Abstract
Tick-borne viruses infect humans through the bite of infected ticks during opportunistic feeding or through crushing of ticks by hand and, in some instances, through contact with infected viremic animals. The Ijara District, an arid to semiarid region in northern Kenya, is home to a pastoralist community for whom livestock keeping is a way of life. Part of the Ijara District lies within the boundaries of a Kenya Wildlife Service–protected conservation area. Arbovirus activity among mosquitoes, animals, and humans is reported in the region, mainly because prevailing conditions necessitate that people continuously move their animals in search of pasture, bringing them in contact with ongoing arbovirus transmission cycles. To identify the tick-borne viruses circulating among these communities, we analyzed ticks sampled from diverse animal hosts. A total of 10,488 ticks were sampled from both wildlife and livestock hosts and processed in 1520 pools of up to eight ticks per pool. The sampled ticks were classified to species, processed for virus screening by cell culture using Vero cells and RT-PCR (in the case of Hyalomma species), followed by amplicon sequencing. The tick species sampled included Rhipicephalus pulchellus (76.12%), Hyalomma truncatum (8.68%), Amblyomma gemma (5.00%), Amblyomma lepidum (4.34%), and others (5.86%). We isolated and identified Bunyamwera (44), Dugbe (5), Ndumu (2), Semliki forest (25), Thogoto (3), and West Nile (3) virus strains. This observation constitutes a previously unreported detection of mosquito-borne Semliki forest and Bunyamwera viruses in ticks, and association of West Nile virus with A. gemma and Rh. pulchellus ticks. These findings provide additional evidence on the potential role of ticks and associated animals in the circulation of diverse arboviruses in northeastern Kenya, including viruses previously known to be essentially mosquito borne.
Key Words: Ijara District, Kenya; Arboviruses; Tick species; Rhipicephalus pulchellus; Hyalomma truncatum; Amblyomma gemma; Amblyomma lepidum
Introduction
Tick-borne viruses have a significant impact on human and animal health. They are responsible for some of the most serious emerging and re-emerging infectious disease problems facing the world today that frequently occur in epidemic form. They fall within six different viral families (Asfarviridae, Reoviridae, Rhabdoviridae, Orthomyxoviridae, Bunyaviridae, and Flaviviridae) and at least nine genera. Some as yet unassigned tick-borne viruses may belong to a seventh family, the Arenaviridae. With one exception (African swine fever virus), all tick-borne viruses (as well as all other arboviruses) are RNA viruses (Karabatsos 1985).
The majority of human infections by tick-borne viruses are asymptomatic or may result in a nonspecific flu-like syndrome, and only a small proportion of infected patients progress to severe disease. In severe infections, tick-borne viruses may cause systemic illness ranging from hemorrhagic fever associated with capillary leakage, shock, jaundice, liver damage, and mild aseptic meningitis to encephalitis with coma, paralysis, and death (Chin 2000). Examples include Crimean–Congo hemorrhagic fever (CCHF), Dugbe, Hazara, and Kyasanur forest disease viruses. Humans are incidental hosts because they do not produce significant viremia (Weaver and Reisen 2010). They are infected through the bite of infected ticks during opportunistic blood feeding, through crushing of ticks by hand, and through contact with tissue fluids of infected viremic animals. Nosocomial infections can also occur after handling of infected tissues and body fluids from infected persons (Calisher 1994). These viruses can be diagnosed by serology, virus isolation in cell culture, and molecular-based assays (Hall et al. 2012).
The Ijara District of Kenya is home to a pastoralist community for whom keeping livestock is a way of life. The animals are highly valued and are often maintained in enclosures close to human dwellings or temporary nomadic sheds; small ruminants are sometimes held inside homes overnight to secure them from wild animals. Such practices increase the risk of tick-borne virus transmission. Poor husbandry, value systems, and grazing practices put great pressure on land resources, which results in the need to continuously move large numbers of animals, especially cattle, in search of pasture. This often brings livestock to share pasture with wild animals in forest ecosystem. With this in mind, a tick-borne arbovirus survey was conducted in a pastoral ecozone where intense livestock farming is practiced and where previous reports have indicated arbovirus activity among mosquitoes, animals, and humans. Our aim was to improve understanding of the role of ticks in arbovirus circulation in such ecosystems as a means of preventing virus emergence and dissemination.
Materials and Methods
Study design
This was a field-based descriptive cross-sectional and laboratory-based study conducted between October, 2010, and January, 2012.
Study area
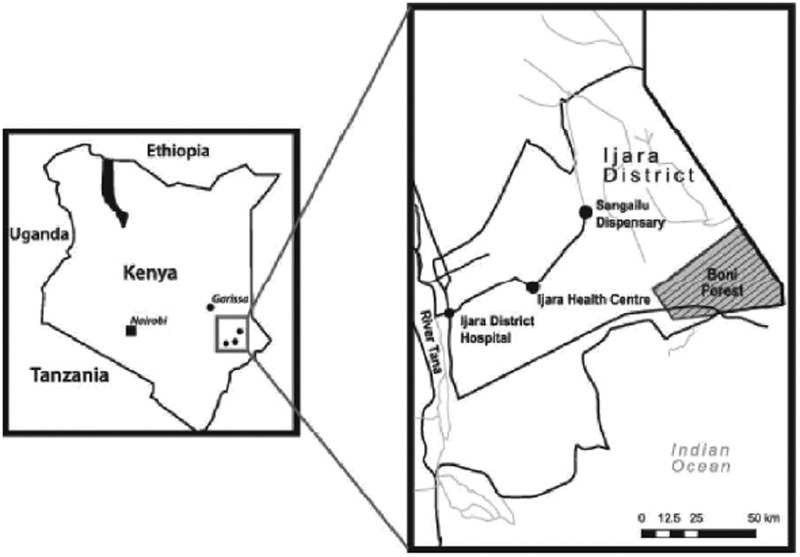
The study was conducted in the Ijara District of the North Eastern Province of Kenya (Fig. 1). This is an arid and semiarid region where 90% of the people practice nomadic pastoralism, keeping indigenous cattle, goats, sheep, donkeys, and camels. Approximately one-quarter of the district is covered by the Boni forest, which borders the Indian Ocean and is an indigenous open canopy forest that forms part of the Northern Zanzibar–Inhamdare Coastal Forest Mosaic (Antipa et al. 2007). The Boni National Reserve, a section of the forest, is under the management of the Kenya Wildlife Service. It is a protected conservation area and is home to a range of wildlife species, including hirola antelope (also known as Hunter's hartebeest), reticulated giraffe, elephant, buffalo, lion, leopard, cheetah, African wild dog, lesser kudu, desert warthog, and bushbuck. Rainfall is unreliable in the Ijara District and does not follow a seasonal pattern, hence the district is prone to frequent droughts. The district is at low altitude (ranging between 0 and 90 meters above sea level) and annual temperature varies from 20°C to 38°C. Prolonged dry seasons trigger the movement of people and livestock to the Tana River delta and the Boni forest area near the Indian Ocean coastline, where water and pasture are abundant long after the rains have gone (Antipa et al. 2007). This migration pattern facilitates the movement of potentially virus-infected ticks across great distances, presenting the opportunity for exchange of diverse tick species between the domestic, wild animals, and even human populations, hence risk of exposure to tick-borne diseases.
FIG. 1.
A map of the study sites within Ijara District in North Eastern Province, Kenya.
Ethical Considerations
Approval to carry out this study was obtained from the Kenya Medical Research Institute (KEMRI) National Ethical Review Committee.
Tick collection and transport
Sampling of ticks from both domestic animals and wildlife was undertaken at various sites of the Ijara District, including the Boni National Game Reserve. Qualified animal handlers who wore the necessary protective gear (such as gloves, coveralls with trouser cuffs taped to shoes, high-top shoes, socks pulled over trouser cuffs, and long-sleeved shirts) performed the tick collections. Livestock (goats, sheep, and cattle) were physically restrained, whereas Kenya Wildlife Service veterinarians immobilized the wild animals (giraffe, warthog, lesser kudu, and zebra) using a combination of etorphine hydrochloride (M99R, Novartis, South Africa) and xylazine hydrochloride (Kyron, South Africa). Both livestock and wild animals were visually examined for ticks, with special attention to the abdomen, back, anal area, and hind legs. If found, the ticks were pulled off manually, placed in sterile plastic vials, and transported to the laboratory in dry ice.
Tick processing and identification
The sampled ticks were washed twice with sterile water to remove excess particulate contamination from animal skin, rinsed once with 70% ethanol, and then rinsed twice with minimum essential medium (MEM) containing antimicrobial agents (100 U/mL penicillin, 100 μg/mL streptomycin, and 1 μL/mL amphotericin B). Tick identification was performed using appropriate identification keys (Matthysse and Colbo 1987, Okello-Onen et al. 1999). The ticks were transferred to sterile vials and stored at −80°C until processed for virus isolation. Ticks were later thawed in ice (4°C), identified, and pooled into groups of one to eight (depending on size) by species, sex, and animal host. Each pool was homogenized using 90-mesh alundum in a prechilled, sterile mortar and pestle with 1.6–2 mL ice-cold MEM containing 15% fetal bovine serum (FBS), 2% glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 1 μL/mL amphotericin B. The homogenates were clarified by low-speed centrifugation at 1500 rpm for 15 min at 4°C, and supernatants aliquoted and stored at −80°C. In the case of Hyalomma species, the primary vectors of Crimean–Congo hemorrhagic fever virus (CCHFV), each pool was prescreened for CCHF by reverse transcription-polymerase chain reaction (RT-PCR) to exclude this virus prior to cell culture screening.
Virus isolation
Vero cells were grown in 25-cm2 cell culture flasks to 80% confluency in MEM containing 10% FBS, 2% glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 1 μL/mL amphotericin B. The cells were then rinsed with sterile phosphate-buffered saline (PBS), and 0.2 mL of clarified tick homogenate was added followed by incubation at 37°C for 45 min to allow virus adsorption. After incubation, MEM supplemented with 2% FBS, 2% glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 1 μL/mL amphotericin B was added into the flasks and the cells allowed to incubate at 37°C for 14 days while observing cytopathic effects (CPE) on a daily basis. The supernatants of virus-infected Vero cell cultures exhibiting CPE of approximately 70% were harvested from the flasks for virus identification. The pooled infection rate program (PooledInfRat, Centers for Disease Control and Prevention, Fort Collins, CO; http://www.cdc.gov/ncidod/dvbid/westnile/software.htm/) was used to compare virus infection rates in the tick species collected and processed in this study.
Reverse transcriptase polymerase chain reaction
Viral RNA was extracted from the culture isolates using a TRIzol Plus RNA Purification Kit (Invitrogen) according to the manufacturer's recommended protocol. Extracted RNA was reverse transcribed to cDNA with the Transcriptor First Strand cDNA Synthesis Kit (Roche Applied Science) using Random hexamers followed by PCR using Phusion High-Fidelity PCR Kit (Finnzyme OY, Espoo, Finland) with primers targeting key arboviruses (Table 1). The following PCR cycling conditions were used: 98°C for 2 min, followed by 40 cycles of 98°C for 30 s of 60°C for 30 s, and 72°C for 30 s. Reactions were terminated with a final extension step at 72°C for 7 min. The PCR products were visualized on a 2% agarose gel stained with ethidium bromide (0.5 μg/mL) and purified using shrimp alkaline phosphatase-exonucleaseI (ExoSapI) (USB Corp, Cleveland, OH) according to the manufacturer's instructions.
Table 1.
Reverse Transcription Polymerase Chain Reaction Primers Used for Isolation of Key Arboviruses and Known African Tick-Borne Viruses
| Virus target | Target gene | Primer designation | Primer sequences 5′- 3′ |
|---|---|---|---|
| Alphaviruses | gp1 | Vir 2052 For | TGGCGCTATGATGAAATCTGGAATGTT |
| Vir 2052 Rev | TACGATGTTGTCGTCGCCGATGAA | ||
| Orthobunyavirus | gp2 | OrthoBun For | CTGCTAACACCAGCAGTACTTTTGAC |
| OrthoBun Rev | TGGAGGGTAAGACCATCGTCAGGAACTG | ||
| Thogoto | N | THO NF | CCTGCAGGGGCGGAAGTTATG |
| THO NR | AAAATCCTCGCAGTTGGCTATCA | ||
| Dugbe | N | DG S1 | TCTCAAAGACAAACGTGCCGCAG |
| DG S5 | TGCAACAACTGGATGTGTGA | ||
| Flaviviruses | NS5 | FLAVI fu2 | GCTGATGACACCGCCGGCTGGGACAC |
| FLAVI cfd3 | AGCATGTCTTCCGTGGTCATCCA |
Results
Tick collection
A total of 10,488 ticks were collected and processed for virus isolation in 1520 pools. Species of ticks collected and their proportions are shown in (Table 2). The predominant species collected was Rhipicephalus pulchellus (76.12%) followed by Hyalomma truncatum (8.68%), Amblyomma gemma (5.00%), Amblyomma lepidum (4.34%), Hyalomma marginatum (2.24%), Hyalomma spp. (0.92%), Rhipicephalus appendiculatus (0.59%), Hyalomma dromedarii (0.59%), Boophilus annulatus (0.53%), Amblyomma hebraem (0.39%), Rhipicephalus pravus (0.20), D. rhinocerous (0.07%), and an unidentified nymph (0.20%). However, the calculated virus pooled infection rate was highest in Hyalomma spp. (142.86) (Table 2).
Table 2.
Virus Isolates Obtained from Ticks Collected from Animal Hosts in Ijara District, Kenya
| |
|
|
Cattle |
|
Goat |
|
Sheep |
|
Giraffe |
|
Warthog |
|
|
|
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Total # tick pools | # tick pools | BU | DU | ND | SE | TH | WN | # tick pools | BU | DU | ND | SE | TH | WN | # tick pools | BU | DU | ND | SE | TH | WN | # tick pools | BU | DU | ND | SE | TH | WN | # tick pools | BU | DU | ND | SE | TH | WN | # virusisolated | Infected pools | Pooled IR |
| A.gemma | 76 | 29 | 2 | 0 | 0 | 0 | 0 | 0 | 14 | 0 | 1 | 0 | 0 | 0 | 0 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 21 | 0 | 0 | 0 | 1 | 0 | 1 | 6 | 5 | 65.79 |
| A.hebraem | 6 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| A.lepidum | 66 | 29 | 1 | 0 | 0 | 1 | 0 | 0 | 30 | 1 | 0 | 0 | 1 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 3 | 45.45 |
| B.annulatus | 8 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D.rhinocerinus | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H.dromedarii | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H.marginatum | 34 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 12 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H.truncatum | 132 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 37 | 1 | 0 | 0 | 1 | 0 | 0 | 61 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 14 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 2 | 15.15 |
| Hyalomma spp | 14 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 142.86 |
| Rh.Pravus | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rh.appendiculatus | 9 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rh.evertsi evertsi | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rh.pulchellus | 1157 | 283 | 10 | 2 | 1 | 6 | 1 | 1 | 486 | 11 | 1 | 0 | 6 | 1 | 0 | 275 | 13 | 1 | 0 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 90 | 1 | 0 | 1 | 1 | 1 | 1 | 67 | 40 | 34.57 |
| Unidentified nymph | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 1520 | 375 | 13 | 2 | 1 | 7 | 1 | 1 | 591 | 14 | 2 | 0 | 8 | 1 | 0 | 365 | 15 | 1 | 0 | 8 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 137 | 1 | 0 | 1 | 2 | 1 | 2 | 82 | 52 | |
BU, Bunyamwera virus; DU, Dugbe virus; ND, Ndumu virus; SE, Semliki Forest virus; TH, Thogoto virus; WN, West Nile virus; IR, infection rate.
Virus isolation and identification
A total of 155 tick pools showed CPE in Vero cells yielding virus isolates. Although all isolates were subjected to RT-PCR with primers targeting key arboviruses (Table 1), only 82 isolates were identified, distributed among the tick species as follows: Rh. pulchellus (67), A. gemma (6), A. lepidum (4), H. truncatum (3) and Hyalomma spp. (2). There was no virus isolated from H. marginatum, Rh. appendiculatus, H. dromedarii, B. annualtus, A. hebraem, Rh. pravus, D. rhinocerous, and the unidentified nymph pools. Rh. pulchellus had the highest number of virus-infected pools (46) followed by A. gemma (5), A. lepidum (3), H. truncatum (2), and Hyalomma spp (2) (Table 2).
The 82 virus strains were obtained from 52 tick pools, of which 27 had single viral infection, 22 contained mixed infections of two different viruses, two contained three different viruses, and one contained five different viruses (Table 2). The observed onset of CPE among the isolates ranged from 3 to 12 days postinfection. The identified isolates included Bunyamwera (44), Dugbe (5), Ndumu (2), Semliki forest (25), Thogoto (3), and West Nile (3) virus strains, respectively. Of all the tick species processed for virus isolation, Rh. pulchellus had the highest infection with Bunyamwera (35), Semliki (21), Dugbe (4), Ndumu (2), Thogoto (3), and West Nile (2) viruses. West Nile virus was also isolated from the A. gemma and Rh. pulchellus species sampled from cattle and warthogs. Thogoto virus was isolated from Rh. pulchellus species sampled from cattle, goats, and warthogs. Dugbe virus was isolated from Rh. pulchellus and A. gemma species sampled from cattle, goats, and sheep.
Bunyamwera and Semliki forest viruses were predominantly isolated from Rh. pulchellus. Ticks sampled from livestock had the highest number of virus isolates. Bunyamwera virus was isolated from A. gemma species sampled from giraffe and Rh. pulchellus species sampled from warthog. A. gemma and Rh. pulchellus species were the major virus carriers amongst wildlife (Table 2). There was no virus isolated from ticks sampled from lesser kudu and zebra.
Discussion
Tick vectors have been implicated as important routes for virus transmission and dissemination where one host may act as a reservoir of infection, pass it via the tick to a more vulnerable host, which then suffers disease (Hudson and Greenman 1998). Tick-borne viruses cause significant morbidity/mortality and economic loss to humans, livestock, and wildlife hosts in the tropics (Sonenshine and Mather 1994). Wildlife serves as potential reservoirs for tick-borne pathogens of livestock and humans. Domestic animals are infected when livestock and wild animals share pasture and water during adverse weather conditions (Jongejan and Uilenberg 1994).
The overall prevalence of tick-borne viruses in this study was 5.4%, similar to a study carried out by Sang et al. (2006) on tick-borne arbovirus surveillance in market livestock in Nairobi, Kenya. However, in our study, the predominant species collected was Rh. Pulchellus, whereas the highest calculated virus pooled infection rate was in Hyalomma spp., which is different from what was observed in the previous study where the predominant species collected was Rh. Pulchellus; however, the calculated virus pooled infection rate was highest for A. gemma. This could be attributed to origin of the tick samples in the two studies. Animals slaughtered in abattoirs in Nairobi originate from diverse geographical regions in Kenya, unlike this collection, which was localized in Ijara only. Some of the viruses found to be prevalent in this survey are of significant public health importance and this puts communities living in the Ijara District (who are pastoralists and interact closely with their animals) at great risk of exposure to these tick-borne viruses.
Despite detecting a higher number of virus isolates, in ticks sampled from livestock, similar viruses (such as Bunyamwera, Semliki, and Ndumu) were also isolated from wildlife, signifying the potential of arbovirus transmission across animal species. Among ticks collected from livestock, cattle (6.6%) and sheep (6.57%) showed the highest prevalence of virus-positive ticks, whereas tick sampled from warthogs had the highest prevalence among wildlife. In the Ijara District, warthogs live in close proximity to households and interact closely with livestock (especially sheep and goats), providing increased opportunity for transfer of ticks between domestic animals and warthogs in the villages.
Two classical tick-borne viruses were isolated during this survey, namely Thogoto and Dugbe viruses. These viruses have been isolated in previous surveys conducted in Kenya (Burt et al. 1996, Sang et al. 2006). Thogoto virus was first isolated in Kenya from Rhipicephalus species and Boophilus decoloratus in the 1930s (Karabatsos 1985) and has been isolated repeatedly from various tick species in Kenya, West Africa, Europe, and Asia (Calisher et al. 1987, Sang et al. 2006). Two Thogoto virus infections have been reported in humans, with one fatality (Moore et al. 1975). There is a need to assess the public health impact of this virus in Kenya.
Dugbe virus has also been commonly isolated in surveillance studies conducted in Africa (Burt et al. 1996, Camicas 1980, Johnson et al. 1980, Sang et al. 2006). The implications of Dugbe circulation to public health have not been evaluated in Kenya, although reports from South Africa suggest that Dugbe virus causes human infection (Karabatsos 1985, Burt et al. 1996) resulting in severe disease.
In an earlier study conducted around Lake Victoria, Dugbe virus was isolated more commonly than any other virus from Rh. pulchellus with a single isolate from A. gemma; it was observed that more tick pools from dry scrubland were infected with Dugbe virus than pools from the swamp edge (Johnson et al. 1980). This is consistent with our findings because most of our Dugbe isolates were from Rh. pulchellus and a single isolate from A. gemma.
In this study Rh. pulchellus was the predominant tick species collected and had the highest number of virus isolates. Bunyamwera, Dugbe, Ndumu, Semliki, West Nile, and Thogoto viruses were isolated from Rh. pulchellus collected from cattle, goats, sheep, and warthogs. A previous survey carried out in abattoirs in Kenya also demonstrates the importance of this species in arbovirus transmission and maintenance (Sang et al. 2006). Rh. pulchellus is a known ectoparasite of both livestock and wildlife in savannah habitats east of the Rift Valley (Hopla et al. 1994). It is also a vector of several viruses such as Nairobi sheep disease, Dugbe, Barur, and CCHF (Butenko et al. 1996). The abundance of Rh. pulchellus within the study region might be due to favorable ecological conditions, which range from semiarid to arid zone with predominant acacia, Commiphora shrubs interspersed with grassy bushes, and close proximity to the Tana Delta and the Indian Ocean. This tick is able to survive diverse climatic conditions where arbovirus populations may be found. Rh. pulchellus is also known to be a three-host tick requiring three hosts to complete its cycle, a situation that provides multiple opportunities for acquiring, transmitting, and disseminating more virus strains.
The most significant finding in the current study is the number of mosquito-borne virus strains isolated from pooled engorged ticks. Three of the West Nile virus isolates in this study were isolated from ticks (A. gemma and Rh. pulchellus) sampled from cattle and warthogs. However, there is no documented role of these animals in West Nile virus transmission and maintenance.
Although ticks may be involved in the transmission and dissemination of the viruses detected in this study, there is also a possibility that ticks sampled in this study fed on viremic animals and, therefore, could have picked up the viruses from host blood (Weaver and Reisen 2010). Further investigations using actual animal (domestic and wild) samples are needed to conclusively determine their role as reservoirs of the viruses in question. Similarly, vector competence studies should be performed to investigate the role of implicated tick species in the natural transmission cycle of the viruses isolated from the ticks in this study.
Conclusions
Our findings suggest a significant role for ticks in the maintenance, spread, and possible transmission of viruses normally associated with mosquitoes in Africa. The observed circulation of multiple arbovirus strains among pooled engorged ticks may provide opportunities for genetic recombinations and reassortments that could result in emergence of new arbovirus strains, some of which could be serious human pathogens (such as Ngari virus) (Bowen et al. 2001, Nichol et al. 2005). Therefore, continued tick-based arbovirus surveillance among diverse host systems is valuable for monitoring arbovirus emergence.
Acknowledgments
We thank the families in the Ijara District who participated in the study by allowing ticks to be collected from their animals; Dunston Beti, John Gachoya, and Reuben Lugalia for assisting in tick collection from domestic animals; Arbovirus Incidence and Diversity Project consortium led by International Centre of Insect Physiology and Ecology (icipe) and including Kenya Medical Research Institute, Kenya Wildlife Service, International Livestock Research Institute, Kenya Agricultural Research Institute, Ministry of Livestock Development-Central Veterinary Laboratories and Ministry of Public Health and Sanitation; the icipe Director General, Prof. Christian Borgemeister, for providing the needed support for project implementation. This work received financial support from Google.org, the philanthropic arm of Google.
Author Disclosure Statement
No competing financial interests exist.
References
- Antipa RS. Ali MH. Hussein AA. National Environmental Management Authority; Kenya: 2007. Assessment of the Potential of Eco/Cultural Tourism as Viable Enterprises in Southern Garissa, Ijara and Lamu Districts: A Community Conservation and Enterprise Support Initiative. [Google Scholar]
- Bowen MD. Trappier SG. Sanchez AJ. Meyer RF, et al. A reassortant bunyavirus isolated from acute hemorrhagic fever cases in Kenya and Somalia. Virology. 2001;291:185–190. doi: 10.1006/viro.2001.1201. [DOI] [PubMed] [Google Scholar]
- Butenko AM. Gromashevsky VL. L'Vov DK. Popov VF. First isolations of Barur virus (Rhabdoviridae) from ticks (Acari: Ixodidae) in Africa. J Med Entomol. 1996;18:232–234. doi: 10.1093/jmedent/18.3.232. [DOI] [PubMed] [Google Scholar]
- Burt FJ. Spencer DC. Leman PA. Patterson B, et al. Investigation of tick-borne viruses as pathogens of humans in South Africa and evidence of Dugbe virus infection in a patient with prolonged thrombocytopenia. Epidemiol Infect. 1996;116:353–361. doi: 10.1017/s0950268800052687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calisher CH. Medically important arboviruses of the United States and Canada. Clin Microbiol Rev J. 1994;7:89–116. doi: 10.1128/cmr.7.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calisher CH. Karabatsos N. Filipe AR. Antigenic uniformity of topotype strains of Thogoto virus from Africa, Europe, and Asia. Am J Trop Med Hyg. 1987;37:670–673. doi: 10.4269/ajtmh.1987.37.670. [DOI] [PubMed] [Google Scholar]
- Camicas JL. Les arbovirus à tiques en zone tropicale. Med Trop. 1980:40. [PubMed] [Google Scholar]
- Chin J. Heymann D. Control of Communicable Diseases Manual. Washington, DC: American Public Health Association; 2000. Arthropod-borne viral diseases; pp. 28–36. [Google Scholar]
- Hall A. Bradley J. Blitvich CA. Blacksell SD. Advances in arbovirus surveillance, detection and diagnosis. J Biomed Biotechnol. 2012;10:1155. doi: 10.1155/2012/512969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopla CE. Durden LA. Keirans JE. Ectoparasites and classification. Rev Sci TechReview Off Int Epiz. 1994;13:985–1017. doi: 10.20506/rst.13.4.815. [DOI] [PubMed] [Google Scholar]
- Hudson PJ. Greenman JV. Parasite mediated competition. Biological and theoretical progress. Trends Ecol Evol. 1998;13:387–390. doi: 10.1016/s0169-5347(98)01475-x. [DOI] [PubMed] [Google Scholar]
- Johnson BK. Chanas AC. Squires EJ. Shockley P, et al. Arbovirus isolations from ixodid ticks infesting livestock, Kano Plain, Kenya. Trans R Soc Trop Med Hyg. 1980;74:732–737. doi: 10.1016/0035-9203(80)90188-1. [DOI] [PubMed] [Google Scholar]
- Jongejan F. Uilenberg G. Ticks and control methods. In: Blancou J, editor. Ectoparasites of Animals and Control Methods. Vol. 13. Paris, France: Scientific and Technical Review, Office Internationale des Epizooties (OIE); 1994. pp. 1201–1226. [DOI] [PubMed] [Google Scholar]
- Karabatsos N, editor. San Antonio, Texas: American Society of Tropical Medicine and Hygiene; 1985. International Catalogue of Arboviruses Including Certain Other Viruses of Vertebrates. [DOI] [PubMed] [Google Scholar]
- Matthysse JG. Colbo MH. College Park, MD: Entomological Society of America; 1987. The Ixodid Ticks of Uganda Together with Species Pertinent to Uganda because of Their Present Known Distribution. [Google Scholar]
- Moore DL. Causey OR. Carey DE. Reddy S, et al. Arthropod-borne viral infections of man in Nigeria, 1964–1970. Ann Trop Med Parasitol. 1975;69:49–64. doi: 10.1080/00034983.1975.11686983. [DOI] [PubMed] [Google Scholar]
- Nichol ST. Beaty B. Elliott RM. Goldbach R, et al. Bunyaviridae. In: Fauquet CM, editor; Mayo MA, editor; Maniloff J, editor; Desselberger U, et al., editors. Virus Taxonomy: Eighth Report of the International Committee on Taxonomy of Viruses. Vol. 8. San Diego, California: Academic Press; 2005. pp. 695–716. [Google Scholar]
- Okello-Onen J. Hassan SM. Essuman S. Taxonomy of African Ticks. Nairobi: icipe Science Press, Nairobi; 1999. [Google Scholar]
- Sang R. Onyango C. Gachoya J. Mabinda E, et al. Tickborne arbovirus surveillance in market livestock, Nairobi, Kenya. Emerg Infect Dis J. 2006;12:1074–1080. doi: 10.3201/eid1207.060253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshine DE. Mather TN. Ecological Dynamics of Tick-borne Zoonoses. New York: Oxford University Press; 1994. [Google Scholar]
- Weaver SC. Reisen WK. Present and future arboviral threats. Antivir Res. 2010;85:328–345. doi: 10.1016/j.antiviral.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]