Abstract
Agenesis of dorsal pancreas is an extremely rare congenital anomaly that occurs due to failure of the dorsal pancreatic bud to form the body and tail of the pancreas. We report the sonographic appearance of this condition in six cases.
Keywords: Agenesis of dorsal pancreas, diabetes mellitus, sonography
Introduction
Agenesis of dorsal pancreas (ADP) is an extremely rare congenital anomaly of the pancreas in which the dorsal pancreas fails to develop either completely or partially. A few case reports have been published in the literature.[1,2,3,4,5,6] However, ultrasonographic features of this condition have not been reported to the best of our knowledge. The patients with ADP can be asymptomatic or present with abdominal pain, weight loss, pancreatitis, diabetes mellitus, bile duct obstruction, duodenal obstruction, or rarely pancreatic exocrine insufficiency and pancreatic adenocarcinoma.[7]
Case Series
Sonographic diagnosis of ADP was made in six patients over a period of 6 years (2005-2011). The mean age was 19 years (range 15-24 years). Of the six patients, four were males and two were females of whom one was pregnant. These patients presented with varied symptoms [Table 1]. ADP was diagnosed in all these patients. Of the six patients reported, only two patients had insulin-dependent diabetes mellitus (IDDM). One was a known diabetic and the other patient was diagnosed to have diabetes subsequent to the diagnosis of ADP. Ultrasonography (USG) was performed with an HDI5000 or IU22 scanner (Philips Ultrasound, Bothell, WA, USA). Convex (1-5 MHz) and linear (5-12 MHz) probes were used. In all the six patients, the body and tail of pancreas were absent and the pancreatic bed anterior to the splenic vein was replaced by the stomach or bowel loops [Figures 1A and 2A]. This is described as the dependent stomach and dependent intestine sign on the computed tomography (CT) scan.[1] Uncinate process and part of head of the pancreas were seen in all these patients [Figures 1B and 2B]. When the stomach and intestinal loops are air filled, they appear as echogenic structures in the pancreatic bed that may interfere with adequate visualization of body and tail of the pancreas on USG. The same may be misinterpreted as normal body and tail of the pancreas on USG examination. This can be avoided by repeating the scan after giving oral fluid which distends the stomach or intestine in the pancreatic bed and observe for peristalsis [Video 1]. This suggests the absence of body and tail of the pancreas. The sagittal scan rules out superior or inferior position of the body in relation to the splenic vein [Figures 1C and 2C]. ADP was confirmed by CT [Figures 1D, E and 2D] in all these patients.
Table 1.
Clinical presentation of patients
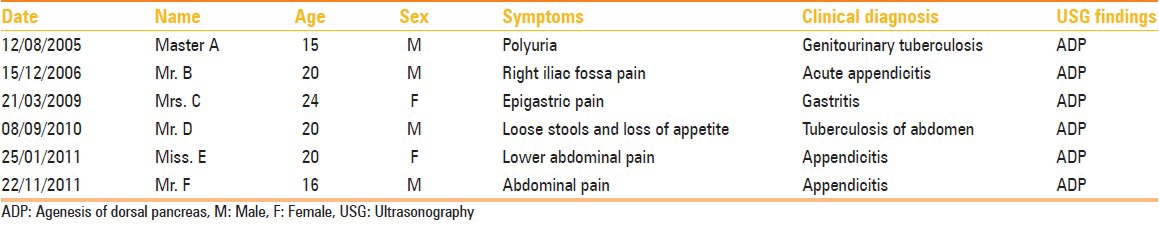
Figure 1 (A-E).
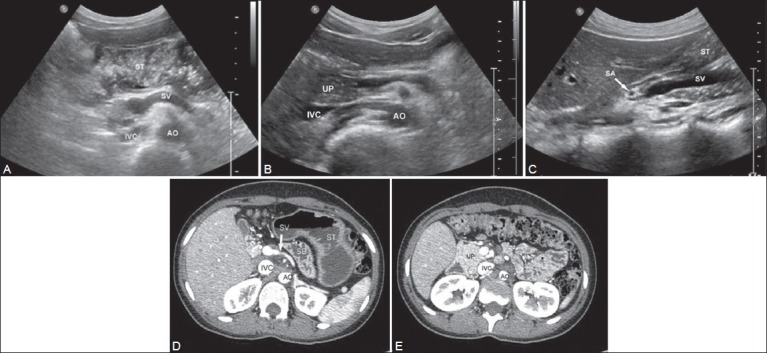
Transverse scans of epigastrium in one of the patients showing absence of body and tail of the pancreas with the fluid distended stomach (ST) seen in the pancreatic bed (A). A slightly caudal section (B) Shows the uncinate process (UP) of the pancreas. (C) Sagittal scan through the superior mesenteric vein (SMV) confirming that the pancreas is not seen superiorly or inferiorly. (D, E) Images of CT scan confirming agenesis of dorsal pancreas (AO: Aorta, IVC: Inferior vena cava, SA: Splenic artery, ST: Stomach, SV: Splenic vein)
Figure 2 (A-D).
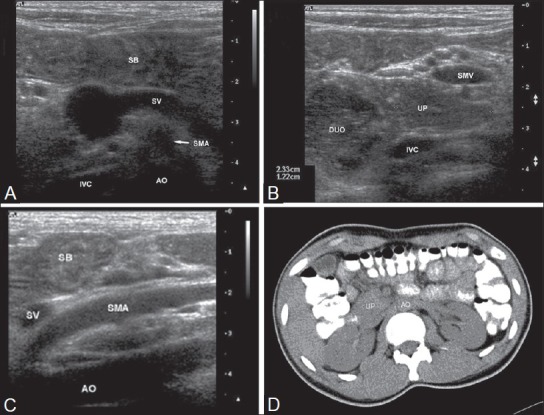
High-resolution images of similar sections in another patient. (A) Shows absence of body and tail of the pancreas. (B) Shows the uncinate process (UP) of the pancreas. (C) Sagittal scan through the superior mesenteric artery (SMA) confirming that the pancreas is not seen superiorly or inferiorly. (D) Image of CT scan confirming agenesis of dorsal pancreas (AO: Aorta, DUO: Duodenum, IVC: Inferior vena cava, SMA: Superior mesenteric artery, SMV: Superior mesenteric vein, SV: Splenic vein, SB: Small bowel)
Discussion
The development of the pancreas begins at the fourth week of gestation. Two separate buds arise from the caudal region of the embryonic foregut (duodenum). The ventral bud originates initially, followed by the dorsal bud. The ventral bud gives rise to the lower portion of the head and the uncinate process of the pancreas. The dorsal bud elongates to form the upper head, neck, body, and tail of the pancreas. The dorsal bud drains through duct of Wirsung[8] and the ventral bud drains through duct of Santorini (accessory pancreatic duct). The dorsal bud duct joins the duct of Santorini and forms the major pancreatic duct which in turn joins the common bile duct and drains into major duodenal papilla (ampulla of Vater). The duct of Santorini drains into minor duodenal papilla. The ventral and dorsal portions fuse at approximately the eighth week of gestation. Complete agenesis of the pancreas and agenesis of the ventral pancreas are incompatible with life.[9] ADP is an extremely rare congenital anomaly. It can be complete or partial. In patients with complete agenesis of the dorsal pancreas, the neck, body, and tail of the pancreas, accessory pancreatic duct (duct of Santorini), and the minor duodenal papilla are absent. While in partial ADP, there is a remnant of the duct of Santorini and the minor duodenal papilla.[8]
The cause of ADP is unknown. Ischemic events in the developing pancreas or primary dysgenesis are the possible explanations.[2] ADP is usually sporadic. It can occur as an autosomal dominant or X-linked dominant inheritance or with polysplenia/heterotaxy syndrome.[10] Recently, five patients with hepatocyte nuclear factor 1B (HNF1B) gene mutation had agenesis of the pancreatic body and tail on magnetic retrograde cholangiopancreatography (MRCP) studies.[11] The author concluded that HNF1B has a critical role to play in the development and differentiation of the dorsal pancreas. ADP can be associated with annular pancreas or with congenital defects such as horseshoe kidney, congenital hydrocephalus, hepato-mesenteric trunk, and retro-aortic left renal vein.
Patients with ADP can be asymptomatic[1,3] or symptomatic with pancreatitis or diabetes mellitus. Two possible mechanisms in the pathogenesis of pancreatitis are sphincter of Oddi dysfunction[12] and hypertrophy of the remnant ventral gland with higher intrapancreatic duct pressures.[13] In isolation, these changes may not result in pancreatitis. The role of genes in the pathogenesis of pancreatitis is being increasingly recognized.[14] The development of diabetes may be due to the absence of the islet cells which usually reside in the body and tail of the pancreas.[3] This, however, is yet to be established. Exocrine function is usually preserved as long as there is 10% of normal pancreatic tissue. Only very few cases with exocrine dysfunction due to ADP have been reported in the medical literature.[4,5,15] In our series, the diagnosis of ADP was incidental in all the patients except the first one. The first patient was referred for frequency of micturition with clinical suspicion of urinary tuberculosis. After diagnosis of ADP on USG, the patient was detected to have diabetic mellitus and the finding was confirmed by CT scan. This case was the eye opener. Subsequently, the other cases were diagnosed incidentally since pancreas is routinely looked for in all abdominal scans. Hence, they were all incidentally diagnosed cases. In the case reports quoted as references in this article, 7 cases were asymptomatic out of the total of 15 cases.[1,3,4,5,6,7,13,15,16,17,18,19]
USG is the first imaging modality for evaluation of abdominal pain and various other abdominal symptoms. Hence, it would be good if this rare condition can be diagnosed on USG. On USG, ADP is diagnosed when the part of head and uncinate process of the pancreas are seen while the body and tail of the pancreas are not visualized, and the pancreatic bed anterior to the splenic vein is replaced by the stomach or bowel loops. This is described as the dependent stomach and dependent intestine sign on the CT scan.[1] Confirmation of the absence of body and tail of the pancreas is done by endoscopic USG, CT, or magnetic resonance imaging. Absolute confirmation of the presence or absence of the dorsal pancreatic duct is done by endoscopic retrograde cholangiopancreatography (ERCP) or MRCP. MRCP is preferred over ERCP as it is noninvasive and has no risk of radiation.[6,17]
Differential diagnoses to be considered with ADP are congenital short pancreas, pseudo-agenesis, pancreatic divisum, pancreatic lipomatosis, and obstructing pancreatic tumors. Atrophy of the body and tail of the pancreas with sparing of pancreatic head which occurs secondary to chronic pancreatitis is called pseudo-agenesis.[19] Diagnosis of pseudo-agenesis would be confirmed in a patient with previous history of abdominal pain and pancreatitis with the presence of calcifications in CT scan and dorsal duct in MRCP scan unlike in ADP. Pancreatic divisum can be differentiated from ADP by the presence of normal body and tail of the pancreas and MRCP shows ventral and dorsal ducts draining separately into the duodenum in contrast to the absence of dorsal duct in ADP.[20] In fatty replacement of the pancreas, the entire gland is usually involved and the pancreatic duct is present. It is usually seen in later life.
In conclusion, ADP is a rare finding on USG and should be considered when the body and tail of the pancreas are not seen.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Mohapatra M, Mishra S, Dalai PC, Acharya SD, Nahak B, Ibrarullah M, et al. Imaging findings in agenesis of the dorsal pancreas. Report of three cases. JOP. 2012;13:108–14. [PubMed] [Google Scholar]
- 2.Macari M, Giovanniello G, Blair L, Krinsky G. Diagnosis of agenesis of the dorsal pancreas with MR pancreatography. AJR Am J Roentgenol. 1998;170:144–6. doi: 10.2214/ajr.170.1.9423620. [DOI] [PubMed] [Google Scholar]
- 3.Pasaoglu L, Vural M, Hatipoglu HG, Tereklioglu G, Koparal S. Agenesis of the dorsal pancreas. World J Gastroenterol. 2008;14:2915–6. doi: 10.3748/wjg.14.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakpal SV, Sexcius L, Babel N, Chamberlain RS. Agenesis of the dorsal pancreas and its association with pancreatic tumors. Pancreas. 2009;38:367–73. doi: 10.1097/MPA.0b013e318196c401. [DOI] [PubMed] [Google Scholar]
- 5.Klein WA, Dabezies MA, Friedman AC, Caroline DF, Boden GH, Cohen S. Agenesis of dorsal pancreas in a patient with weight loss and diabetes mellitus. Dig Dis Sci. 1994;39:1708–13. doi: 10.1007/BF02087781. [DOI] [PubMed] [Google Scholar]
- 6.Balakrishnan V, Narayanan VA, Siyad I, Radhakrishnan L, Nair P. Agenesis of the dorsal pancreas with chronic calcific pancreatitis. Case report, review of the literature and genetic basis. JOP. 2006;7:651–9. [PubMed] [Google Scholar]
- 7.Ulusan S, Yakar T, Koc Z, Kayaselcuk F, Torer N. Adenocarcinoma of the pancreas associated with dorsal agenesis. Pancreas. 2006;33:437–9. doi: 10.1097/01.mpa.0000236728.23994.27. [DOI] [PubMed] [Google Scholar]
- 8.Fukuoka K, Ajiki T, Yamamoto M, Fujiwara H, Onoyama H, Fujita T, et al. Complete agenesis of the dorsal pancreas. J Hepatobiliary Pancreat Surg. 1999;6:94–7. doi: 10.1007/s005340050090. [DOI] [PubMed] [Google Scholar]
- 9.Voldsgaard P, Kryger-Baggesen N, Lisse I. Agenesis of pancreas. Acta Paediatr. 1994;83:791–3. doi: 10.1111/j.1651-2227.1994.tb13144.x. [DOI] [PubMed] [Google Scholar]
- 10.Sener RN, Alper H. Polysplenia syndrome: A case associated with transhepatic portal vein, short pancreas, and left inferior vena cava with hemiazygous continuation. Abdom Imaging. 1994;19:64–6. doi: 10.1007/BF02165866. [DOI] [PubMed] [Google Scholar]
- 11.Haldorsen IS, Vesterhus M, Raeder H, Jensen DK, Søvik O, Molven A, et al. Lack of pancreatic body and tail in HNF1B mutation carriers. Diabet Med. 2008;25:782–7. doi: 10.1111/j.1464-5491.2008.02460.x. [DOI] [PubMed] [Google Scholar]
- 12.Nishimori I, Okazaki K, Morita M, Miyao M, Sakamoto Y, Kagiyama S, et al. Congenital hypoplasia of the dorsal pancreas: With special reference to duodenal papillary dysfunction. Am J Gastroenterol. 1990;85:1029–33. [PubMed] [Google Scholar]
- 13.Gold RP. Agenesis and pseudo-agenesis of the dorsal pancreas. Abdom Imaging. 1993;18:141–4. doi: 10.1007/BF00198051. [DOI] [PubMed] [Google Scholar]
- 14.Whitcomb DC. Genes means pancreatitis. Gut. 1999;44:150–1. doi: 10.1136/gut.44.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doxey BW, Jackson WD, Adler DG. A unique presentation: Dorsal agenesis of the pancreas manifesting as pancreatic exocrine insufficiency in the absence of diabetes mellitus in an 8-year-old boy. Dig Dis Sci. 2008;53:2005–6. doi: 10.1007/s10620-007-0094-9. [DOI] [PubMed] [Google Scholar]
- 16.Maier M, Wiesner W, Mengiardi B. Annular pancreas and agenesis of the dorsal pancreas in a patient with polysplenia syndrome. AJR Am J Roentgenol. 2007;188:W150–3. doi: 10.2214/AJR.05.1859. [DOI] [PubMed] [Google Scholar]
- 17.Uygur-Bayramicli O, Dabak R, Kilicoglu G, Dolapcioglu C, Oztas D. Dorsal pancreatic agenesis. JOP. 2007;8:450–2. [PubMed] [Google Scholar]
- 18.Lang K, Lasson A, Muller MF, Thorlacius H, Toth E, Olsson R. Dorsal agenesis of the pancreas-A rare cause of abdominal pain and insulin-dependent diabetes. Acta Radiol. 2012;53:2–4. doi: 10.1258/ar.2011.110480. [DOI] [PubMed] [Google Scholar]
- 19.Suda K, Matsumoto Y, Fujii H, Miura K, Nobukawa B. Clinicopathologic differentiation of atrophy of the pancreatic body and tail aplasia. Int J Pancreatol. 1998;24:227–35. doi: 10.1007/BF02788426. [DOI] [PubMed] [Google Scholar]
- 20.Bret PM, Reinhold C, Taourel P, Guibaud L, Atri M, Barkun AN. Pancreas divisum: Evaluation with MR cholangiopancreatography. Radiology. 1996;199:99–103. doi: 10.1148/radiology.199.1.8633179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


