Abstract
Real-time fluorescence resonance energy transfer (FRET) PCR and melting curve analysis using newly developed fluorophore-labeled hybridization probes were applied for the detection of Trichinella spiralis DNA in muscle of mice following oral inoculation with 300 T. spiralis larvae. The developed assay could detect and differentiate T. spiralis, Trichinella papuae, and Trichinella pseudospiralis DNAs by the different melting temperatures (Tm). The assay had a detection limit of 5×102 positive control plasmid copies, which was equivalent to 1 ng of T. spiralis DNA spiked into 250 mg of muscle sample. No fluorescence signal was detected when the technique was applied to the DNA of 27 parasites other than Trichinella spp. The assay could detect T. spiralis DNA in muscle at 7, 14, and 21 days postinoculation. The range, mean±standard deviation, and median of the Tm values of all positive muscle tissue samples were 60.4–60.8, 60.6±0.2, and 60.5, respectively. This assay provides an effective tool for the specific, sensitive, and high-throughput detection of T. spiralis DNA in muscle during the early stage of infection. In addition, the technique can be useful for epidemiologic surveillance in naturally infected wildlife.
Key Words: Trichinella spiralis, Real-time FRET PCR, Detection, Muscle
Introduction
Trichinellosis is a worldwide zoonotic disease caused by the ingestion of insufficiently cooked meat containing nematodes of the genus Trichinella. Trichinella spiralis is the most pathogenic and cosmopolitan species that causes human trichinellosis. It is estimated that approximately 10,000 people per year are infected with Trichinella worldwide, with a mortality rate of 0.2% for severe infections (Dupouy-Camet and Murell 2007, Gottstein et al. 2009). In Thailand, three Trichinella species, including T. spiralis (Pozio and Khamboonruang 1989), T. pseudospiralis (Jongwutiwes et al. 1998), and T. papuae (Chotmongkol et al. 2005, Khumjui et al. 2008, Intapan et al. 2011), have been reported as etiologic agents of human trichinellosis.
The accurate diagnosis of T. spiralis infection in animal reservoir hosts is important for the prevention and control of human trichinellosis. The direct detection of muscle larva by artificial digestion methods, compression techniques, and trichinoscopy are routinely used for the detection of Trichinella larva in meat. However, these methods are labor intensive and time consuming and have a low sensitivity. Alternatively, serological tests have been useful for surveillance and epidemiological studies, but these tests cannot substitute for the direct detection methods for meat inspection (Dupouy-Camet and Murell 2007).
To overcome the limitations of conventional and serological methods, several molecular techniques, i.e., conventional PCR (cPCR) (Dick et al. 1992, Wu et al. 1998, Golab et al. 2009, Wang et al. 2012), random amplified polymorphic DNA-PCR (Bandi et al. 1995), PCR-based single-strand conformation polymorphism (Gasser et al. 1998), PCR-restriction fragment length polymorphism (Wu et al. 1999, 2007), and multiplex PCR (Zarlenga et al. 1999, Borsuk et al. 2003), have been developed to detect and diagnose Trichinella infection. Presently, real-time PCR is becoming more widely used for routine diagnostic purposes because it is accurate, sensitive, and fast, allowing the rapid quantitative analysis of a specific DNA in a biological sample. In addition, the various species or strains of various medically pathogenic microorganisms can be differentiated by melting curve analysis (Lyon and Wittwer 2009). Recently, SYBR Green detection-based (Guenther et al. 2008, Cuttell et al. 2012) and Taqman probe-based (Atterby et al. 2009) real-time PCR approaches have been reported as a diagnostic tool for the detection of T. spiralis DNA in muscle tissue. Moreover, high-resolution melting (HRM) assay in a single tube real-time PCR reaction was used to detection of inter- and intraspecies polymorphisms of four Trichinella species—T. pseudospiralis, T. spiralis, T. britovi, and T. nativa (Masny et al. 2012). Real-time fluorescence resonance energy transfer (FRET) PCR and melting curve analysis has application potential in differentiating nonencapsulated larvae of T. papuae from T. spiralis and T. pseudospiralis in tissues of infected humans and animals (Tantrawatpan et al. 2012). However, the melting temperature (Tm) values for T. spiralis and T. pseudospiralis were notably similar, which caused the species discrimination to be difficult.
In this study, a newly developed probes-based real-time FRET PCR combined with a melting curve analysis was developed to detect the T. spiralis DNA sequence for the mitochondrial small-subunit ribosomal RNA (rRNA) directly in muscle tissue from mice experimentally infected with T. spiralis in the early stage of infection. The different Tm values were used to differentially detect T. spiralis, T. papuae, and T. pseudospiralis. The analytical sensitivity and specificity of the real-time FRET PCR and melting curve analysis have been evaluated.
Materials and Methods
Experimental infection
Isolates of the T. spiralis strain that caused an outbreak in Mae Hong Son Province in 1986 (Pozio and Khamboonruang 1989), the reference strain of T. pseudospiralis (code ISS13), and T. papuae isolated from a patient in 2005 (Chotmongkol et al. 2005, Intapan et al. 2011) were used in this study. The muscles of Trichinella larvae-infected mice were digested with pepsin-HCl 1 month after oral inoculation. T. spiralis larvae were harvested using a modified Baermann technique (Justus and Morakote 1981) and were used for subsequent experimental infection. The remaining pooled larva sample was stored at −20°C for DNA extraction.
For experimental infection, 12 mice were orally inoculated with 300 T. spiralis larvae per mouse (lpm). These mice were then divided into three groups. Four mice of each group were killed on days 7, 14, and 21 postinoculation (PI). Pooled muscle samples from the hind limbs, abdominal muscle, and diaphragm from each mouse were separately collected for DNA extraction. All animal procedures in this study were approved by the Animal Ethics Committee of Khon Kaen University, based on the Ethics of Animal Experimentation of the National Research Council of Thailand (reference no. 0514.1.12.2/70).
Extraction of genomic DNA from muscle
Each infected muscle sample (250 mg) and pooled Trichinella larvae were homogenized with a disposable a polypropylene pestle, followed by DNA extraction using a NucleoSpin Tissue Kit (Macherey-Nagel GmbH & Co., Duren, Germany) according to the manufacturer's protocols. Genomic DNA was eluted in 50 μL of distilled water, of which 1 μL was used in the real-time FRET PCR.
Primer and probe design
Primers targeting rRNA gene for the small subunit of the mitochondrial ribosome of Trichinella spp. (Tantrawatpan et al. 2012) and the TSpMito_LC 640 and TSpMito_FL probes, which are specific for T. spiralis (GenBank accession no. AF293969), were newly designed using the LightCycler probe design software (Roche Applied Science, Mannheim, Germany). The genus-specific primers TSMito_F (5′-AAT AGT GTG CCA GCT ATC G-3′) and TSMito_R (5′-TTA GGG GGT AAT TAG CGA GG-3′; Sigma-Proligo, Singapore) amplified a 289-bp fragment of the mitochondrial small-subunit rRNA gene sequence. A pair of adjacent probes, one labeled at the 5′ end with the LightCycler Red 640 fluorophore (TSpMito_LC 640: 5′ Red 640–GAT ACC CTT CTA TCC TAA ACT TAA ATT AAC CAA GAA G–Phosphate 3′) and the other at the 3′ end with 530 fluorescein (TSpMito_FL: 5′-ACA TCT AAA TCA CCA AAA GTT AAA CAA GAA ACA AGG A–Fluo 530 3′; TIB Molbiol, Berlin, Germany), were used to detect the T. spiralis-specific product.
Real-time FRET PCR
The real-time PCRs were performed using a LightCycler PCR and detection system (LightCycler 2.0, Roche Applied Science) following the protocol described previously by Tantrawatpan et al. (2012). Briefly, amplification reactions were set up in 20-μL volumes in glass capillaries. The reaction mixtures contained 1× LightCycler FastStart DNA Master HybProbe (Roche Applied Science), 3 mM MgCl2, 0.2 μM each of TSMito_F and TSMito_R primers as well as TSpMito_FL and TSpMito_LC640 probes, and the extracted DNA sample. Each PCR consisted of 45 cycles of repeated denaturation (10 s at 95°C), annealing (30 s at 50°C), and extension (15 s at 72°C). The temperature transition rate was 20°C/s. The amplification program was followed by a melting curve program of 95°C for 10 s, 48°C for 20 s, and then 48°C to 85°C at a transition rate of 0.2°C/s with continuous monitoring of the fluorescence. The color compensation for the fluorescence signals was performed according to Technical Note no. LC19/2004 of Roche Applied Science. Each sample was analyzed in duplicate. Sterile distilled water and T. spiralis-positive control plasmid DNA (5×105 copies/reaction) were used as negative and positive controls, respectively, and for the evaluation of the within-determination (intraassay) and between-determination (interassay) variations in the Tm value. The cycle number (Cn) indicating the target sequence copy number was presented as the PCR cycles needed for the fluorescence signal of the amplicons to exceed the detection threshold value. Melting curves were applied to assess the hybridization of probes and the specific PCR products.
The detection limit was obtained by analyzing 5 μL of 10-fold serial dilutions of T. spiralis–positive control plasmid DNA ranging from 5×108 copies to 5 copies. One microliter of 20 ng, 2 ng, 1 ng, 2.5×10−1 ng, and 1.25×10−1 ng genomic DNA extracted from muscle larvae was spiked into 250 mg of uninfected mouse muscle. Differential detection was evaluated with each of 5×107 copies plasmid controls and 3 ng of genomic DNAs of T. spiralis, T. pseudospiralis, and T. papuae.
The specificity of this real-time FRET PCR was evaluated using 1 ng of genomic DNA isolated from each of 27 parasites, i.e., Schistosoma mekongi, Schistosoma japonicum, Brugia malayi, Brugia pahangi, Wuchereria bancrofti, Babesia spp., Ehrlichia canis, Hepatozoon canis, Plasmodium vivax, Plasmodium falciparum, Fasciola gigantica, Paragonimus heterotremus, Stellantchasmus spp., Echinostoma malayanum, Clonorchis sinensis, Opisthorchis viverrini, human hookworms, Strongyloides stercoralis, Taenia spp., Ascaris lumbricoides, Trichuris trichiura, Trichostrongylus spp., Giardia lamblia, Haplorchis taichui, Capillaria philippinensis, lecithodendriid flukes, and Isospora belli. Human genomic DNA and DNA extracted from muscular tissue of uninfected mice were also tested.
Plasmid Control Preparations
Plasmid controls for T. spiralis, T. papuae, and T. pseudospiralis DNAs were constructed by cloning each of the homologous PCR products corresponding to the mitochondrial small-subunit rRNA genes into the pGEM-T easy vector (Promega, WI) according to manufacturer's instructions. Each PCR product was obtained by cPCR using the TSMito_F and TSMito_R primers. Each recombinant plasmid was propagated in Escherichia coli. For validation, the nucleotide sequences of the inserted genes were sequenced in both directions.
Results
The analytical sensitivity and specificity of the real-time FRET PCR assay
The assay could differentially detect T. spiralis, T. papuae, and T. pseudospiralis DNAs and showed the different mean±standard deviation (SD) of Tm values at 60.6±0.1, 52.2±0.2, and 53.9±0.2, respectively (Fig. 1). In terms of the analytical specificity, no fluorescence signal (Cn values were all >40) was observed when the DNAs from 27 parasites other than Trichinella (see Materials and Methods), genomic DNA from humans, or muscle samples of uninfected mice were tested (Fig. 2). Despite the fact that nonspecific bands were present when the genomic DNAs from some control parasites were amplified (Fig. S1), no specific fluorescence signal was detected during melting curve analysis. (Supplementary Data are available at www.liebertonline/vbz/.)
FIG. 1.
A representative melting curve analysis of two fluorophore-labeled probes hybridized to the amplification products of the mitochondrial rRNA gene from 5×107 copies of plasmid DNA (a, c, e) and 3 ng of genomic DNA (b, d, f) from three species of Trichinella. The melting curves of this experiment shown as −(d/dT) fluorescence (640/530). T. spiralis plasmid DNA control (a; Tm=60.8), genomic DNA of T. spiralis (b; Tm=60.6), T. pseudospiralis plasmid DNA control (c; Tm=54.0), genomic DNA of T. pseudospiralis (d; Tm=54.0), T. papuae plasmid DNA control (e; Tm=52.4), and genomic DNA of T. papuae (f; Tm=52.4). Distilled water (g) was used as negative control.
FIG. 2.
The melting curves for the T. spiralis positive control plasmid (5×105 copies) (a, cycle number, Cn=25.9) and for the 27 parasites other than Trichinella (see Materials and Methods), the genomic DNA from humans and muscle tissue from uninfected mice (b, Cn>40).
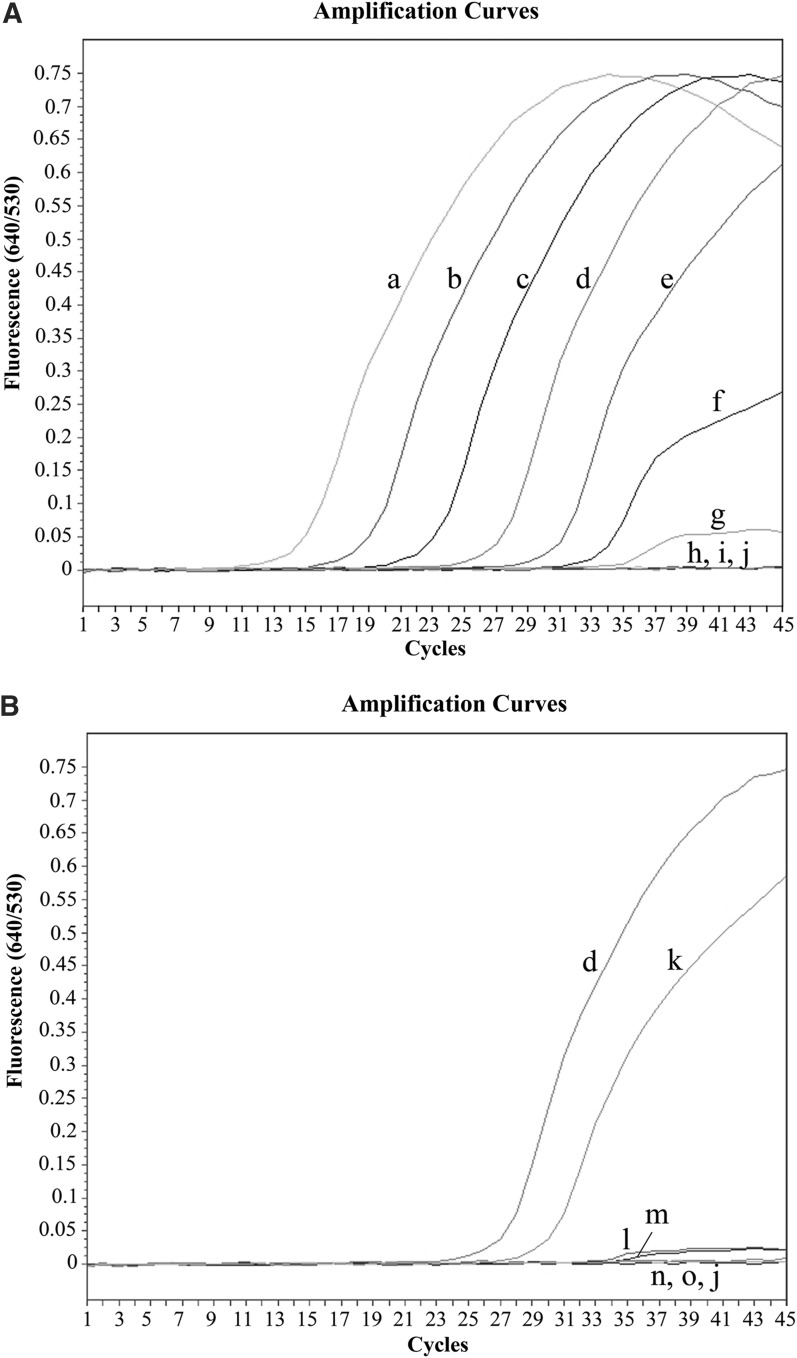
To determine the analytical sensitivity of the real-time FRET PCR for T. spiralis DNA, serial 10-fold dilutions of T. spiralis-positive control plasmid DNA and genomic DNA from T. spiralis muscle larvae diluted in uninfected mouse muscle samples were used. The detection limit of real-time FRET PCR for the positive control plasmid was found to be 5×102 copies (Cn=32.9) (Fig. 3A), whereas the detection limit for the T. spiralis larva DNA was 1 ng (Cn=31.6) spiked into 250 mg of muscle (Fig. 3B) when using 40 cycle numbers as the cutoff between positivity and negativity. The mean±SD/median of the Tm values (intraassay and interassay) for the positive control plasmid and the genomic DNA were 60.5±0.1/60.5 and 60.5±0.2/60.5, respectively. The coefficients of variation of this assay were within 15%, demonstrating acceptable day-to-day variations (data not shown).
FIG. 3.
Amplification plots of fluorescence (y axis) versus cycle numbers (x axis) show the detection limit of real-time fluorescence resonance energy transfer (FRET) PCR for detecting 10-fold dilutions of T. spiralis plasmid DNA or genomic DNA from T. spiralis larvae spiked into 250 mg of mouse muscle. (A) Plasmid DNA control: 5×108 copies (a, Cn=13.6), 5×107 copies (b, Cn=17.6), 5×106 copies (c, Cn=21.8), 5×105 copies (d, Cn=25.9), 5×104 copies (e, Cn=29.5), 5×103 copies (f, Cn=31.7), 5×102 copies (g, Cn=32.9), 5×101 copies (h, Cn>40), 5 copies (i, Cn>40), and distilled water (j, Cn>40). (B) Genomic DNA of T. spiralis larvae spiked into 250 mg of mouse muscle at concentrations of 20 ng (k, Cn=28.6), 2 ng (l, Cn=30.7), 1 ng (m, Cn=31.6), 2.5×10−1 ng (n, Cn >40), and 1.25×10−1 ng (o, Cn >40); 5×105 copies of T. spiralis plasmid DNA control (d, Cn=25.9) and distilled water (j, Cn >40) were used as positive and negative controls, respectively.
Detection of T. spiralis DNA in muscle of experimentally infected mice
All muscle samples obtained at days 7, 14, and 21 PI were positive (Table 1 and Fig. 4). The range, mean±SD, and median of the Tm values of all positive muscle tissue samples were 60.4–60.8, 60.6±0.2, and 60.5, respectively. The 289-bp positive PCR amplicons for all muscle samples from infected mice were sequenced in both directions, and the nucleotide sequence of the amplicons revealed sequences identical to that of the mitochondrial small-subunit rRNA gene of T. spiralis in the GenBank database (AF293969).
Table 1.
Results of Real-Time FRET PCR for Detection of T. spiralis DNA in Muscle Tissue of Experimentally Mice Infected with 300 Larvae of T. spiralis
| |
Day postinoculation |
||
|---|---|---|---|
| Sample/code no. | 7 | 14 | 21 |
| Tissue/Ia | + (38.3) | + (33.4) | + (24.9) |
| Tissue/IIa | + (33.0) | + (32.9) | + (26.9) |
| Tissue/IIIa | + (35.6) | + (32.9) | + (28.4) |
| Tissue/IVa | + (33.6) | + (31.7) | + (27.9) |
The cycle number (Cn) of positive samples by the real-time FRET PCR was indicated in the parenthesis. + indicates positive.
Each of 4 mice were killed on days 7, 14, and 21 postinoculation.
FIG. 4.
Representative amplification plots (A) and melting curve analysis (B) of two fluorophore-labeled probes hybridized to the amplification products of T. spiralis the mitochondrial small-subunit rRNA gene in muscle tissues. The melting curves of this experiment are shown as −(d/dT) fluorescence (640/530): 5×105 copies of T. spiralis plasmid control (a); muscle tissue from infected mice at 7 days postinoculation (PI) (d), 14 days PI (c), and 21 days PI (b), and distilled water (e).
Discussion
The life cycle of Trichinella in the host body is characterized by two phases, the intestinal phase in the duodenum and the systemic and muscular phase in the lymph and blood vessels and then in the striated muscle cells (Pozio 2007). The Trichinella genus-specific primers and species-specific T. spiralis probes were designed from mitochondrial rRNA gene sequences and used for developing a real-time FRET PCR combined with melting curve analysis for detection of T. spiralis infection during the early period of the muscular phase. Mitochondrial rRNA gene sequences have previously been demonstrated to be suitable for the species identification of cestodes (Dinkel et al. 1998, von Nickisch-Rosenegk et al. 1999a, b). Recently, Trichinella mitochondrial rRNA gene sequences were used to design primers for the detection of Trichinella spp. in muscle samples by using a classical type of real-time PCR (SYBR Green–based real-time PCR) combined with melting curve analysis of the amplicon products (Guenther et al. 2008). However, the SYBR Green–based real-time PCR method can result on nonspecific fluorescence signals by primer dimers effect or other PCR artifacts.
The TSMito_F and TSMito_R primers used in this assay were designed to detect all three Trichinella spp., whereas the TSpMito_LC 640 and TSpMito_FL probes are specific for T. spiralis. Our developed assay was the powerful tool for differential detection of T. spiralis, T. papuae, and T. pseudospiralis DNAs, which showed the different mean of Tm±SD at 60.6±0.1, 52.2±0.2, and 53.9±0.2, respectively. Compared with our previous study (Tantrawatpan et al. 2012), the previous probes designed based on the mitochondrial small-subunit rRNA gene sequence of T. papuae are difficult for species discrimination between T. spiralis and T. pseudospiralis because the Tm values of both species were nearly similar. This real-time PCR also showed no fluorescence signal in response to the DNA of any of the other tested parasites, indicating a high specificity. Previous studies have shown that the detection limits of real-time PCR assays for T. spiralis larvae in muscle were 0.01 larva per gram (lpg) (Atterby et al. 2009) and 0.1 lpg (Guenther et al. 2008). Interestingly, our real-time FRET PCR assay could detect T. spiralis DNA in the muscle tissues of infected mice as early as 7 days PI, with positive results through 21 days PI during the muscular phase and a detection limit of 1 ng of T. spiralis DNA spiked into a 250-mg muscle sample (equivalent to a 1:20 dilution of the genomic DNA extracted from one T. spiralis muscle larva; data not shown). The positive result at day 7 PI by this method may result from newborn larvae circulating into capillaries infiltrating muscle. However, to diagnose clinical trichinellosis in humans, the sensitivity of the test must be as low as 1–3 lpg in muscle tissue (Zimmermann 1983, Gamble et al. 2000). Thus, our present assay may be used in the future for the detection of T. spiralis in tissue samples from infected wildlife.
In conclusion, the rapid, sensitive, and specific real-time FRET PCR assay using specific primers and newly developed fluorophore-labeled probes based on detection of the mitochondrial small-subunit rRNA gene was developed and could be used for interspecies differentiation of T. spiralis, T. pseudospiralis, and T. papuae by the different Tm values. It offers an alternative approach for T. spiralis detection and for the early diagnosis of trichinellosis using muscle samples. The technique also eliminates the need for well-trained staff to perform the laborious and time-consuming routine microscopic procedure. A large number of samples can be analyzed at the same time. This technique can be applied to herd surveillance and epidemiological surveys of wildlife populations, which will contribute to the effective control of human trichinellosis and the prevention of outbreaks in endemic areas.
Supplementary Material
Acknowledgments
This research was supported by grants from the National Science and Technology Development Agency (Discovery Based Development Grant); the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission; and Khon Kaen University, Thailand. The authors thank Dr. Zhiliang Wu for kindly provided Trichinella pseudospiralis DNA. Wanchai Maleewong was supported by TRF Senior Research Scholar Grant, Thailand Research Fund (Grant no. RTA5580004).
Author Disclosure Statement
No competing financial interests exist.
References
- Atterby H. Learmount J. Conyers C. Zimmer I, et al. Development of a real-time PCR assay for the detection of Trichinella spiralis in situ. Vet Parasitol. 2009;161:92–98. doi: 10.1016/j.vetpar.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Bandi C. La Rosa G. Bardin MG. Damiani G, et al. Random amplified polymorphic DNA fingerprints of the eight taxa of Trichinella and their comparison with allozyme analysis. Parasitology. 1995;110:401–407. doi: 10.1017/s003118200006474x. [DOI] [PubMed] [Google Scholar]
- Borsuk P. Moskwa B. Pastusiak K. Cabaj W. Molecular identification of Trichinella spiralis and Trichinella britovi by diagnostic multiprimer large mitochondrial rRNA amplification. Parasitol Res. 2003;91:374–377. doi: 10.1007/s00436-003-0971-x. [DOI] [PubMed] [Google Scholar]
- Chotmongkol V. Intapan PM. Koonmee S. Kularbkaew C, et al. Case report: Acquired progressive muscular hypertrophy and trichinosis. Am J Trop Med Hyg. 2005;72:649–650. [PubMed] [Google Scholar]
- Cuttell L. Corley SW. Gray CP. Vanderlinde PB, et al. Real-time PCR as a surveillance tool for the detection of Trichinella infection in muscle samples from wildlife. Vet Parasitol. 2012;188:285–293. doi: 10.1016/j.vetpar.2012.03.054. [DOI] [PubMed] [Google Scholar]
- Dick TA. Lu MC. deVos T. Ma K. The use of the polymerase chain reaction to identify porcine isolates of Trichinella. J Parasitol. 1992;78:145–148. [PubMed] [Google Scholar]
- Dinkel A. von Nickisch-Rosenegk M. Bilger B. Merli M, et al. Detection of Echinococcus multilocularis in the definitive host: Coprodiagnosis by PCR as an alternative to necropsy. J Clin Microbiol. 1998;36:1871–1876. doi: 10.1128/jcm.36.7.1871-1876.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupouy-Camet J. Murell KD. FAO/WHO/OIE; Paris: 2007. FAO/WHO/OIE guidelines for the surveillance, management, prevention and control of trichinellosis; pp. 1–119. [Google Scholar]
- Gamble HR. Bessonov AS. Cuperlovic K. Gajadhar AA, et al. International Commission on Trichinellosis: Recommendations on methods for the control of Trichinella in domestic and wild animals intended for human consumption. Vet Parasitol. 2000;93:393–408. doi: 10.1016/s0304-4017(00)00354-x. [DOI] [PubMed] [Google Scholar]
- Gasser RB. Zhu XQ. Monti JR. Dou L, et al. PCR-SSCP of rDNA for the identification of Trichinella isolates from mainland China. Mol Cell Probes. 1998;12:27–34. doi: 10.1006/mcpr.1997.0142. [DOI] [PubMed] [Google Scholar]
- Golab E. Rozej W. Wnukowska N. Rabczenko D, et al. Detection of Trichinella spiralis DNA in mouse faeces during the early stage of infection. J Microbiol Methods. 2009;78:213–215. doi: 10.1016/j.mimet.2009.05.019. [DOI] [PubMed] [Google Scholar]
- Gottstein B. Pozio E. Nockler K. Epidemiology, diagnosis, treatment, and control of trichinellosis. Clin Microbiol Rev. 2009;22:127–145. doi: 10.1128/CMR.00026-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther S. Nockler K. von Nickisch-Rosenegk M. Landgraf M, et al. Detection of Trichinella spiralis, T. britovi and T. pseudospiralis in muscle tissue with real-time PCR. J Microbiol Methods. 2008;75:287–292. doi: 10.1016/j.mimet.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Intapan PM. Chotmongkol V. Tantrawatpan C. Sanpool O, et al. Molecular identification of Trichinella papuae from a Thai patient with imported trichinellosis. Am J Trop Med Hyg. 2011;84:994–997. doi: 10.4269/ajtmh.2011.10-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongwutiwes S. Chantachum N. Kraivichian P. Siriyasatien P, et al. First outbreak of human trichinellosis caused by Trichinella pseudospiralis. Clin Infect Dis. 1998;26:111–115. doi: 10.1086/516278. [DOI] [PubMed] [Google Scholar]
- Justus DE. Morakote N. Mast cell degranulation associated with sequestration and removal of Trichinella spiralis antigens. Int Arch Allergy Appl Immunol. 1981;64:371–384. doi: 10.1159/000232718. [DOI] [PubMed] [Google Scholar]
- Khumjui C. Choomkasien P. Dekumyoy P. Kusolsuk T, et al. Outbreak of trichinellosis caused by Trichinella papuae, Thailand, 2006. Emerg Infect Dis. 2008;14:1913–1915. doi: 10.3201/eid1412.080800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon E. Wittwer CT. LightCycler technology in molecular diagnostics. J Mol Diagn. 2009;11:93–101. doi: 10.2353/jmoldx.2009.080094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masny A. Jagiello A. Plucienniczak G. Golab E. Ribo HRM—Detection of inter- and intra-species polymorphisms within ribosomal DNA by high resolution melting analysis supported by application of artificial allelic standards. J Microbiol Methods. 2012;78:336–341. doi: 10.1016/j.mimet.2012.06.012. [DOI] [PubMed] [Google Scholar]
- Pozio E. Taxonomy, biology and epidemiology of Trichinella parasites. In: Dupouy-Camet J, editor; Murrell KD, editor. En FAO/WHO/OIE Guidelines for the Surveillance, Management, Prevention and Control of Trichinellosis. World Organisation for Animal Health; Rome: 2007. pp. 1–36. [Google Scholar]
- Pozio E. Khamboonruang C. Trichinellosis in Thailand: epidemiology and biochemical identification of the aethiological agent. Trop Med Parasitol. 1989;40:73–74. [PubMed] [Google Scholar]
- Tantrawatpan C. Intapan PM. Thanchomnang T. Lulitanond V, et al. Differential detection of Trichinella papuae, T. spiralis and T. pseudospiralis by real-time fluorescence resonance energy transfer PCR and melting curve analysis. Vet Parasitol. 2012;185:210–215. doi: 10.1016/j.vetpar.2011.09.043. [DOI] [PubMed] [Google Scholar]
- von Nickisch-Rosenegk M. Lucius R. Loos-Frank B. Contributions to the phylogeny of the Cyclophyllidea (Cestoda) inferred from mitochondrial 12S rDNA. J Mol Evol. 1999a;48:586–596. doi: 10.1007/pl00006501. [DOI] [PubMed] [Google Scholar]
- von Nickisch-Rosenegk M. Silva-Gonzalez R. Lucius R. Modification of universal 12S rDNA primers for specific amplification of contaminated Taenia spp. (Cestoda) gDNA enabling phylogenetic studies. Parasitol Res. 1999b;85:819–825. doi: 10.1007/s004360050638. [DOI] [PubMed] [Google Scholar]
- Wang ZQ. Li LZ. Jiang P. Liu LN, et al. Molecular identification and phylogenetic analysis of Trichinella isolates from different provinces in mainland China. Parasitol Res. 2012;110:753–757. doi: 10.1007/s00436-011-2549-3. [DOI] [PubMed] [Google Scholar]
- Wu Z. Nagano I. Takahashi Y. The detection of Trichinella with polymerase chain reaction (PCR) primers constructed using sequences of random amplified polymorphic DNA (RAPD) or sequences of complementary DNA encoding excretory-secretory (E-S) glycoproteins. Parasitology. 1998;117:173–183. doi: 10.1017/s0031182098002881. [DOI] [PubMed] [Google Scholar]
- Wu Z. Nagano I. Pozio E. Takahashi Y. Polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) for the identification of Trichinella isolates. Parasitology. 1999;118:211–218. doi: 10.1017/s0031182098003679. [DOI] [PubMed] [Google Scholar]
- Wu Z. Snabel V. Pozio E. Hurníkova Z, et al. Genetic relationships among Trichinella pseudospiralis isolates from Australian, Nearctic, and Palearctic regions. Parasitol Res. 2007;101:1567–1573. doi: 10.1007/s00436-007-0677-6. [DOI] [PubMed] [Google Scholar]
- Zarlenga DS. Chute MB. Martin A. Kapel CM. A multiplex PCR for unequivocal differentiation of all encapsulated and non-encapsulated genotypes of Trichinella. Int J Parasitol. 1999;29:1859–1867. doi: 10.1016/s0020-7519(99)00107-1. [DOI] [PubMed] [Google Scholar]
- Zimmermann WJ. Control II: Surveillance in swine and other animals by muscle examination. In: Campbell WC, editor. Trichinella and Trichinosis. Plenum Press; New York: 1983. pp. 515–528. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.