Abstract
Background
Carbamazepine is a commonly used antiepileptic drug in elderly patients. This study analyzed prospective data collected as part of a randomized, double-blinded trial of newly diagnosed epilepsy patients. The aims of this study were to determine the pharmacokinetic parameters and their variability of carbamazepine in elderly patients and to quantify the effect of covariates on these parameters.
Methods
Prospectively collected carbamazepine concentrations from 121 patients aged 60 years or older were used to develop a population pharmacokinetic model. Data were analyzed by a nonlinear mixed effects model (NONMEM). A one-compartment model with first order absorption and elimination was used to characterize the time course of carbamazepine concentration. Model evaluation and the predictive performance of the final model were assessed using the nonparametric bootstrap approach.
Results
The apparent clearance (CL/F) of carbamazepine in this community-dwelling elderly population was estimated to be 3.59 L/hr with an inter-individual variability of 18.1%. The CL/F increases 23% in patients co-medicated with phenytoin. The volume of distribution (V/F) was estimated to be 102 L with an inter-individual variability of 74.7%.
Conclusions
Carbamazepine clearance was not associated with body weight or any parameterization of body size, nor was age or race, nor any marker of hepatic or renal function. Patients on concurrent phenytoin therapy can be expected to require a 23% higher dose on average.
Keywords: Carbamazepine, anticonvulsants, pharmacokinetics, aged
Background
Carbamazepine is approved for the treatment of both partial and generalized tonic-clonic seizures as well as bipolar disorder and trigeminal neuralgia [1, 2]. The incidence of epilepsy is increased in those over 60 years of age [3]. Although lamotrigine and gabapentin have been shown to be more tolerable than carbamazepine in the elderly population [4], carbamazepine is used frequently in the elderly epilepsy population [5, 6]. In addition, elderly patients who are on carbamazepine and considered stable will most likely not switch to another antiepileptic drug. The pharmacokinetics of carbamazepine is complex, including high variability in absorption, high protein binding, low therapeutic index, and enzyme induction which may be different in the elderly versus younger adult patients. Not only is carbamazepine inducible by other enzyme-inducing co-medications, it also induces its own metabolism making this medication a challenge when administered as part of a polytherapy regimen.
Physiological changes in the elderly may affect the pharmacokinetics of drugs. Carbamazepine is extensively metabolized in man by the cytochrome P450 3A4 (CYP3A4) isoenzyme. There is evidence that CYP3A4 shows a decrease in activity with increasing age [7]. Moreover, it has been suggested that the inducible effect is less sensitive in the elderly than in younger patients [8, 9]. Evidence from studies that compared therapeutic drug monitoring data in younger and elderly outpatients show that the elderly may require lower doses of carbamazepine than younger adults [10, 11]. For all of these reasons, the dose of carbamazepine selected when initiating therapy is open to a great deal of interpretation. An understanding of the causes of altered pharmacokinetics and quantification of their effects would allow clinicians to more effectively choose initial doses of carbamazepine and potentially reduce the frequency of therapeutic drug monitoring. The aims of this analysis were to determine the relevant population pharmacokinetic parameters of carbamazepine in a population of community dwelling elderly subjects and to evaluate the impact of eighteen commonly available covariates on drug dosing requirements through an effect on drug clearance.
Methods
Patients and Data Collection
Carbamazepine concentration data were obtained from VA Cooperative Study 428, a randomized, double-blinded, parallel group, monotherapy comparison study of lamotrigine, gabapentin, and carbamazepine in elderly patients with epilepsy (N=593)[4]. The VA Cooperative Study 428 was approved by the central VA Human Rights Committee and all local institutional review boards. This study was approved by the University of Minnesota’s Human Subject’s Committee.
Patients aged 60 years or older with newly diagnosed seizures were randomly assigned to one of the three treatment groups. Data from the carbamazepine arm were used in this analysis. Carbamazepine was titrated by 200 mg every 2 weeks to 600 mg/day. It should be noted that all carbamazepine prescribed to these patients were immediate release formulation Tegretol. Coadministration of other antiepileptic medications included phenytoin (n=20 patients), phenobarbital (n=1 patient) and valproic acid (n=2 patients). Concomitant therapy on any one nonantiepileptic drug known to be an inducer or inhibitor of carbamazepine were in less than 10% of the population and therefore, these drugs were not tested. Patients were allowed to enter the study if they were receiving phenytoin. However, the phenytoin doses were gradually withdrawn during the first 6–8 weeks of carbamazepine therapy. Blood collection and clinical evaluations were carried out at enrollment, biweekly to week 8, monthly to week 28, and bimonthly to week 52. The time of blood collection was not controlled and reflected convenience samples. As carbamazepine concentrations were obtained at least 2 weeks after a change of carbamazepine dose, carbamazepine concentrations obtained at each visit were assumed to be at steady statein the post-induction phase. Dosing history including the actual time the dose was received and drug amount of the last three doses before sampling time, time of blood draw, and updated co-medications were obtained at each clinic visit. Pill counts indicated that medications were taken as prescribed by 93% of the patients in the study.
Population Pharmacokinetic Analysis
The population pharmacokinetic analyses were performed using NONMEM (version VI, NONMEM Project Group, UCSF/Globomax and PDx-Pop version 2.0). A one-compartment model with first order absorption and elimination (subroutine ADVAN2 TRANS2) was used [12]. Therefore, CL/F, V/F, and Ka were obtained from the model. The inter-individual variability (IIV) was modeled using an exponential error model for all parameters as given in the following equation.
| equation 1 |
Pj is the pharmacokinetic parameter for subject j, TVP is the typical value of the pharmacokinetic parameter in the population, and η is the inter-individual variability which is assumed to be normally distributed with a mean of zero and variance of ω2. The residual unexplained variability (RUV) which includes other unexplained variability (e.g., model misspecification, assay errors, and dosing history errors) was modeled using a proportional error model which can be described by the following equation.
| equation 2 |
where Cobs, ij and Cpred, ij are the ith observed and predicted concentrations in the individual and εij is the residual unexplained variability which is normally distributed with a mean of zero and variance of σ2.
The first-order conditional estimation method with interaction (FOCE-I) was used for all analyses. A graphical assessment of the goodness of fit was performed by Xpose 3.1[13] and S-PLUS 2000 (Insightful Corp, Seattle, WA, USA).
The model was initially developed without including patient specific covariates (base pharmacokinetic model). To identify the significant covariates, 18 available covariates that could be important in elderly patients were considered in the covariate model building step. These covariates included measures of body size (weight, body mass index (BMI), body surface area (BSA), ideal body weight (IBW), lean body weight (LBW)) to determine if body size is a better predictor of pharmacokinetic parameters in elderly patients; demographic factors (race, age, study center); co-medication use of known metabolic inducers (phenytoin use); factors that could effect liver function (alcohol use, smoking, age, aspartate aminotransferase (AST), alanine aminotransferase (ALT)); factors that could affect protein binding (albumin (ALB), total protein); and kidney function indicators (blood urea nitrogen (BUN), serum creatinine (CR), creatinine clearance (CRCL) which was calculated using Cockroft-Gault equation). Due to a small sample size of some race categories, race was evaluated as a dichotomous covariate (White and non-White). Age was evaluated as both a continuous and categorical covariate (age ≤ 80, and age > 80 years). Age 80 years was considered as a possible break point based on the initial graphical analysis. Each covariate was added to the base model sequentially using a stepwise technique. All continuous covariates were introduced into the base model in a linear and non-linear manner. The significance of each covariate was evaluated by the likelihood ratio test and visual inspection of diagnostic plots. The difference in NONMEM objective function value (OFV) of at least 6.64 (corresponding to a nominal p-value of 0.01) and 10.83 (corresponding to a nominal p-value of 0.001) were used as cutoff values for forward selection and backward deletion, respectively.
A covariate randomization procedure was used to assess the actual significance levels for a covariate effect in the final model [14, 15]. A randomization test was performed for each covariate remaining in the final model separately. Each covariate vector in the final model was randomly permuted 2000 times using Wings for NONMEM (Version 600, http://wfn.sourcefourge.net). A full model (a model including a parameter-covariate relationship) was fitted to each of the permuted data sets. The difference in the objective function value (dOFV) between a full model and a reduced model (a model without a parameter-covariate relationship) was calculated. Two thousand dOFV were obtained and rank ordered. The dOFV values corresponding to the 0.1th and 1stpercentile were used as a cutoff dOFV for the actual p-value of 0.001 and 0.01, respectively.
Model evaluation
Model evaluation was assessed using the nonparametric bootstrap. The bootstrap re-sampling technique was applied to assess model reliability of the parameter estimates and their 95% confidence interval (CI) from the final model [16, 17]. One thousand bootstrap data sets were generated by repeatedly sampling with replacement from the original data set using Wings for NONMEM (Version 600, http://wfn.sourcefourge.net). The final model was fitted to each of 1000 bootstrap data sets and parameter estimates were obtained. These parameter estimates were rank ordered. The values at the 2.5th, 50th (median), and 97.5th percentile were obtained and compared with the parameter estimates and their 95% CI obtained from NONMEM.
The predictive performance of the final model was assessed using the bootstrap approach previously illustrated [17, 18]. In this study, two prediction error metrics, mean square error (MSE), defined as the squared difference between the observed and model predicted concentrations and mean absolute error (MAE), defined as the absolute difference between the observed and model predicted concentrations, were used to assess predictive performance. Two hundred bootstrap data sets were generated using Wings for NONMEM (Version 600, http://wfn.sourcefourge.net). The final model was fitted to each of the bootstrap data sets obtaining a bootstrap model. In this step the coefficients were allowed to be estimated from each of the 200 bootstrap data sets. Therefore, 200 bootstrap models were obtained. Each of the bootstrap models was applied to the original data set by fixing the coefficients and random effects for the bootstrap model. The difference between the MSE obtained from fitting the bootstrap model to the bootstrap data set and MSE obtained from fitting the bootstrap model to the original data set, defined as the “optimism” (OPT1) was calculated. The difference between the MAE obtained from fitting the bootstrap model to the bootstrap data set and MAE obtained from fitting the bootstrap model to the original data set defined as OPT2 was also calculated. The mean OPT1 and OPT2 from200 paired runs were obtained. The MSEimp, the improved estimate of MSE, and MAEimp, the improved estimate of MAE, were calculated by adding the mean OPT1 and OPT2 to the original MSE and MAE obtained from fitting the final model to the original data set.
Results
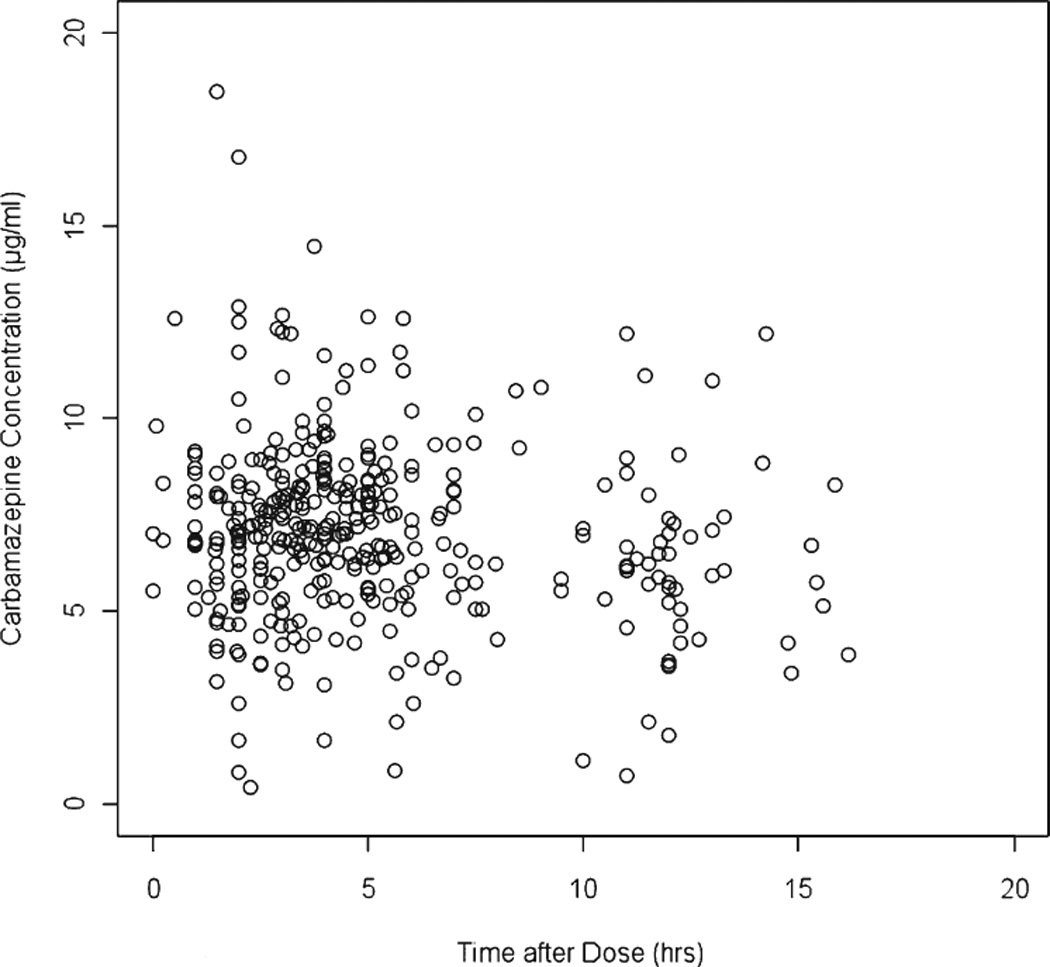
A total of 555 plasma concentrations from 121 patients were used in this analysis. A summary of the patient characteristics is shown in Table 1. All concentrations were below 20 µg/ml with a majority of concentrations below 10 µg/ml. A plot of carbamazepine plasma concentrations (µg/ml) versus the time after last dose (hours) of patients taking carbamazepine 600 mg/day is shown in Figure 1.
Table 1.
Summary of Patient Characteristics (N=121)
| Patient Characteristics | Frequencies (%) |
|---|---|
| Gender | |
| Male | 118 (97.5) |
| Female | 3 (2.5) |
| Race | |
| American Indian | 3 (2.5) |
| Black | 30 (24.8) |
| Hispanic | 4 (3.3) |
| White | 83 (68.6) |
| Other | 1 (0.8) |
| Alcohol use | 36 (29.8) |
| Smokers | 31 (25.6) |
| PHT use* | 20 (16.5) |
| mean (range) | |
| Age (year) | 70.5 (60-96) |
| Weight (kg) | 81.4 (50-129) |
| IBW (kg) | 68.6 (37-82) |
| BMI (kg) | 27 (17-38) |
| LBW (kg) | 61 (39-80) |
| BSA (m2) | 1.96 (1.44-2.5) |
| Height (inches) | 68.6 (52-76) |
| Creatinine Clearance (ml/min) | 76.45 (5.45-174) |
| BUN (mg%) | 18.4 (3-92) |
| Serum creatinine (mg%) | 1.15 (0.6-11.5) |
| Albumin (mg%) | 3.8 (2.1-5) |
| Aspartate aminotransferase (mU/ml) | 24 (6-361) |
| Alanine aminotransferase (mU/ml) | 27 (3-266) |
| Total protein (gm%) | 7 (5.5-9) |
The patients used PHT before enrollment and PHT dose was gradually withdrawn during the 5-week titration phase of CBZ.
IBW, ideal body weight; BMI, body mass index; LBW, lean body weight; BSA, body surface area; BUN, blood urea nitrogen; PHT, phenytoin.
Figure 1.
As is often the case with sparse data sets in relatively small populations, interindividual variability cannot be obtained for all parameters of interest. In this analysis, the IIV of Ka could not be estimated and only the typical value of Ka in the population is reported. This does not mean that there is no between-subject variability in Ka, but that there is not sufficient information in the available data to uniquely identify the variability parameter. During the forward selection process, the significant covariates for CL/F were phenytion use and ALT; whereas, phenytoin use and alcohol use were the significant covariates for V/F. When backward deletion was performed, ALT failed to reach a significance level for CL/F. Similarly, alcohol use and phenytoin use failed to reach a significance level for V/F. Finally, only phenytoin use was a significant covariate for CL/F; whereas, none of the covariates were significant for V/F. The final model can be represented by the following equations:
| equation 3.1 |
| equation 3.2 |
| equation 3.3 |
The final parameter estimates from NONMEM are summarized in Table 2. The goodness-of-fit plots from the final model are presented in Figure 2.
Table 2.
The final parameter estimates and 95% CI from NONMEM and the bootstrap analyses§
| Parameter | NONMEM | Bootstrap Analysis | ||
|---|---|---|---|---|
| Estimate | 95% CI* | Median | 95% CI† | |
| CL/F (L/hr) | 3.59 | [3.39–3.79] | 3.58 | [3.39–3.80] |
| If taking PHT | 1.23 | [0.961–1.50] | 1.26 | [0.963–1.50] |
| V/F (L) | 102 | [37.1–167] | 92.1 | [19–189.6] |
| Ka (hr−1) | 0.197 | [0.0933–0.301] | 0.19 | [0.03–0.314] |
| IIV of CL/F (%CV) | 18.1 | [12.37–22.34] | 17.92 | [13.02–22.98] |
| IIV of V/F (%CV) | 74.7 | [21.1–103.44] | 77.84 | [1.06–121.24] |
| RUV, proportional (%CV) | 25.1 | [21.47–28.2] | 24.6 | [21.74–28.68] |
From 747 bootstrap runs with a successful minimization and successful covariance step
(Estimate) ± 1.96 × (standard error of the estimate)
2.5th and 97.5th percentile of the ranked bootstrap parameter estimates
Figure 2.
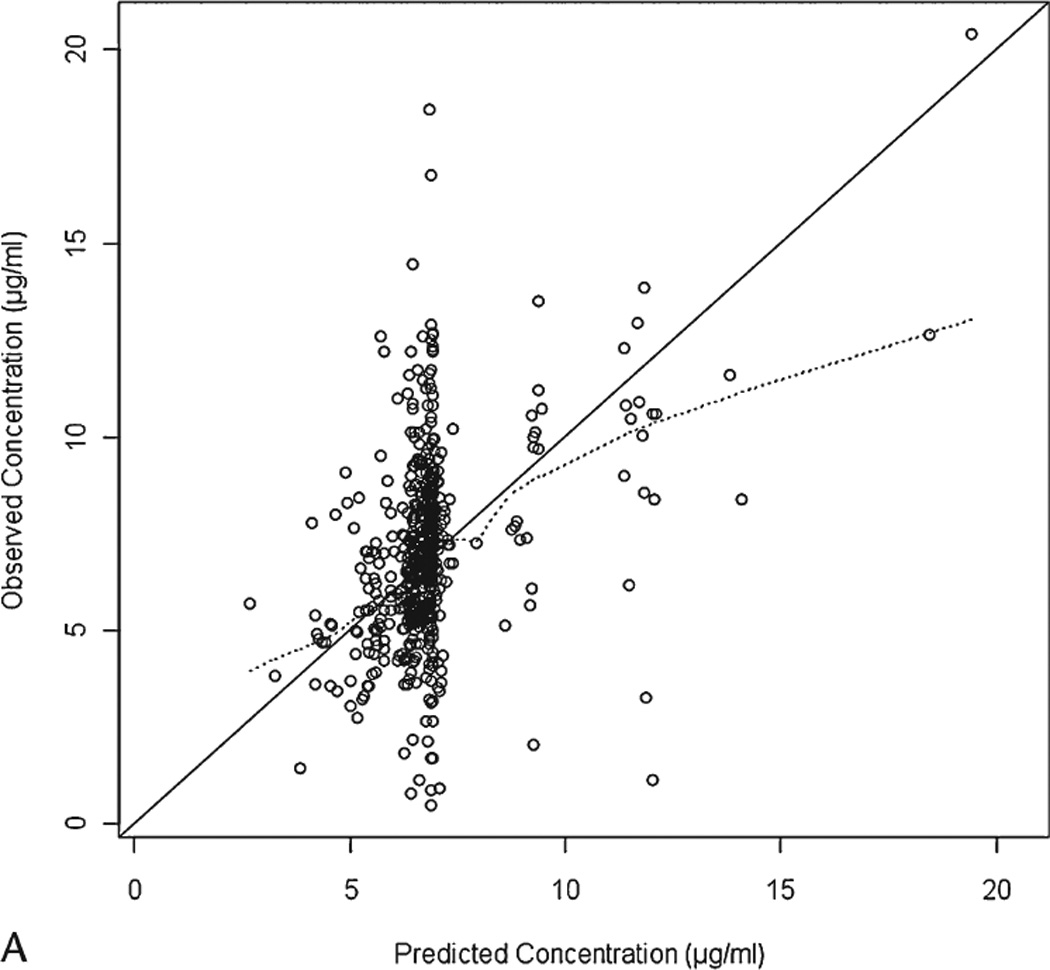
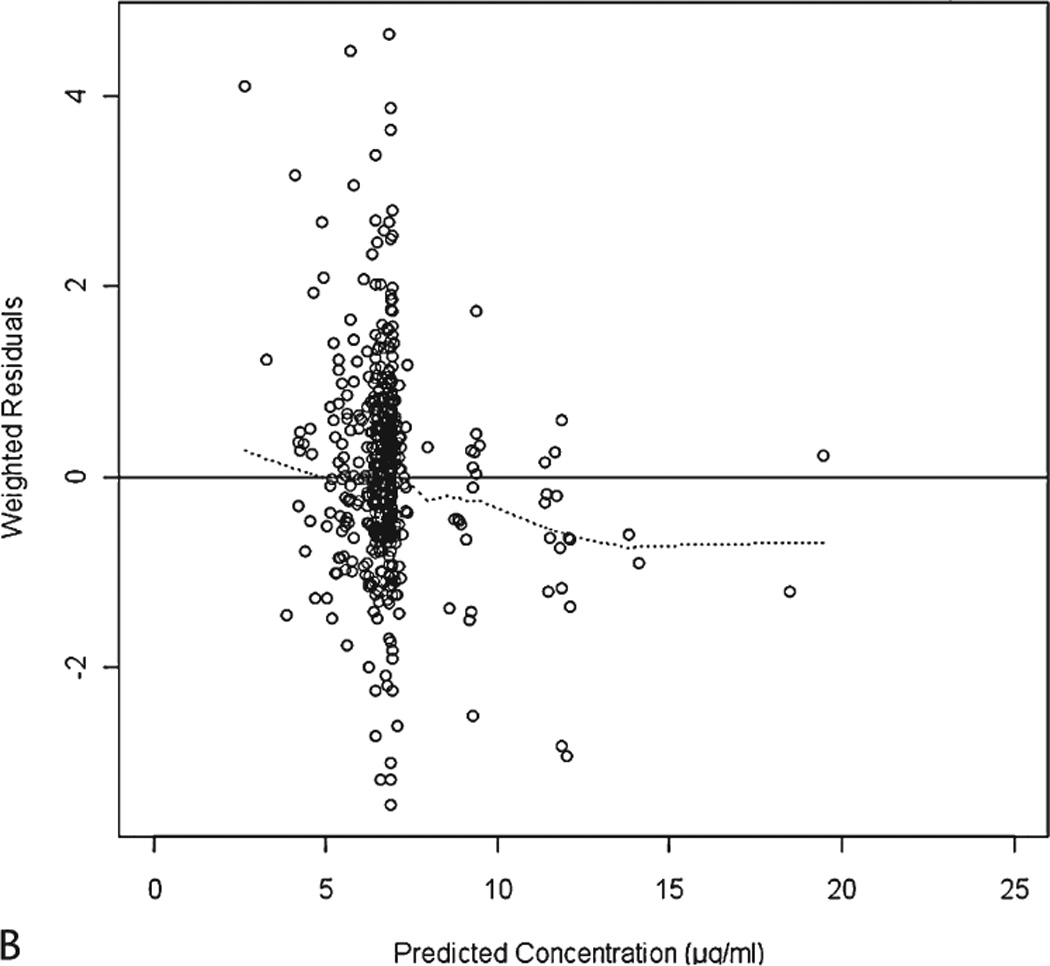
The cutoff dOFVs corresponding to the actual p-value of 0.01 and 0.001 based on a 2000 randomization procedure were 9.763 and 6.379, respectively. The results from the randomization test show that the actual significance levels are similar to the nominal levels.
Model Evaluation
From 1000 bootstrap runs, 747 runs had a successful minimization and successful covariance step and were included in the bootstrap analysis. Table 2 shows the parameter estimates and 95% CI obtained from NONMEM compared with the values obtained from the bootstrap approach. The mean parameter estimates for the fixed and random effects from the bootstrap approach were comparable and within 10% of the estimates from NONMEM. Therefore, we determined the parameter estimates from NONMEM to be reliable. The 95% CI for all parameters from the bootstrap approach were generally comparable with the estimates from NONMEM, except for IIV of V/F.
When the bootstrap approach was applied to assess predictive performance, the average OPT1 and OPT2 from 200 bootstrap runs were 0.03 µg/ml and 0.009 µg2/ml2, respectively. The original MSE and MAE obtained from fitting the final model to the original data set were 5.29 µg2/ml2 and 1.63 µg/ml; therefore, the MSEimp and MAEimp were 5.33 µg2/ml2 and 1.64 µg/ml, respectively. The average OPT1 and OPT2 were small and less than 0.15 times MSEimp and MAEimp. Therefore, the model is considered to be without substantial deficiencies.
Discussion
This study presents the development of an equation for and an estimation of the carbamazepine pharmacokinetic parameters as well as the relationship of these parameters with patient specific covariates in a particular patient population, community-dwelling elderly patients. The population mean CL/F estimated from the model was 3.59 L/hr in patients receiving carbamazepine monotherapy with an interindividual variability of 18.1%. Among 18 covariates considered in this analysis, phenytoin use was the only covariate found to be significant for CL/F although it should be noted that the increase in carbamazepine CL/F with the co-administration of phenytoin was to a smaller extent than that seen with younger adults. The estimates of V/F and Ka were 102 L (IIV = 74%) and 0.197 hr−1, respectively. Based on the parameter estimates from the final model, carbamazepine half-life was approximately 19.7 hours in this community dwelling elderly patients which was consistent with the values estimated from adult patients of 10–20 hours [19, 20]. These values may provide a more population specific estimate of maintenance and loading doses in the community-dwelling elderly population.
The estimate of CL/F and the variability between patients from our study are comparable with previous reports from population pharmacokinetic studies of carbamazepine in adults and elderly patients [21–24]. Carbamazepine is primarily metabolized by CYP3A4 yielding an active carbamazepine-10, 11-epoxide. CYP3A4 is known to decrease its capacity with increasing age [7]. Whereas there is evidence of decreasing CL/F of carbamazepine in elderly patients compared with adult patients [6, 22]; some studies show no age-dependent changes in CL/F of carbamazepine [23, 25]. These previous studies included small numbers of elderly patients. The effect of age on CL/F of carbamazepine was not observed in our study and therefore, indicates that clearance may not differ significantly once an individual reaches 60 years of age. However, this data set consists of only elderly patients aged 60–96 years old. In order to investigate the effect of age on carbamazepine pharmacokinetics and the difference of pharmacokinetic parameters between elderly and younger adults, further study in a data set with a wider range of age is required.
The metabolism of carbamazepine is highly inducible by other antiepileptic drugs including phenytoin, primidone, and phenobarbital [26, 27]. In this study, phenytoin increases carbamazepine clearance, but to a lesser extent (23% compared to 44 and 42%) than in previous studies in adult patients [22, 23]. This difference in the effect on carbamazepine clearance in the presence of phenytoin may be due to a decreased sensitivity to enzyme induction in elderly patients [8, 9]. This finding suggests that doses may need to be changed to a lesser degree in elderly patients than younger adults who are having phenytoin added or withdrawn from their dosing regimens.
Previous population pharmacokinetic studies show a positive association between CL/F and total daily dose (TDD) [23, 24]. Several explanations were given to discuss this association such as a change in bioavailability, hepatic enzyme activity, or the degree of autoinduction of carbamazepine metabolism [23, 24]. In medications where doses are adjusted based on therapeutic drug monitoring, clinical responses, or adverse effects, there is an inherent correlation between dose and drug clearance; this correlation does not necessarily imply a true relationship [28]. The clinical management of carbamazepine in this elderly population would introduce an inherent correlation between dose and clearance in this data set. Therefore, total daily dose was not considered in covariate model building in this study.
The results from the randomization test show that the actual significance levels are similar to the nominal levels which is expected when FOCE-I is used [15]. The results from the bootstrap analysis show that the median parameter estimates obtained from 1000 bootstrap data sets were generally comparable to the estimates from NONMEM indicating that the final parameter estimates are reliable [17]. However, 95% CI of IIV for V/F obtained from the bootstrap approach was wider than the one obtained from NONMEM. This may be a result of the sparse sampling design used in this data. With a limited number of samples per individual, the ability to obtain an accurate and a precise estimate of the interindividual variability of the parameter estimates is often problematic [29]. This contributes to a wider bootstrap 95% CI of IIV for V/F.
The predictive performance of the final model was assessed by the bootstrap approach. When the model is applied to the data from which it was derived, the predictive metrics (e.g., mean absolute error) tend to be smaller than when the model is applied to the external data. Therefore, the “optimism” (OPT) is calculated and is added to obtain the improved metrics. The improved metrics will then reflect the values obtained when the model is applied to the external data. In this study, the mean OPT for both MAE and MSE are less than 0.15 times the improved metric, indicating that the model is without substantial deficiency [17].
There were some limitations in this study. First, the dosing history (the actual time and drug amount of the last three doses before sampling time) was based on patients’ self-report which commonly may not be exact in a large clinical study. Additionally, only one blood sample was taken for each individual at each clinic visit. These factors may impact the accuracy and precision of the parameter estimates. However, a previous simulation study shows that the clearance estimate and its IIV are robust for sampling time error [30]. Moreover, a limited number of samples have minimal effect on the fixed effect parameter estimates [29]. Therefore, the parameters in this study should be reasonably estimated. Finally, the effect of other co-medications interacting with carbamazepine was not quantified, as phenytoin was the only interacting co-medication that was present in a sufficient number of patients to be tested in this study population.
Conclusion
This study provides estimates of population pharmacokinetic parameters from a community-dwelling elderly population. An important result from the covariate analysis is that of the commonly available covariates, only concurrent phenytoin use had a significant impact on carbamazepine clearance and can potentially guide the selection of the initial dose. Carbamazepine clearance was not associated with body weight or any parameterization of body size, nor was age or race, nor any marker of hepatic or renal function. Hence, there is little to be gained from weight-based doses or modifying initial doses based on age, race, renal insufficiency, or laboratory markers of hepatic function in community-dwelling elderly. Patients on concurrent phenytoin therapy can be expected to require a 23% higher dose on average. Clinicians dosing to a targeted concentration range will likely need to continue monitoring plasma concentrations and adjusting doses as needed.
Acknowledgements
This work was supported by NIH/NINDS P50-NS16308 from the National Institute of Neurological Disorders and Stroke (NINDS) and NIH/NIA R01-AG026390 from the National Institute on Aging (NIA). The content is solely the responsibility of the authors and does not necessarily represent the official views of NINDS, NIA, or the National Institutes of Health. The authors thank the VA Cooperative Study 428 for providing the data set used in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Johannessen Landmark C. Antiepileptic drugs in non-epilepsy disorders: relations between mechanisms of action and clinical efficacy. CNS Drugs. 2008;22(1):27–47. doi: 10.2165/00023210-200822010-00003. [DOI] [PubMed] [Google Scholar]
- 2.Brodie MJ, Dichter MA. Antiepileptic drugs. N Engl J Med. 1996;334(3):168–175. doi: 10.1056/NEJM199601183340308. [DOI] [PubMed] [Google Scholar]
- 3.Hauser WA, Annegers JF, Kurland LT. Prevalence of epilepsy in Rochester, Minnesota: 1940–1980. Epilepsia. 1991;32(4):429–445. doi: 10.1111/j.1528-1157.1991.tb04675.x. [DOI] [PubMed] [Google Scholar]
- 4.Rowan AJ, Ramsay RE, Collins JF, et al. New onset geriatric epilepsy: a randomized study of gabapentin, lamotrigine, and carbamazepine. Neurology. 2005;64(11):1868–1873. doi: 10.1212/01.WNL.0000167384.68207.3E. [DOI] [PubMed] [Google Scholar]
- 5.Savica R, Beghi E, Mazzaglia G, et al. Prescribing patterns of antiepileptic drugs in Italy: a nationwide population-based study in the years 2000–2005. Eur J Neurol. 2007;14(12):1317–1321. doi: 10.1111/j.1468-1331.2007.01970.x. [DOI] [PubMed] [Google Scholar]
- 6.Cloyd JC, Lackner TE, Leppik IE. Antiepileptics in the elderly. Pharmacoepidemiology and pharmacokinetics. Arch Fam Med. 1994;3(7):589–598. doi: 10.1001/archfami.3.7.589. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka E. In vivo age-related changes in hepatic drug-oxidizing capacity in humans. J Clin Pharm Ther. 1998;23(4):247–255. doi: 10.1046/j.1365-2710.1998.00164.x. [DOI] [PubMed] [Google Scholar]
- 8.Salem SA, Rajjayabun P, Shepherd AM, et al. Reduced induction of drug metabolism in the elderly. Age Ageing. 1978;7(2):68–73. doi: 10.1093/ageing/7.2.68. [DOI] [PubMed] [Google Scholar]
- 9.Twum-Barima Y, Finnigan T, Habash AI, et al. Impaired enzyme induction by rifampicin in the elderly. Br J Clin Pharmacol. 1984;17(5):595–597. [PMC free article] [PubMed] [Google Scholar]
- 10.Battino D, Croci D, Rossini A, et al. Serum carbamazepine concentrations in elderly patients: a case-matched pharmacokinetic evaluation based on therapeutic drug monitoring data. Epilepsia. 2003;44(7):923–929. doi: 10.1046/j.1528-1157.2003.62202.x. [DOI] [PubMed] [Google Scholar]
- 11.Birnbaum AK, Conway JM, Hardie NA, et al. Carbamazepine dose-concentration relationship in elderly nursing home residents. Epilepsy Res. 2007;77(1):31–35. doi: 10.1016/j.eplepsyres.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Beal SL, Sheiner LB. NONMEM Users Guide. 1994 [Google Scholar]
- 13.Jonsson EN, Karlsson MO. Xpose--an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed. 1999;58(1):51–64. doi: 10.1016/s0169-2607(98)00067-4. [DOI] [PubMed] [Google Scholar]
- 14.Wahlby U, Bouw MR, Jonsson EN, et al. Assessment of type I error rates for the statistical sub-model in NONMEM. J Pharmacokinet Pharmacodyn. 2002;29(3):251–269. doi: 10.1023/a:1020254823597. [DOI] [PubMed] [Google Scholar]
- 15.Wahlby U, Jonsson EN, Karlsson MO. Assessment of actual significance levels for covariate effects in NONMEM. J Pharmacokinet Pharmacodyn. 2001;28(3):231–252. doi: 10.1023/a:1011527125570. [DOI] [PubMed] [Google Scholar]
- 16.Efron B, Gong G. A leisurely look at the bootstrap, the jacknife, and cross-validation. Am Stat. 1983;37:36–48. [Google Scholar]
- 17.Ette EI, Williams PJ, Kim YH, et al. Model appropriateness and population pharmacokinetic modeling. J Clin Pharmacol. 2003;43(6):610–623. [PubMed] [Google Scholar]
- 18.Ette EI. Stability and performance of a population pharmacokinetic model. J Clin Pharmacol. 1997;37(6):486–495. doi: 10.1002/j.1552-4604.1997.tb04326.x. [DOI] [PubMed] [Google Scholar]
- 19.Bertilsson L. Clinical pharmacokinetics of carbamazepine. Clin Pharmacokinet. 1978;3(2):128–143. doi: 10.2165/00003088-197803020-00003. [DOI] [PubMed] [Google Scholar]
- 20.Punyawudho B, Cloyd JC, Leppik IE, et al. Characterization of the time course of carbamazepine deinduction by an enzyme turnover model. Clin Pharmacokinet. 2009;48(5):313–320. doi: 10.2165/00003088-200948050-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bondareva IB, Jelliffe RW, Gusev EI, et al. Population pharmacokinetic modelling of carbamazepine in epileptic elderly patients: implications for dosage. J Clin Pharm Ther. 2006;31(3):211–221. doi: 10.1111/j.1365-2710.2006.00717.x. [DOI] [PubMed] [Google Scholar]
- 22.Graves NM, Brundage RC, Wen Y, et al. Population pharmacokinetics of carbamazepine in adults with epilepsy. Pharmacotherapy. 1998;18(2):273–281. [PubMed] [Google Scholar]
- 23.Jiao Z, Shi XJ, Zhao ZG, et al. Population pharmacokinetic modeling of steady state clearance of carbamazepine and its epoxide metabolite from sparse routine clinical data. J Clin Pharm Ther. 2004;29(3):247–256. doi: 10.1111/j.1365-2710.2004.00557.x. [DOI] [PubMed] [Google Scholar]
- 24.Reith DM, Hooper WD, Parke J, et al. Population pharmacokinetic modeling of steady state carbamazepine clearance in children, adolescents, and adults. J Pharmacokinet Pharmacodyn. 2001;28(1):79–92. doi: 10.1023/a:1011569703060. [DOI] [PubMed] [Google Scholar]
- 25.Hockings N, Pall A, Moody J, et al. The effects of age on carbamazepine pharmacokinetics and adverse effects. Br J Clin Pharmacol. 1986;22(6):725–728. doi: 10.1111/j.1365-2125.1986.tb02965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christiansen J, Dam M. Influence of phenobarbital and diphenylhydantoin on plasma carbamazepine levels in patients with epilepsy. Acta Neurol Scand. 1973;49(4):543–546. doi: 10.1111/j.1600-0404.1973.tb01327.x. [DOI] [PubMed] [Google Scholar]
- 27.Rambeck B, May T, Juergens U. Serum concentrations of carbamazepine and its epoxide and diol metabolites in epileptic patients: the influence of dose and comedication. Ther Drug Monit. 1987;9(3):298–303. doi: 10.1097/00007691-198709000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Ahn JE, Birnbaum AK, Brundage RC. Inherent correlation between dose and clearance in therapeutic drug monitoring settings: possible misinterpretation in population pharmacokinetic analyses. J Pharmacokinet Pharmacodyn. 2005;32(5–6):703–718. doi: 10.1007/s10928-005-0083-6. [DOI] [PubMed] [Google Scholar]
- 29.al-Banna MK, Kelman AW, Whiting B. Experimental design and efficient parameter estimation in population pharmacokinetics. J Pharmacokinet Biopharm. 1990;18(4):347–360. doi: 10.1007/BF01062273. [DOI] [PubMed] [Google Scholar]
- 30.Sun H, Ette EI, Ludden TM. On the recording of sample times and parameter estimation from repeated measures pharmacokinetic data. J Pharmacokinet Biopharm. 1996;24(6):637–650. doi: 10.1007/BF02353484. [DOI] [PubMed] [Google Scholar]


