Abstract
β-sitosterol is an important component in food and herbal products and beneficial in hyperlipidemia. Its higher concentrations in serum may lead to coronary artery disease in case of sitosterolemia. Therefore, it is essential to determine the quantity of β-sitosterol in food and herbal drugs. Saraca asoca and its preparations have been widely used by traditional healers are also a source of β-sitosterol. In the present study, quantitative estimation of β-sitosterol present in hot and cold water extracts of bark, regenerated bark, leaves and flowers of the S. asoca and Ashokarista drugs were carried out first time using high performance liquid chromatography coupled (HPLC) with quadrupole time-of-flight mass spectrometry. Different concentrations of β-sitosterol and crude extracts were estimated by HPLC and targeted mass spectrometry. Standard curve for β-sitosterol was prepared from the intensities of transitions (397.50 → 147.0987 m/z) having regression coefficient (r2) 0.9952. Out of eight extracts and two drugs used in the study bark water, leaves water and leaves hot water extracts were found to have a considerable quantity of β-sitosterol, i.e. 170, 123.5 and 19.3 ng/mL, respectively. The results showed significant differences in the distribution of β-sitosterol among different organs of S. asoca and drugs prepared from its bark. HPLC/electrospray ionizationmass spectroscopy method is accurate, reproducible and requires less specimen, sample preparation and analysis time over HPLC assay. This type of approaches could be helpful for the quality control of herbal medicines and provides necessary information for the rational utilization of plant resources.
Keywords: Liquid chromatography-mass spectrometry, natural products, quantitative analysis, Saraca asoca, β-sitosterol
INTRODUCTION
Drug regulation authorities in several countries do not regulate dietary supplements/nutraceuticals. Herbal unprocessed formulations, also used as dietary supplements/nutraceuticals play a significant role in health-care programs. Saraca asoca De. Wild (Caesalpinaceae) is an important medicinal plant used in raw form as well as in several commercially available herbal formulations. The bark and leaves of S. asoca are used for the treatment of menorrhagia, leucorrhea, ulcers and fevers.[1] The flowers also reported for medicinal property and used in diabetes, cancer and hemorrhagic dysentery.[2,3,4] Antimicrobial property of different plant parts including bark, flower and flower buds are reported in the literature.[5]
β-sitosterol constitutes about 90% of phytosteroles present in plants. β-sitosterol is reported to have anti-oxidant, anti-inflammatory and anti-arthrosclerosis activities.[6,7,8] It is also found to be useful in the treatment of benign prostatic hyperplasia.[9] β-sitosterol is absorbed only in trace amounts and inhibits the absorption of intestinal cholesterol including re-circulating endogenous biliary cholesterol, a key step in cholesterol elimination.[10] β-sitosterol and cholestanol in human serum are good markers of sterol absorption since they are not synthesized in the human body. It has been reported that the absorption rate of phytosterols in individuals is variable and hereditary.[11]
β-sitosterol alone or in combination with β-sitosterol glucoside is used in treating hypercholesterolemia.[12] At the same time, it is considered as the major risk for the coronary heart problems and some disease conditions like sitosterolemia.[11] Herbal dietary products being recommended for any cause also contains unknown levels of β-sitosterol. Therefore, it is essential to quantize β-sitosterol in herbal supplements. Quadrupole time-of-flight mass spectrometry (QTOFMS) is a technique to analyze multi-components in herbal extracts due to accurate mass measurement, high resolution and ion separation.[13,14] These processed data have been used to study various pharmacophysiological studies such as disease diagnostics, human nutritional science and drug discovery.[13,15]
In the current study, high performance liquid chromatography coupled to quadrupole time-of-flight mass spectrometry (HPLC-QTOFMS) has been used for non-targeted and targeted analysis of β-sitosterol in various sample of S. asoca. The technique used is reliable, reproducible and accurate, don’t require processed sample and can be used further for the quality control purpose.
MATERIALS AND METHODS
Plant Material
Various samples (bark, regenerated bark, leaves and flowers) of S. asoca were collected in February, 2012 from the Garden of National Research Institute of Basic Ayurvedic Sciences, Central Council for Research in Ayurveda and Siddha (Department of Ayurveda, Yoga, Naturopathy, Unani, Siddha and Homeopathy), Nehru Garden, Kothrud, Pune. The collected plant materials were identified and voucher specimens (No. 207) were kept at the medicinal plant museum of the Institute. The Ashokarista formulations of Baidyanath Pvt., Ltd. (Batch No 110085, mfg April 2011) and Dabur Pvt., Ltd. (Batch No BD1049, mfg Sept 2010) were purchased from authorized medical stores.
Chemicals
Standard compounds and solvents, i.e. lidocaine, D-camphor, 5, 7-isoflavone, β-sitosterol, acetonitrile, tri-fluroacetic acid and water were purchased from Sigma Aldrich.
Extraction
The plant materials (10 g each) were washed with deionized water and crushed. The crushed materials were extracted overnight with equal quantity of deionized water (Direct-Q, Millipore) with continuous shaking at 25°C and 70°C (water extract and hot water extracts) and this step was repeated three times to ensure complete recovery. The sample were named as bark water extract (BWE), bark hot water extract (BHWE), re-generated bark water extract (RBWE), regenerated bark hot water extract (RBHWE), leaves water extract (LWE), leaves hot water, flower water and flower hot water extract. Extracts were centrifuged at 10,000 g for 10 min for complete removal of suspended particles. The extracts were lyophilized using lyophilizer (Freezone 4.5 Labconco) and stored at −80°C until further use. The plant extracts were reconstituted in liquid chromatography-mass spectrometry (LC/MS) grade water (5 mg/ml) and filtered through 0.22 μ filters (Hi-media) for further analytical study. Baidyanath (BA) and Dabur (DA) ashokarista formulations were centrifuged at 10,000 g for 20 min at 4°C and filtered through 0.22 μ membrane before analysis.
Mass Spectrometry
Auto MSn experiments were performed on an Agilent 1290 infinity series rapid resolution liquid chromatography interfaced to an Agilent 6538 accurate-mass QTOF mass spectrophotometer (LCMS). A volume of 20 μl of each sample was injected into ZORBAX 300SB reversed phase column (C18, 4.5 mm × 250 mm) of 5 μm particle size. The column temperature was maintained at 40°C. Mobile phase comprised solvent A (water containing 0.1% formic acid) and solvent B (acetonitrile containing 0.1% formic acid) used in gradient mode (time/concentration [min/%]) for solvent B 8/5%; 15/10%; 22/45%; 30/65%; 35/90%; 40/5%, with a flow rate of 0.4 ml/min. The mass spectrometer was operated in positive ion polarity mode in the extended dynamic range (1700 m/z, 2 GHz) with following parameters: Gas temperature 350°C, nebulizer 50 Psi, gas flow 8 L/Min, capillary voltage 3500 V, nozzle 500 V and fragmentor voltage 175 V. To assure the mass accuracy of recorded data, continuous internal calibration was performed. Lidocaine (234.3 m/z), 5, 7-isoflavone (284.3 m/z) were infused with samples as external standards.
MSn Data Analysis
Initial processing of LCMS data, i.e. baseline correction, noise reduction, background contaminants removal; were performed by MassHunter Qualitative Analysis Software (Version 3.1, Agilent Technologies). The MSn spectrums were extracted using auto MS/MS extraction tool. The specific molecular formulas of β-sitosterol and β-sitosterol-6-O-glucoside were searched using the formula search option in the software. MSn spectrum of β-sitosterol and β-sitosterol-6-O-glucoside were compared with the standards.
Targeted Mass Spectrometery
Standard solutions of β-sitosterol (ranging from 5-500 ng/ml) and three different dilutions (5, 0.5 and 0.05 mg/ml) of crude extracts were injected into the mass spectrometer operated in the positive mode. The standards and sample were analyzed using the same solvent system and parameters as described above in MS mode. The collision energy (CE) for β-sitosterol was fixed 35 V.
Targeted MS Data Analysis
Standards curve was prepared from spectra of β-sitosterol solutions using Agilent Mass Hunter Quantitative Analysis Software B 2.0.0.2. A window of 100 ppm was set for fragment identification. Standards and their corresponding metabolites in the extracts were quantified by using the peak size of fragments of extracted ion chromatogram function.
RESULTS AND DISCUSSION
β-sitosterol is a widely known component of several daily use foods and herbal medicines and used to treat hypercholesterolemia and other disease conditions.[12] It was observed that bark of S. asoca, which is most widely used component of this plant in various pharmacophysiological applications, contains good amounts of β-sitosterol. Therefore, β-sitosterol and related sterols in S. asoca and two herbal drugs were analyzed by non-targeted and targeted MS/MS.
Sample processing, which is a time consuming work can be omitted because of high sensitivity on LC-QTOFMS being the hybrid of the mass spectrometer and TOF. Time period of chromatography run can also be reduced, but in the present study we employed crude extracts of plant, run time was kept to minimum but in a course to separate most of the metabolites. The reversed phase chromatographic system was employed for rapid separation of a wide range of polar compounds. On a C-18 column, all metabolites ionizable/detectable with the (±) electrospray ionization were eluted in 40 min [Figure 1]. Additional 5 min run were given to ensure the complete removal of sample from the column. Retention time variability across the samples was found to be 2 s or a relative standard deviation (RSD) of less than 3% (n = 15). The QTOFMS was calibrated before analysis and the mass accuracy was achieved below 2.0 ppm, calculated by using reference ions. Internal standards were employed throughout the analysis to ensure the mass accuracy. Total ion current (TIC) chromatograms, recorded in positive ion mode, showed high variability in the contents of samples [Figure 1]. Variability across the samples may be because different organs have specific roles in the plant survival in order to maintain daily requirements and fight infections.
Figure 1.
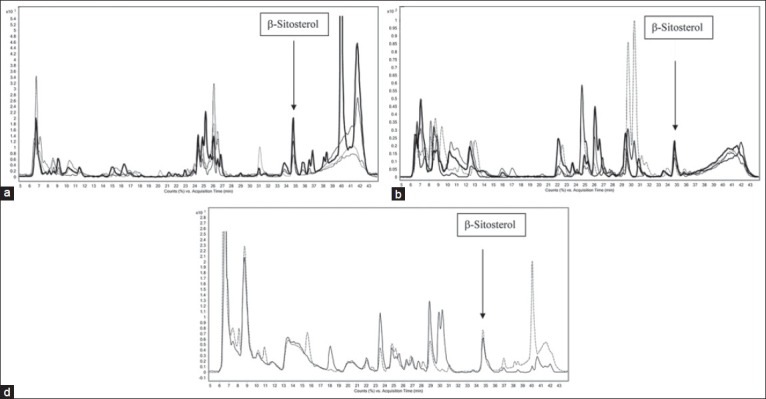
Total ion chromatograms of (a) bark water extract, bark hot water extract, regenerated bark water extract, regenerated bark hot water extract, (b) flower water extract, flower hot water extract, leaves water extract, leaves hot water extract and (c) Dabur and Baidyanath ashokarista showing the variation among the samples
MSn data was processed by using qualitative MassHunter (B.04.00 Version). Compounds from TIC were identified by using command find by auto MS/MS in the software. On an average, more than 400 compounds at a threshold 5000 cpu were detected in each sample. Molecular formulas of the compounds were generated using the formula generator in built software. Extracts were found to be highly complex in nature as on an average 3-10 compounds of same m/z ratio were observed. Therefore, specific pattern of mass fragmentation was searched among the compounds to identify the specific compounds. An in-house generated library specific for S. asoca was used to identify β-sitosterol, β-sitosterol-6-O-glucoside, stigmasterol and campesterol present in the different extracts. A retention time of β-sitosterol-6-O-glucoside was observed to be 25.509 min in all the samples. The MSn spectrums of β-sitosterol-6-O-glucoside were compared with its standard to confirm the structure. β-sitosterol-6-O-glucoside was found to be present across the sample, but highest amount was observed in regenerated bark and LWE extracts [Figure 2]. Stigmasterol and campesterol were found to be negligible in all the samples.
Figure 2.
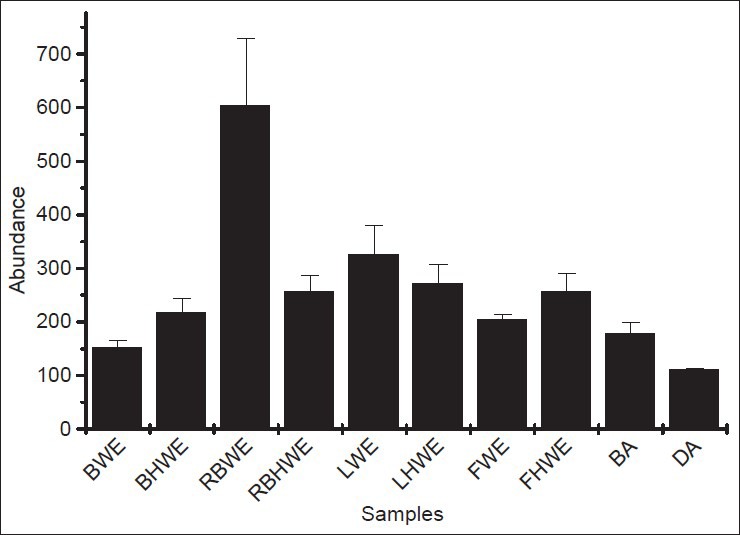
Abundance of β-sitosterol-6-O-glucoside in the sample and drugs
Retention time of β-sitosterol was found to be 35.596 min. The spectra generated for the compound in positive ion detection gave the protonated molecule [M H] + (m/z 397.70). The product ion scan spectrum of 397.70 m/z generate the daughter ion of 147.0987 m/z. Quantization of β-sitosterol in samples was performed by injection of extracts in the HPLC-MS/MS system in targeted mode. Identification of β-sitosterol was performed on the basis of retention time and intensity of peaks in the samples by comparing with those of the standards [Figure 3]. Regression coefficient was observed to 0.9952 shows the accuracy of the method. The highest concentrations of β-sitosterol were found to be in BWE and LWE extracts [Figure 4]. In other sample, it was found to be in non-significant amounts. In a recent finding, it is reported that leaves have anti-diabetic activity.[4] However, our data on the bark and leaves extracts support the hypolipidemic activity owing to the presence of high amounts of β-sitosterol and β-sitosterol-6-O-glucoside. Intensities of fragments and its pattern depend upon the several factors including CE. Auto MS/MS was used with variable (sloped) CE shows a different pattern in the intensities as compared with fixed CE in case of targeted MS/MS. But in the complex extracts it is better to use CE slope due to unknown molecular weights of compounds.
Figure 3.
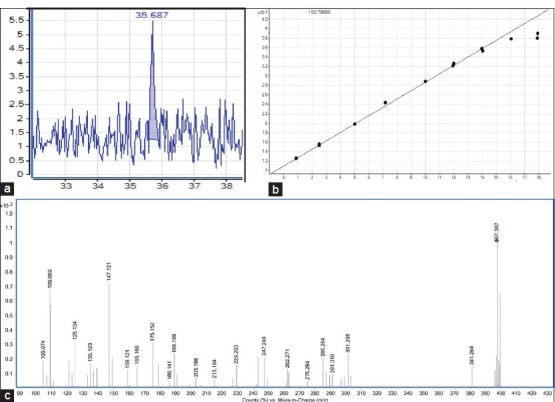
(a) Total ion chromatogram of β-sitosterol with retention time, (b) standard curve for β-sitosterol (R2 = 0.9971) (c) product ion spectrum of β-sitosterol
Figure 4.
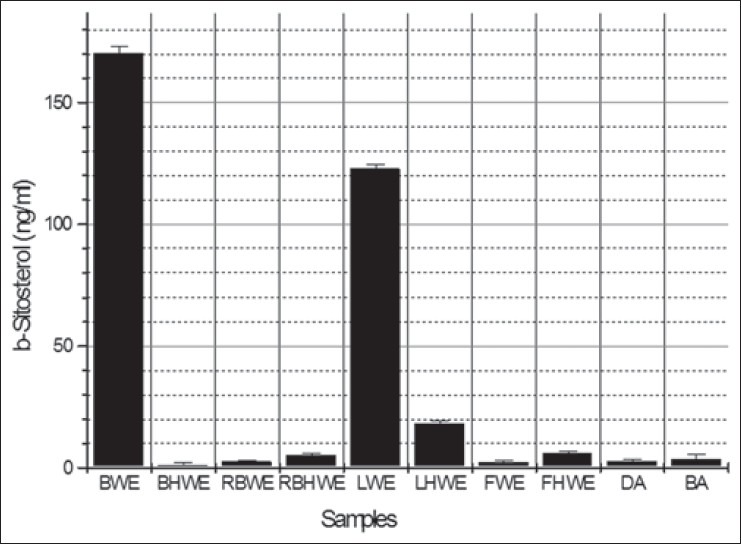
Concentration of β-sitosterol (ng/ml) in various samples
CONCLUSION
HPLC-QTOFMS technique was successfully used first time for qualitative analysis of β-sitosterol-6-O-glucoside and quantitative analysis of β-sitosterol in various crude extracts of S. asoca and related drugs. Bark and LWE extract were found to have higher quantities of β-sitosterol as compared to other parts. Both drugs from various sources were found to have less quantity of β-sitosterol compare to the plant part from which these drugs were prepared. At the same time, Baidyanath asokarista sample showed 5 fold more β-sitosterol than Dabur asokarista. Significant differences observed in the distribution of β-sitosterol among different organs of S. asoca could be helpful for the quality control of herbal medicines and provides necessary information for the rational utilization of plant resources. Considering the encouraging results obtained in this study, we propose the application of HPLC-QTOFMS in authentication of crude and processed herbal drugs over HPLC assay. Furthermore, this technique seems to promising because of its accuracy and reproducibility.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.Pradhan P, Joseph L, Gupta V, Chulet R, Arya H, Verma R, et al. Saraca asoca (Ashoka): A review. J Chem Pharma Res. 2009;1:62–71. [Google Scholar]
- 2.Saravanan S, Babu NP, Pandikumar P, Ignacimuthu S. Therapeutic effect of Saraca asoca (Roxb.) Wilde on lysosomal enzymes and collagen metabolism in adjuvant induced arthritis. Inflammopharmacology. 2011;19:317–25. doi: 10.1007/s10787-011-0091-7. [DOI] [PubMed] [Google Scholar]
- 3.Cibin TR, Devi DG, Abraham A. Chemoprevention of two-stage skin cancer in vivo by Saraca asoca. Integr Cancer Ther. 2012;11:279–86. doi: 10.1177/1534735411413264. [DOI] [PubMed] [Google Scholar]
- 4.Kumar S, Narwal S, Kumar D, Singh G, Arya R. Evaluation of antihyperglycemic and antioxidant activities of Saraca asoca (Roxb.) de Wild leaves in streptozotocin induced diabetic mice. A Pas J Trop Dis. 2012;2:170–6. [Google Scholar]
- 5.Dabur R, Gupta A, Mandal TK, Singh DD, Bajpai V, Gurav AM, et al. Antimicrobial activity of some Indian medicinal plants. Afr J Tradit Complement Altern Med. 2007;4:313–8. doi: 10.4314/ajtcam.v4i3.31225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta R, Sharma AK, Dobhal MP, Sharma MC, Gupta RS. Antidiabetic and antioxidant potential of β-sitosterol in streptozotocin-induced experimental hyperglycemia. J Diabetes. 2011;3:29–37. doi: 10.1111/j.1753-0407.2010.00107.x. [DOI] [PubMed] [Google Scholar]
- 7.Ntanios FY, Jones PJ. Effects of variable dietary sitostanol concentrations on plasma lipid profile and phytosterol metabolism in hamsters. Biochim Biophys Acta. 1998;1390:237–44. doi: 10.1016/s0005-2760(97)00196-3. [DOI] [PubMed] [Google Scholar]
- 8.Maruthappan V, Shree KS. Hypolipidemic activity of haritaki (terminalia chebula) in atherogenic diet induced hyperlipidemic rats. J Adv Pharm Technol Res. 2010;1:229–35. [PMC free article] [PubMed] [Google Scholar]
- 9.Berges RR, Windeler J, Trampisch HJ, Senge T. Randomised, placebo-controlled, double-blind clinical trial of beta-sitosterol in patients with benign prostatic hyperplasia. Beta-sitosterol Study Group. Lancet. 1995;345:1529–32. doi: 10.1016/s0140-6736(95)91085-9. [DOI] [PubMed] [Google Scholar]
- 10.Ostlund RE., Jr Phytosterols in human nutrition. Annu Rev Nutr. 2002;22:533–49. doi: 10.1146/annurev.nutr.22.020702.075220. [DOI] [PubMed] [Google Scholar]
- 11.Lee MH, Lu K, Patel SB. Genetic basis of sitosterolemia. Curr Opin Lipidol. 2001;12:141–9. doi: 10.1097/00041433-200104000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mironova VN, Kalashnikova LA. Hypolipidemic action of beta-D-glycoside beta-sitosterol in the rat. Farmakol Toksikol. 1982;45:45–7. [PubMed] [Google Scholar]
- 13.Shirolkar A, Gahlaut A, Hooda V, Dabur R. Phytochemical composition changes in untreated stem juice of Tinospora cordifolia (W) Mier during refrigerated storage. J Pharma Res. 2013;7:1–6. [Google Scholar]
- 14.Gahlaut A, Taneja P, Shirolkar A, Nale A, Hooda V, Dabur R. Principal component and partial least square discriminant based analysis of methanol extracts of bark and regenerated bark of Saraca asoca. Int J Pharm Pharm Sci. 2012;4:331–5. [Google Scholar]
- 15.Shirolkar A, Gahlaut A, Chhillar AK, Dabur R. Quantitative analysis of catechins in Saraca asoca and correlation with antimicrobial activity. J Pharma Anal. 2013. [Last accessed on 2013 Jan 7]. Available from: http://www.dx.doi.org/10.1016/j.jpha . [DOI] [PMC free article] [PubMed]


