Abstract
Objective:
To study the clinical characteristics and 28-days mortality in patients with ventilator-associated pneumonia (VAP) due to carbapenem-resistant Acinetobacter (CRA).
Design:
Retrospective, observational, cohort study.
Setting:
Intensive care unit (ICU) of a university hospital.
Materials and Methods:
Microbiologically confirmed VAP due to CRA infection.
Intervention:
None.
Results:
Out of 87 patients with VAP due to CRA, 60 (69%) were male; whose median age was 51 years; 73 (84%) patients were medical; 26 (30%) had history of hospitalization in last 3 months; median acute physiology and chronic health evaluation (APACHE) II was 15 and median SOFA 9 at admission; primary reason for ICU admission was respiratory failure (34%); 46 (53%) patients had more than 2 organ failure at ICU admission; median length of ICU stay was 19 days; 66 (76%) patients need vasoactive agents during ICU stay, whereas 55 (63%) patients had renal failure; median duration of mechanical ventilation was 17 days; 22 (25%) patients had acute respiratory distress syndrome (ARDS) during ICU stay; 72 (83%) patients had exposure to carbapenem before inclusion in the study; 33 (38%) patients had same organism at other sites. In the follow-up, 47 (54%) patient survived at 28 days after having VAP; whereas only 40 (46%) patients were discharged from the hospital.
Conclusions:
CRA-VAP has high crude mortality. Advanced age; severity of illness and presence of pneumonia at ICU admission; and presence of shock, ARDS and renal failure have impact on outcome in these patients.
Keywords: Acinetobacter, carbapenem, intensive care unit, ventilator-associated pneumonia
Introduction
Nosocomial infections due to Acinetobacter species, especially multidrug-resistant, have been emerged as a great concern to the clinicians worldwide. These aerobic, gram-negative, non-motile, non-lactose-fermenting oxidase-negative, catalase-positive coccobacilli microorganisms may colonize humans and survive on dry or moist environment including soil, water, food, or inanimate objects for many weeks. This notorious pathogen has been implicated in a wide spectrum of nosocomial infections like bacteremia, nosocomial pneumonia, urinary tract infections etc., with its extraordinary ability to develop multiple resistance mechanisms against many antibiotics including carbapenems; and making these infections difficult to treat.[1,2] The attributable mortality for nosocomial infection due to Acinetobacter was 8.4-36.5% in recent studies.[3]
Acinetobacter infections are predominant in critically ill patients, particularly in intensive care units (ICUs), and become one of the leading causes of ventilator-associated pneumonia (VAP) in recent years.[1,4,5] Since first reported case in 1991 from the United States, the prevalence of carbapenem-resistant Acinetobacter (CRA) has increased alarmingly up to 85% in the ICUs.[4,6,7,8,9]
In the present study, we aimed to study clinical characteristics, course of illness and outcome of ICU patients who developed VAP due to Acinetobacter species, which was resistant to both carbapenems, i.e., imipenem and meropenem in culture growth sensitivity report.
Materials and Methods
The study was carried out in a 12-bedded ICU of a tertiary care university hospital of India. In this retrospective study, medical record of all ICU patients with microbiologically confirmed VAP due to CRA infection, who were admitted for over 2 years, i.e., from July 2008 to June 2010, were reviewed.
Definitions
CRA-VAP was defined as presence of both: (1) diagnostic criteria for VAP; and (2) growth of CRA showed ++ or +++ semi quantitative culture on a good quality endotracheal aspirate. Diagnosis of VAP was considered clinically, after 48 h of endotracheal intubation, if a new or progressive and persistent infiltrate was present in the chest X-ray together with at least two signs of systemic inflammation, such as fever with temperature >38°C or hypothermia with a temperature <36°C, leukocytosis (>11,000 WBC/mm3) or leucopenia (<4000 WBC/mm3), and with at least one sign of local inflammation such as purulent or increased amount of tracheal aspirate.
Data collected
After ethical clearance from the Institute ethics committee, who waived the need for informed consent, data were retrieved from individual case records of diagnosed CRA-VAP and collected on a structured proforma. The primary outcome was defined as post-CRA-VAP 28-days mortality. Secondary outcome was mortality at ICU discharge, as well as at hospital discharge. The form included age, sex, diagnosis, date of admission to hospital and to the ICU, type of admission (medical/surgical/other hospital), primary reason for admission (respiratory, cardiovascular, neurological, gastrointestinal, renal, trauma and others), history of previous hospitalization, i.e., within the last 3 months, history of immunosuppression (use of steroids, malignancy, chemotherapy), number of co-morbid illness (nil, single, two, three or more), number of organ failures at ICU admission (The organ dysfunction definitions were adapted from the sequential organ failure assessment score (SOFA) score cut-offs: Kidney, Cr > 2 mg/dL or urine < 400 cc/day; lung, PaO2 /FiO2 < 300; liver, total bilirubin >2 mg/dL; coagulation, platelet count <100,000/mm3 ; and hemodynamic, need for vasopressor.), history of pneumonia (community- acquired or hospital-acquired) during current illness, ICU scores, i.e., sequential organ failure assessment (SOFA) and acute physiology and chronic health evaluation (APACHE) II at time of ICU admission, total duration of mechanical ventilation, presence of acute respiratory distress syndrome after CRA-VAP, renal failure, and need of vasoactive agents, total length of ICU stay, were noted.
The number of hospital days prior to CRA-VAP, pre-and post-CRA-VAP duration of mechanical ventilation, history of antibiotics used, history of carbapenem exposure and days of exposure, presence of CRA at others sites, i.e., blood, skin or soft tissue infections, urinary tract infection, as well as concurrent other organisms in the lungs at time of CRA-VAP were also noted.
Statistics
Continuous variables described as median (25th-75th percentile) and categorical variables are described as n (%). For comparative test on continuous variables, Mann–Whitney U-test was applied. For categorical variables, the Pearson Chi-square test or Fisher's exact test were used as appropriate. The response variable used in mortality analysis was vital status (survivor versus non-survivor) 28 days after diagnosis of CRA-VAP. In patients with multiple episodes of VAP, only the first microbiologically confirmed CRA-VAP was retained for further analysis.
Overall, predictors showing a P < 0.05 association with in-hospital mortality in univariate analysis were incorporated in regression analysis. Logistic regression analysis was used to asses the multivariate relation between multiple patient characteristics and outcome of CRA-VAP. Statistical analysis was done using the SPSS-17 software, P < 0.05 was considered significant.
Results
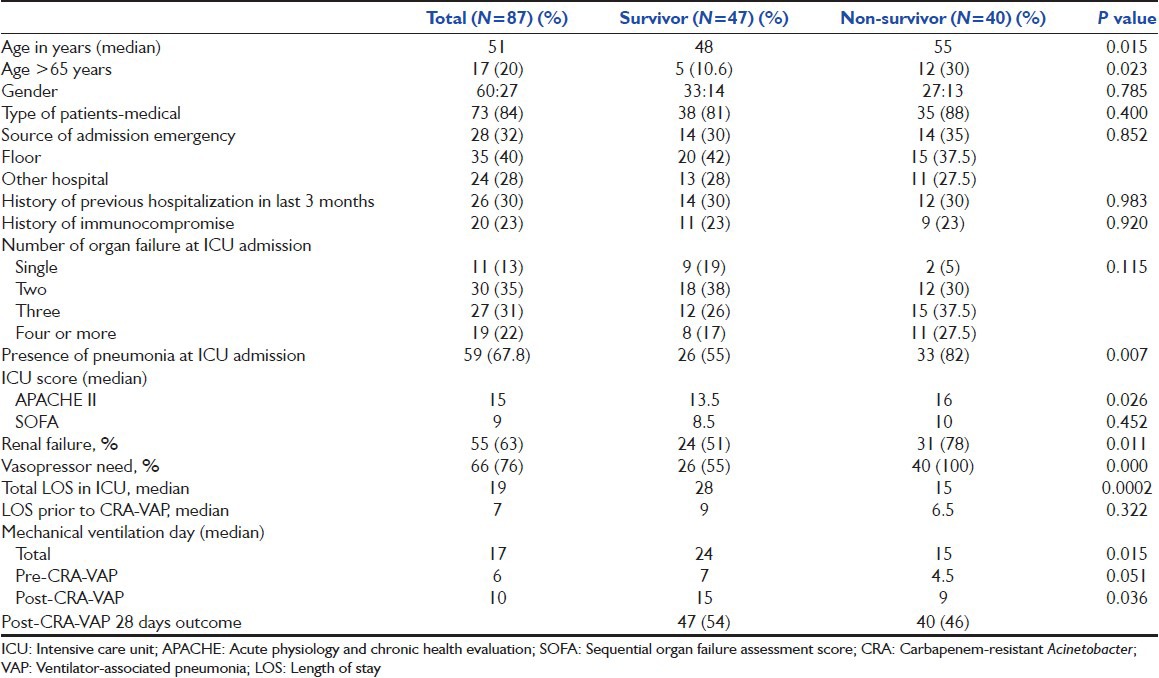
During the study period, out of 576 patients who were treated in the ICU, 118 patients (20.5%) were found to have VAP. Among these, 87 (about 75%) patients who had VAP due to CRA (A. baumannii in 84 cases, while A. lwoffii in 3 cases) were included in this study. Sixty (69%) patients were male; median age was 51 years; 17 (20%) patients were > 65 years of age; 73 (84%) patients were medical; 54 (62%) patients had history of co-morbid illness; 26 (30%) patients had history of hospitalization in last 3 months; severity score (median) at ICU admission were: APACHE II 15 and SOFA 9; at ICU admission 59 (68%) patients had pneumonia; primary reason for ICU admission was respiratory failure (34%); 46 (53%) patients had more than 2-organ failure at ICU admission; median length of ICU stay was 19 days; 66 (76%) patients need vasoactive agents during ICU stay, whereas 55 (63%) patients had renal failure; median duration of mechanical ventilation was 17 days; 22 (25%) patients had ARDS during ICU stay [Table 1].
Table 1.
Clinical characteristics and course of patients with carbapenem-resistant Acinetobacter ventilator-associated pneumonia
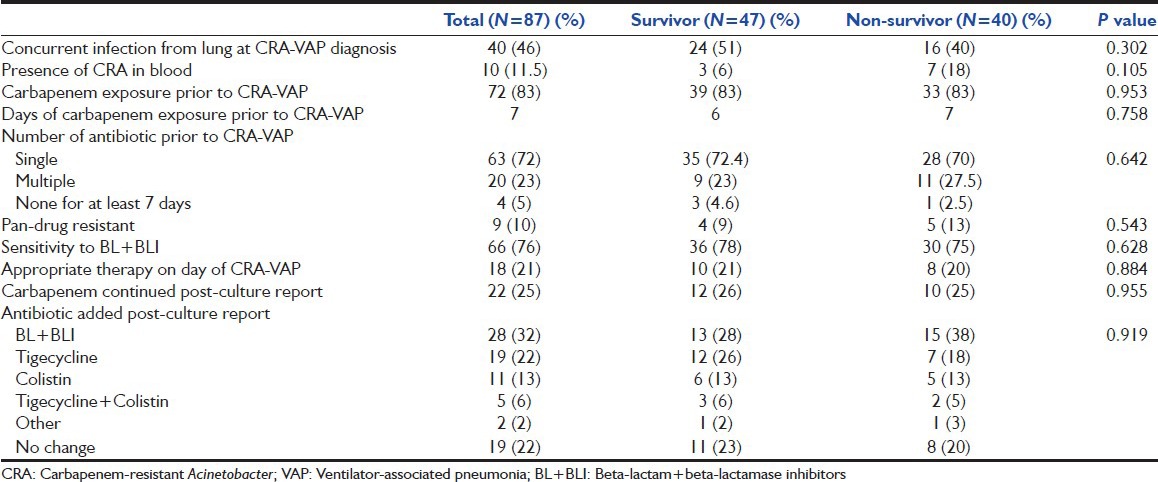
There was growth of gram-negative bacteria in endotracheal aspirate from 54 (62%) patients before diagnosis of CRA-VAP; whereas 39 (45%) patients had concurrent other bacterial growth from the endotracheal aspirate at the time of CRA-VAP diagnosis and 33 (38%) patients had been found with CRA from the other sites. There was a history of exposure to carbapenem, before inclusion in the study, in 72 (83%) patients (30 patients culture guided and, in 42 patients empirically used) with average 7 days (median) of exposure. The sensitivity pattern of CRA had shown pan-drug resistance pattern, including colistin, in 9 (10%) patients; whereas it was sensitive to only one drug in 21 (24%) patients [Table 2].
Table 2.
Microbiological and antibiotics data among patients with carbapenem-resistant Acinetobacter ventilator-associated pneumonia
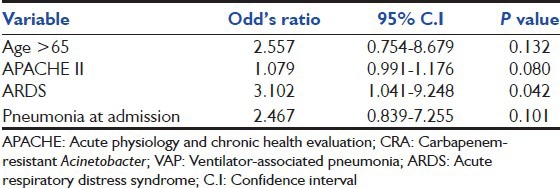
During follow-up, 40 (46%) patients died at 28 days after having CRA-VAP; whereas an overall of 47 (54%) patients died in further follow-up till ICU/hospital discharge in this group of patients. During the study period, among all (118) VAP patients, overall mortality was found to be 46.6% in their follow-up till hospital discharge. A total of 136 patients (28%) died among the remaining 489 ICU patients during the study period. Significant predictors of the outcome were: Age (P = 0.01); age >65 years (P = 0.02); APACHE II score at ICU admission (P = 0.01); presence of pneumonia at ICU admission (P = 0.006); need of vasopressor (P = 0.000); presence of ARDS (P = 0.01) and renal failure (P = 0.01) [Table 1]. While, only presence of ARDS was found to be significant predictor of outcome on multivariate analysis in this cohort group of patients[Table 3].
Table 3.
Predictors of 28-days mortality on multivariate analysis in patients with CRA-VAP
Discussion
Acinetobacter was first described by a Dutch microbiologist, a century ago, as Micrococcus calcoaceticus, but became known as Acinetobacter only in 1950's and till now, more than 25 species have been identified.[1,10] Acinetobacter infections have increasingly been reported during the past decades.[1,2,11] This pathogen has commonly been isolated from hospital environment including equipments, hands of the hospital staff, and patients, more often from the ICUs. Risk factors for Acinetobacter infections include stay in the ICU, recent surgery, mechanical ventilation, indwelling catheters, antibiotic exposures including carbapenem, previous hospitalization, and underlying chronic disease.[1,7] The main clinical syndromes due to Acinetobacter infections in ICU patients include pneumonia and bacteremia.[6,7]
This pathogen has the ability to develop resistance to a wide range of antimicrobial agents including carbapenem. The nosocomial occurrence of CRA is strongly related to an ICU stay and duration of hospital stay, and may be favored by the selection pressure of previously used antibiotics.[12] The limitation of our retrospective study is that we did not obtain data from carbapenem-sensitive Acinetobacter infection that would have allowed us to find out risk factors, as well as attributable mortality rate for CRA-VAP.
In our case series of 87 patients with VAP due to CRA, most patients were <55 years of age with median age being 51 years. In other studies also, the mean age of patients with CRA infection was 43 to 58 years.[8,12,13,14] Age is one of the factors that have a bearing on survival, however, age >65 years was found to be associated with poorer prognosis as in another study.[8,14,15,16] The prevalence of CRA infection was more in male patients in other studies, like in our study.[8,17,18] We did not find that type of patients whether medical or surgical, and source of admission either from emergency, in-hospital from wards or from other hospital have effect on the outcome in these patients. Though in our study, about two-thirds of the patients had co-morbid illness, but this has no impact on the survival of patients with CRA-VAP; whereas in a another study also, in-patient population of imipenem-resistant Acinetobacter bacteremia, presence of co-morbidity had no impact on the outcome.[14] Neither primary reason nor number of organ failures at the time of ICU admission had impact on the outcome. ICU survival scores have been used to assess prognosis in all ICU patients. In this study also, we calculated both APACHE II and SOFA score at the time of ICU admission, but only APACHE II score was significantly higher among non-survivors as shown in another study also, this suggests that the non-survivors were the sicker group of patients.[16,19]
Patients with history of previous hospitalization (in the last 3 months) and immunocompromise did not have a bearing on the outcome in our study group, whereas immunosuppressive status was found to have an impact on outcome in patients with CRA bacteremia.[14] In our study, history of alcohol intake was associated with poor outcome in CRA-VAP patients with possible explanation that alcohol intake leads to free radical formation and oxidant lung injury. Those patients, who had pneumonia either CAP or health care-associated pneumonia at the time of ICU admission, were associated with poor prognosis. Sixty-six patients (76%) had septic shock and out of these, 40 (60%) died. Three recently published studies also had found that the presence of septic shock is a significant prognostic factor in patients with CRA infection.[16,17,20,21]
In a matched cohort study by Eberle et al. among trauma patients, incidence of ARDS was more frequent in the Acinetobacter group compared to the control group having other microorganisms (35% vs. 15%).[22] In our study, we had found that the presence of ARDS in patients with CRA-VAP is associated with poor outcome; 22 patients had developed ARDS and only seven survived. The median duration of mechanical ventilation prior to development of CRA-VAP was 4.5 days in non-survivors as compared to 7 days in survivors. This is in contrast to the belief that early VAP is associated with better prognosis as compared to late VAP. Median duration of mechanical ventilation post-CRA-VAP was 24 days in survivors as compared to 9 days in non-survivors, which was statistically significant. Similarly, length of ICU stay was also longer in survivors as compared to non-survivors. Average length of ICU stay have been found to be variable among different ICU population, like in burn patients 49 days, whereas among general ICU patients it was 15 days.[8,23]
Common concurrent infections from the lungs at the time of diagnosis of CRA-VAP, were Klebsiella, Pseudomonas, Staphylococcus and their presence did not affect the outcome of patients in our study. Apart from the lungs, CRA was found mainly in the blood (n = 23), followed by abdomen (n = 7), and other sites (n = 3). Presence of CRA from other sites did not have prognostic significance.
In our study, prevalence of pan-drug resistant, (including colistin), Acinetobacter was 10%; sensitivity of CRA to single drug was 25%; two drugs was 70%; and it had no significant impact on outcome of the patient. At the time of the diagnosis of CRA-VAP, 21% of patients (20% in survivor vs. 21% in non-survivor) were on appropriate therapy.
Antibiotics were changed or added as per culture sensitivity like beta-lactam + beta-lactamase inhibitors (BL + BLI), colistin, tigecycline, or others (aminoglycoside/quinolones). Most commonly used agents were BL + BLI (32%), followed by tigecycline (22%). In further analysis, we found that around 35 patients received colistin and/or tigecycline, of them 21 (60%) survived, thought not statistically significant, but showed a trend that the use of these two antibiotics in patients with CRA-VAP leads to a better outcome. Other recent studies also conclude that those patients with CRA-VAP should receive either of these two antibiotics along with broad spectrum antibiotic (BL + BLI like sulbactam) for a better outcome.[15,16,24,25]
Factors found significant on univariate analysis were compared on multivariate analysis; only the presence of ARDS was found to be a significant factor on multivariate analysis to predict post-VAP 28 days mortality among patients with CRA-VAP [Table 3]. Overall mortality among hospitalized patients with CRA infections was found to be 45% and 46% in two different studies.[5,26] In ICU patients with CRA infection, 30-days mortality was found to be 47% by Prates et al., and this is comparable to our study (46%).[19]
There are some limitations in this study as being a retrospective, observational, cohort study; there was no control group of patients such as VAP due to carbapenem-sensitive Acinetobacter or other gram-negative organism to assess differences in mortality or patient characteristics.
Conclusion
CRA-VAP has high crude mortality (54%). Advanced age; history of alcohol intake; severity of illness and presence of pneumonia at ICU admission; and presence of shock, ARDS and renal failure have impact on the outcome in these patients. However, on multivariate analysis only presence of ARDS was found to be a significant predictor of outcome in this cohort of patients. In future, a matched cohort study is warranted to investigate risk factors and treatment options for CRA-VAP and their impact on outcome.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Munoz-Price LS, Weinstein RA. Acinetobacter infection. N Engl J Med. 2008;358:1271–81. doi: 10.1056/NEJMra070741. [DOI] [PubMed] [Google Scholar]
- 2.Karageorgopoulos DE, Falagas ME. Current control and treatment of multidrug-resistant Acinetobacter baumannii infections. Lancet Infect Dis. 2008;8:751–62. doi: 10.1016/S1473-3099(08)70279-2. [DOI] [PubMed] [Google Scholar]
- 3.Falagas ME, Rafailidis PI. Attributable mortality of Acinetobacter baumannii: No longer a controversial issue. Crit Care. 2007;11:134. doi: 10.1186/cc5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dizbay M, Tunccan OG, Sezer BE, Hizel K. Nosocomial imipenem-resistant Acinetobacter baumannii infections: Epidemiology and risk factors. Scand J Infect Dis. 2010;42:741–6. doi: 10.3109/00365548.2010.489568. [DOI] [PubMed] [Google Scholar]
- 5.Schimith Bier KE, Luiz SO, Scheffer MC, Gales AC, Paganini MC, Nascimento AJ, et al. Temporal evolution of carbapenem-resistant Acinetobacter baumannii in Curitiba, southern Brazil. Am J Infect Control. 2010;38:308–14. doi: 10.1016/j.ajic.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Go ES, Urban C, Burns J, Kreiswirth B, Eisner W, Mariano N, et al. Clinical and molecular epidemiology of acinetobacter infections sensitive only to polymyxin B and sulbactam. Lancet. 1994;344:1329–32. doi: 10.1016/s0140-6736(94)90694-7. [DOI] [PubMed] [Google Scholar]
- 7.Manchanda V, Sinha S, Singh NP. Multidrug-resistant Acinetobacter. J Global Infect Dis. 2010;2:291–304. doi: 10.4103/0974-777X.68538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trottier V, Segura PG, Namias N, King D, Pizano LR, Schulman CI. Outcomes of Acinetobacter baumannii infection in critically ill burned patients. J Burn Care Res. 2007;28:248–54. doi: 10.1097/BCR.0B013E318031A20F. [DOI] [PubMed] [Google Scholar]
- 9.Mera RM, Miller LA, Amrine-Madsen H, Sahm DF. Acinetobacter baumannii 2002-2008: Increase of carbapenem-associated multiclass resistance in the United States. Microb Drug Resist. 2010;16:209–15. doi: 10.1089/mdr.2010.0052. [DOI] [PubMed] [Google Scholar]
- 10.Beijerinck MW. Uber pigmentbildungbei essigbakterien. Cent Bakteriol Parasitenk. 1911;29:169–76. [Google Scholar]
- 11.Gaynes R, Edwards JR. National Nosocomial Infections Surveillance System. Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis. 2005;41:848–54. doi: 10.1086/432803. [DOI] [PubMed] [Google Scholar]
- 12.Baran G, Erbay A, Bodur H, Ongürü P, Akinci E, Balaban N, et al. Risk factors for nosocomial imipenem-resistant Acinetobacter baumannii infections. Int J Infect Dis. 2008;12:16–21. doi: 10.1016/j.ijid.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Lee SO, Kim NJ, Choi SH, Hyong Kim T, Chung JW, Woo JH, et al. Risk factors for acquisition of imipenem-resistant Acinetobacter baumannii: A case-control study. Antimicrob Agents Chemother. 2004;48:224–8. doi: 10.1128/AAC.48.1.224-228.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwon KT, Oh WS, Song JH, Chang HH, Jung SI, Kim SW, et al. Impact of imipenem resistance on mortality in patients with Acinetobacter bacteraemia. J Antimicrob Chemother. 2007;59:525–30. doi: 10.1093/jac/dkl499. [DOI] [PubMed] [Google Scholar]
- 15.Curcio D, Fernández F, Vergara J, Vazquez W, Luna CM. Late onset ventilator-associated pneumonia due to multidrug-resistant Acinetobacter spp.: Experience with tigecycline. J Chemother. 2009;21:58–62. doi: 10.1179/joc.2009.21.1.58. [DOI] [PubMed] [Google Scholar]
- 16.Oliveira MS, Prado GV, Costa SF, Grinbaum RS, Levin AS. Ampicillin/sulbactam compared with polymyxins for the treatment of infections caused by carbapenem-resistant Acinetobacter spp. J Antimicrob Chemother. 2008;61:1369–75. doi: 10.1093/jac/dkn128. [DOI] [PubMed] [Google Scholar]
- 17.Hernández-Torres A, García-Vázquez E, Gómez J, Canteras M, Ruiz J, Fernández-Rufete A, et al. [Carbapenem and multidrug-resistant Acinetobacter baumannii colonisation/infection: Epidemiology and factors associated with infection] Med Clin (Barc) 2010;135:389–96. doi: 10.1016/j.medcli.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 18.Asensio A, Cantón R, Vaqué J, Calbo-Torrecillas F, Herruzo R, Arribas JL, et al. [Prevalence of infection by carbapenem-resistant Acinetobacter baumannii in Spain (1999-2005)] Enferm Infecc Microbiol Clin. 2008;26:199–204. doi: 10.1016/s0213-005x(08)72691-0. [DOI] [PubMed] [Google Scholar]
- 19.Prates CG, Martins AF, Superti SV, Lopes FS, Ramos F, Cantarelli VV, et al. Risk factors for 30-day mortality in patients with carbapenem-resistant Acinetobacter baumannii during an outbreak in an intensive care unit. Epidemiol Infect. 2011;139:411–8. doi: 10.1017/S0950268810001238. [DOI] [PubMed] [Google Scholar]
- 20.del Mar Tomas M, Cartelle M, Pertega S, Beceiro A, Llinares P, Canle D, et al. Hospital outbreak caused by a carbapenem-resistant strain of Acinetobacter baumannii: Patient prognosis and risk-factors for colonisation and infection. Clin Microbiol Infect. 2005;11:540–6. doi: 10.1111/j.1469-0691.2005.01184.x. [DOI] [PubMed] [Google Scholar]
- 21.Munoz-Price LS, Zembower T, Penugonda S, Schreckenberger P, Lavin MA, Welbel S, et al. Clinical outcomes of carbapenem-resistant Acinetobacter baumannii bloodstream infections: Study of a 2-state monoclonal outbreak. Infect Control Hosp Epidemiol. 2010;31:1057–62. doi: 10.1086/656247. [DOI] [PubMed] [Google Scholar]
- 22.Eberle BM, Schnüriger B, Putty B, Barmparas G, Kobayashi L, Inaba K, et al. The impact of Acinetobacter baumannii infections on outcome in trauma patients: A matched cohort study. Crit Care Med. 2010;38:2133–8. doi: 10.1097/CCM.0b013e3181f17af4. [DOI] [PubMed] [Google Scholar]
- 23.Playford EG, Craig JC, Iredell JR. Carbapenem-resistant Acinetobacter baumannii in intensive care unit patients: Risk factors for acquisition, infection and their consequences. J Hosp Infect. 2007;65:204–11. doi: 10.1016/j.jhin.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Chan JD, Graves JA, Dellit TH. Antimicrobial treatment and clinical outcomes of carbapenem-resistant Acinetobacter baumannii ventilator-associated pneumonia. J Intensive Care Med. 2010;25:343–8. doi: 10.1177/0885066610377975. [DOI] [PubMed] [Google Scholar]
- 25.Dizbay M, Altuncekic A, Sezer BE, Ozdemir K, Arman D. Colistin and tigecycline susceptibility among multidrug-resistant Acinetobacter baumannii isolated from ventilator-associated pneumonia. Int J Antimicrob Agents. 2008;32:29–32. doi: 10.1016/j.ijantimicag.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 26.Sheng WH, Liao CH, Lauderdale TL, Ko WC, Chen YS, Liu JW, et al. A multicenter study of risk factors and outcome of hospitalized patients with infections due to carbapenem-resistant Acinetobacter baumannii. Int J Infect Dis. 2010;14:e764–9. doi: 10.1016/j.ijid.2010.02.2254. [DOI] [PubMed] [Google Scholar]


