Abstract
Background and Study Aims:
Our aim was to assess the efficacy and tolerability of drug-eluting beads-transarterial chemoembolization (DEB-TACE) in the treatment of hepatocellular carcinoma (HCC), evaluating the response to the treatment after 1, 6, 12, and 24 months with multidetector computed tomography (MDCT) comparing European Association for the study of the Liver (EASL) and modified Response Evaluation Criteria in Solid Tumors (mRECIST) criteria.
Materials and Methods:
We enrolled 154 patients with uni- or multifocal HCC who underwent a DEB-TACE. A total of 278 HCC nodules were treated. CT follow-up was performed at 1, 6, 12, and 24 months after the procedure according to the EASL and RECIST criteria evaluating overall target and target nodule response. We also analyzed the shrinking of nodules in relation to response to treatment.
Results:
A total of 278 nodules of HCC underwent TACE by using DC-Beads: At 24, months complete response was similar for EASL and RECIST criteria (112 vs. 121 nodules) with optimal accordance between methods and readers with k = 0.9. Partial Response resulted significantly different among the two methods within the first month, otherwise was similar after 24-month follow-up. Similar results in both methods were found for nodules classified as Stable Disease (P > 0.05). Progressive Disease results were similar in both the groups according to both the classification criteria without any significant difference (P > 0.05).
Conclusion:
Our study confirmed that EASL and mRECIST criteria are both effective methods for patient follow-up, however with some technical differences.
Keywords: Chemoembolization, computed tomography, hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is the most frequent primitive liver neoplasm. Its annual incidence is 3-7 cases in 100,000 in North and South America, northern and central Europe, and Australia. However, the highest rate is in Taiwan, and in southern China, where its incidence can go up to 150/100,000. Among cirrhotic patients, the risk of HCC ranges from 1,500 to 7,800 cases every 100,000 patients a year.[1,2]
At present, drug-eluting beads-transarterial chemoembolization (DEB-TACE) represents a precious resource of treatment for patients with HCC at intermediate stage.[2] The advantage of DEB-TACE is the possibility to deliver higher doses of chemotherapy carried by microspheres within the tumor nodule, determining a significant reduction in liver toxicity and drug-related adverse events as compared to conventional TACE (cTACE).[2,3]
Survival is considered the straightforward endpoint to assess treatment efficacy, nevertheless radiological responses have been widely used as surrogate endpoints in phase II trials.[4,5]
Radiological response was traditionally evaluated by Response Evaluation Criteria in Solid Tumors (RECIST) criteria which considered the tumor size shrinking (sum of unidimensional measurements) as the sole positive response criteria.
In 2000, European Association for the Study of Liver (EASL) recommended measuring change in the area of tumor enhancement on contrast-enhanced imaging as the optimal method to assess treatment response.[6] More recently the American Association for the Study of Liver Diseases (AASLD) has proposed a formal amendment of the RECIST criteria to take into consideration changes in the degree of tumor arterial enhancement - the modified RECIST criteria (mRECIST).[7]
Nowadays, success rate of improvement of patients’ survival and radiological follow-up of DEB-TACE represent highly debatable issues. The aim of this study was to evaluate the response to the TACE treatment after 1, 6, 12, and 24 months comparing EASL criteria and the most recent modified RECIST (mRECIST) criteria. Secondary endpoint was to assess the efficacy and tolerability of DEB-TACE for the treatment of hepatocellular carcinoma (HCC).
Materials and Methods
Criteria of admission to the treatment
Patients included in the study had the following features according to the Barcelona Clinic Liver Cancer (BCLC) classification:[8]
Patients with early stage HCC (stage A) who were not candidates for resection, transplantation, thermal ablation, or had failed/bad response to the mentioned interventions
Patients with HCC at intermediate stage (stage B).
Clinical characteristics of the treated population were as follows:
Chronic hepatic disease either with Child-Pugh B or A patients who are not suitable for other surgical or interventional procedures
Single nodules >5 cm, multiple nodules (two or three with maximum diameter >3 cm) or more than three nodules.
Contraindication to the treatment
Absolute contraindications were: Class C of Child-Pugh, complete portal thrombosis, severe liver encephalopathy (class III-IV), portal-systemic shunts (TIPS), portal hepatofugal flow, total bilirubin >5 mg/dl, extra-liver localizations, renal failure, leuko-deficiency, <3.000/mm3
Relative contraindications: Partial portal thrombosis, mild hepatic encephalopathy, refractory ascites, platelets deficiency (<50.000 mm3), prothrombin activity <50%, cardiac ejection fraction <40%.
Radiologic pretreatment assessment
Prior to the treatment, all patients underwent ultrasound examination (US), multidetector computed tomography (MDCT), and in selected cases magnetic resonance imaging (MRI). Imaging exam was performed no later than 1 month before the chemoembolization.
Radiological assessment of response to the treatment
Treatment response was blindly assessed by two experienced radiologists in liver imaging, each applying either EASL or mRECIST criteria. EASL criteria defined viable tumor according to the uptake of contrast in the arterial phase of dynamic CT or MRI and are based on the product of bidimensional diameters of the enhancing area of measurable lesions. Otherwise, mRECIST assessment is based on the sum of unidimensional measurements of arterially enhancing lesions.
For each of the two radiological methods, responses were recorded according to both target lesion response and overall response. Target responses for mRECIST are defined as those that assess up to two measurable lesions in the liver whereas EASL target response refers to all measurable arterially-enhancing lesions in the liver. Target responses do not take into consideration changes in nontarget lesions, the appearance of new lesions and any change in extrahepatic sites. By contrast, overall responses assess up to two measurable lesions in the liver (all enhancing lesions for EASL) but also take into consideration nontarget lesions as well as the appearance of new lesions and extrahepatic disease sites.
Moreover, we evaluated the changes over time only of target nodules treated with TACE in order to analyze the differences of measurement parameters between EASL and mRECIST criteria; we defined such changes as “target nodule response” to distinguish it from the target response that refers to patients.
At 1, 6, 12, and 24 months after the treatment with DEB-TACE, all patients underwent CT and the images were compared with all the previous ones. Our follow-up used the following parameters to evaluate the response to the TACE treatment:
Number of tumor nodules treated,
Distribution and extension of the necrotic area,
Vascularization of the residual tumor, and
Shrinking of the lesion dimensions.
Necrosis
Necrosis was radiologically defined as an area of very low X-ray beam attenuation. Necrotic areas do not show postcontrast enhancement in arterial phase nor in portal and late phase; unlike what happens in nearby normal hepatic tissue.
The density, expressed in Hounsfield unit (HU), was measured at CT exam on all the lesions, both before and after treatment. Review of images was performed in precontrast, arterial, portal, and late phases.
The absence of significant differences of density between the phases was defined as presence of coagulative necrosis and therefore as factor of positive response to treatment.
Size change
This finding was assessed measuring the major perpendicular diameters of treated areas found in checks at 1, 6, 12, and 24 months and comparing them with their previous values in order to determine the variation. A reduction of those diameters was considered a factor of positive response to the treatment; otherwise, the entity of response to the treatment was rated according to the size of such residual tissue. The following formula was used to calculate the percentage variation of size in the treated areas.
X = 100 × [(A × B) - (A′ × B′)]/(A × B)
Where, A × B is the product of perpendicular diameters of the nodule prior to the treatment and A′ × B′ is the product of the diameters of the nodule after the treatment.
Definition of the response to the treatment
All criteria embraced the following four response categories: Complete Response (CR), Partial Response (PR), Stable Disease (SD), and Progressive Disease (PD). Objective response included both CR and PR. The four response categories according either to EASL or mRECIST criteria were defined as follows:
Group CR: Complete response. Broad necrotic area and absence of vital tumor tissue at CT scan
Group PR-EASL: At least a 50% decrease in the sum of the product of bidimensional diameters of viable (enhancement in the arterial phase) target lesions, taking as reference the baseline sum of the diameters of target lesions
Group PR-mRECIST: At least a 30% decrease in the sum of unidimensional diameters of viable (enhancement in the arterial phase) target lesions, taking as reference the baseline sum of the diameters of target lesions
Group SD: Stable disease. Any cases that do not qualify for either partial response or progressive disease
Group PD: Progressive disease. EASL: An increase of at least 25% in the sum of the diameters of viable (enhancing) target lesions, taking as reference the smallest sum of the diameters of viable (enhancing) target lesions recorded since treatment started
mRECIST: An increase of at least 20% in the sum of the diameters of viable (enhancing) target lesions, taking as reference the smallest sum of the diameters of viable (enhancing) target lesions recorded since treatment started.
Finally, we correlated the size of the lesions with the criteria of response, to check if there may be a positive relationship.
DEB-TACE technique
Among the 334 nodules found, 278 were treated by DEB-TACE. The selective angiography either of the celiac trunk or the superior mesenteric artery was performed with a transfemoral approach under local anesthesia with a 4 Fr sheath and 4 Fr SIM1 catheter.
After the preliminary angiographic panoramic exam aimed to reveal possible anatomic variations, the catheter was placed in the hepatic artery in order to perform a detailed study of the liver vascularity, thereby to exactly define the location of the tumor and its feeding arteries. Two types of embolizing microspheres (DC-Beads®, C, C, Biocompatibles, UK Ltd.) were deployed in each session: 100-300 μm and 300-500 μm in size.
All patients were treated with an average of 70 ± 20 mg of doxorubicin. In case of extended or multifocal lobar HCC, DEB-TACE was performed by injection in the lobar hepatic artery, using the same diagnostic catheter. In case of single lesion or in case of multiple lesions, a superselective catheterization of the arterial feeders was preferred and performed using a microcatheter (2.7 Fr Progreat®, Terumo, Japan) inserted in the diagnostic catheter with coaxial technique.
Criteria of successful embolization were the occlusion of the arterial feeder and lack of enhancement of the nodule.
Statistics
All analyses were carried out using SPSS for Windows (SPSS, Chicago, IL, USA) and Excel for Mac 2008. Data analysis was made either per patient or per nodule when considered appropriate. Continuous data are presented as the mean ± standard deviation (SD) or, if adequate, as the median and range. Survival rates and curves were determined using the Kaplan-Meier method, and compared using the log-rank test. A P value less than 0.05 was considered significant. Interreader reliability for each category of response was calculated by k-statistics.
Results
Patients’ population
Beginning March 2008, 154 patients (98 males and 56 females of age 47-83, average 65 ± 7.8) with uni- or multifocal HCC, of which 60 in Child-Pugh class A and 94 in class B, underwent a single DEB-TACE procedure.
Out of 154 patients, 72 revealed multifocal HCC with more than 3 nodules, all less than 3 cm of diameter, 38 patients had uni- or multifocal HCC, with at least one of the diameters larger than 5 cm, 36 patients had two or three lesions with at least one above 3 cm.
The remaining patients showed under radiological exam unifocal HCC below 5 cm (three patients), or other two or three lesions all less than 3 cm (five patients) for which treatment was not possible to perform with thermal ablation due to the risk of damages to surrounding organs by radiofrequencies. Overall HCC nodules were encountered in the amount of 334, of which 278 were treated with DC-Bead injection. The average diameter of those was 27 mm (with lesion ranging from 8-106 mm).
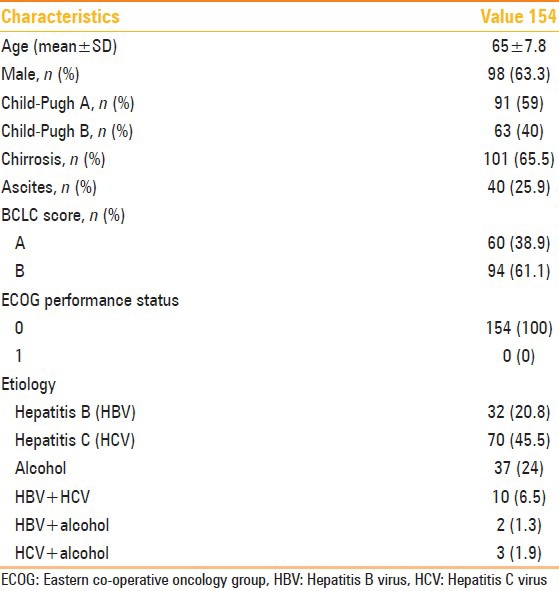
The patients had serum levels of total bilirubin averaging 1.02 ± 0.5 mg/dl and α-fetoprotein (VN < 10 ng/ml) lower than 10 ng/ml in two patients between 20 and 200 ng/ml in 16 patients and over 200 ng/ml in 10 patients. Main features of the patients are reported in Table 1.
Table 1.
Main clinical features of the patient population
Assessment of response to the treatment
A total of 278 nodules of HCC were treated with DEB-TACE.
Overall response at first month resulted similar in EASL and mRECIST criteria: 65 patients (42.2%) and 135 nodules (48.5%) showed a complete response in EASL criteria and 66 (42.8%) patients and 141 nodules (50.7%) according to mRECIST criteria (P = 0.13).
For Partial Response, according to EASL criteria we found: 48 patients (31.1%), 76 nodules (27.3%) were classified as group PR.
Otherwise, according to mRECIST criteria we classified as group PR: 46 patients (29.8%), 72 nodules (25.8%), P = 0.045; 26 patients (16.8%), 42 nodules (15.1%) were classified in group SD for EASL criteria; 27 patients (17.5%), 40 nodules (14.3%) according to mRECIST criteria; and 15 patients (9.7%), 25 nodules (8,9%) were classified in the PD group according to both the systems.
Since mRECIST takes only into account the long axis measurement of enhancing tumor whilst EASL looks at the product of the two diameters of enhancing tumor, some differences were encountered: For overall response one patient classified as having a partial response by EASL criteria was classified as stable disease by mRECIST as well as another patient considered partial response fell in the complete response group (CR). Behavior of overall response is represented in Figure 1.
Figure 1.
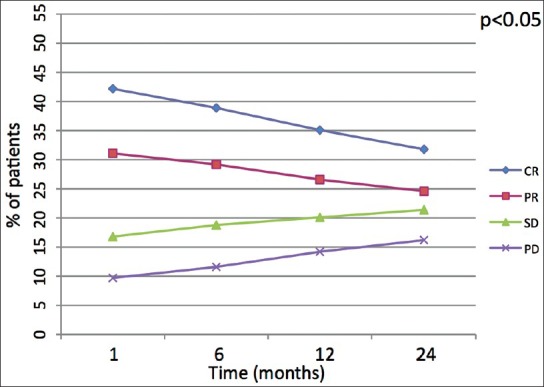
Linear diagram shows behavior of overall response among the four groups of response (CR: Complete response, PR: Partial response, SD: Stable disease, PD: Progressive disease) over the time. The P value indicates if the shift of values after 24 months is either statistically signifi cant (<0.05) or not (>0.05)
Target response analysis showed five patients in whom the results differed at 1 month follow-up. Three patients resulted as complete “target” responders by mRECIST but only partial “target” responders by EASL. Also, two patients were classified as partial “target” response by EASL criteria and as stable “target” disease by mRECIST. Behavior of target response over the time has been resumed in Table 2 after 1 month follow-up and in Figure 2 during the entire follow-up period. Comparisons between mRECIST and EASL criteria according to overall, target response and target nodule response are showed in Tables 2, 3, and 4, respectively.
Table 2.
Overall response at 1 month CT follow-up (number of patients)
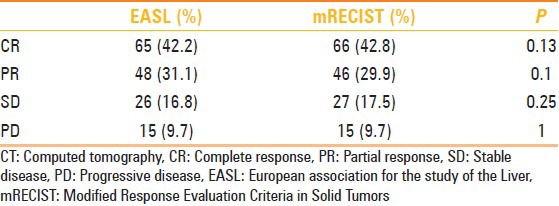
Figure 2.
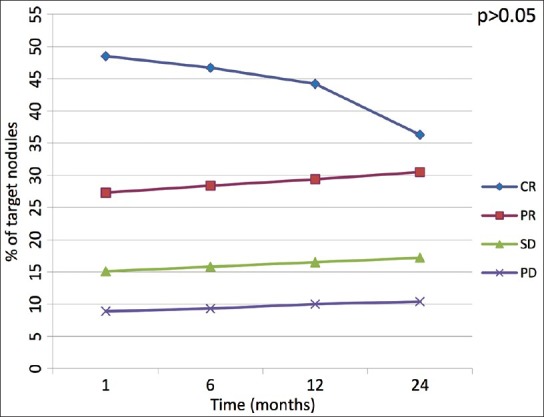
Linear diagram shows behavior of target lesion response among the four groups of response (CR: Complete response, PR: Partial response, SD: Stable disease, PD: Progressive disease) over the time. The P value indicates if the shift of values after 24 months is either statistically signifi cant (<0.05) or not (>0.05)
Table 3.
Target lesion response 1 month after TACE (number of patients)
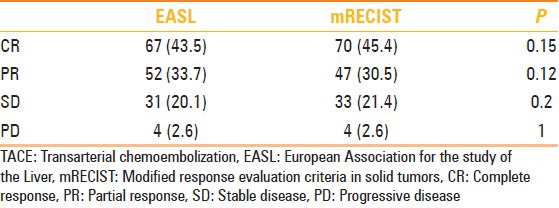
Table 4.
Target nodule response at 1 month follow-up (number of nodules)
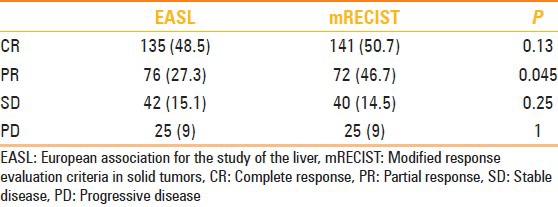
According to EASL criteria after 24 month follow-up, we noted significant decrease of nodules which presented initially CR 135 to 101 nodules, (P = 0.01). Also for mRECIST criteria, we found a consistent decrease of nodules with CR over time from 141 to 101 nodules (P = 0.01).
For PR, no statistical difference was noted during follow-up for nodules evaluated with EASL criteria (from 76 to 86 nodules at 24 months; P = 0.45) as well as for nodules evaluated with mRECIST criteria (from 72 nodules PR to 84 at 24 months; P = 0.56). However, when comparing EASL with mRECIST at 1 month we noted significant difference in evaluation of PR (EASL n = 72 vs. 76 nodules for mRECIST; P = 0.045) with a good accordance between readers (k = 0.8). Otherwise at 24 months, we observed nonstatistical difference between the two criteria (P > 0.05) with optimal accordance with k = 0.91.
According to EASL criteria, we evaluated 42 nodules as SD at 1 month which reached 53 after 24 months (P = 0.49); according to mRECIST criteria 40 nodules were classified as SD at 1 month, then they have been 53 at 24 months (P = 0.35). Increase of nodules classified as PD in both groups (from 25 nodules at 1 month to 27 nodules at 24 months) was found without any significant difference between both classification criteria (>0.05). Changes of target nodules over time are reported in Figure 3.
Figure 3.
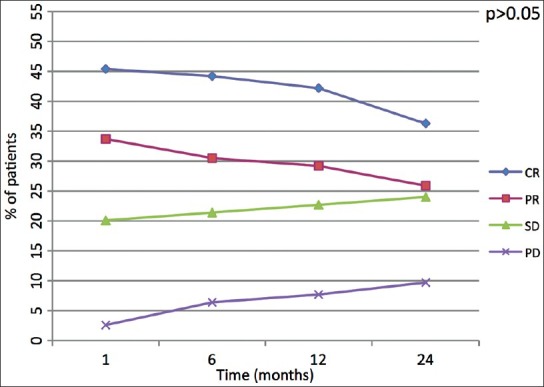
Linear diagram shows behavior of target lesion response among the four groups of response (CR: Complete response, PR: Partial response, SD: Stable disease, PD: Progressive disease) over the time. The P value indicates if the shift of values after 24 months is either statistically signifi cant (<0.05) or not (>0.05)
Analysis of changes of dimensions of nodules over time showed mean reduction in responders of about 32 and 24% in nonresponders (P > 0.05). However, changing of size of nodules did not correlate with a better response to treatment as we did not find a significant difference between responders and nonresponders to DEB-TACE.
Overall survival and association with survival
After a maximum follow-up of 24 months (24 ± 1), 123 patients were alive, 31 patients had died, and two had received transplantation. The 1, 6, 12, and 24 month survival of the whole cohort was 98.3, 92.6, 84.5, and 74.8%; with a mean overall survival (OS) of 19.7 months (95% CI: 12.4-23.2) [Figure 1]. The 1, 6, 12, and 24-month survival for Child-Pugh A patients was 98.9, 95.6, 91.2, and 85.7%; with a mean OS of 20.2 months (95% CI: 14.3-24.1), while for Child-Pugh B patients it was 98.4, 90.4, 82.5, and 71.4%, with a mean OS of 17.7 months (95% CI: 10.1-19.2) [Figures 4A and B].
Figure 4 (A, B).
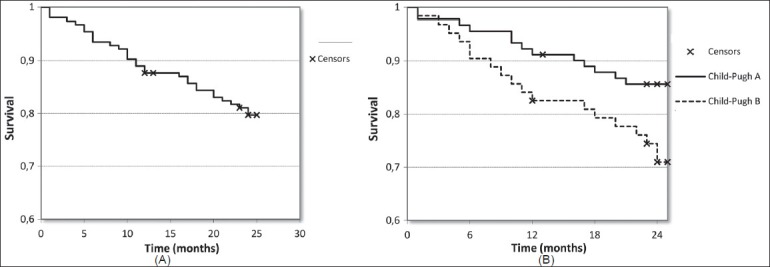
Kaplan-Meier curves show overall survival (A) and survival rates among Child-Pugh A and B patients (B) over the time (P = 0.029)
Kaplan-Meier methods were used to calculate the median survival times and 1-year survival probabilities for the responders and nonresponders in both criteria: The 1-year survival for EASL responders was 85% (95% CI: 67-92%) and nonresponders 64% (95% CI: 45-78%). mRECIST was very similar with 83% (95% CI: 66-92%) of responders and 62% (95% CI: 47-79%) of nonresponders surviving to 1-year.
Complications
Following the treatment, minor complications were found (postembolization syndrome) in 31 patients (20%) without extending the hospitalization that lasted an average of 2 days. Twelve patients (7.8%) required acetaminophen or tramadole due to abdominal pain right after the procedure. Eight patients (5%) had moderate fever (<38°C) and six patients (3.9%) vomited and nausea. None of the patients had halopecia, bone marrow toxicity, cardiac toxicity, dyspnea, or lung embolism.
Furthermore, after the chemoembolization, we observed variations in levels of total bilirubin in all the patients: A slight increase during the first 3 days (average 2.3 ± 0.2 mg/dl), followed by a return to basal value of preembolization in about 7-10 days.
Lastly, the levels of albumin and prothrombin activity showed a temporary reduction (average albumin 32.2 ± 1.2; prothrombin activity 68 ± 3%), with a full restore of basal value in about 4 weeks (average albumin 40.8 ± 4.0; prothrombin activity 84 ± 2%).
An event of spleen embolization occurred and was probably related to the presence of a hepatogastric trunk; another case involved the embolization of a nontargeted hepatic area. However, in both cases no symptoms related to the complication were observed.
Discussion
TACE represents an effective therapeutic alternative in treating HCC patients at intermediate and early stage where surgical resection and percutaneous ablation are not an option.[2,8,9,10,11,12,13] The extension of patients’ life is strictly related to the objective response to the treatment which is assessed according to the extension of the necrotic area after the chemoembolization.[6,7,10,14]
Main disadvantage of cTACE for CT follow-up is the use of hyperdense iodized oil as drug carrier usually hints a correct imaging interpretation to detect recurrence of disease. Hence, the use of embolizing microspheres as drug carrier instead of iodized oil allowed to discern the hypervascular part of the treated lesion from the hypodense area of necrosis [Figure 5].[3,15,16,17,18,19]
Figure 5 (A-H).
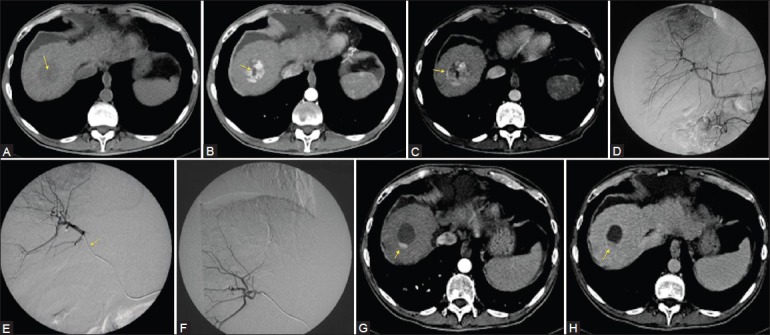
Fifty-year-old male with chronic HBV-related infection. A computed tomography (CT) exam shows the presence of a solid nodule in the VIII segment with a central necrotic area in precontrast phase (arrow) (A). After contrast injection, arterial phase (B) show enhancement of the solid part of the nodule, and confi rmed the central necrotic area (arrow). In venous phase, wash-out with a pseudocapsule formation typical of an HCC nodule (C, arrow) are noted. Angiographic exam confi rmed a correspondent hypervascular area within the VIII segment (D). After superselective catheterization, the nodule with a microcatheter (E, arrow) has been embolized with 4 ml of DC-Beads (dimensions of 100-300 μm and 300-500 μm loaded with 25 mg/ml of doxorubicin cloridrate) (F). After 1 month of follow-up, CT showed residual hypervascular area considered as pathologic finding due to the enhancement in arterial phase (G, arrow) and wash-out in late phase (H, arrow) as compared with the original CT-exam, reduction of 75% of the hypervascular area was calculated: The patient was considered in Partial Response (PR) for both EASL and mRECIST criteria
In regard to this, recent posttreatment assessment criteria have been developed by EASL[6] and mRECIST[7] which can take into account the hypervascular part of the lesion and not only the size of the lesion as used to consider by the previous RECIST 1.1 criteria. Current studies addressed the comparison between mRECIST and EASL criteria founding almost identical results in overall and target response after TACE.[20,21]
We compared the two follow-up criteria after DEB-TACE considering overall response and target nodule response over the time and also analyzing the interreader agreement. When considering overall response, no significant difference was found between the two follow-up methods both in responders and non-responders. Both methods resulted highly comparable in assessment of overall response of patients treated with DEB-TACE as similarly reported in other recent studies.[20,21] Despite minor differences, target response resulted almost identical among the two response criteria, in fact not statistical difference was found among all the response classes of EASL and mRECIST after 1 month [Table 3] and alongside the follow-up period [Figure 2].
Differences in target response can be explained by the fact that EASL takes into consideration all measurable arterial-enhancing nodules whereas mRECIST only considered up to two target lesions. Thus, persistent vascularization in any of the tumors outside the mRECIST range would have been recorded as a partial response by EASL. Same matter can occur when a patient has a partial response for EASL, but can fall within the stable disease class according to mRECIST criteria.
When considering target nodule analysis, we noted that at 1 month, six nodules classified as complete response for EASL criteria were not included in partial response by mRECIST criteria. At 24 months such interpretation was confirmed with slight difference among the two criteria. Since the definition of CR is identical in both criteria, such different interpretation in few cases may be related to the judgment of the hypervascular area around the lesion as disease recurrence instead of blood flow alteration; nevertheless, this difference was not statistically significant with an optimal interobserver agreement (k = 0.9).
When we analyze the PR at 1 month, we observed a significant difference between number of nodules therein considered (EASL n = 72 vs. 76 nodules for mRECIST; P = 0.045) with a good accordance between readers (k = 0.8). Otherwise at 24 months, we observed nonstatistical difference between the two criteria (P > 0.05) and optimal accordance with k = 0.91. Such behavior is probably related to the different criteria and measurement systems established by EASL and mRECIST to define partial response. In particular, both criteria lie on measurement of the enhancing area of measurable lesions; however, we noted that a smaller and more irregular enhancing area is measurable, as the accuracy in measurement decreases. Therefore at 24 months, we registered an improvement of accordance between the two criteria and the observers due to the change in dimensions and morphology of the enhancing areas. Observing this behavior, a weakness of both criteria is actually to measure the enhancing area and from that obtain the percentage of response to treatment. Stable disease (SD) is defined as follows in both criteria: Any cases that do not qualify for either partial response or progressive disease; therefore it can be considered a diagnosis by exclusion, so no significant differences were observed among the two criteria (overall and target response results) neither between readers (k = 0.95).
Similarly, in case of nodules or patients classified as PD, we encountered nonsignificant differences between methods with optimal interobserver agreement (k = 0.88) over the time. Overall survival values and low mortality and complication rates in our study resulted in line with other recent studies[22,23] thus advocating DEB-TACE as a safe and effective treatment of patients with HCC in intermediate and early clinical stage.
Statistical difference was reached when comparing mortality between Child-Pugh A of 14.2% vs. Child-Pugh B patients mortality of 28.5% at 24 months with overall survival respectively of 85.7 and 71.4% at 24 months (P = 0.029). The 1-year survival of responders and nonresponders was similar in both criteria without significant difference.
Moreover, we found that the original concept of decrease of dimension of nodule considered as a positive response to treatment is not actually confirmed in our results. In fact, a nodule could have similar dimensions to another one with complete necrosis, however presenting areas of postcontrast enhancement so that it is considered partial response and not complete response.
Our study confirmed that EASL and mRECIST responses are both effective methods to assess overall response to TACE treatment in patients with HCC treated with DEB-TACE, however some differences have been found for target nodule assessment.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Rampone B, Schiavone B, Martino A, Viviano C, Confuorto G. Current management strategy of hepatocellular carcinoma. World J Gastroenterol. 2009;15:3210–6. doi: 10.3748/wjg.15.3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol. 2012;56:908–43. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: Efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474–81. doi: 10.1016/j.jhep.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: A randomised controlled trial. Lancet. 2002;359:1734–9. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 5.Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–71. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 6.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–30. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 7.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 8.Lencioni R. Chemoembolization for hepatocellular carcinoma. Semin Oncol. 2012;39:503–9. doi: 10.1053/j.seminoncol.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: An update. Hepatology. 2011;53:1020–2. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paul SB, Gamanagatti S, Sreenivas V, Chandrashekhara SH, Mukund A, Gulati MS, et al. Trans-arterial chemoembolization (TACE) in patients with unresectable Hepatocellular carcinoma: Experience from a tertiary care centre in India. Indian J Radiol Imaging. 2011;21:113–20. doi: 10.4103/0971-3026.82294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis AL, Gonzalez MV, Lloyd AW, Hall B, Tang Y, Willis SL, et al. DC Bead: In vitro characterization of a drug-delivery device for transarterial chemoembolization. J Vasc Interv Radiol. 2006;17:335–42. doi: 10.1097/01.RVI.0000195323.46152.B3. [DOI] [PubMed] [Google Scholar]
- 12.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 13.Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: Rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62–7. doi: 10.1002/hep.510290145. [DOI] [PubMed] [Google Scholar]
- 14.de Lope CR, Tremosini S, Forner A, Reig M, Bruix J. Management of HCC. J Hepatol. 2012;56(Suppl 1):S75–87. doi: 10.1016/S0168-8278(12)60009-9. [DOI] [PubMed] [Google Scholar]
- 15.Song MJ, Chun HJ, Song DS, Kim HY, Yoo SH, Park CH, et al. Comparative study between doxorubicin-eluting beads and conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Hepatol. 2012;57:1244–50. doi: 10.1016/j.jhep.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 16.Lewis AL, Gonzalez MV, Leppard SW, Brown JE, Stratford PW, Phillips GJ, et al. Doxorubicin eluting beads-1. Effects of drug loading on bead characteristics and drug distribution. J Mater Sci Mater Med. 2007;18:1691–9. doi: 10.1007/s10856-007-3068-8. [DOI] [PubMed] [Google Scholar]
- 17.Lewis AL, Taylor RR, Hall B, Gonzalez MV, Willis SL, Stratford PW. Pharmacokinetic and safety study of doxorubicin-eluting beads in a porcine model of hepatic arterial embolisation. J Vasc Interv Radiol. 2006;17:1335–43. doi: 10.1097/01.RVI.0000228416.21560.7F. [DOI] [PubMed] [Google Scholar]
- 18.Hong K, Khwaja A, Liapi E, Torbenson MS, Georgiades CS, Geschwind JF. New intra-arterial drug delivery system for the treatment of liver cancer: Preclinical assessment in a rabbit model of liver cancer. Clin Cancer Res. 2006;12:2563–7. doi: 10.1158/1078-0432.CCR-05-2225. [DOI] [PubMed] [Google Scholar]
- 19.Poon RT, Tso WK, Pang RW, Ng KK, Woo R, Tai KS, et al. A phase I/II trial of chemoembolisation for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol. 2007;5:1100–8. doi: 10.1016/j.cgh.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 20.Shim JH, Lee HC, Kim SO, Shin YM, Kim KM, Lim YS, et al. Which response criteria best help predict survival of patients with hepatocellular carcinoma following chemoembolization? A validation study of old and new models. Radiology. 2012;262:708–18. doi: 10.1148/radiol.11110282. [DOI] [PubMed] [Google Scholar]
- 21.Gillmore R, Stuart S, Kirkwood A, Hameeduddin A, Woodward N, Burroughs AK, et al. EASL and mRECIST responses are independent prognostic factors for survival in hepatocellular cancer patients treated with transarterial embolization. J Hepatol. 2011;55:1309–16. doi: 10.1016/j.jhep.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Burrel M, Reig M, Forner A, Barrufet M, de Lope CR, Tremosini S, et al. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using Drug Eluting Beads. Implications for clinical practice and trial design. J Hepatol. 2012;56:1330–5. doi: 10.1016/j.jhep.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: A randomised controlled trial. Lancet. 2002;359:1734–9. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]


