Abstract
Mixed dyslipidemia is characterized by increased low-density lipoprotein cholesterol (LDL-C) elevated triglycerides (TGs) and decrease high-density lipoprotein cholesterol (HDL-C). It is more common in diabetes and is associated with an increased risk of coronary artery disease. Monotherapy with statins or fibrates may not effectively control all lipid parameters. The atorvastatin-fenofibrate combination has been shown to have highly beneficial effect on lipid parameters in type 2 diabetes associated with combined hyperlipidemia (CHL). In an open-label study, we evaluated the efficacy of atorvastatin alone and in combination with fenofibrate in 60 types 2 diabetes mellitus patients associated with hyperlipidemia. Patients were randomly assigned to receive atorvastatin 10 mg (Group 1) or combination of atorvastatin 10 mg and fenofibrate 145 mg (Group 2) once daily for 12 weeks. The effect of drugs on lipid profile was evaluated before and after treatment. After 12 weeks, the reduction in total cholesterol (TC), TGs, LDL-C, VLDL-C was 28%, 20%, 37% and 20% in Group 1 (P < 0.001 for all) as compared with 31%, 39%, 33% and 40% in Group 2 (P < 0.001 for all). There was insignificant rise in HDL-C in Group 1 (P = 0.71) and insignificant decrease in HDL-C (P = 0.70) in Group 2. During the combination therapy, the decrease in TC, TGs and VLDL-C was greater than atorvastatin alone. The combination of atorvastatin with fenofibrate in type 2 diabetes patients with CHL may have a favorable effect on some major coronary artery disease risk factors.
Keywords: Atorvastatin, fenofibrate, hyperlipidemia
INTRODUCTION
Combined hyperlipidemia (CHL), a highly atherogenic lipid disorder characterized by increased low-density lipoprotein cholesterol (LDL-C), elevated triglycerides (TGs) and decreased high-density lipoprotein cholesterol (HDL-C) is common in patients with type 2 diabetes mellitus.[1] Moreover, metabolic abnormalities such as predominance of small dense LDL particles and increased glycation of LDL raise the atherogenic risk in these patients.[2,3] Glycemic control appears to improve, but does not normalize these abnormalities.[4]
Cardiovascular disease or coronary artery disease is the major cause of death in both men and women in the developed countries.[5] Atherosclerosis remains a major risk factor for cardiovascular disease.
Statins or fibrates can be used in this condition. Statins have been shown to reduce atherosclerosis related morbidity and mortality in patients with diabetes mellitus[6,7] and fibrates can decrease TG concentrations and elevate HDL-C, thus reducing the cardiovascular morbidity and mortality.[8,9]
Recent studies showed that statin or fibrate monotherapy can improve the lipid profile in patients with type 2 diabetes mellitus.[10,11] However, these affect different aspects of lipoprotein metabolism. Hence, it is difficult to modify the lipid profile of patients with type 2 diabetes mellitus using the monotherapy with either a statin or a fibrate according to the recent investigations of the American Diabetes Association.[12]
Hypolipidemic effect of atorvastatin is due to inhibition of hydroxymethylglutaryl-CoA reductase and decrease in LDL-C is also due to up regulation of LDL receptor activity.[13] Outcome trials of statins have proved conclusively that these classes of drugs decrease LDL-C levels, resulting in a significant reduction of cardiovascular events in many high-risk patients.[14,15] It has been shown that statins significantly reduced circulating levels of all major LDL subspecies; light, intermediate and dense.[16] Statins have been reported to produce “pleiotropic” effects such as vasodilatation, plaque stabilization, antioxidant, anti-inflammatory and antithrombotic effects.[17]
Fibrates, commonly referred to as peroxisome proliferator activated receptor-α (PPAR-α) agonists, are subfamily of the nuclear receptor superfamily that have been noted to be naturally activated by ligands such as free fatty acids and eicosanoids.[18,19,20] PPAR-α expression is present in liver, kidney, endothelium and vascular smooth muscle. They significantly decrease TG levels, LDL-C and increase HDL-C and hence help in reducing the cardiovascular events.[21] Fibrates decrease the production of TG-rich lipoproteins and increase the catabolism of TGs by the inducing lipoprotein lipase and also reduces expression of apolipoprotein C-III through activation of PPAR-α.
An effective therapeutic approach for CHL is a combination of statin and fibrate.[22,23,24,25,26] Studies comparing lipid lowering therapies have shown that atorvastatin reduces all LDL sub fractions, whereas fenofibrate reduces LDL density. This is due to a more pronounced reduction of the densest LDL fraction (LDL-6) by fenofibrate compared with atorvastatin. The literature on statin-fibrate combinations in patients with type 2 diabetes and CHL is limited. Hence, an open label, prospective, randomized, parallel study was undertaken to investigate the hypothesis that combination therapy with atorvastatin and fenofibrate will be more effective than monotherapy with a statin/fibrate to prevent coronary heart disease in patients with type 2 diabetes mellitus.
MATERIALS AND METHODS
Study Design
An open-labeled, randomized, prospective study was conducted from March 2009 to June 2010 in patients of type 2 diabetes mellitus associated with hyperlipidemia, attending the out-patient Department of Endocrinology, Sri Venkateswara Institute of Medical Sciences, Tirupati. The study was conducted by the Department of Pharmacology, Sri Venkateswara Medical College, Tirupati.
Patients of either gender in the age group of 30-70 years diagnosed as type 2 diabetes mellitus with hyperlipidemia (total cholesterol [TC] >200 mg/dl, TGs > 150 mg/dl, LDL-C >100 mg/dl, HDL-C < 40 mg/ dl) based on criteria laid down by National Cholesterol Education Program (NCEP Adult Treatment Panel III) guidelines were included in the study. Only newly diagnosed hyperlipidemic patients of diabetes mellitus were included in the study.
Patients on concurrent therapy with beta blockers, thiazides, oral contraceptives pills, and cyclosporine, erythromycin and azole antifungals were excluded. Patients having liver dysfunction (levels of transaminases 1.5 or more times the upper limit of normal, renal dysfunction (serum creatinine greater than 1.6 mg/dl) and pregnant and lactating women were also excluded from the study.
The Institutional Ethical Committee approved the study protocol and informed consent was obtained from all patients before enrollment after detailed explanation of possible adverse effects of the drug combinations.
Patients were on the National Cholesterol Expert Panel step 1 hypolipedemic diet, which limits dietary intake of cholesterol to 300 mg/day, saturated fats 10% of total energy intake and total fats to 30% total energy intake during the study. Anti-diabetic drugs used remained unchanged throughout the study.
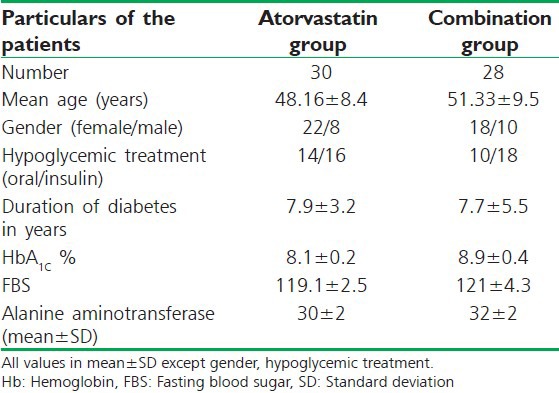
Patients who fulfilled the inclusion/exclusion criteria were randomly divided into two groups of 30 each by simple randomization using computer generated random numbers. All laboratory investigations were obtained after the patients had fasted for 12 h overnight and included the estimation of serum lipid parameters (TC, HDL-C, LDL-C,TGs and VLDL-C) as well as fasting blood sugar (FBS), glycated hemoglobin (HbA1c), Hb%, renal function tests and liver function tests were measured during screening in the same laboratory. Baseline characteristics of the study population are listed in Table 1.
Table 1.
Patient demographic and baseline characteristics
Treatment Schedule
Group 1 received atorvastatin 10 mg/day and Group 2 received combination of atorvastatin 10 mg/day and fenofibrate 145 mg/day for 12 weeks orally at night. All patients purchased the drugs from the hospital pharmacy as per prescription. Patients were assessed after 12 weeks and were asked to report immediately if they developed unusual muscle soreness or pain throughout the study. Lipid profile, FBS was done after 12 weeks. In case of history of muscle pain, creatine phospho kinase was also evaluated at the end of the study.
Serum cholesterol and TGs were determined using an enzymatic colorimetric assay and HDL-C was determined by phosphotungstic acid method. LDL-C was calculated using Friedewald's formula (LDL = TC - [TG/5 + HDL]).
Sample Size Calculation and Statistical Analysis
Sample size was calculated taking into consideration the mean values and standard deviation from the study done by Athyro et al.[27] Power of the study = 90%, α = 0.05 and β = 0.1. The data obtained were analyzed using descriptive statistics and paired and unpaired Student t-test to compare results within the group and between groups.
RESULTS
From the initial 30 patients in Group 2, one patient terminated the study for personal reasons and one patient interrupted the study because of mild muscle pain. Thus, 58 patients completed the study. They received atorvastatin (n = 30) and combination of atorvastatin and fenofibrate (n = 28) for a period of 3 months. These patients tolerated both study medications well and completed the study. No significant adverse events were recorded during the study.
Changes in the Lipid Profile
There was a significant reduction (P < 0.001) in TC (28%), TGs (20%), LDL-C (37%), VLDL-C (20%) and insignificant increase in HDL-C in atorvastatin group at the end of 3 months as compared to pretreatment values. There was a significant reduction (P < 0.001) in TC (31%), TGs (39%), LDL-C (33%), VLDL-C (40%) and insignificant decrease in HDL-C in combination treatment group at the end of 3 months as compared with pretreatment values as represented in Table 2.
Table 2.
Comparison of changes in the lipid profile between the two treatment groups after 12 weeks
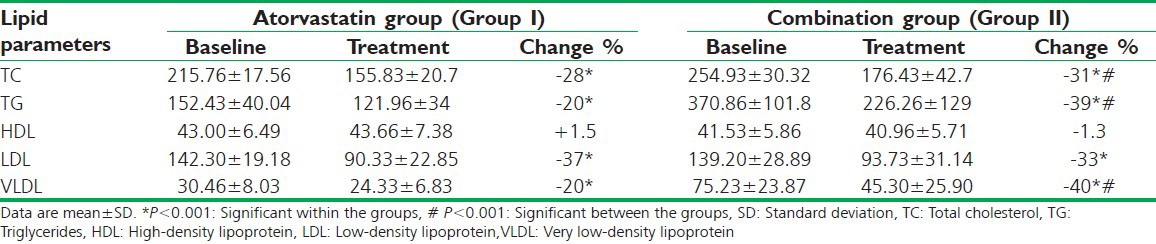
The reduction in TC, TGs, VLDL-C in combination treatment group was statistically significant (P < 0.001) when compared to atorvastatin group alone. The observed difference in a decrease in LDL-C and change in HDL-C was not statistically significant between the two treatment groups [Table 2].
DISCUSSION
Type 2 diabetes mellitus is one of the most common chronic disease and is associated with co-morbidities such as obesity, hypertension, hyperlipidemia and cardiovascular disease, which together, constitute metabolic syndrome.[28]
There is evidence to suggest that diabetes is more common in females than males. In recent years an increase in number of diabetic males resulted in an equal prevalence rates for both males and females in some societies.[29]
In our study, we have analyzed the effect of atorvastatin 10 mg once daily and combination of atorvastatin 10 mg and fenofibrate 145 mg once daily in type 2 diabetes mellitus patients associated with hyperlipidemia. Patients were in the age group of 30-70 years in both groups. Both treatments had decreased TC, TG, LDL-C, VLDL-C, but the reduction of TC, TG, VLDL-C was more and statistically significant in combination treatment group when compared with atorvastatin group alone at the end of 12 weeks. The observed difference in decrease in LDL-C and change in HDL-C was not statistically significant between the two treatment groups.
A similar study, which compared the effect of atorvastatin (20 mg/day) alone with micronized fenofibrate (200 mg/day) monotherapy and in combination with fenofibrate in type 2 diabetes mellitus patients with CHL where in the patients were in the age group of 44-69 years.[27] The combination treatment had reduced TC by 37%, LDL-C by 46%, TGs by 50% and increased HDL-C by 46% (P < 0.0001 for all) and these changes were significantly better than those of monotherapy. No significant adverse events were recorded during the study.
In another study, combination of simvastatin (20 mg/day)and bezafi brate (400 mg/day) had reduced TC by 23% and LDL-C by 29% but had significantly reduced TG levels by 42% and increased HDL-C by 25%. Of the 148 patients from this study, 2 had presented with myopathy.[30]
In Action to Control Cardiovascular Risk in Diabetes lipid trial it was found that combination therapy with the use of fenofibrate and simvastatin (at a daily dose of 40 mg or less) did not reduce rates of cardiovascular disease as compared with simvastatin alone.[31]
The primary risk of using statins in combination with fibrates is believed to be hepatotoxicity and myopathy.[25] In most studies combination therapy was no more hepatotoxic than the statin itself.[32] However, due to limited number of patients and the short term follow-up, we cannot draw any definite conclusions. Hence, further studies in a large number of patients and for longer duration are necessary to assess the long-term safety of this combination.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.Farnier M, Picard S. Diabetes: Statins, fibrates, or both? Curr Atheroscler Rep. 2001;3:19–28. doi: 10.1007/s11883-001-0006-y. [DOI] [PubMed] [Google Scholar]
- 2.Packard C, Caslake M, Shepherd J. The role of small, dense low density lipoprotein (LDL): A new look. Int J Cardiol. 2000;74(Suppl 1):S17–22. doi: 10.1016/s0167-5273(99)00107-2. [DOI] [PubMed] [Google Scholar]
- 3.Bucala R, Makita Z, Vega G, Grundy S, Koschinsky T, Cerami A, et al. Modification of low density lipoprotein by advanced glycation end products contributes to the dyslipidemia of diabetes and renal insufficiency. Proc Natl Acad Sci U S A. 1994;91:9441–5. doi: 10.1073/pnas.91.20.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stern MP, Mitchell BD, Haffner SM, Hazuda HP. Does glycemic control of type II diabetes suffice to control diabetic dyslipidemia? A community perspective. Diabetes Care. 1992;15:638–44. doi: 10.2337/diacare.15.5.638. [DOI] [PubMed] [Google Scholar]
- 5.Pyŏrälä K, Pedersen TR, Kjekshus J, Faergeman O, Olsson AG, Thorgeirsson G. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian simvastatin survival study (4S) Diabetes Care. 1997;20:614–20. doi: 10.2337/diacare.20.4.614. [DOI] [PubMed] [Google Scholar]
- 6.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and recurrent events trial investigators. N Engl J Med. 1996;335:1001–9. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 7.Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease: The bezafibrate infarction prevention (BIP) study. Circulation. 2000;102:21–7. doi: 10.1161/01.cir.102.1.21. [DOI] [PubMed] [Google Scholar]
- 8.Robins SJ, Collins D, Wittes JT, Papademetriou V, Deedwania PC, Schaefer EJ, et al. Relation of gemfibrozil treatment and lipid levels with major coronary events: VA-HIT: A randomized controlled trial. JAMA. 2001;285:1585–91. doi: 10.1001/jama.285.12.1585. [DOI] [PubMed] [Google Scholar]
- 9.Frost RJ, Otto C, Geiss HC, Schwandt P, Parhofer KG. Effects of atorvastatin versus fenofibrate on lipoprotein profiles, low-density lipoprotein subfraction distribution, and hemorheologic parameters in type 2 diabetes mellitus with mixed hyperlipoproteinemia. Am J Cardiol. 2001;87:44–8. doi: 10.1016/s0002-9149(00)01270-4. [DOI] [PubMed] [Google Scholar]
- 10.Rustemeijer C, Schouten JA, Voerman HJ, Hensgens HE, Donker AJ, Heine RJ. Pravastatin compared to bezafibrate in the treatment of dyslipidemia in insulin-treated patients with type 2 diabetes mellitus. Diabetes Metab Res Rev. 2000;16:82–7. doi: 10.1002/(sici)1520-7560(200003/04)16:2<82::aid-dmrr89>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 11.Haffner SM. Management of dyslipidemia in adults with diabetes. Diabetes Care. 2003;26:83–6. doi: 10.2337/diacare.26.2007.s83. [DOI] [PubMed] [Google Scholar]
- 12.Haffner SM. Management of dyslipidemia in adults with diabetes. Diabetes Care. 2002;25:74–7. doi: 10.2337/diacare.21.1.160. [DOI] [PubMed] [Google Scholar]
- 13.Ellen RL, McPherson R. Long-term efficacy and safety of fenofibrate and a statin in the treatment of combined hyperlipidemia. Am J Cardiol. 1998;81:60B–5. doi: 10.1016/s0002-9149(98)00040-x. [DOI] [PubMed] [Google Scholar]
- 14.Bakker-Arkema RG, Davidson MH, Goldstein RJ, Davignon J, Isaacsohn JL, Weiss SR, et al. Efficacy and safety of a new HMG-CoA reductase inhibitor, atorvastatin, in patients with hypertriglyceridemia. JAMA. 1996;275:128–33. [PubMed] [Google Scholar]
- 15.Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 16.LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–35. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
- 17.Guerin M, Lassel TS, Le Goff W, Farnier M, Chapman MJ. Action of atorvastatin in combined hyperlipidemia: Preferential reduction of cholesteryl ester transfer from HDL to VLDL1 particles. Arterioscler Thromb Vasc Biol. 2000;20:189–97. doi: 10.1161/01.atv.20.1.189. [DOI] [PubMed] [Google Scholar]
- 18.Goldfine AB, Kaul S, Hiatt WR. Fibrates in the treatment of dyslipidemias – Time for a reassessment. N Engl J Med. 2011;365:481–4. doi: 10.1056/NEJMp1106688. [DOI] [PubMed] [Google Scholar]
- 19.Stolarczyk M, Gutman W, Derlacz RA. Nuclear receptors PPAR as a drug target in metabolic disorders. Postepy Biochem. 2011;57:207–14. [PubMed] [Google Scholar]
- 20.Tyagi S, Gupta P, Saini AS, Kaushal C, Sharma S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J Adv Pharm Technol Res. 2011;2:236–40. doi: 10.4103/2231-4040.90879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao JK. Effects of statins on 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition beyond low-density lipoprotein cholesterol. Am J Cardiol. 2005;96:24F–33. doi: 10.1016/j.amjcard.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans affairs high-density lipoprotein cholesterol intervention trial study group. N Engl J Med. 1999;341:410–8. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 23.Athyros VG, Papageorgiou AA, Hatzikonstandinou HA, Didangelos TP, Carina MV, Kranitsas DF, et al. Safety and efficacy of long-term statin-fibrate combinations in patients with refractory familial combined hyperlipidemia. Am J Cardiol. 1997;80:608–13. doi: 10.1016/s0002-9149(97)00430-x. [DOI] [PubMed] [Google Scholar]
- 24.Kiortisis DN, Millionis H, Bairaktari E, Elisaf MS. Efficacy of combination of atorvastatin and micronised fenofibrate in the treatment of severe mixed hyperlipidemia. Eur J Clin Pharmacol. 2000;56:631–5. doi: 10.1007/s002280000213. [DOI] [PubMed] [Google Scholar]
- 25.Pierce LR, Wysowski DK, Gross TP. Myopathy and rhabdomyolysis associated with lovastatin-gemfibrozil combination therapy. JAMA. 1990;264:71–5. [PubMed] [Google Scholar]
- 26.Davidson MH, Dembowski E. Statin-fibrate combination therapy for the treatment of dyslipidemia. Eur Cardiol. 2008;4:10–1. [Google Scholar]
- 27.Athyros VG, Papageorgiou AA, Athyrou VV, Demitriadis DS, Kontopoulos AG. Atorvastatin and micronized fenofibrate alone and in combination in type 2 diabetes with combined hyperlipidemia. Diabetes Care. 2002;25:1198–202. doi: 10.2337/diacare.25.7.1198. [DOI] [PubMed] [Google Scholar]
- 28.Mokdad AH, Ford ES, Bowman BA, Nelson DE, Engelgau MM, Vinicor F, et al. Diabetes trends in the U.S.: 1990-1998. Diabetes Care. 2000;23:1278–83. doi: 10.2337/diacare.23.9.1278. [DOI] [PubMed] [Google Scholar]
- 29.Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The third national health and nutrition examination survey, 1988-1994. Diabetes Care. 1998;21:518–24. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 30.Gavish D, Leibovitz E, Shapira I, Rubinstein A. Bezafibrate and simvastatin combination therapy for diabetic dyslipidaemia: Efficacy and safety. J Intern Med. 2000;247:563–9. doi: 10.1046/j.1365-2796.2000.00646.x. [DOI] [PubMed] [Google Scholar]
- 31.ACCORD Study Group. Ginsberg HN, Elam MB, Lovato LC, Crouse JR, 3rd, Leiter LA, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–74. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murdock DK, Murdock AK, Murdock RW, Olson KJ, Frane AM, Kersten ME, et al. Long-term safety and efficacy of combination gemfibrozil and HMG-CoA reductase inhibitors for the treatment of mixed lipid disorders. Am Heart J. 1999;138:151–5. doi: 10.1016/s0002-8703(99)70261-9. [DOI] [PubMed] [Google Scholar]


