Abstract
Background
The use of acupuncture in the treatment of pain conditions has been extensively investigated. However, the influence of dietary ingredients on acupuncture-induced analgesia (AA) remains unexplored. Recently, the role of adenosine receptors in AA has been shown, and caffeine, one of the world's most commonly consumed dietary ingredients, is an antagonist of these receptors. In this study, the postincisional pain model was used to investigate caffeine's influence on AA.
Method
Mice submitted to plantar incision surgery were treated with acupuncture needling after administration of acute or chronic caffeine. Acupuncture needling was performed using two different types of stimuli, manual acupuncture and electroacupuncture bilaterally in the acupoint SP6.
Results
We found that acute preadministration of caffeine (10 mg/kg, i.p.) completely reversed AA in both types of acupuncture. In the chronic preadministration, we used two doses that mimicked the average daily caffeine consumption in Western countries and China. Interestingly, the Western dose of caffeine (70 mg/kg/day) administered during 8 days in the drinking water reversed AA and the Chinese dose (4 mg/kg/day) administered during the same period did not.
Conclusions
These results indicate that the use of caffeine can inhibit the analgesic effect of different forms of acupuncture. In addition, our findings suggest that doses of caffeine relevant to dietary human intake levels could be a confounding factor in the context of acupuncture research.
Introduction
The use of acupuncture in the treatment of pain conditions has been extensively investigated in different models of basic science and clinical research.1 Moreover, due to increasing concerns regarding the side effects of analgesics and anti-inflammatory drugs, acupuncture has been widely used clinically.2 Despite the widespread use of acupuncture for pain management, explaining the relationship between acupuncture's clinical effects and its neurobiological mechanisms is still a challenge to modern science.3 Several studies have shown that acupuncture modulates the endogenous analgesic pathways by activating the opioid, serotonergic, and noradrenergic systems.1 Recently, there has been growing interest in the role of the adenosine receptors in the analgesic effects of acupuncture.4 Caffeine, a commonly consumed dietary component, is an antagonist at these receptors and has the potential to affect analgesia (AA).5
Caffeine is naturally found in coffee beans, cacao beans, and tea leaves, and is one of the most commonly consumed dietary ingredients throughout the world.6 It is also available as a drug, used as a stimulant, or added to analgesics in over-the-counter formulations.5 Moderate caffeine consumption is considered safe and its use as a food ingredient has been approved, within certain limits, by numerous regulatory agencies.6 Many studies confirm that caffeine has some beneficial effects, such as the ability to enhance mood, alertness, and exercise performance.7,8 Additionally, meta-analyses of adjuvant actions of caffeine suggest that it may be useful for enhancing relief of headache pain.9 However, the use of caffeine as an adjuvant to treat other pain conditions has been questioned by preclinical studies that show that caffeine blocks the antinociceptive effect of several drugs, including acetaminophen, amitriptyline, and other antidepressants, and carbamazepine, which are currently used to treat pain in humans.10–13
In clinical practice, since ancient times, most acupuncturists use manual acupuncture (MA) (lifting-thrusting and twisting-rotating needle manipulations) to treat pain conditions. In the past 50 years, electroacupuncture (EA) (adding electric current to the acupuncture needles) was introduced to acupuncture treatments and its use is becoming a popular type of stimuli.1 The clinical efficacy of both modalities of stimuli has been show in different studies and many authors suggest that MA and EA work through different neural mechanisms, based on the fact that the two modalities have significantly different effects on brain image studies and distinct patterns of neurotransmitter release in the central nervous system.1
In the present study, using a translational research perspective, we investigated the possible influence of acute and chronic caffeine preadministration in two of the most common forms of acupuncture stimuli, MA and EA, using a postoperative pain model in mice.
Materials and Methods
Animals
Experiments were conducted using adult male Swiss mice (25–35 g) from the animal facility of the Universidade Federal de Santa Catarina (UFSC, Florianópolis, SC, Brazil) and properly housed in cages (six animals per cage) at 22±1°C under a 12-hour light/12-h dark cycle (lights on at 06:00 hours) at 60–80% humidity with free access to food and tap water. All animal care and experimental procedures were carried out in accordance with the National Institutes of Health Animal Care Guidelines (NIH publications No. 80–23), and conducted following the protocol approved by the Committee of the Ethical Use of Animals of the Universidade Federal de Santa Catarina (CEUA/UFSC protocol number PP00574). Animals were habituated to the laboratory conditions for at least 1 hour before testing and all experiments were performed during the light phase of the cycle. All efforts were made to minimize the number of animals used and their suffering.14
Acupuncture needling
Mice were anesthetized with 1–2% isoflurane delivered via a nose cone to minimize restraint-induced stress. Then, stainless needles (0.18 mm/diameter and 8 mm/length) were inserted bilaterally into Sanyinjiao acupoint (SP6) at a depth of 3 mm. In mice, the SP6 acupoint has been used in the study of pain conditions and is located 2 mm proximal to the upper border of the medial malleolus, between the posterior border of the tibia and the anterior border of the Achilles tendon.15–17 Needles inserted at the SP6 acupoint stimulate fibers of the flexor digitorum longus muscle and branches of the tibial nerve, which is related to the innervation of the plantar surface of the hindpaw.18 For EA, electrical current was passed along the inserted needle for 10 min using a NKL EL-608 electrostimulator (NKL produtos eletronicos, Brusque, SC). Previously determined EA parameters of low frequency (10 Hz) at 3 mA and 0.1 ms pulse width, which showed significant anti-hyperalgesic effects in a rat model of paw inflammation (CFA) were chosen for the present study stimulation.19 For MA, animals underwent the same procedures of the EA treatment, but instead of electrical stimulation, the needle was manually rotated at a rate of two spins per second for 15 seconds each in a total of 30 seconds and was left inserted for an additional 10 minutes.20 Animals appeared fully recovered from the anesthetic, 5 minutes after the end of the needling procedures.
It is important to mention that mice were treated under light isoflurane anesthesia. This method that has been validated by other authors and it is extensively used in different acupuncture studies.21,22 Moreover, we carefully used control groups to exclude the possible influences of isoflurane and caffeine in the hyperalgesia induced by the postoperative model. In addition, we performed an open field test to verify if there was any influence of isoflurane or caffeine in the motor activity of mice and no difference was found compared to naive animals (data not shown).
Mouse model of postincisional pain
The plantar incision surgery was performed as previously described.23 Briefly, mice were anesthetized with 1–2% isoflurane delivered via a nose cone. After sterile preparation of the right hind paw, a 5-mm longitudinal incision was made through the skin and fascia of the plantar surface using a number 11 scalpel blade. The incision started 2 mm from the proximal edge of the heel and extended toward the toes. The underlying muscle was elevated with a curved forceps, leaving the muscle origin and insertion intact. After wound homeostasis, the skin was opposed with an 8.0 nylon mattress suture, and the wound was covered with a 10% povidone–iodine solution. Control animals were anesthetized, but no incision was made.
Mechanical hyperalgesia
The mechanical hyperalgesia was measured as described previously.24 Mice were acclimated in individual clear boxes (9×7×11 cm) on an elevated wire mesh platform to allow access to the ventral surface of the hind paws. The mechanical sensitivity (Von Frey) test consists of thin calibrated plastic filaments that are applied to the plantar surface of the right hindpaw. The withdrawal response frequency was determined by 10 applications of 0.4 g von Frey filament (Stoelting, Chicago, IL), each 5 seconds in duration, and expressed as a percentage of the withdrawal response to nociceptive behavior. Mechanical hyperalgesia was tested 24 hours after plantar incision and 30 minutes after adenosine, EA or MA treatment.
Drug administration
To evaluate the influence of the acute administration of caffeine on acupuncture-induced AA, 24 hours after the plantar incision, postsurgery mice were treated with an intraperitoneal (i.p.) injection of caffeine (a nonselective adenosine receptor antagonist, 10 mg/kg) or saline (10 mL/kg), and after 30 minutes, animals were treated with EA or MA. Another group of mice was treated with saline (10 mL/kg, i.p.) or caffeine (10 mg/kg, i.p.), and after 30 minutes, animals were treated with adenosine (nonselective adenosine receptor agonist, 50 mg/kg, i.p.) or vehicle (saline 10 mL/kg, i.p.). These last two groups received adenosine and saline treatment under 1–2% isoflurane anesthesia delivered via a nose cone during 10 minutes (same time length of EA and MA treatment). Mechanical hyperalgesia was assessed in all groups 30 minutes after adenosine administration or acupuncture needling procedures. To evaluate the influence of chronic caffeine administration, animals received drinking water, which contained 0.015 (0.0015%) or 0.3 g/L (0.03%) caffeine for 8 days. Twenty-four hours after the plantar incision surgery, animals were treated with adenosine (50 mg/kg, i.p.), saline (10 mL/kg, i.p.), MA or EA. Another group of mice received drinking water without caffeine for 8 days and were treated with adenosine (50 mg/kg, i.p.), saline (10 mL/kg, i.p.), MA or EA. Mechanical hyperalgesia was assessed in all groups 30 minutes after adenosine administration or acupuncture needling procedures.
Average fluid consumption of the drinking water was 7 mL/day/mouse. If all consumption resulted in caffeine intake, this would correspond to doses of ∼4 mg/kg/day or ∼70 mg/kg/day, using the caffeine concentration of 0.0015% or 0.03%, respectively.11,25
Previous studies have related the dosage of 0.03% to moderate human dietary caffeine consumption.11,25 Based on the average caffeine consumption in different countries described by Fredholm et al.,26 we noticed that an average of daily caffeine consumption in China is approximately twenty times lower than in Western countries (Table 1). Using a translational approach, we considered the concentration of 0.03% a Western dose of caffeine and the concentration of 0.0015% a Chinese dose of caffeine.
Table 1.
Average Levels of Daily Caffeine Consumption in Different Countries
| Country | mg/person/day |
|---|---|
| China | 16 |
| United States | 168 |
| United Kingdom | 202 |
| Canada | 210 |
| Italy | 210 |
| Australia | 232 |
| France | 239 |
| Brazil | 300 |
| Germany | 313 |
| Sweden | 407 |
Data adapted from 1995 food balance sheets of the Food and Agriculture Organization of the United Nations.26
It is important to notice, as previously described in this drug administration section, that we performed all treatments (vehicle, adenosine, and acupuncture) using light isoflurane anesthesia. We included the groups vehicle+saline and vehicle+caffeine in all the sets of experiment to control the possible influence of the pharmacokinetic interaction between caffeine and isoflurane in animal pain behavior. Moreover, a previous study has demonstrated that isoflurane anesthesia did not influence the pain behavior in animals that were pretreated with caffeine.27
Drugs
Caffeine and adenosine were purchased from Sigma Chemical Co., USA. For i.p. injections, drugs were diluted in saline. Caffeine was dissolved in drinking water for the chronic administration. Drug dosages were selected on the basis of previous studies,11,28 except the caffeine dose of 0.0015% in the drinking water, that was chosen to mimic the average Chinese consumption of caffeine.
Statistical analysis
The results are presented as mean±standard errors of the mean (SEM) for each group and were statistically evaluated by analysis of variance (ANOVA) for repeated measures followed by Bonferroni multiple comparisons as the post hoc test using GraphPad Prism version 5.0 (GraphPad Software, Inc., San Diego, CA). p-Values of <0.05 were considered as indicative of significance.
Results
Acute caffeine administration prevents antihyperalgesic effect of acupuncture or adenosine
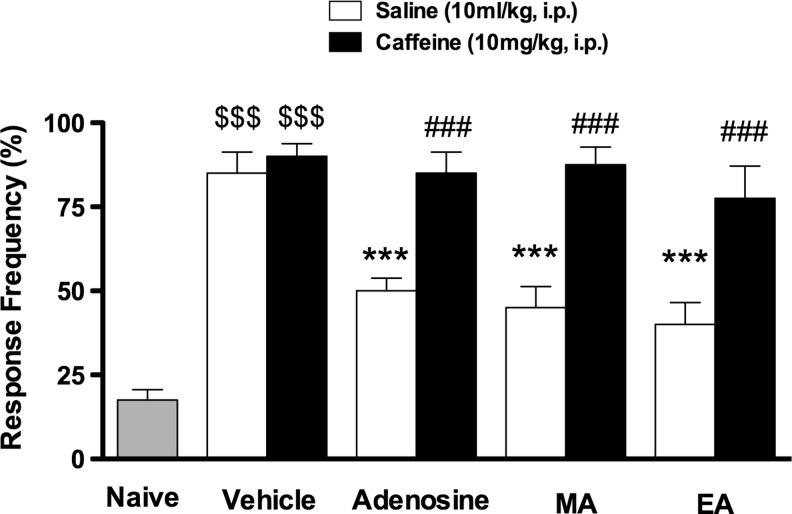
The results presented in Figure 1 show that the plantar incision in mice produced marked mechanical hyperalgesia when measured using the von Frey filament and compared with a naive response (p<0.001). In addition, treatment of mice with adenosine (50 mg/kg, i.p.) or acupuncture needling, using both manual (MA) and electrical stimulation (EA), inhibited the hyperalgesia caused by plantar incision. Furthermore, we found that acute pretreatment of mice with caffeine (10 mg/kg, i.p.), produced a significant inhibition of the antihyperalgesic effect induced by adenosine (50 mg/kg, i.p.), EA and MA in this postoperative pain model when assessed with the von Frey test (Fig. 1).
FIG. 1.
Effect of acute caffeine preadministration on analgesia (AA). Evaluation of mechanical hypersensitivity in the postincisional pain model in mice. Each group represents the mean of eight animals, and the vertical error bars indicate the SEM. $$$p<0.001 when compared with the naive group; ***p<0.001 when compared with the vehicle group; ###p<0.001 when compared with respective saline+treatment groups. EA, electroacupuncture; MA, manual acupuncture.
Western dose of caffeine prevents antihyperalgesic effect of acupuncture or adenosine
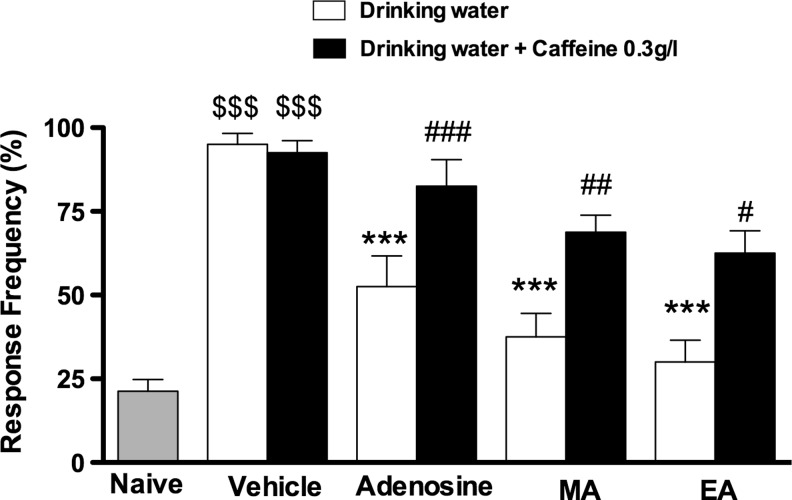
The results presented in Figure 2 show that chronic administration of caffeine, 0.03% in drinking water for 8 days, reversed the antihypersalgesic effect caused by EA or MA, when assessed with the von Frey test. Caffeine also reverted the antihyperalgesic effect of adenosine (50 mg/kg, i.p.).
FIG. 2.
Effect of the Western dose (0.03%) of caffeine on AA. Evaluation of mechanical hypersensitivity in the postincisional pain model in mice. Each group represents the mean of eight animals, and the error bars indicate the SEM. $$$p<0.001 when compared with naive group; ***p<0.001 when compared with vehicle group; #p<0.05, ##p<0.01 and ###p<0.001 when compared to respective drinking water+treatment groups.
Chinese dose of caffeine does not prevent antihyperalgesic effect of acupuncture or adenosine
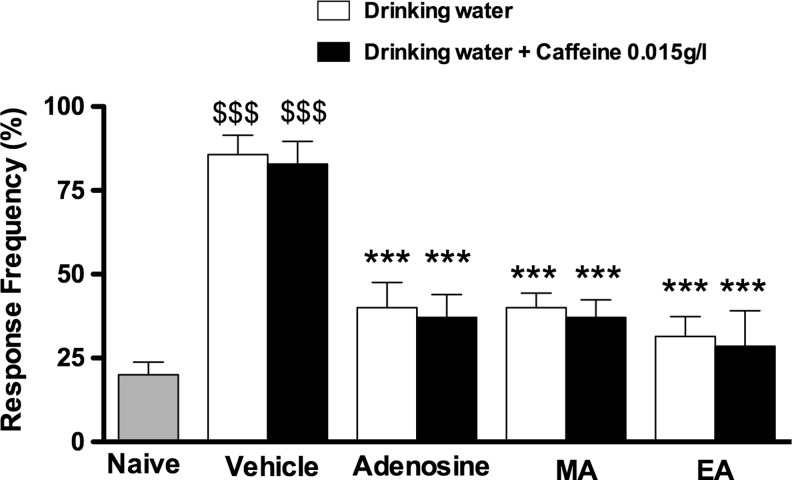
The results presented in Figure 3 show that chronic administration of caffeine, 0.0015% in drinking water for 8 days, did not influence the antihyperalgesic effect induced by adenosine (50 mg/kg, i.p.) or by acupuncture when assessed in the von Frey test.
FIG. 3.
Effect of the Chinese dose (0.0015%) of caffeine on AA. Evaluation of mechanical hypersensitivity in the postincisional pain model in mice. Each group represents the mean of eight animals, and the error bars indicate the SEM. $$$p<0.001 when compared with the naive group; ***p<0.001 when compared with the vehicle group.
Discussion
The present study confirms and largely extends previous data from the literature by demonstrating that adenosine receptors are involved in acupuncture-induced AA. In addition, we demonstrated that acute and chronic administration of caffeine 0.03%, but not 0.0015%, in drinking water, prevented the antihyperalgesic effect caused by acupuncture needling, using both manual and electrical stimulation, and adenosine in a mouse model of postoperative pain. These observations suggest that endogenous adenosine is involved in AA because the pharmacological properties of caffeine involve the blockade of adenosine receptors.5 Another study has previously reported the involvement of adenosine receptors in the antinociceptive effect of acupuncture in mouse models of inflammatory and neuropathic pain.4 Moreover, a local increase of adenosine after acupuncture needling was recently described in human subjects.29 Collectively, these reports suggest that adenosine is an important endogenous mediator of the analgesic properties of acupuncture needling.
The mechanism by which acupuncture needling interacts with the adenosinergic system is likely through ATP release after the insertion of an acupuncture needle into the muscle cell.4 ATP can be metabolized extracellularly to ADP, AMP, and adenosine by ectonucleotidases; extracellular adenosine then activates A1 receptors (A1Rs) located in the nociceptive neuron.30 Furthermore, the participation of A1Rs in AA has been shown by Goldman and colleagues with the demonstration that MA did not change the pain behavior of A1R knockout mice.4
In this study, we show that adenosine, administered systemically, induces anti-hyperalgesic effect in the postoperative pain model and this effect is similar to acupuncture needling. Additionally, acute and chronic preadministration of caffeine blocks this antihyperalgesic effect. Consistent with this data is the observation that, in different pain models, adenosine analogs produce pain relieving effects,31–33 which can be blocked by caffeine preadministration.28,34 However, adenosine or caffeine administration can produce either analgesic or pronociceptive effects depending on many variables, including dose, administration site, and the modality of pain treated.5,35,36 This duality observed in the effects of adenosine and caffeine in pain conditions may be related to the expression of adenosine receptors in the nervous system.37 Both drugs activate A1 and A2A receptors and, while the first inhibits nociception, the second can facilitate nociception.36
Human caffeine intake levels vary between countries, and generally range from 16 mg/day in China to 150–400 mg/day in Western countries (Table 1).26,38 Based on this pattern of caffeine consumption, we chose the dosing levels for mice. Initially, using a chronic oral Western dosing regimen of caffeine (0.3 g/L in the drinking water for 8 days), we observed a blockade of AA comparable to the blockade observed with an acute preadministration of caffeine (10 mg/kg, i.p.), given 30 minutes before acupuncture. On the other hand, we demonstrated that the Chinese dosing regimen of caffeine (0.015 g/L for 8 days, in the drinking water) did not affect AA. In addition, it is important to notice that the effect of these different doses of caffeine had a similar effect on both MA and EA, which are the two most common forms of acupuncture stimuli used in clinical practice and research.
Acute ingestion of two cups of strong coffee (100 mg of caffeine) by a man weighing 70 kg equates to a dose of approximately 3.6 mg/kg.38 At first glance, the doses of caffeine used in this study seem much higher than the ones observed in human consumption. However, direct extrapolation between mice and humans may not be entirely appropriate due to pharmacokinetics and other variables.11 One example is the use of morphine in animal studies. While humans need doses of approximately 0.5 mg/kg of morphine to control pain after a surgical procedure,39 in the mouse model of postoperative pain, a dose of morphine twenty times higher (10 mg/kg) than the human dose needs to be administered to inhibit the nociceptive behavior.18 In addition, other basic science studies have related the same doses of caffeine used in this study to human dietary consumption levels.11,34 Of note, in 1994, Liu and colleagues demonstrated that caffeine could influence the analgesic effect of EA in rats; however, it only used acute administration of caffeine and teophyline.40 In 2010, Zylka published a commentary using part of the data from the above-mentioned article and suggested that caffeine could influence AA in clinical practice.30 Thus, our study is the first in the literature to demonstrate that caffeine can influence the effect of MA stimuli and that chronic caffeine intake, in doses relevant to human consumption, can inhibit AA.
Interestingly, other treatment modalities used to manage pain conditions have their effects inhibited by caffeine. The antinociceptive effects of amitriptyline, oxcarbazepine, and acetaminophen are blocked by caffeine in animal models10,11,28; while the effect of transcutaneous electrical nerve stimulation has been reported to be inhibited by caffeine in humans.41 All of these treatment modalities modulate endogenous adenosine metabolism and it is not surprising that the use of an antagonist of adenosine receptors may inhibit their effects.
The differences in the results of acupuncture clinical trials conducted in China compared to those conducted in Western countries are intriguing. The number of studies in China favoring acupuncture treatment is much higher than in the Western country studies.42 While some authors suggest that publication bias, cultural factors, and poor methodology of the Chinese studies are related to this high proportion of studies favoring acupuncture,42–44 the results of our study suggest that caffeine consumption may be related, at least partially, to the difference observed in Chinese and Western trials.
Caffeine is the most widely consumed psychoactive agent in the world and, given its ability to interfere with AA, as shown in this study, it is necessary to consider the effects of caffeine intake in acupuncture practice. At acupuncture clinics, it is common to observe prospective clients drinking coffee or tea in the reception area while they wait for treatment. In the research field, significant variations of caffeine consumption can be expected in patients that are participating in clinical trials. Our observations that acute caffeine administration inhibits AA and that Western and Chinese doses of caffeine have different effects, raise two questions (1) should acupuncturists advise their patients not to use caffeine before their treatment? (2) should researchers add a caffeine consumption item to research questionnaires, to evaluate the influence of this consumption on the study outcome? Further studies with detailed consideration of caffeine–acupuncture interactions are warranted to precisely answer these questions.
Finally, we should point out some limitations of the present study. We did not perform a dose–response curve to determine what was the lowest dose of caffeine to inhibit the AA, and previous studies have demonstrated that lower doses of caffeine (3.75 mg/kg) can inhibit the effect of drugs that influence the adenosine receptors, like amitriptyline.10 Moreover, we did not use other methods, like histology and electrophysiological recordings, to support our findings. However, as mentioned before in this article, we designed the experiments using a translational perspective, using doses that are considered relevant to the human intake levels of caffeine by different authors,11,25 and our behavior tests clearly demonstrated the influence of acute and chronic caffeine administration in the AA. It will be interesting in future basic science studies to investigate more details about caffeine's influence in AA.
Conclusion
In conclusion, the results of this study confirm that the antinociceptive effect of acupuncture needling is mediated, in part, through interactions with adenosine receptors. This antinociceptive effect occurs following both manual and EA and can be reversed by acute and chronic preadministration of caffeine. Moreover, we demonstrate for the first time that caffeine consumption in a dietary context can influence the efficacy of acupuncture-induced AA. In addition, our findings raise the possibility that doses of caffeine relevant to dietary human intake levels could be a confounding factor in the context of acupuncture research. Further studies with detailed consideration of caffeine–acupuncture interactions in humans are warranted.
Acknowledgments
This work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo á Pesquisa e Inovação do Estado de Santa Catarina (FAPESC), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil. The authors are grateful to Professor Jana Sawynok and Allison Reid for their critical review of the manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Zhao ZQ. Neural mechanism underlying acupuncture analgesia. Prog Neurobiol. 2008;85:355–375. doi: 10.1016/j.pneurobio.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Meng CF. Wang D. Ngeow J, et al. Acupuncture for chronic low back pain in older patients: a randomized, controlled trial. Rheumatology (Oxford) 2003;42:1508–1517. doi: 10.1093/rheumatology/keg405. [DOI] [PubMed] [Google Scholar]
- 3.Han JS. Acupuncture analgesia: areas of consensus and controversy. Pain. 2011;152:S41–S48. doi: 10.1016/j.pain.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Goldman N. Chen M. Fujita T, et al. Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture. Nat Neurosci. 2010;13:883–888. doi: 10.1038/nn.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sawynok J. Caffeine and pain. Pain. 2011;152:726–729. doi: 10.1016/j.pain.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Heckman MA. Weil J. Gonzalez de Mejia E. Caffeine (1, 3, 7-trimethylxanthine) in foods: a comprehensive review on consumption, functionality, safety, and regulatory matters. J Food Sci. 2010;75:R77–R87. doi: 10.1111/j.1750-3841.2010.01561.x. [DOI] [PubMed] [Google Scholar]
- 7.Doherty M. Smith PM. Effects of caffeine ingestion on exercise testing: a meta-analysis. Int J Sport Nutr Exerc Metab. 2004;14:626–646. doi: 10.1123/ijsnem.14.6.626. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan GB. Greenblatt DJ. Ehrenberg BL, et al. Dose-dependent pharmacokinetics and psychomotor effects of caffeine in humans. J Clin Pharmacol. 1997;37:693–703. doi: 10.1002/j.1552-4604.1997.tb04356.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhang WY. A benefit-risk assessment of caffeine as an analgesic adjuvant. Drug Saf. 2001;24:1127–1142. doi: 10.2165/00002018-200124150-00004. [DOI] [PubMed] [Google Scholar]
- 10.Esser MJ. Sawynok J. Caffeine blockade of the thermal antihyperalgesic effect of acute amitriptyline in a rat model of neuropathic pain. Eur J Pharmacol. 2000;399:131–139. doi: 10.1016/s0014-2999(00)00336-8. [DOI] [PubMed] [Google Scholar]
- 11.Sawynok J. Reid AR. Caffeine inhibits antinociception by acetaminophen in the formalin test by inhibiting spinal adenosine A(1) receptors. Eur J Pharmacol. 2012;674:248–254. doi: 10.1016/j.ejphar.2011.10.036. [DOI] [PubMed] [Google Scholar]
- 12.Tomic MA. Vuckovic SM. Stepanovic-Petrovic RM, et al. The anti-hyperalgesic effects of carbamazepine and oxcarbazepine are attenuated by treatment with adenosine receptor antagonists. Pain. 2004;111:253–260. doi: 10.1016/j.pain.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Yaba G. Sezer Z. Tekol Y. Interaction between venlafaxine and caffeine on antinociception in mice. Pharmazie. 2006;61:60–62. [PubMed] [Google Scholar]
- 14.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 15.Chen XH. Geller EB. Adler MW. Electrical stimulation at traditional acupuncture sites in periphery produces brain opioid-receptor-mediated antinociception in rats. J Pharmacol Exp Ther. 1996;277:654–660. [PubMed] [Google Scholar]
- 16.Silva MD. Guginski G. Werner MF, et al. Involvement of interleukin-10 in the anti-inflammatory effect of sanyinjiao (SP6) acupuncture in a mouse model of peritonitis. Evid Based Complement Alternat Med. 2011;2011:217946. doi: 10.1093/ecam/neq036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin CS. Jeong HS. Park HJ, et al. A proposed transpositional acupoint system in a mouse and rat model. Res Vet Sci. 2008;84:159–165. doi: 10.1016/j.rvsc.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Pogatzki EM. Gebhart GF. Brennan TJ. Characterization of Adelta- and C-fibers innervating the plantar rat hindpaw one day after an incision. J Neurophysiol. 2002;87:721–731. doi: 10.1152/jn.00208.2001. [DOI] [PubMed] [Google Scholar]
- 19.Lao L. Zhang G. Wei F. Berman BM. Ren K. Electro-acupuncture attenuates behavioral hyperalgesia and selectively reduces spinal Fos protein expression in rats with persistent inflammation. J Pain. 2001;2:111–117. doi: 10.1054/jpai.2001.19575. [DOI] [PubMed] [Google Scholar]
- 20.Cidral-Filho FJ. da Silva MD. More AO, et al. Manual acupuncture inhibits mechanical hypersensitivity induced by spinal nerve ligation in rats. Neuroscience. 2011;193:370–376. doi: 10.1016/j.neuroscience.2011.07.076. [DOI] [PubMed] [Google Scholar]
- 21.Fais RS. Reis GM. Rossaneis AC, et al. Amitriptyline converts non-responders into responders to low-frequency electroacupuncture-induced analgesia in rats. Life Sci. 2012;91:14–19. doi: 10.1016/j.lfs.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Koo ST. Lim KS. Chung K. Ju H. Chung JM. Electroacupuncture-induced analgesia in a rat model of ankle sprain pain is mediated by spinal alpha-adrenoceptors. Pain. 2008;135:11–19. doi: 10.1016/j.pain.2007.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pogatzki EM. Raja SN. A mouse model of incisional pain. Anesthesiology. 2003;99:1023–1027. doi: 10.1097/00000542-200310000-00041. [DOI] [PubMed] [Google Scholar]
- 24.Bortalanza LB. Ferreira J. Hess SC, et al. Anti-allodynic action of the tormentic acid, a triterpene isolated from plant, against neuropathic and inflammatory persistent pain in mice. Eur J Pharmacol. 2002;453:203–208. doi: 10.1016/s0014-2999(02)02428-7. [DOI] [PubMed] [Google Scholar]
- 25.Yang JN. Bjorklund O. Lindstrom-Tornqvist K, et al. Mice heterozygous for both A1 and A(2A) adenosine receptor genes show similarities to mice given long-term caffeine. J Appl Physiol. 2009;106:631–639. doi: 10.1152/japplphysiol.90971.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fredholm BB. Battig K. Holmen J. Nehlig A. Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- 27.Martins DF. Mazzardo-Martins L. Cidral-Filho FJ. Stramosk J. Santos AR. Ankle joint mobilization affects postoperative pain through peripheral and central adenosine A1 receptors. Phys Ther. 2013;93:401–412. doi: 10.2522/ptj.20120226. [DOI] [PubMed] [Google Scholar]
- 28.Sawynok J. Reid AR. Fredholm BB. Caffeine reverses antinociception by oxcarbazepine by inhibition of adenosine A1 receptors: insights using knockout mice. Neurosci Lett. 2010;473:178–181. doi: 10.1016/j.neulet.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 29.Takano T. Chen X. Luo F, et al. Traditional acupuncture triggers a local increase in adenosine in human subjects. J Pain. 2012;13:1215–1223. doi: 10.1016/j.jpain.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zylka MJ. Needling adenosine receptors for pain relief. Nat Neurosci. 2010;13:783–784. doi: 10.1038/nn0710-783. [DOI] [PubMed] [Google Scholar]
- 31.Cui JG. Sollevi A. Linderoth B. Meyerson BA. Adenosine receptor activation suppresses tactile hypersensitivity and potentiates spinal cord stimulation in mononeuropathic rats. Neurosci Lett. 1997;223:173–176. doi: 10.1016/s0304-3940(97)13435-8. [DOI] [PubMed] [Google Scholar]
- 32.Khandwala H. Zhang ZZ. Loomis CW. Inhibition of strychnine-allodynia is mediated by spinal adenosine A(1)- but not A(2)-receptors in the rat. Brain Res. 1998;808:106–109. doi: 10.1016/s0006-8993(98)00752-5. [DOI] [PubMed] [Google Scholar]
- 33.Lee YW. Yaksh TL. Pharmacology of the spinal adenosine receptor which mediates the antiallodynic action of intrathecal adenosine agonists. J Pharmacol Exp Ther. 1996;277:1642–1648. [PubMed] [Google Scholar]
- 34.Esser MJ. Chase T. Allen GV. Sawynok J. Chronic administration of amitriptyline and caffeine in a rat model of neuropathic pain: multiple interactions. Eur J Pharmacol. 2001;430:211–218. doi: 10.1016/s0014-2999(01)01276-6. [DOI] [PubMed] [Google Scholar]
- 35.Jacobson KA. Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sawynok J. Adenosine receptor activation and nociception. Eur J Pharmacol. 1998;347:1–11. doi: 10.1016/s0014-2999(97)01605-1. [DOI] [PubMed] [Google Scholar]
- 37.Sawynok J. Liu XJ. Adenosine in the spinal cord and periphery: release and regulation of pain. Prog Neurobiol. 2003;69:313–340. doi: 10.1016/s0301-0082(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 38.Barone JJ. Roberts HR. Caffeine consumption. Food Chem Toxicol. 1996;34:119–129. doi: 10.1016/0278-6915(95)00093-3. [DOI] [PubMed] [Google Scholar]
- 39.Macintyre PE. Safety and efficacy of patient-controlled analgesia. Br J Anaesth. 2001;87:36–46. doi: 10.1093/bja/87.1.36. [DOI] [PubMed] [Google Scholar]
- 40.Liu C. Zhao F. Zhu L. [Involvement of purines in analgesia produced by weak electro-acupuncture] Zhen Ci Yan Jiu. 1994;19:59–62. 54. [PubMed] [Google Scholar]
- 41.Marchand S. Li J. Charest J. Effects of caffeine on analgesia from transcutaneous electrical nerve stimulation. N Engl J Med. 1995;333:325–326. doi: 10.1056/NEJM199508033330521. [DOI] [PubMed] [Google Scholar]
- 42.Vickers A. Goyal N. Harland R. Rees R. Do certain countries produce only positive results? A systematic review of controlled trials. Control Clin Trials. 1998;19:159–166. doi: 10.1016/s0197-2456(97)00150-5. [DOI] [PubMed] [Google Scholar]
- 43.Sandford P. Completeness of reporting of trials published in languages other than English. Lancet. 1996;347:907–908. doi: 10.1016/s0140-6736(96)91393-8. [DOI] [PubMed] [Google Scholar]
- 44.Schulz KF. Chalmers I. Hayes RJ. Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–412. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]