Abstract
Aims:
To evaluate the role of radiofrequency ablation (RFA) as an ablative technique in patients with unresectable hepatocellular carcinoma (HCC).
Settings and Design:
A tertiary care center, prospective study.
Materials and Methods:
The subjects comprised 31 patients (30 males, one female; age range 32-75 years) with HCC (41 lesions) who were treated with image-guided RFA. The follow-up period ranged from 3 months to 6 years, and included a multiphasic computed tomography (CT) at 1, 3 and 6 months post-RFA, and every 6 months thereafter. Patient outcome was evaluated and the tumor recurrence, survival and complications were assessed.
Statistical Analysis Used:
Discrete categorical data were presented as n (%) and continuous data as mean ± SD. Pearson correlation coefficient was used to determine the relationship between the different variables. Kaplan–Meier survival curve and Log-rank test were used to test the significance of difference between the survival time of the different groups.
Results:
The ablation success rate was 80.5% (33/41 HCC lesions). 12.2% (5/41) of the lesions were managed with repeat RFA due to tumor residue. 4.9% (2/41) of the lesions were managed with repeated RFA and transarterial chemoembolization. Eight patients had tumor recurrence (five patients (16.1%) had local recurrence and three patients (9.6%) had distant recurrence). Eleven patients died within 3.5-20 months post-RFA. The survival rate at 1 year in patients who completed at least 1 year of follow-up was 63.3%. There was one major complication (1/31, 3.2%) in a patient with a subcapsular lesion and ascites. This patient developed hemoperitoneum in the immediate postprocedure period and was managed with endovascular treatment. She, however, had hepatic decompensation and died 48 h post-RFA.
Conclusion:
RFA is an effective and safe treatment for small unresectable HCC.
Keywords: HCC, RFA, small, unresectable
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignant neoplasm in the world and a major cause of mortality worldwide.[1,2] The mortality rate of HCC is almost the same as the incidence rate, which was projected to be around 14,120 patients in 2001.[3,4] This figure is around 18-times lesser than the figure of 250,000 projected deaths by international organizations.[5] The different therapeutic options include surgical resection, orthotopic liver transplantation (OLT) and the different locoregional therapies.[6,7,8,9] The locoregional therapies include endovascular and percutaneous interventions. The various endovascular interventions include transarterial chemoembolization (TACE), including the use of drug-eluting beads, transarterial embolization (TAE) and transarterial radionuclide therapy (TART) using yttrium microspheres. The percutaneous interventions use either thermal or chemical ablation techniques. The various thermal ablation therapies used are radiofrequency ablation (RFA), cryotherapy, interstitial laser therapy and microwave coagulation. Ethanol and acetic acid are used in the chemical ablation techniques. The different therapeutic options are used according to the availability and expertise and are based on the general clinical status, underlying diseases, tumor staging and nodule location within the liver.
Only 9-27% of the patients with HCC are eligible for surgical resection and, without treatment, the 5-year survival rate is less than 5%. The factors limiting the surgical resection include severe impairment of hepatic functional reserve, bilobar distribution of the tumors, extrahepatic metastasis or involvement of the main portal vein. Patients not candidates for surgery are considered eligible for locoregional therapies, among which RFA seems to be the most effective for small tumors and is currently considered the best technique to obtain the destruction of the neoplastic nodules. The main advantages of RFA include low morbidity and mortality rates and effective tumor ablation with preservation of maximal normal liver parenchyma.[6]
The purpose of this study was to evaluate the role of RFA as an ablative technique in patients with small HCCs who are not candidates for surgery. To the best of our knowledge, there is no published English literature on this from the Indian subcontinent.
Materials and Methods
Study population
From 2006 to 2012, a prospective study was performed in 31 consecutive patients with concurrent HCC who were treated with image-guided RFA at our institute. The study was approved by the institute ethics committee. The patients were diagnosed to have HCC based on the American Association for the Study of Liver Diseases (AASLD) practice guidelines,[7] and most of them were selected for RFA treatment based on the Barcelona Clinic Liver Cancer Staging System (BCLC) guidelines.[10]
RFA protocol and technique
All the procedures were performed with the patient under conscious sedation on an inpatient basis in the interventional radiology suite using a commercial available system (Radionics, Cool-Tip System, Burlington, MA, USA). Single/clustered needle electrodes were used with the length of the burning tip of the radiofrequency (RF) probe ranging from 1 cm to 2.5 cm. A 12-min RF cycle was given in auto mode and 1 min cycle was given for tract ablation. The procedure was performed under ultrasound (US) guidance in 30 patients (96.8%) and computed tomography (CT) guidance in 1 patient (3.2%). Five percent dextrose was used to create artificial ascites in three patients (9.7%).
Post-RFA follow-up
Patient outcome was evaluated and the tumor recurrence, survival and complications were assessed. The tumors were considered as ablated completely if no viability was found on dynamic contrast-enhanced CT done at 3 months after RFA.
Two types of tumor recurrence were looked for: Local tumor progression (LTR), which occurs along the peripheral margin of the ablative lesion, and intrahepatic distant recurrence (IDR), which is a new HCC tumor remote from the margin of the ablative lesion.
The follow-up period ranged from 3 months to 6 years, and included a multiphasic CT at 1, 3 and 6 months post-RFA, and every 6 months thereafter.
Statistical analysis
All analyses were conducted using SPSS for Windows (version 15.0; SPSS Inc., Chicago, IL, USA). Discrete categorical data are presented as n (%); continuous data are given as mean ± SD. To determine the relationship between the different variables, Pearson correlation coefficient was calculated. Kaplan–Meier survival curve and Log-rank test were applied to test the significance of difference between the survival time of the different groups (age, sex, alfa-fetoprotein levels, size, number of lesions and Child score categories). All statistical tests were two-sided and performed at a significance level of α = 0.05.
Results
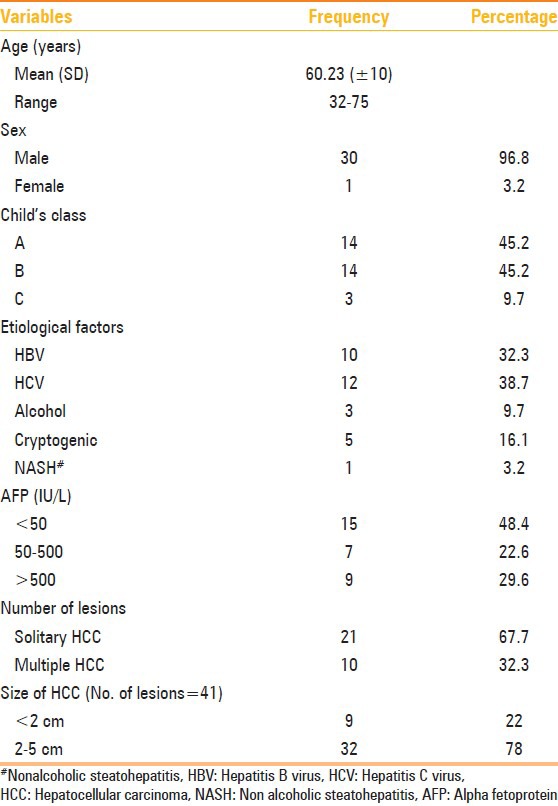
There were 31 patients, of which 30 were males. The age range of the study group was 32-75 years, having a mean age of 60.23 years. A total of 41 HCCs were seen in 31 patients. Twenty-one patients (67.7%) had single lesion, while each of the remaining 10 patients (32.3%) had two lesions. The tumor size ranged from 1 cm to 5 cm (mean 3.17 + 1.08 cm).
Of the 31 patients, 28 (90.3%) were cirrhotics. Cirrhosis was related to hepatitis B virus (HBV) infection in 10 cases (35.7%), hepatitis C virus (HCV) in 12 cases (42.9%), alcoholism in three cases (10.7%) and cryptogenic cirrhosis in three cases (10.7%).
According to the Child–Pugh scoring system, the series included 14 (45.2%) class A, 14 (45.2%) class B and three (9.7%) class C patients. The characteristics of the study population and HCC are summarized in Table 1.
Table 1.
Demographic profile and clinical features of HCC patients (N=31)
The technical success rate was 100%. RFA efficacy, defined as primary complete ablation on nodular basis, was 80.4% (33/41 HCC lesions). A total of 41 sittings and 75 treatments were performed in 41 nodules of 31 patients (1.83 treatments on average per nodule).
Absolute alcohol (100% ethanol) was used along with RFA in five (12.2%) lesions in four (12.9%) patients.
12.2% (5/41 HCC lesions) tumors were managed with repeated RFA due to residual tumor. 4.9% (2/41 HCC lesions) tumors were managed with repeated RFA and TACE.
During the follow-up period, eight patients had tumor recurrence. Of these, five (16.1%) had local recurrence while three (9.6%) had a distant recurrence. All local recurrences occurred in the second year post-RFA. Extrahepatic dissemination was seen in two (6.5%) patients, one of whom had pulmonary metastases while the other one had retroperitoneal lymphadenopathy.
Kaplan–Meier test was used to demonstrate the correlation between the survival and factors such as age, sex, AFP levels, size of lesion, number of lesions and Child score of the patients [Figure 1A-F]. However, the correlation was not statistically significant (P-values were greater than 0.05).
Figure 1 (A-F).
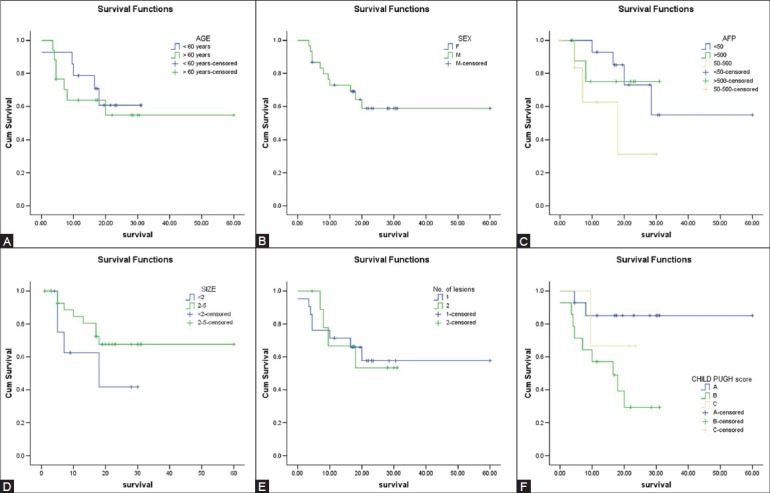
Kaplan-Meier curves demonstrating the correlation between the survival and age (A), sex (B), alfa-fetoprotein levels (C), size of lesions (D), number of lesions (E) and Child score (F) of patients. The correlation is not statistically significant
Eleven patients died within 3-20 months (9.59 + 5.17 months) of the post-RFA follow-up period. The survival rate at 1 year in patients who completed at least 1-year follow-up was 63.3% [Figure 2].
Figure 2 (A, B).
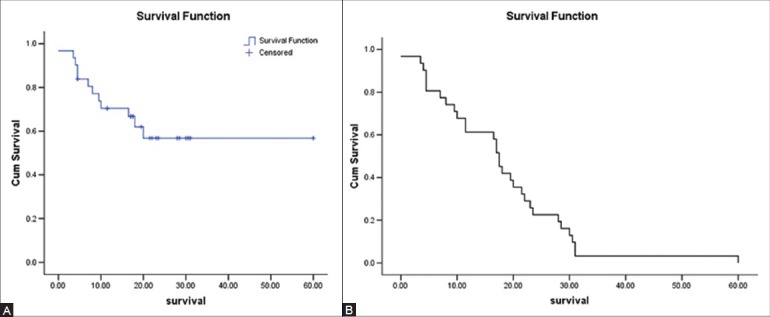
Kaplan-Meier survival curves demonstrating the survival (A) and the survival rate (B) during the follow-up period
Pain requiring analgesics was the most frequent complication encountered in all the patients (100%), while bile duct injury and skin burn were seen in one case each. One of the patients having a lesion in a subcapsular location in segment VIII and ascites developed hemoperitoneum in the immediate postprocedure period and was managed with endovascular treatment. This patient, however, had hepatic decompensation and died 48 h post-RFA.
Discussion
RFA is a minimally invasive procedure and is accepted as one of the treatment options for patients with small HCC who are not suitable for surgery. Among the various local percutaneous ablative therapies, there is an immense interest for RFA because of its effectiveness and safety in the treatment of small HCCs. Recent evidence supports percutaneous local ablation therapy for small HCC, and it is considered as effective as surgical resection.[11,12]
A major limitation of RFA is the small volume of tumor that it can treat. The rate of complete ablative necrosis decreases with the size of the tumor, particularly those larger than 3 cm. In our study, the lesions larger than 3 cm were also offered RFA as a minimal invasive treatment option to improve the survival. Short-term follow-up results in terms of tumor recurrence in smaller HCC treated with RFA have been shown to be comparable with larger lesions treated with RFA.[13] There is general consensus that complete response of RFA therapy in patients is associated with improved outcome.[14,15,16]
The developing world has a peculiar epidemiological variation in terms of etiology and the stage of HCC at diagnosis; more than 80% of the HCC occurs in Asia and Africa. In our study, HCV infection was the most common background causal factor for HCC. This is in contrast to the observations of published studies from India, in which HBV infection was the most common causal factor.[17,18] This could be because of the small sample size in our study.
Studies have shown that RFA combined with percutaneous ethanol injection (PEI) facilitates better local tumor control and long-term survival, compared with RFA alone.[19] During the course of our study, we also gave combined treatment of RFA and PEI in four (12.9%) patients. Of these, one patient died within 4 months of the follow-up period while another patient developed local recurrence.
Two types of tumoral recurrence are seen in patients with HCC after RFA. They are LTR and IDR. LTR occurs along the peripheral margin of the ablative lesion and IDR is a new HCC tumor remote from the margin of the ablative lesion. Local progression rates vary widely between 2% and 60%.[20] Shiina et al. recorded the lower local progression rate of 2% at 3 years,[21] while in our study, we encountered local progression in five (16.1%) cases. All the local recurrences in our study occurred during the second year of follow-up. The distant progression was seen in three (9.6%) patients only.
The survival rate at 1 year in patients who completed at least 1 year follow-up was 63.3% [Figure 3].
Figure 3 (A-F).
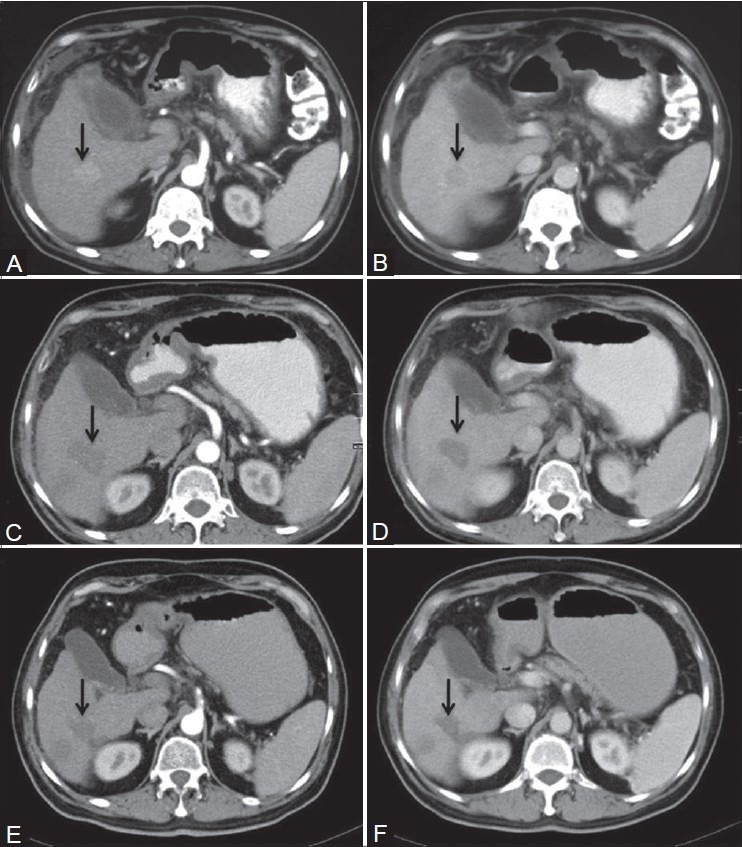
Axial computed tomography images of a patient who completed 1-year follow-up. Preradiofrequency ablation (RFA) images showing hypervascular lesion in segment VI with washout (arrows in A and B). Follow-up post-RFA imaging at 3 months and 1 year showing no hypervascularity (C and E) or washout (D and F) to suggest recurrence. There is associated capsular retraction
The correlation between the survival and the factors such as age, sex, AFP levels, size of lesion, number of lesions and Child score of patients was not statistically significant (P-values were greater than 0.05). This is contrary to the previously published study by Zhang et al.[19] They demonstrated patient sex and tumor diameter as the significant prognostic factors for the overall survival. The likely cause could be the small patient group in our study. However, they also had a small number of female patients recruited in their study, which could be the possible cause for the patient sex as a significant prognostic factor.
According to the BCLC guidelines, only symptomatic treatment is offered to patients of class C. In our study, three patients of Child's class C were offered RFA as a possible treatment modality to improve survival. RFA has been shown to provide a possible treatment modality in patients with poor liver function, especially patients of Child score C.[22]
The lungs, intraabdominal lymph nodes and bones are the most common sites of extrahepatic metastatic HCC.[23] We encountered lung metastases in one patient and retroperitoneal lymphadenopathy in another patient.
The incidence of adverse events of RFA shows mortality rates ranging from 0% to 1.2% and major morbidity rates ranging from 1.7% to 12%.[24,25,26,27,28,29,30] Our patients tolerated the procedure well. According to the Accordion Severity Grading System of Surgical Complications,[31] grade I complication in the form of analgesics requirement for alleviation of pain was seen in all the patients (100%). Grade III complication in the form of skin burn was seen in one (3.3%) case. Grade IV complications were seen in two (6.7%) cases, of which bile duct injury was seen in one case. The other grade IV complication was seen in the form of hemoperitoneum, in which HCC was located in a subcapsular location in segment VIII. This patient underwent endovascular treatment, but developed hepatic failure and died within 48 h. We did not encounter any of the grade II complications.
There were limitations in our study. We consider the small sample size as one of the major limitations of our study. A higher number of patients are required with long-term follow-up in future studies.
To conclude, RFA is as an effective and safe treatment for small unresectable HCC.
Acknowledgments
We thank Dr. Nripen Puri and Dr. Vikram Singh Bhinder, Junior Research Fellows, for organizing the data. We also thank Mr. Raman, Mrs. Poonam and Mrs. Geeta for their administrative support.
Footnotes
Source of Support: This study was partly funded by the ICMR
Conflict of Interest: No
References
- 1.El-Serag HB. Hepatocellular carcinoma: An epidemiologic view. J Clin Gastroenterol. 2002;35:S72–8. doi: 10.1097/00004836-200211002-00002. [DOI] [PubMed] [Google Scholar]
- 2.Padma S, Martinie JB, Iannitti DA. Liver tumor ablation: Percutaneous and open approaches. J Surg Oncol. 2009;100:619–34. doi: 10.1002/jso.21364. [DOI] [PubMed] [Google Scholar]
- 3.Dhir V, Mohandas KM. Epidemiology of digestive cancer in India-III. Liver. Indian J Gastroenterol. 1998;17:100–3. [PubMed] [Google Scholar]
- 4.Mohandas KM. Hepatitis B associated hepatocellular carcinoma: Epidemiology, diagnosis and treatment. Hep B Annual. 2004;1:140–52. [Google Scholar]
- 5.Miller MA, McCann L. Policy analysis of the use of Hepatitis B, Hemophilus influenzae type B, Streptococcus pneumoniae-conjugate and Rotavirus vaccines in the National Immunization Schedules. Health Econ. 2000;9:19–35. doi: 10.1002/(sici)1099-1050(200001)9:1<19::aid-hec487>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 6.Zytoon AA, Ishii H, Murakami K, El-Kholy MR, Furuse J, El-Dorry A, et al. Recurrence-free survival after radiofrequency ablation of hepatocellular carcinoma. A registry report of the impact of risk factors on outcome. Jpn J Clin Oncol. 2007;37:658–72. doi: 10.1093/jjco/hym086. [DOI] [PubMed] [Google Scholar]
- 7.Bruix J, Sherman M. Management of hepatocellular carcinoma: An update. Hepatology. 2011;53:1021–3. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dodd G, Soulen M, Kane R, Livraghi T, Lees WR, Yamashita Y, et al. Minimally invasive treatment of malignant hepatic tumors: At the threshold of a major breakthrough. Radiographics. 2000;20:9–27. doi: 10.1148/radiographics.20.1.g00ja019. [DOI] [PubMed] [Google Scholar]
- 9.Poon RT, Fan ST, Tsang FH, Wong J. Locoregional therapies for hepatocellular carcinoma: A critical review from the surgeon's perspective. Ann Surg. 2002;235:466–86. doi: 10.1097/00000658-200204000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: The BCLC update and future prospects. Semin Liver Dis. 2010;30:61–74. doi: 10.1055/s-0030-1247133. [DOI] [PubMed] [Google Scholar]
- 11.Kaido T, Uemoto S. Recent evidence in the treatment of small hepatocellular carcinoma. Hepatogastroenterology. 2008;55:1460–2. [PubMed] [Google Scholar]
- 12.Guglielmi A, Ruzzenante A, Valdegamberi A, Pachera S, Campagnaro T, D’Onofrio M, et al. Radiofrequency ablation versus surgical resection for the treatment of hepatocellular carcinoma in cirrhosis. J Gastrointest Surg. 2008;12:192–8. doi: 10.1007/s11605-007-0392-8. [DOI] [PubMed] [Google Scholar]
- 13.Poon RT, Ng KK, Lam CM, Ai V, Yuen J, Fan ST. Effectiveness of radiofrequency ablation for hepatocellular carcinomas larger than 3 cm in diameter. Arch Surg. 2004;139:281–87. doi: 10.1001/archsurg.139.3.281. [DOI] [PubMed] [Google Scholar]
- 14.Sala M, Llovet JM, Vilana R, Bianchi L, Solé M, Ayuso C, et al. Barcelona clínic liver cancer group. Initial response to percutaneous ablation predicts survival in patients with hepatocellular carcinoma. Hepatology. 2004;40:1352–60. doi: 10.1002/hep.20465. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi S, Kudo M, Chung H, Inoue T, Ishikawa E, Kitai S, et al. Initial treatment response is essential to improve survival in patients with hepatocellular carcinoma who underwent curative radiofrequency ablation therapy. Oncology. 2007;72:98–103. doi: 10.1159/000111714. [DOI] [PubMed] [Google Scholar]
- 16.Guglielmi A, Ruzzenente A, Sandri M, Pachera S, Pedrazzani C, Tasselli S, et al. Radio frequency ablation for hepatocellular carcinoma in cirrhotic patients: Prognostic factors for survival. J Gastrointest Surg. 2007;11:143–9. doi: 10.1007/s11605-006-0082-y. [DOI] [PubMed] [Google Scholar]
- 17.Acharya SK, Madan K, Dattagupta S, Panda SK. Viral hepatitis in India. Natl Med J India. 2006;19:203–17. [PubMed] [Google Scholar]
- 18.Paul SB, Chalamalasetty SB, Vishnubhatla S, Madan K, Gamanagatti SR, Batra Y, et al. Clinical profile, etiology and therapeutic outcome in 324 hepatocellular carcinoma patients at a tertiary care center in India. Oncology. 2009;77:162–71. doi: 10.1159/000231886. [DOI] [PubMed] [Google Scholar]
- 19.Zhang YJ, Liang HH, Chen MS, Guo RP, Li JQ, Zheng Y, et al. Hepatocellular carcinoma treated with radiofrequency ablation with or without ethanol injection: A prospective randomized trial. Radiology. 2007;244:599–607. doi: 10.1148/radiol.2442060826. [DOI] [PubMed] [Google Scholar]
- 20.Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS, et al. Small hepatocellular carcinoma: Treatment with radio-frequency ablation versus ethanol injection. Radiology. 1998;210:655–61. doi: 10.1148/radiology.210.3.r99fe40655. [DOI] [PubMed] [Google Scholar]
- 21.Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129:122–30. doi: 10.1053/j.gastro.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Jin-yu WU, Wei Y, Ming C, Shan-shan Y, Wen G, Wei W, et al. Efficacy and feasibility of radiofrequency ablation for decompensated cirrhotic patients with hepatocellular carcinoma. Chin Med J. 2010;123:1967–72. [PubMed] [Google Scholar]
- 23.Katyal S, Oliver JH, Peterson MS, Ferris JV, Carr BS, Baron RL. Extrahepatic metastases of hepatocellular carcinoma. Radiology. 2000;216:698–703. doi: 10.1148/radiology.216.3.r00se24698. [DOI] [PubMed] [Google Scholar]
- 24.Buscarini L, Buscarini E, Di Stasi M, Vallisa D, Quaretti P, Rocca A. Percutaneous radiofrequency ablation of small hepatocellular carcinoma: Long-term results. Eur Radiol. 2001;11:914–21. doi: 10.1007/s003300000659. [DOI] [PubMed] [Google Scholar]
- 25.Lencioni R, Cioni D, Crocetti L, Franchini C, Pina CD, Lera J, et al. Early-stage hepatocellular carcinoma in patients with cirrhosis: Long-term results of percutaneous image-guided radiofrequency ablation. Radiology. 2005;234:961–7. doi: 10.1148/radiol.2343040350. [DOI] [PubMed] [Google Scholar]
- 26.Raut CP, Izzo F, Marra P, Ellis LM, Vauthey JN, Cremona F, et al. Significant long-term survival after radio-frequency ablation of unresectable hepatocellular carcinoma in patients with cirrhosis. Ann Surg Oncol. 2005;12:616–28. doi: 10.1245/ASO.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 27.Machi J, Bueno RS, Wong LL. Long-term follow-up outcome of patients undergoing radiofrequency ablation for unresectable hepatocellular carcinoma. World J Surg. 2005;29:1364–73. doi: 10.1007/s00268-005-7829-6. [DOI] [PubMed] [Google Scholar]
- 28.Tateishi R, Shiina S, Teratani T, Obi S, Sato S, Koike Y, et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer. 2005;103:1201–9. doi: 10.1002/cncr.20892. [DOI] [PubMed] [Google Scholar]
- 29.Cabassa P, Donato F, Simeone F, Grazioli L, Romanini L. Radiofrequency ablation of hepatocellular carcinoma: Long-term experience with expandable needle electrodes. Am J Roentgenol. 2006;186:S316–21. doi: 10.2214/AJR.05.0243. [DOI] [PubMed] [Google Scholar]
- 30.Choi D, Lim HK, Rhim H, Kim YS, Lee WJ, Paik SW, et al. Percutaneous radiofrequency ablation for early-stage hepatocellular carcinoma as a first-line treatment: Long-term results and prognostic factors in a large single-institution series. Eur Radiol. 2007;17:684–92. doi: 10.1007/s00330-006-0461-5. [DOI] [PubMed] [Google Scholar]
- 31.Strasberg SM, Linehan DC, Hawkins WG. The accordion severity grading system of surgical complications. Ann Surg. 2009;250:177–86. doi: 10.1097/SLA.0b013e3181afde41. [DOI] [PubMed] [Google Scholar]


