membrane of human intestine and Caco-2 cells. Transport mediated by ZnT5 in Xenopus oocytes suggests bi-directional transport [15, 16]. This was the first transporter to be suggested with bidirectional properties. Perhaps such activity could serve a buffering function. A ZnT5 knockout mouse possesses an altered phenotype [17] , but an influence on intestinal absorption has not been reported in this model.
Zip11 mRNA is expressed along the entire gastrointestinal tract from stomach to the colon (Maki and Cousins, unpublished data). In all regions examined, Zip11 is up-regulated with zinc restriction. Expression of Zip4 in the stomach responds similarly to Zip11, suggesting ZIP11 has an apical localization, but this has yet to be demonstrated. Zip14 is expressed in the mouse intestine [18] to a greater extent than in the liver where it proposed to have a major function in the acute phase response [19]. Of particular interest is that intestinal Zip14 is very responsive to endotoxin treatment of mice. Furthermore, ZIP14 is localized to both the apical and basolateral membranes of enterocytes. The phenotypic outcome of intestinal ZIP14 function is not known, but it may relate to an intestinal response to infection.
Transcellular Zinc Movement in Enterocytes
ZnT2 and ZnTs 4 – 7 have all been localized to intracellular structures in enterocytes [20]. They exhibit localization to vesicles and/or Golgi apparatus, representing various phases of membrane trafficking. Since the function of the ZnT proteins is to lower intracellular zinc levels, these proteins function either to promote cellular efflux of zinc or to move zinc into vesicles, secretory granules, and endosomes as well as into the Golgi complex. We have proposed much earlier that ZnT1 was localized to the basolateral membrane of enterocytes [21] but also to adjacent endosomes (vesicles), although these have not been extensively studied.
The clearest transcellular zinc trafficking mechanism has been that shown for ZnT7, which is localized to the Golgi complex [22, 23]. ZnT7 knockout mice exhibit phenotypic changes that are not typical of zinc deficiency, such as alopecia or dermatitis. This is despite reduced zinc uptake into numerous organs including liver, bone, and kidney in the mutant mice. Body composition was altered in these mice. Most notable was a decrease in body fat. This suggests that ZnT7 has an unrecognized function that explains the phenotype produced in ZnT7−/− mice. ZnT7 has been shown to activate one of the alkaline phosphatases, enzymes that require zinc for their catalytic function [24]. Within the context of zinc absorption, ZnT7−/− mice show zinc malabsorption with reduced tissue accumulation of orally administered 65Zn. A vesicular mode of transcellular zinc movement has been proposed, which is a counterpart of that described for calcium [25].
Enterocyte to Plasma Zinc Transport
The major transporter controlling cellular zinc efflux is ZnT1 [1]. It was the first zinc transporter to receive major attention as the protein responsible for zinc efflux from enterocytes [21]. The transporter showed greatest abundance in cells at the villus tip. The distribution was primarily at the basolateral membrane. As noted above, the vesicular-localized ZnT1 is most likely a manifestation of association with early endosomes or secretory vesicles. These relationships have not been investigated.
The mechanism of ZnT1 regulation is under control of the transcription factor MTF-1, which is zinc-responsive [26, 27]. ZnT1 is not dramatically influenced by zinc restriction as are other MTF-1 regulated genes. This is fortuitous, as a reduction in ZnT1 expression in enterocytes would limit zinc transfer to the systemic circulation. The refractory nature of the MTF-1 mediated ZnT1 gene regulation during zinc restriction has not been explored. ZIP5 is also localized to the enterocyte basolateral membrane [5, 7]. Its role in zinc homeostasis has not been investigated. Expression of Zip5 mRNA is not zinc-responsive to zinc depletion, but is responsive to acute administration of zinc. ZIP5 is internalized and degraded with zinc depletion. The localization and its responsiveness to zinc suggest that ZIP5 could serve as a monitor of body zinc status [7]. The transport of zinc by the intestine in the serosal to mucosal direction has been demonstrated [2]. Hence ZIP5 activity could monitor body zinc and with an appropriate stimulus, activate MTF-1 in enterocytes.
Endogenous Zinc Excretion and Reutilization
The pancreas as a major excretory route for zinc has been studied for decades [6, 28]. Clearly the pancreas is a conduit for endogenous zinc. The acinar cells produce zymogen granules, where zinc metalloenzymes are packaged with zinc ions in the acidic environment of the granule. Numerous older studies do not support a role for the pancreas in zinc excretion [27]. Nevertheless, those studies preceded knowledge of the tissue specificity of zinc transporters. For example, ligation of the pancreatic duct would produce a transient increase in systemic zinc, thereby producing MTF-1 mediated activation of genes including ZnT1, ZnT2, and metallothionein (MT). This would certainly perturb zinc absorption mechanisms. The pancreas has the potential to act as a key component of zinc homeostasis. Acinar cells express ZIP5, which localizes to the basolateral membrane of those polarized cells [7] and could act as a passive carrier of zinc destined for secretion. Acinar cells also produce ZnT1 and ZnT2 by a MTF-1 mediated process [28]. These participate in zinc release at the plasma membrane and zymogen secretory granules, respectively. Certainly the pancreas has a role in zinc homeostasis, but all components may not have been identified.
The other potential route for endogenous zinc release into the gastrointestinal tract is in the serosal to mucosal transport of zinc, with eventual release into the intestinal lumen. Zinc transfer in this direction has been shown in rats [2]. Isotopic tracer studies with humans also support the release of large amounts of endogenous zinc being secreted into the intestine [29]. It is not possible to establish with those tracer studies whether zinc is transported out of the intestinal cells or the pancreatic acinar cells.
While the origin of the endogenous intestinal zinc is not certain, it nevertheless provides a source of bioavailable zinc for reutilization by the host or for incorporation into the resident microbiota.
Immune Responses and Zinc Transport
It has been shown that concurrent systemic inflammation, as demonstrated by increased acute phase bio-markers, prevents the increase in plasma zinc in response to a micronutrient supplement containing 15 mg zinc/day [30]. This could result from either decreased intestinal zinc uptake, increased endogenous zinc loss via the intestinal or pancreatic secretions, or enhanced hepatic zinc accumulation. Recently it was observed that alcohol creates pro-oxidant conditions in the intestine, as demonstrated by increased reactive oxygen species that are concomitant with decreased intestinal zinc concentrations and increased blood endotoxin [31]. Endotoxin is known to enhance the absorption and/or retention of orally administered65 Zn [32, 33]. This has been interpreted as representing an influence of proinflammatory cytokines on zinc absorption. Indeed, the Zip14 transporter gene is induced by endotoxin along the entire gastrointestinal tract (Guthrie and Cousins, unpublished observations) as it is in the liver [19, 34]. A challenge in this area is to identify culture systems that mimic the gut during immune responses at the cell culture level. The latter is necessary to delineate effects that are at the enterocyte level from those produced by hepatic or renal zinc processing.
Conclusions
Understanding the transport properties, cellular location, cell-specific expression, and regulation of the zinc transporters involved in enteric zinc absorption is extremely important for developing an accurate appraisal of zinc bioavailability. Steady-state levels of the various transporters are important, but no more so than that many are regulated by physiologic and immune mediators that in turn influence absorption. Future issues that will need to be investigated to more fully understand zinc bioavailability include:
Immune status of the gastrointestinal tract
Reduced zinc intake may enhance gastric acid production and impair zinc solubility
Differential absorbability of zinc along the gastrointestinal tract
Influence of the intestinal microbiota on zinc absorption
Competition of the host and resident microbiota for available zinc
These challenges for the future will place greater emphasis on integrative models such as mutant mice, but also on state-of-the-art sequencing methods to explore influences of gut microbiota on zinc bioavailability.
Figure 1.
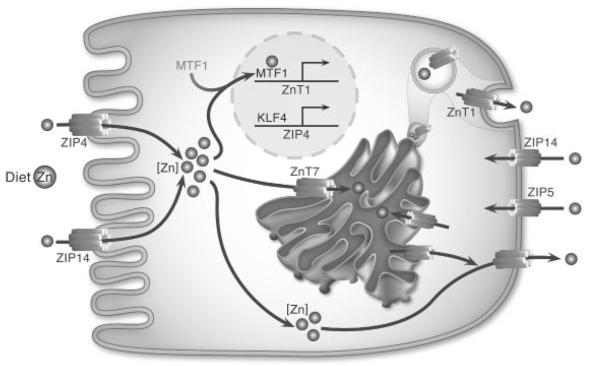
Overview of major zinc transporters expressed in intestinal epithelial cells. ZIP4 is a major importer and is regulated by zinc. ZIP14 is responsive to proinflammatory conditions and is postulated to be at both the apical and basolateral surface of enterocytes. ZnT1 and ZIP5 influence zinc traffi cking at the basolateral membrane. ZnT7 influences the apparent transcellular movement of zinc.
Acknowledgements
Research from the author's laboratory as cited here is supported by NIH Grant DK 31127.
References
- 1.Palmiter RD, Findley SD. Cloning and functional characterization of a mammalian zinc transporter that confers resistance to zinc. EMBO J. 1995;14:639. doi: 10.1002/j.1460-2075.1995.tb07042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cousins RJ. Theoretical and practical aspects of zinc uptake and absorption. Adv. Exp. Med. Biol. 1989;249:3. doi: 10.1007/978-1-4684-9111-1_1. [DOI] [PubMed] [Google Scholar]
- 3.Lichten LA, Cousins RJ. Mammalian zinc transporters: nutritional and physiologic regulation. Annu. Rev. Nutr. 2009;29:153. doi: 10.1146/annurev-nutr-033009-083312. [DOI] [PubMed] [Google Scholar]
- 4.Wang K, Zhou B, Kuo YM, Zemansky J, Gitschier J. A novel member of a zinc transporter family is defective in acrodermatitis enteropathica. Am. J. Hum. Genet. 2002;71:66. doi: 10.1086/341125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dufner-Beattie J, Wang F, Kuo YM, Gitschier J, Eide D, Andrews GK. The acrodermatitis enteropathica gene ZIP4 encodes a tissue-specific, zinc-regulated zinc transporter in mice. J Biol. Chem. 2003;278:33474. doi: 10.1074/jbc.M305000200. [DOI] [PubMed] [Google Scholar]
- 6.Liuzzi JP, Bobo JA, Lichten LA, Samuelson DA, Cousins RJ. Responsive transporter genes within the murine intestinal-pancreatic axis form a basis of zinc homeostasis. Proc. Natl. Acad. Sci. USA. 2004;101:14355. doi: 10.1073/pnas.0406216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dufner-Beattie J, Kuo YM, Gitschier J, Andrews GK. The adaptive response to dietary zinc in mice involves the differential cellular localization and zinc regulation of the zinc transporters ZIP4 and ZIP5. J. Biol. Chem. 2004;279:49082. doi: 10.1074/jbc.M409962200. [DOI] [PubMed] [Google Scholar]
- 8.Weaver BP, Dufner-Beattie J, Kambe T, Andrews GK. Novel zinc-responsive post-transcriptional mechanisms reciprocally regulate expression of the mouse Slc39a4 and Slc39a5 zinc transporters (Zip4 and Zip5) Biol. Chem. 2007;388:1301. doi: 10.1515/BC.2007.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liuzzi JP, Guo L, Chang SM, Cousins RJ. Kruppel-like factor 4 regulates adaptive expression of the zinc transporter Zip4 in mouse small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296:G517. doi: 10.1152/ajpgi.90568.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizumori M, Ham M, Guth PH, Engel E, Kaunitz JD, Akiba Y. Intestinal alkaline phosphatase regulates protective surface microclimate pH in rat duodenum. J. Physiol. 2009;587:3651. doi: 10.1113/jphysiol.2009.172270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huesca M, Lock LS, Khine AA, Viau S, Peralta R, Cukier IH, Jin H, Al-Qawasmeh RA, Lee Y, Wright J, Young A. A novel small molecule with potent anticancer activity inhibits cell growth by modulating intracellular labile zinc homeostasis. Mol. Cancer Ther. 2009;8:2586. doi: 10.1158/1535-7163.MCT-08-1104. [DOI] [PubMed] [Google Scholar]
- 12.Shah YM, Matsubara T, Ito S, Yim SH, Gonzalez FJ. Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell Metab. 2009;9:152. doi: 10.1016/j.cmet.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim BE, Wang F, Dufner-Beattie J, Andrews GK, Eide DJ, Petris MJ. Zn2+-stimulated endocytosis of the mZIP4 zinc transporter regulates its location at the plasma membrane. J. Biol. Chem. 2004;279:4523. doi: 10.1074/jbc.M310799200. [DOI] [PubMed] [Google Scholar]
- 14.Mao X, Kim BE, Wang F, Eide DJ, Petris MJ. A histidine-rich cluster mediates the ubiquitination and degradation of the human zinc transporter, hZIP4, and protects against zinc cytotoxicity. J. Biol. Chem. 2007;282:6992. doi: 10.1074/jbc.M610552200. [DOI] [PubMed] [Google Scholar]
- 15.Jackson KA, Helston RM, McKay JA, O'Neill ED, Mathers JC, Ford D. Splice variants of the human zinc transporter ZnT5 (SLC30 A5) are differentially localized and regulated by zinc through transcription and mRNA stability. J. Biol. Chem. 2007;282:10423. doi: 10.1074/jbc.M610535200. [DOI] [PubMed] [Google Scholar]
- 16.Valentine RA, Jackson KA, Christie GR, Mathers JC, Taylor PM, Ford D. ZnT5 variant B is a bidirectional zinc transporter and mediates zinc uptake in human intestinal Caco-2 cells. J. Biol. Chem. 2007;282:14389. doi: 10.1074/jbc.M701752200. [DOI] [PubMed] [Google Scholar]
- 17.Inoue K, Matsuda K, Itoh M, Kawaguchi H, Tomoike H, Aoyagi T, Nagai R, Hori M, Nakamura Y, Tanaka T. Osteopenia and male-specific sudden cardiac death in mice lacking a zinc transporter gene, Znt5. Hum. Mol. Genet. 2002;11:1775. doi: 10.1093/hmg/11.15.1775. [DOI] [PubMed] [Google Scholar]
- 18.Liuzzi JP, Aydemir F, Nam H, Knutson MD, Cousins RJ. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc. Natl. Acad. Sci. USA. 2006;103:13612. doi: 10.1073/pnas.0606424103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liuzzi JP, Lichten LA, Rivera S, Blanchard RK, Aydemir TB, Knutson MD, Ganz T, Cousins RJ. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc. Natl. Acad. Sci. USA. 2005;102:6843. doi: 10.1073/pnas.0502257102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Zhou B. Dietary zinc absorption: A play of Zips and ZnTs in the gut. IUBMB Life. 62:176. doi: 10.1002/iub.291. year. [DOI] [PubMed] [Google Scholar]
- 21.McMahon RJ, Cousins RJ. Regulation of the zinc transporter ZnT-1 by dietary zinc. Proc. Natl. Acad. Sci. USA. 1998;95:4841. doi: 10.1073/pnas.95.9.4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirschke CP, Huang L. ZnT7, a novel mammalian zinc transporter, accumulates zinc in the Golgi apparatus. J. Biol. Chem. 2003;278:4096. doi: 10.1074/jbc.M207644200. [DOI] [PubMed] [Google Scholar]
- 23.Huang L, Yu YY, Kirschke CP, Gertz ER, Lloyd KK. Znt7 (Slc30a7)-deficient mice display reduced body zinc status and body fat accumulation. J. Biol. Chem. 2007;282:37053. doi: 10.1074/jbc.M706631200. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki T, Ishihara K, Migaki H, Nagao M, Yamaguchi-Iwai Y, Kambe T. Two different zinc transport complexes of cation diffusion facilitator proteins localized in the secretory pathway operate to activate alkaline phosphatases in vertebrate cells. J. Biol. Chem. 2005;280:30956. doi: 10.1074/jbc.M506902200. [DOI] [PubMed] [Google Scholar]
- 25.Fleet JC, Turnbull AJ, Bourcier M, Wood RJ. Vitamin D-sensitive and quinacrine-sensitive zinc transport in human intestinal cell line Caco-2. Am. J. Physiol. 1993;264:G1037. doi: 10.1152/ajpgi.1993.264.6.G1037. [DOI] [PubMed] [Google Scholar]
- 26.Langmade SJ, Ravindra R, Daniels PJ, Andrews GK. The transcription factor MTF-1 mediates metal regulation of the mouse ZnT1 gene. J. Biol. Chem. 2000;275:34803. doi: 10.1074/jbc.M007339200. [DOI] [PubMed] [Google Scholar]
- 27.Lonnerdal B. Intestinal Absorption of Zinc. Springer-Verlag; London, New York: 1988. p. 33. [Google Scholar]
- 28.Guo L, Lichten LA, Ryu MS, Liuzzi JP, Wang F, Cousins RJ. STAT5-glucocorticoid receptor interaction and MTF-1 regulate the expression of ZnT2 (Slc30a2) in pancreatic acinar cells. Proc. Natl. Acad. Sci. USA. 2010;107:2818. doi: 10.1073/pnas.0914941107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.King J, Cousins RJ. Zinc. 10th ed Lippincott, Williams & Wilkins; Philadelphia: 2006. p. 271. [Google Scholar]
- 30.Mburu AS, Thurnham DI, Mwaniki DL, Muniu EM, Alumasa FM. The influence of inflammation on plasma zinc concentration in apparently healthy, HIV+ Kenyan adults and zinc responses after a multi-micronutrient supplement. Eur. J. Clin. Nutr. 2010;64:510. doi: 10.1038/ejcn.2010.33. [DOI] [PubMed] [Google Scholar]
- 31.Zhong W, McClain CJ, Cave M, Kang YJ, Zhou Z. The role of zinc deficiency in alcohol-induced intestinal barrier dysfunction. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;298:G625. doi: 10.1152/ajpgi.00350.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pekarek RS, Evans GW. Effect of acute infection and endotoxemia on zinc absorption in the rat. Proc. Soc. Exp. Biol. Med. 1975;150:755. doi: 10.3181/00379727-150-39119. [DOI] [PubMed] [Google Scholar]
- 33.Sas B, Bremner I. Effect of acute stress on the absorption and distribution of zinc and on Znmetallothionein production in the liver of the chick. J. Inorg. Biochem. 1979;11:67. doi: 10.1016/s0162-0134(00)80055-0. [DOI] [PubMed] [Google Scholar]
- 34.Lichten LA, Liuzzi JP, Cousins RJ. Interleukin-1beta contributes via nitric oxide to the upregulation and functional activity of the zinc transporter Zip14 (Slc39a14) in murine hepatocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296:G860. doi: 10.1152/ajpgi.90676.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


