Abstract
Wallerian degeneration (WD) is the process of demyelination and disintegration of the distal axonal segment following the interruption of the axonal integrity or damage to the neuron. We report a patient having WD of middle cerebellar peduncles due to pontine infarction caused by basilar artery thrombosis. We review the anatomy and discuss the pathogenesis of this condition.
Keywords: Basilar artery thrombosis, demyelination, diffusion-weighted imaging, middle cerebellar peduncle, Wallerian degeneration
Introduction
Infarction is the most common event resulting in Wallerian degeneration (WD) in the central nervous system (CNS).[1] We report a case showing symmetrical restricted diffusion in middle cerebellar peduncles (MCPs) after 21 days of the onset of symptoms, which was interpreted as WD. Further, magnetic resonance imaging (MRI) after 12 weeks revealed high signal on diffusion-weighted images (DWI) and apparent diffusion coefficient (ADC) maps. Transient signal abnormalities in MCPs on DWI following brain stem infarction may be seen and should not be mistaken for a new ischemic event.
Case Report
A 59-year-old male patient with known arterial hypertension, hypercholesterolemia, and diabetes mellitus came to our hospital with accentuated dysarthria, skew deviation, double vision, and right hemisyndrome since 2 days. Before that the patient had acute vertigo for 9 days as the initial symptom and he went to a local hospital where MRI of the brain was performed, which showed acute tegmental infarction of left paramedian pons.
Upon admission, the patient was drowsy and markedly dysarthric. Neurological examination showed abnormal horizontal movements of the extraocular muscles and spontaneous nystagmus; however, vertical gaze was maintained. Left facial palsy as well as weakness in the upper and lower extremities on both sides was noted. The total National Institute Health Stroke Scale (NIHSS) score was 8/42.
MRI at this time [Figure 1] revealed small bilateral foci of restricted diffusion in the ventral pons. The central paramedian pons and the midbrain showed lesser hyperintense signal on DWI and contrast enhancement suggestive of subacute infarct. MR angiography (MRA) revealed no flow void in intracranial right vertebral artery and basilar artery [Figure 2], suggesting thrombosis. Consensus decision was taken to avoid catheter cerebral angiography and thrombolysis, as the patient had history of bleeding per rectum 1 month prior. Intravenous unfractionated heparin was administered. Extensive work-up to rule out any cause of vasculitis was done; however, these were normal. Four days later, he was in full control of his spaciotemporal orientation; however, he developed new ataxia on the left side. The oculomotor and motoric deficits were unchanged. Intravenous heparin was continued and the patient was managed conservatively. On day 12, he further developed diminished facial sensation in trigeminal distribution, dysmetria, and dysdiadokinesia of the lower left extremity and the NIHSS score was 5/42.
Figure 1 (A-C).
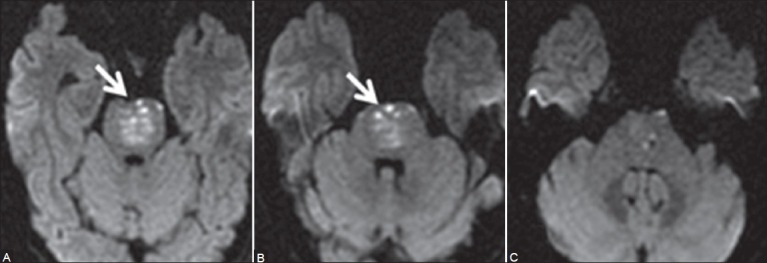
Initial MRI of brain on admission: Axial diffusion-weighted images (A, B) New tiny hyperintense signal foci in ventral and paramedian pons on both sides (arrows) suggestive of acute infarcts. The central paramedian pons reveals lesser hyperintensity due to subacute infarct. At this time, MCPs were normal (C)
Figure 2 (A-C).
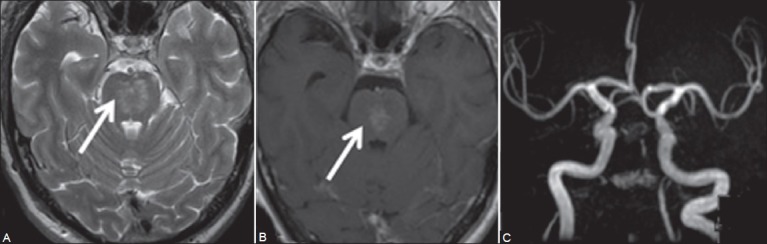
Axial T2W image (A) Hyperintense signal in paramedian midbrain and pons with contrast enhancement (B) Suggestive of subacute infarct. Coronal MRA image (C) No flow in intracranial right vertebral and basilar arteries
MRI [Figure 3] performed at this time (day 12) revealed restricted diffusion in both the MCPs with hyperintense signal on fluid-attenuated inversion recovery (FLAIR) images symmetrically. The new lesions were interpreted as WD due to bilateral paramedian pontine infarction following the basilar artery thrombosis. No new focus of acute infarct was seen. At this time, intravenous heparin was stopped and prophylactic aspirin 100mg daily was considered. Patient was transferred to neurorehabilitation clinic and gradually regained power in extremities.
Figure 3 (A-C).
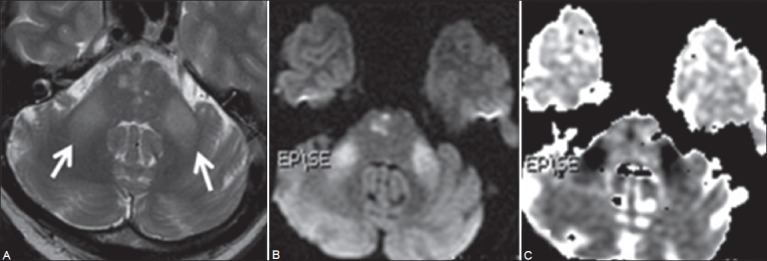
Twelfth day follow-up MRI: Axial T2W images (A) shows symmetrical hyperintense signal in both the MCPs (arrow). Few hyperintense foci are also seen in pons. Diffusion-weighted image (B) shows hyperintense signal in both MCPs and in right paramedian pons. Corresponding ADC map (C) reveals reduced signal suggestive of restricted diffusion
Last follow-up MRI [Figure 4] was done 12 weeks after the onset showed gliosis in midbrain and ventral pons. Persistent T2-hyperintense signal was noted in both the MCPs with decreased fractional anisotropy. Paucity of fibers of MCP and transverse pontine fibers was also seen. Presently, the patient has persistent mild dysarthria, dysmetria, and inability to converge eyes, but otherwise there are no motor or sensory defects.
Figure 4 (A-F).
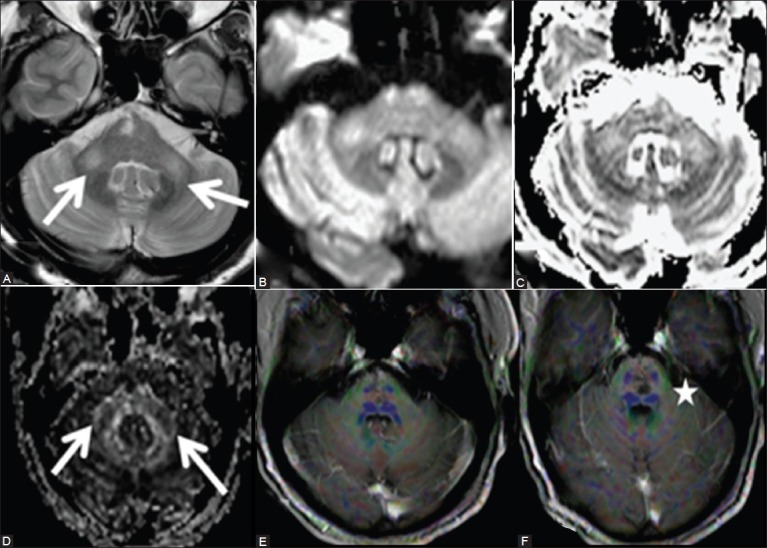
Twelve weeks follow-up MRI shows persistent hyperintense signal in both the MCPs (arrows) on axial T2W. Note the gliotic change in tegmentum of pons on the right side (A) both MCPs reveal mild hyperintense signal on diffusion image (B) and ADC maps (C) with decreased fractional anisotropy (D) 2D diffusion tensor image (DTI) superimposed on T1 post-contrast images shows paucity of fibers in MCP (E) and also of transverse pontine fibers (F)
Discussion
WD is a pathological event occurring after neuronal injury. It was first described by Waller in experiments with frogs in 1850. WD has been extensively described as affecting the corticospinal tract (CST) in association with pathologies of the cerebrum and internal capsule.[1]
Kuhn et al.,[1] described four stages of WD in the cerebrum and they can be observed after 4 weeks on T2W images, however, as early as 12 days with DWI on CST.[2,3] Castillo et al.,[4] found increased signal on DWI suggestive of WD of CST within 72 h after the symptoms in 20% of cases.
Neuroanatomically, the brachium pontis, or MCPs are a large bundle of fibers that connect the pons with the cerebellum. The pontine nuclei receive input from the cerebral cortex via the corticopontine tract and project almost exclusively to the contralateral cerebellum via the transverse pontine fibers and MCPs, which constitute the pontocerebellar tract. The pontocerebellar fibers cross at an upper pontine level;[5] hence, in unilateral pontine infarction, there is damage to local pontine nuclei resulting in ipsilesional involvement of the MCP, and to axons of more lateral neurons which will have to cross midline to reach contralateral MCP [Figure 5].
Figure 5.
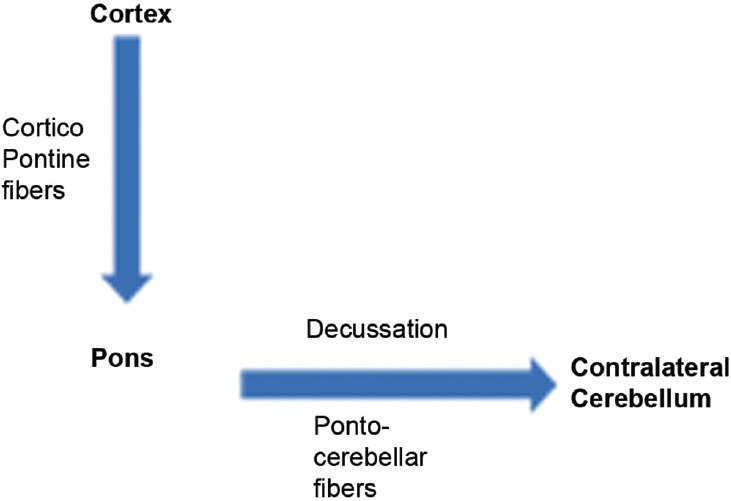
Corticopontine and pontocerebellar tract with synapses in the pontine nuclei. Unilateral pontine lesions damage the local pontine neurons, thus affecting ipsilateral MCP after its fibers have crossed, and also damage the ipsilateral lateral fibers which have to cross the midline to form opposite MCP
Most of the papers report T2-hyperintense signal in MCPs due to unilateral pontine infarction[6,7,8] at or after 3 months of the onset of symptoms. In our patient, there were bilateral small paramedian pontine infarctions due to basilar artery thrombosis, resulting in increased signal in both MCPs. These MCPs revealed restricted diffusion as early as 21 days from the onset of symptoms, suggesting acute WD and facilitated diffusion with paucity of fibers at 12 weeks follow-up. To the best of our knowledge, no case report showing sequential evolution of WD in MCPs due to basilar artery thrombosis has been described. This may be due to poor clinical outcome of patients with basilar artery thrombosis leading to bilateral brainstem infarcts.[9]
Similar signal abnormalities are also seen in central pontine myelinosis, in neurodegenerative disorders like olivopontocerebellar atrophy, spinocerebellar ataxia, adrenoleukodystropy, alcoholic liver disease, Wilson disease, hypoglycemic coma,[10] or progressive multifocal leukoencephalopathy (PML).[11] However, clinical and biochemical investigations help in differentiation. Presence of restricted diffusion in brainstem with no flow in basilar artery suggests the primarily vascular etiology, rather than a metabolic abnormality.
Acute occlusion of the basilar artery is a neurologic emergency. MRI and MRA help in assessing the extent of brainstem infarction and the length of the occluded artery. Patients with no or only relatively small DWI lesions may have a favorable outcome, if reperfusion is achieved with intra-arterial or intravenous thrombolysis. However, the extent of DWI abnormalities is highly variable. Mechanical embolectomy with a clot retrieval device has been used in selected cases. The common etiological factors are local thrombosis in basilar artery which also commonly affects vertebral artery origin, artery-to-artery thromboembolism, cardiac emboli, and vertebral artery dissections. In an acute event, basilar thrombolysis with stenting of vertebral artery due to atheromatous vertebrobasilar stenosis may be considered.[12] For high-grade symptomatic basilar artery stenosis refractory to medical therapy (warfarin or aspirin), balloon angioplasty and stenting have also been described.
Conclusion
In conclusion, restricted diffusion of the MCPs following pontine infarction is transient and when imaged during that duration; it may mimic a new infarction focus. Knowledge of sequential evolution of WD of both MCPs following pontine infarction/basilar artery thrombosis helps to avoid this misdiagnosis.
Footnotes
Source of Support: Nil
Conflict of Interest: No.
References
- 1.Kuhn MJ, Mikulis DJ, Ayoub DM, Kosofsky BE, Davis KR, Taveras JM. Wallerian degeneration after cerebral infarction: Evaluation with sequential MR imaging. Radiology. 1989;172:179–82. doi: 10.1148/radiology.172.1.2740501. [DOI] [PubMed] [Google Scholar]
- 2.Uchino A, Sawada A, Takase Y, Egashira R, Kudo S. Transient detection of early Wallerian degenerationon diffusion-weighted MRI after anacute cerebrovascular accident. Neuroradiology. 2004;46:183–8. doi: 10.1007/s00234-003-1159-x. [DOI] [PubMed] [Google Scholar]
- 3.Kang DW, Chu K, Yoon BW, Song IC, Chang KH, Roh JK. Diffusion-weighted imaging in Wallerian degeneration. J Neurol Sci. 2000;178:167–9. doi: 10.1016/s0022-510x(00)00373-7. [DOI] [PubMed] [Google Scholar]
- 4.Castillo M, Mukherji SK. Early abnormalities related to postinfarction Wallerian degeneration: Evaluation with MR diffusion-weighted imaging. J Comput Assist Tomogr. 1999;23:1004–7. doi: 10.1097/00004728-199911000-00034. [DOI] [PubMed] [Google Scholar]
- 5.Brodal P, Bjaalie JG. Organization of the pontine nuclei. Neurosci Res. 1992;13:83–118. doi: 10.1016/0168-0102(92)90092-q. [DOI] [PubMed] [Google Scholar]
- 6.De Simone T, Regna-Gladin C, Carriero MR, Farina L, Savoiardo M. Wallerian degeneration of thepontocerebellar fibers. AJNR Am J Neuroradiol. 2005;26:1062–5. [PMC free article] [PubMed] [Google Scholar]
- 7.Küker W, Schmidt F, Heckl S, Nägele T, Herrlinger U. Bilateral Wallerian degeneration of the middlecerebellar peduncles due to paramedianpontine infarction: MRI findings. Neuroradiology. 2004;46:896–9. doi: 10.1007/s00234-004-1287-y. [DOI] [PubMed] [Google Scholar]
- 8.Fitzek C, Fitzek S, Stoeter P. Bilateral Wallerian degeneration of the medial cerebellar peduncles after ponto-mesencephalic infarction. Eur J Radiol. 2004;49:198–203. doi: 10.1016/S0720-048X(03)00132-3. [DOI] [PubMed] [Google Scholar]
- 9.Fitzek S, Fitzek C, Urban PP, Marx J, Hopf HC, Stoeter P. Time course of lesion developmentin patients with acute brain stem infarction and correlation with NIHSS score. Eur J Radiol. 2001;39:180–5. doi: 10.1016/s0720-048x(01)00372-2. [DOI] [PubMed] [Google Scholar]
- 10.Okamoto K, Tokiguchi S, Furusawa T, Ishikawa K, Quardery AF, Shinbo S, et al. MR features of diseases involving bilateralmiddle cerebellar peduncles. AJNR Am J Neuroradiol. 2003;24:1946–54. [PMC free article] [PubMed] [Google Scholar]
- 11.Mader I, Herrlinger U, Klose U, Schmidt F, Kuker W. Progressive multifocal leukoencephalopathy: Analysis oflesion development with diffusion-weighted MRI. Neuroradiology. 2003;45:717–21. doi: 10.1007/s00234-003-0966-4. [DOI] [PubMed] [Google Scholar]
- 12.Lin DD, Gailloud P, Beauchamp NJ, Aldrich EM, Wityk RJ, Murphy KJ. Combined stent placement and thrombolysis inacute vertebrobasilar ischemic stroke. AJNR Am J Neuroradiol. 2003;24:1827–33. [PMC free article] [PubMed] [Google Scholar]


