Abstract
Sepsis is the commonest cause of admission to medical ICUs across the world. Mortality from sepsis continues to be high. Besides shock and multi-organ dysfunction occurring following the intense inflammatory reaction to sepsis, complications arising from sepsis-related immunoparalysis contribute to the morbidity and mortality from sepsis. This review explores the basis for sepsis related immune dysfunction and discusses its clinical implications for the treating intensivist. Recent trends indicate that a significant proportion of septic patients succumb to the complications of secondary infections and chronic critical care illness from the initial bout of sepsis. Therefore care-givers in the ICU need to be aware of the impediments posed by sepsis-related immune dysfunction that can impair recovery in patients with sepsis and contribute to sepsis-related mortality.
Keywords: Chronic critical care illness, sepsis, immune dysfunction, pneumonia, ventilator-associated
Introduction
Sepsis develops in 750,000 people annually and remains a major cause of mortality with an estimated 200,000 deaths annually in USA.[1] The understanding and treatment of sepsis has evolved considerably over the last few decades. The cornerstones of sepsis therapy however, remain the localization of the source of infection followed by prompt initiation of antibacterial therapy in conjunction with hemodynamic, respiratory and renal support.[1,2] Despite a vigorous and uncontrollably sustained inflammatory response to infection to septic patients, immune dysfunction is a notable feature of severe sepsis.[1,2] The implications of immune dysfunction in sepsis are considerable. Immune dysfunction predisposes septic patients to secondary infection that can delay recovery.[1] In addition, immune dysfunction may delay restitution of the necessary milieu crucial to healing following severe sepsis and can potentially contribute to ongoing multi-organ dysfunction. Therefore, understanding the mechanisms of immune dysfunction and potential secondary implications are paramount in the management of the severely septic patient.
SIRS vs. CARS
In 1991, the American College of Chest Physicians (ACCP) and the Society of Critical Care Medicine (SCCM) recognized that systemic inflammation accompanied severe infection and sterile trauma and introduced the term ‘systemic inflammatory response syndrome’ (SIRS) to connote the consequent inflammatory response.[3] SIRS is defined when patients have 2 or more of the following clinical criteria: Body temperature >38°C or <36°C; heart rate >90/min; hyperventilation evidenced by a respiratory rate of >20/min or a PaCO2 of <32 mm Hg, and a white blood cell count of >12,000 cell/Ml or <4,000 cells/Ml.[3] Sepsis is defined as documented or suspected infection with evidence of systemic inflammation manifested by 2 or more SIRS criteria.[3] In 1996, the concept of ‘compensatory anti-inflammatory response syndrome’ (CARS) was introduced.[4] CARS was described as an immunological phenomenon occurring in sepsis characterized by the induction of several anti-inflammatory mechanisms.[4,5] However, the time course and intensity of evolution of anti-inflammatory mechanisms following an intense pro-inflammatory reaction is variable although immunoparalysis seems typical in patients surviving the initial hyper-inflammatory response.[6] For understanding sepsis-related immunoparalysis, and the ensuing clinical complications, knowledge of mechanisms that underlie both the initial inflammatory response and anti-inflammatory response are important.
Pro-inflammatory Phase in Sepsis
During sepsis, recognition of the pathogen by the host and initiation of pro-inflammatory response constitute key elements of the innate immune system reaction to the offending pathogen.[7] Recognition of the pathogen by toll-like receptors (TLRs) is the best characterized mechanism for sensing gram-negative endotoxemia.[8] Germ-line-encoded pattern recognition receptors (PRRs) in innate immune cells can identify a variety of pathogen-associated molecular patterns besides endotoxin.[7] Four major categories for PRRs have been recognized: TLRs, Nod-Like receptors, C-type lectin receptors, and retinoic acid-induced gene.[7] Innate immune germ-line coded receptors have therefore an ability to recognize a variety of microbiological molecular signatures that include peptidoglycans, mannans, bacterial DNA, double-stranded RNA and glucans.[8] Following recognition, macrophage and dendritic cells engage in endocytosis by which bacterial products are processed for presentation on antigen-presenting cells.[8] Alternatively, stimulation of TLRs on innate immune cells results in direct activation of cytokine pathways and particularly expression of nuclear factor kappa-light-chain-enhancer of activated B-cells (NF-κB) mediated and stress-kinase cytokine pathways.[8] The signal through TLRs produces various inflammatory cytokines, type 1 interferons, anti-microbial proteins, and chemokines.[7] The exuberant pro-inflammatory response mediated by tumor necrosis factor, interleukin-1 (IL-1), IL-2, IL-6, IL-8, high-mobility group box-1 (HMGB-1), macrophage migratory inhibitory factor (MIF), nitric oxide (NO), platelet-activating factor (PAF), C3a-5a, prostaglandins and leukotrienes results in a horde of systemic effects secondary to diffuse activation of neutrophils with capillary damage, interstitial damage, fibrin deposition and organ damage[9] [Table 1]. The intensity of the initial inflammatory response determines the likelihood of multi-organ dysfunction and shock that accompanies the septic response.[9] Although, variations in TNF, IL-1 and PAI-1 polymorphisms may account for differences in the inflammatory response to sepsis, further work needs to be done to understand the wide spectrum of host human responses to infection.[9]
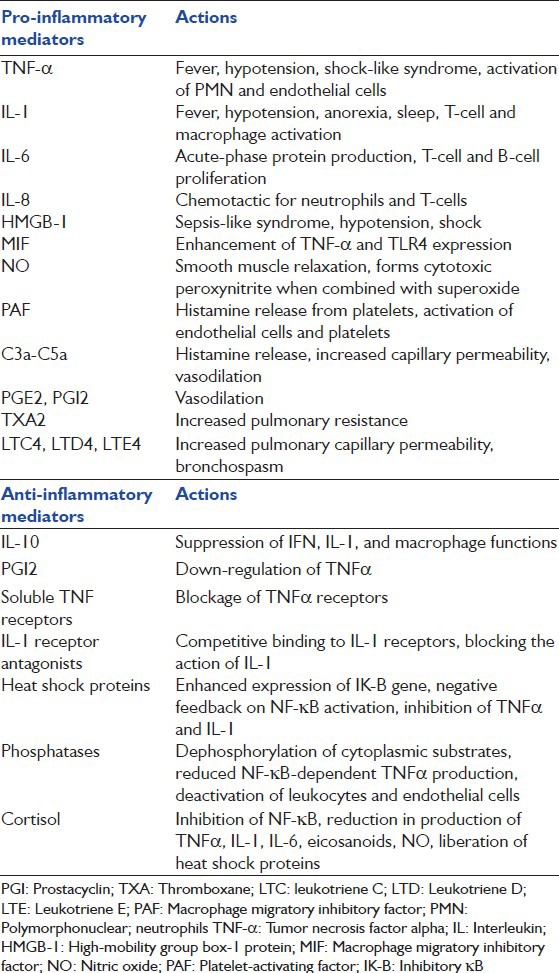
Table 1.
List of principal pro and anti-inflammatory mediators (Reprinted with permission from Jean-Baptiste E. Cellular mechanisms in sepsis. J Intensive Care Med 2007;22:63-72)
Shift to an Anti-Inflammatory Response
Following any systemic inflammatory response, an orderly transition to a hypo-inflammatory state and a restorative phase occurs.[10,11] Multiple mechanisms are believed to bring about the transition to a hypo-inflammatory state that helps limit the unbridled cytokine response that causes organ dysfunction and shock. These include the following
Gene-specific epigenetic reprogramming occurs within hours of TLR activation of the NF-κB-p65 dependent initiation phase to silence pro-inflammatory genes and activate anti-inflammatory pathways.[11]
With TLR-related energy burst, there are changes in NADH/NAD+ ratio that result in changes in cellular metabolism simulating “cellular hibernation” that parallels the adaptive phase of systemic inflammation.[10] These metabolic changes occurring in conjunction with epigenetic reprogramming that follows the acute pro-inflammatory phase pave the way for the hypo-inflammatory phase of sepsis.[10]
Anti-Inflammatory or Immune Dysfunction Phase
Following the onset of hypo-inflammatory phase, a period of immune dysfunction dominates [Figure 1]. Three main processes account for the immune dysfunction
Figure 1.
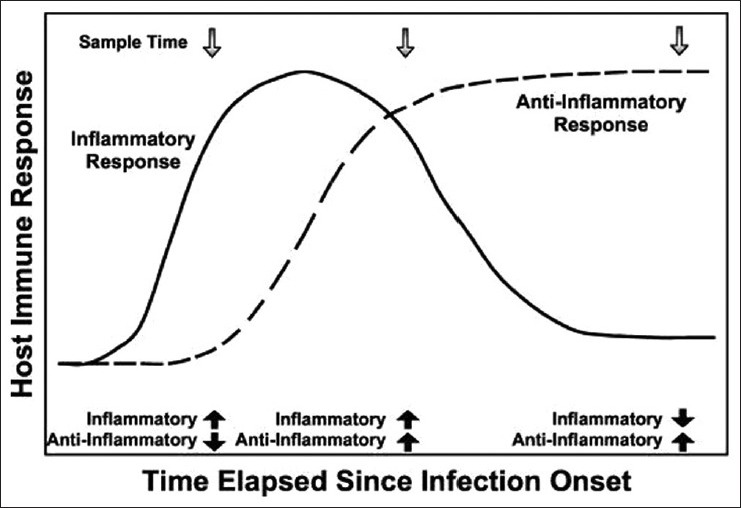
Pro and anti-inflammatory responses in sepsis (Reprinted with permission from Carrigan SD, Scott D, Tabrizian M. Towards resolving the challenges of sepsis diagnosis. Clin Chem 2004;50:1301-14)
Anergy: Impaired response to antigen with decreased release of cytokines in T-cells is a major cause for immune dysfunction occurring during sepsis.[12,13] Aberrant responses to bacterial stimuli are also found in cells of the innate immune system with both splenic and lymph nodal dendritic cells demonstrating a decreased ability for IL-2 synthesis and T-cell activation.[14] In addition, loss of macrophage expression of major histocompatibility complex (MHC)class II and co-stimulatory molecules contribute to monocyte dysfunction and the decrease in CD14/HLA-DR co-expression correlates with degree of immunoparalysis and confers a poorer outcome in severe sepsis.[15]
Shift to anti-inflammatory cytokines: A shift to IL-10 production in monocytes occurs following the pro-inflammatory phase and this shift is initiated by high TNF-α levels.[16] A prominent role for programmed death-1 (PD-1) protein is being ascribed for maintaining the immunoparalysis of sepsis, especially in HIV patients.[17] PD-1 stimulates IL-10 production with subsequent impairment of T-cell proliferative responses and sensing.[17,18] A number of other cytokines have been implicated in the anti-inflammatory response in sepsis-related immunoparalysis [Table 1].
- Death of immune cells: Autopsy studies for patients died of sepsis show a profound, progressive, apoptosis-induced loss of cells of the adaptive immune system.[12] As compared to necrosis that occurs from an acute metabolic disturbance (and consequent ATP depletion), apoptosis represents the execution of an ATP-dependent death program that is triggered following death receptor activation.[19] Apoptosis is not associated with inflammation and therefore induces anergy and an anti-inflammatory state whereas rupture of the necrotic cells incites an inflammatory response that causes immune stimulation.[12,19] Apoptosis is prominent in CD4 T-cells, B-cells, natural killer cells and follicular dendritic cells in septic patients.[12,20] Two pathways for apoptosis have been identified, both of which converge to caspase activation.
- Mitochondrial-mediated pathway: Bcl-2 activation protects against apoptosis and loss of Bcl-2 gene occurs in tumors with consequent loss of apoptotic regulation.[22] Among the Bcl-2 family of proteins, those with BH3 domains can neutralize the anti-apoptotic members and can cause increase in mitochondrial cell membrane permeability with release of cytochrome c and activation of caspase-9. [21,22]
Markers of cell death in the form of caspase-specific proteolytic activity measurements in serum can provide a window into the intensity of ongoing apoptosis vs. necrosis during sepsis.[19] Although, apoptosis may be important in immunoparalysis that accompanies dropout of cells in the innate and adaptive immune system, organ dysfunction as manifested by liver impairment may occur from necrotic mechanisms.[23] This was demonstrated in a study measuring caspase-cleaved and uncleaved cytokeratin-18, a protein marker of cell death originating from hepatocytes, renal, intestine and lung parenchymal cells.[23]
Implications of Immunosuppression
Although apoptosis of monocytes and other immune cells in early sepsis may be a mechanism of abrogating the deleterious effects accompanying the hyper-inflammatory phase,[24] the resulting profound immunoparalysis that follows has its own serious consequences on the septic host. The duration of immunoparalysis depends on various factors including the type, location and severity of infection,[25] age of the patient[8,26] and co-morbidities (with cancer and organ failure prolonging duration of immunoparalysis[27]). Although, the intensity of the initial inflammatory response as measured by the levels of cytokines can be a prognosticator of survival, the key factors affecting recovery remain organ dysfunction and shock in sepsis,[28] and the duration and intensity of immunoparalysis[12] [Figure 1]. Immunoparalysis following sepsis leads to secondary infections that also perpetuates the failure of recovery of organ function following the initial pro-inflammatory response. Whether immunoparalysis has any direct effects on the recovery of organ function following sepsis remains to be shown.
The effects of sepsis-related immunoparalysis may be compounded in the elderly due to a number of reasons. Elderly patients exhibit an increased susceptibility to both infection and to the development of septic shock; these stem from a number of immune defects that occur with aging.[8,26,29]
Abnormalities in innate immune cells include decreased TLR expression, decreased mitogen-activated protein kinase activity with decreased production of TNF-α and IL-6
Reduction in natural killer cell lytic activity with decreased interferon-γ (IFN- γ) production in response to cytokines
Decreased oxidative radical generation in neutrophils and monocytes/macrophages
Shift from Th1 to Th2 cytokine profile, poor helper T-cell function for B-cells and decreased humoral response to neoantigens
Disproportionate increase in apoptosis in elderly septic patients.
Immune senescence has important implications in the care of septic elderly patients. Although, this explains an increased susceptibility to septic shock and mortality in the elderly,[26] there may be modifiable factors specific to elderly that enhance immune function during bouts of sepsis and improve outcomes.[29] Given the rising population of septic elderly patients in intensive care units (ICUs)s,[30] there needs to be further work in understanding immune factors that predispose these patients to chronic critical care illness.[30]
Secondary Infections in Patients with Sepsis-Related Immune Dysfunction
Bacterial infections
Critically ill patients are susceptible to a variety of bacterial infections usually accessing the human body along inserted tubes and catheters. Among various infections, ventilator-associated pneumonia (VAP) remains a secondary complication with a high morbidity and mortality.[31] Given the inevitable immunoparalysis seen following sepsis-related critical illness, measures to reduce the incidence of VAP by decreasing bacterial colonization and entry into the lungs are paramount.[32] The organisms causing VAP range from common commensals in the oropharynx to resistant pathogens such as Pseudomonas, Acinetobacter, methicillin-resistant Staphylococcus aureus and Stenotrophomonas and this attests further to impairment of a broad range of immune defenses.[33] Occurrence of a VAP can add to the lymphocyte and monocyte apoptosis and further the immunoparalysis engendered by the primary bout of sepsis.[34] The occurrence of VAP may be predicted by sequential measurement of IL-6 levels which has been shown to discriminate VAP from other causes of pulmonary infiltrates.[35] Similar to adults, nosocomial pneumonia in children is correlated with degree of immunoparalysis as defined by whole blood ex vivo lipopolysaccharide (LPS) induced tumor necrosis factor α (TNFα) response <200 pg/ml.[36] In the latter study, administration of granulocyte-macrophage colony stimulating factor (GM-CSF) in small group of patients restored lipopolysaccharide-induced TNFα response to more than 200 pg/ml and prevented nosocomial infections.[36] Additionally topical therapy with IFN-γ may improve monocyte anergy[37] and may have the potential to improve local defenses and prevent VAP.
Viral infections
The occurrence of reactivation of cytomegalovirus (CMV) and herpes simplex virus-1 (HSV) infections in critically ill patients is further attestation to the impairment of innate and adaptive defects occurring secondary to sepsis-induced apoptosis.[6] CMV and HSV reactivation are associated with prolonged ICU hospitalizations.[38,39] In addition, HSV and CMV reactivation in ICU patients are markers of poorer ICU outcomes with patients reactivating these viruses having prolonged ventilator stays and increased mortality.[40,41] An increased risk of secondary bacterial infections (especially nosocomial pneumonia) has been noted with both HSV and CMV reactivation that may reflect local injury from herpetic or CMV disease or underlying systemic immune dysfunction secondary to critical illness.[42,43] Although, sepsis-related immunosuppression may be an obvious cause for reactivation of the herpes family of viruses in critically ill patients, animal models of CMV infection show a wide variety of triggers for the reactivation ranging from stress, bacterial sepsis, and TLR4 signaling.[44] Mechanisms of viral reactivation are however, complex with difficulty in predicting at-risk populations and further studies are required to elucidate the pathobiology of HSV and CMV reactivation in ICU patients.[44] Given the high prevalence of herpes reactivation (accounting for 30% of respiratory pathogens in mechanically ventilated patients[45]) and CMV reactivation (in 33% of patients on ventilator >12 days[46]) in ICU patients, understanding and treatment of viral infections are important for improving outcomes.
Fungal infections
Invasive fungal infections from Candida spp. are increasingly common in ICU patients, especially following mechanical ventilation and accompanying infection with gram-negative or gram-positive bacteria.[47] Multiple studies have detailed clinical, microbiological and pharmacological factors predisposing patients to invasive fungal infections.[47,48,49] Besides these, both genetic variations in mucosal immunity[50] and systemic caspase and cytokine activity[51] are being investigated to explain susceptibility to invasive candidiasis. A recent 2-hit murine model of cecal ligation and puncture followed by challenge with C. albicans showed that susceptibility to candidiasis varied with time after initial septic episode and this correlated with the degree of immunosuppression.[52] Further studies on local and systemic immune defenses against fungal infections are needed to develop effective immunotherapy for fungal prophylaxis in septic patients.
Immunomodulating Therapies in Sepsis
In the current era of critical care, majority of patients (except those dying from fulminant meningococcemia or pneumonia) can be supported through their initial hyper-inflammatory phase of sepsis following which they enter into a phase of sepsis-related immunoparalysis.[12] Therefore, current approaches are directed more toward the modulation of cytokine and cellular factors in an attempt to alleviate the degree of immunoparalysis and foster restitution in septic patients. Although, such interventions may be associated with a risk of worsened outcomes as with anti-TNF and anti-IL-6 therapies in early pro-inflammatory phase of sepsis,[53] preliminary studies indicate favorable effects with blockade of anti-inflammatory mechanisms for sepsis-immunoparalysis in both murine and human subjects.
Experimental studies of immune-modulation for sepsis-related immunoparalysis
Studies using models of cecal-ligation and puncture (CLP) and Pseudomonas pneumonia have pursued the basis that apoptosis of lymphocyte and mononuclear cells results in impairment of innate immunity and also of the cross-talk between the adaptive and innate immune systems and that prevention of lymphocyte apoptosis may result in improved survival from sepsis. Given IL-15's pluripotent effects on both the innate and adaptive immune system, administration of IL-15 was shown to improve survival in both CLP and Pseudomonas pneumonia murine models.[54] In addition, IL-15 reduced sepsis-induced NK cell, CD8 T-cell and gut epithelial cell apoptosis along with increasing circulating IFN-Y and anti-apoptotic Bcl-2.[54]
Attempts to modify Bcl-2 anti-apoptotic pathway have used the novel ability of TAT-conjugated pathways to internalize into cell and reduce apoptosis. In this regard, utilization of TAT-conjugated Bcl-xL (particularly it BH4 domain-another member of the anti-apoptotic Bcl-2 pathway) reduced apoptosis in both in vitro CD3 T-cells treated with E. coli and in vivo mice subjected to CLP.[55] Although, caspases are critical to the mediation of apoptosis, the use of caspase inhibitors in sepsis requires careful consideration given the broad array of effects that these agents exert on cellular proliferation and differentiation.[56]
The finding that IL-10 is a key anti-inflammatory molecule[1,57] has led to the demonstration that the use of nontoxic immunomodulator AS101 in CLP models of sepsis.[58,59] This IL-10 mediated immune suppression occurs through specific population of T-CD4+ 25+ regulatory cells and in mice depleted of CD25+ T-cells, there was improved Th1 cytokine release without alteration of pro-inflammatory cytokines following CLP.[60]
Other approaches have utilized the administration of antibodies against critical regulatory proteins that down-regulate cytotoxic T-cell proliferation and activation; both cytotoxic T-lymphocyte antigen 4 (CTLA-4) and PD-1 inhibit CD28 mediated co-stimulation. Antibodies against CTLA-4 and PD-1 in murine CLP models show improved sepsis outcomes.[61,62]
Clinical trials of immune-modulation for sepsis-related immunoparalysis
The initial results with immuno-modulating therapies addressing the TNF and IL-1 pathways have tempered efforts to find biological mediator-based treatments in patients with sepsis.[63] Given the complexity of inflammation in sepsis and temporal variations in immune status, it has been recommended that efforts to understand the immune status in sepsis patients will help substantially in addressing the timing and nature of immune-targeting therapies.[64] Meta-analyses of human studies using granulocyte-colony stimulating factor (G-CSF) and GM-CSF for sepsis have shown no clear-cut benefit in terms of mortality but do demonstrate improved reversal rates from infection.[65,66] Further studies looking at G-CSF or GM-CSF therapy based upon monocyte HLA-DR patterns in sepsis are needed. Similarly, studies on IFN-γ in carefully selected patients with low monocytic HLA-DR expression have shown improvement in the deficient HLA-DR expression and cytokine profile of septic patients.[37] A recent study measured cytokines, monocyte HLA-DR and lymphocyte subset counts following LPS injections to healthy volunteers.[67] In between LPS injections that were given 6 days apart, volunteers received placebo, IFN-γ and GM-CSF.[67] Placebo intervention resulted in immunoparalysis manifested by reduction in LPS-induced TNF-α levels following second LPS injection although IL-10 responses were not significantly changed.[67] IFN-γ treatment between LPS administrations showed improvement in LPS-induced TNF-α responses, reduction in IL-10 levels and improved monocyte HLA-DR expression that were statistically significant; whereas similar patterns were seen with GM-CSF intervention, they did not reach statistical significance.[67]
Conclusion
Understanding of sepsis-induced immune dysfunction offers vast opportunities for improving the mortality and morbidity from prolonged ICU stays and secondary infections. Measurement of cytokine profiles,[68] monocyte HLA-DR expression,[15] whole blood ex vivo LPS-induced TNF-α secretion[36] all afford exciting prospects of estimating the state of relative immune dysfunction in the septic patient. Although, timing of immunostimulant therapy may be critical, a horde of novel therapies are under investigation for improving outcomes from severe sepsis and septic shock.[18,69] Given the compelling evidence that nearly two-thirds of deaths from sepsis occur in the late phase of sepsis due to secondary opportunistic bacterial and fungal infections,[70] there is an urgent need to resolve mechanisms that underlie sepsis-related immunoparalysis. This is especially necessary given the burgeoning population of patients with chronic critical care illness that have prolonged stays in ICUs due to failure of recovery from sepsis and secondary infections.[71]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Skrupky LP, Kerby PW, Hotchkiss RS. Advances in the management of sepsis and the understanding of key immunologic defects. Anesthesiology. 2011;115:1349–62. doi: 10.1097/ALN.0b013e31823422e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 3.Bone RC, Sibbald WJ, Sprung CL. The ACCP-SCCM consensus conference on sepsis and organ failure. Chest. 1992;101:1481–3. doi: 10.1378/chest.101.6.1481. [DOI] [PubMed] [Google Scholar]
- 4.Bone RC. Immunologic dissonance: A continuing evolution in our understanding of the systemic inflammatory response syndrome (SIRS) and the multiple organ dysfunction syndrome (MODS) Ann Intern Med. 1996;125:680–7. doi: 10.7326/0003-4819-125-8-199610150-00009. [DOI] [PubMed] [Google Scholar]
- 5.van der Poll T, Meijers JC. Systemic inflammatory response syndrome and compensatory anti-inflammatory response syndrome in sepsis. J Innate Immun. 2010;2:379–80. doi: 10.1159/000318190. [DOI] [PubMed] [Google Scholar]
- 6.Hotchkiss RS, Coopersmith CM, McDunn JE, Ferguson TA. The sepsis seesaw: Tilting toward immunosuppression. Nat Med. 2009;15:496–7. doi: 10.1038/nm0509-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chong DL, Sriskandan S. Pro-inflammatory mechanisms in sepsis. Contrib Microbiol. 2011;17:86–107. doi: 10.1159/000324022. [DOI] [PubMed] [Google Scholar]
- 8.De Gaudio AR, Rinaldi S, Chelazzi C, Borracci T. Pathophysiology of sepsis in the elderly: Clinical impact and therapeutic considerations. Curr Drug Targets. 2009;10:60–70. doi: 10.2174/138945009787122879. [DOI] [PubMed] [Google Scholar]
- 9.Jean-Baptiste E. Cellular mechanisms in sepsis. J Intensive Care Med. 2007;22:63–72. doi: 10.1177/0885066606297123. [DOI] [PubMed] [Google Scholar]
- 10.McCall CE, El Gazzar M, Liu T, Vachharajani V, Yoza B. Epigenetics, bioenergetics, and microRNA coordinate gene-specific reprogramming during acute systemic inflammation. J Leukoc Biol. 2011;90:439–46. doi: 10.1189/jlb.0211075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCall CE, Yoza BK. Gene silencing in severe systemic inflammation. Am J Respir Crit Care Med. 2007;175:763–7. doi: 10.1164/rccm.200610-1436CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–50. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 13.Heidecke CD, Hensler T, Weighardt H, Zantl N, Wagner H, Siewert JR, et al. Selective defects of T lymphocyte function in patients with lethal intraabdominal infection. Am J Surg. 1999;178:288–92. doi: 10.1016/s0002-9610(99)00183-x. [DOI] [PubMed] [Google Scholar]
- 14.Tinsley KW, Grayson MH, Swanson PE, Drewry AM, Chang KC, Karl IE, et al. Sepsis induces apoptosis and profound depletion of splenic interdigitating and follicular dendritic cells. J Immunol. 2003;171:909–14. doi: 10.4049/jimmunol.171.2.909. [DOI] [PubMed] [Google Scholar]
- 15.Sáenz JJ, Izura JJ, Manrique A, Sala F, Gaminde I. Early prognosis in severe sepsis via analyzing the monocyte immunophenotype. Intensive Care Med. 2001;27:970–7. doi: 10.1007/s001340100962. [DOI] [PubMed] [Google Scholar]
- 16.Volk HD, Reinke P, Döcke WD. Clinical aspects: From systemic inflammation to ‘immunoparalysis’. Chem Immunol. 2000;74:162–77. doi: 10.1159/000058753. [DOI] [PubMed] [Google Scholar]
- 17.Said EA, Dupuy FP, Trautmann L, Zhang Y, Shi Y, El-Far M, et al. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+T cell activation during HIV infection. Nat Med. 2010;16:452–9. doi: 10.1038/nm.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hotchkiss RS, Opal S. Immunotherapy for sepsis: A new approach against an ancient foe. N Engl J Med. 2010;363:87–9. doi: 10.1056/NEJMcibr1004371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bantel H, Schulze-Osthoff K. Cell death in sepsis: A matter of how, when, and where. Crit Care. 2009;13:173. doi: 10.1186/cc7966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, et al. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27:1230–51. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell death. N Engl J Med. 2009;361:1570–83. doi: 10.1056/NEJMra0901217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang KC, Unsinger J, Davis CG, Schwulst SJ, Muenzer JT, Strasser A, et al. Multiple triggers of cell death in sepsis: Death receptor and mitochondrial-Mediated apoptosis. FASEB J. 2007;21:708–19. doi: 10.1096/fj.06-6805com. [DOI] [PubMed] [Google Scholar]
- 23.Hofer S, Brenner T, Bopp C, Steppan J, Lichtenstern C, Weitz J, et al. Cell death serum biomarkers are early predictors for survival in severe septic patients with hepatic dysfunction. Crit Care. 2009;13:R93. doi: 10.1186/cc7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giamarellos-Bourboulis EJ, Routsi C, Plachouras D, Markaki V, Raftogiannis M, Zervakis D, et al. Early apoptosis of blood monocytes in the septic host: Is it a mechanism of protection in the event of septic shock? Crit Care. 2006;10:R76. doi: 10.1186/cc4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gogos C, Kotsaki A, Pelekanou A, Giannikopoulos G, Vaki I, Maravitsa P, et al. Early alterations of the innate and adaptive immune statuses in sepsis according to the type of underlying infection. Crit Care. 2010;14:R96. doi: 10.1186/cc9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turnbull IR, Clark AT, Stromberg PE, Dixon DJ, Woolsey CA, Davis CG, et al. Effects of aging on the immunopathologic response to sepsis. Crit Care Med. 2009;37:1018–23. doi: 10.1097/CCM.0b013e3181968f3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosolem MM, Rabello LS, Lisboa T, Caruso P, Costa RT, Leal JV, et al. Critically ill patients with cancer and sepsis: Clinical course and prognostic factors. J Crit Care. 2012;27:301–7. doi: 10.1016/j.jcrc.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 28.Pierrakos C, Vincent JL. Sepsis biomarkers: A review. Crit Care. 2010;14:R15. doi: 10.1186/cc8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Opal SM, Girard TD, Ely EW. The immunopathogenesis of sepsis in elderly patients. Clin Infect Dis. 2005;41(Suppl 7):S504–12. doi: 10.1086/432007. [DOI] [PubMed] [Google Scholar]
- 30.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Safdar N, Dezfulian C, Collard HR, Saint S. Clinical and economic consequences of ventilator-associated pneumonia: A systematic review. Crit Care Med. 2005;33:2184–93. doi: 10.1097/01.ccm.0000181731.53912.d9. [DOI] [PubMed] [Google Scholar]
- 32.Morrow LE, Kollef MH. Recognition and prevention of nosocomial pneumonia in the intensive care unit and infection control in mechanical ventilation. Crit Care Med. 2010;38:S352–62. doi: 10.1097/CCM.0b013e3181e6cc98. [DOI] [PubMed] [Google Scholar]
- 33.Chastre J. Conference summary: Ventilator-associated pneumonia. Respir Care. 2005;50:975–83. [PubMed] [Google Scholar]
- 34.Pelekanou A, Tsangaris I, Kotsaki A, Karagianni V, Giamarellou H, Armaganidis A, et al. Decrease of CD4-lymphocytes and apoptosis of CD14-monocytes are characteristic alterations in sepsis caused by ventilator-associated pneumonia: Results from an observational study. Crit Care. 2009;13:R172. doi: 10.1186/cc8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramírez P, Ferrer M, Gimeno R, Tormo S, Valencia M, Piñer R, et al. Systemic inflammatory response and increased risk for ventilator-associated pneumonia: A preliminary study. Crit Care Med. 2009;37:1691–5. doi: 10.1097/CCM.0b013e31819fec5f. [DOI] [PubMed] [Google Scholar]
- 36.Hall MW, Knatz NL, Vetterly C, Tomarello S, Wewers MD, Volk HD, et al. Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome. Intensive Care Med. 2011;37:525–32. doi: 10.1007/s00134-010-2088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Döcke WD, Randow F, Syrbe U, Krausch D, Asadullah K, Reinke P, et al. Monocyte deactivation in septic patients: Restoration by IFN-gamma treatment. Nat Med. 1997;3:678–81. doi: 10.1038/nm0697-678. [DOI] [PubMed] [Google Scholar]
- 38.Limaye AP, Kirby KA, Rubenfeld GD, Leisenring WM, Bulger EM, Neff MJ, et al. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA. 2008;300:413–22. doi: 10.1001/jama.300.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sundar KM, Ludwig KA, Alward WT, Pearce MJ, Bishop CT, Hammond RC, et al. Clinical course and spectrum of intensive care unit patients reactivating herpes simplex-1 virus: A retrospective analysis. Indian J Crit Care Med. 2008;12:145–52. doi: 10.4103/0972-5229.45073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osawa R, Singh N. Cytomegalovirus infection in critically ill patients: A systematic review. Crit Care. 2009;13:R68. doi: 10.1186/cc7875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simoons-Smit AM, Kraan EM, Beishuizen A, Strack van Schijndel RJ, Vandenbroucke-Grauls CM. Herpes simplex virus type 1 and respiratory disease in critically-ill patients: Real pathogen or innocent bystander? Clin Microbiol Infect. 2006;12:1050–9. doi: 10.1111/j.1469-0691.2006.01475.x. [DOI] [PubMed] [Google Scholar]
- 42.Chiche L, Forel JM, Roch A, Guervilly C, Pauly V, Allardet-Servent J, et al. Active cytomegalovirus infection is common in mechanically ventilated medical intensive care unit patients. Crit Care Med. 2009;37:1850–7. doi: 10.1097/CCM.0b013e31819ffea6. [DOI] [PubMed] [Google Scholar]
- 43.Luyt CE, Combes A, Deback C, Aubriot-Lorton MH, Nieszkowska A, Trouillet JL, et al. Herpes simplex virus lung infection in patients undergoing prolonged mechanical ventilation. Am J Respir Crit Care Med. 2007;175:935–42. doi: 10.1164/rccm.200609-1322OC. [DOI] [PubMed] [Google Scholar]
- 44.Cook CH, Trgovcich J. Cytomegalovirus reactivation in critically ill immunocompetent hosts: A decade of progress and remaining challenges. Antiviral Res. 2011;90:151–9. doi: 10.1016/j.antiviral.2011.03.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prellner T, Flamholc L, Haidl S, Lindholm K, Widell A. Herpes simplex virus: The most frequently isolated pathogen in the lungs of patients with severe respiratory distress. Scand J Infect Dis. 1992;24:283–92. doi: 10.3109/00365549209061333. [DOI] [PubMed] [Google Scholar]
- 46.Cook CH, Martin LC, Yenchar JK, Lahm MC, McGuinness B, Davies EA, et al. Occult herpes family viral infections are endemic in critically ill surgical patients. Crit Care Med. 2003;31:1923–9. doi: 10.1097/01.CCM.0000070222.11325.C4. [DOI] [PubMed] [Google Scholar]
- 47.Xie GH, Fang XM, Fang Q, Wu XM, Jin YH, Wang JL, et al. Impact of invasive fungal infection on outcomes of severe sepsis: A multicenter matched cohort study in critically ill surgical patients. Crit Care. 2008;12:R5. doi: 10.1186/cc6766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shoham S, Marwaha S. Invasive fungal infections in the ICU. J Intensive Care Med. 2010;25:78–92. doi: 10.1177/0885066609355262. [DOI] [PubMed] [Google Scholar]
- 49.Playford EG, Marriott D, Nguyen Q, Chen S, Ellis D, Slavin M, et al. Candidemia in nonneutropenic critically ill patients: Risk factors for non-albicans Candida spp. Crit Care Med. 2008;36:2034–9. doi: 10.1097/CCM.0b013e3181760f42. [DOI] [PubMed] [Google Scholar]
- 50.Rosentul DC, Plantinga TS, Oosting M, Scott WK, Velez Edwards DR, Smith PB, et al. Genetic variation in the dectin-1/CARD9 recognition pathway and susceptibility to candidemia. J Infect Dis. 2011;204:1138–45. doi: 10.1093/infdis/jir458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosentul DC, Plantinga TS, Scott WK, Alexander BD, van de Geer NM, Perfect JR, et al. The impact of caspase-12 on susceptibility to candidemia. Eur J Clin Microbiol Infect Dis. 2012;31:277–80. doi: 10.1007/s10096-011-1307-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davis CG, Chang K, Osborne D, Walton AH, Dunne WM, Muenzer JT. Increased susceptibility to Candida infection following cecal ligation and puncture. Biochem Biophys Res Commun. 2011;414:37–43. doi: 10.1016/j.bbrc.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freeman BD, Natanson C. Anti-inflammatory therapies in sepsis and septic shock. Expert Opin Investig Drugs. 2000;9:1651–63. doi: 10.1517/13543784.9.7.1651. [DOI] [PubMed] [Google Scholar]
- 54.Inoue S, Unsinger J, Davis CG, Muenzer JT, Ferguson TA, Chang K, et al. IL-15 prevents apoptosis, reverses innate and adaptive immune dysfunction, and improves survival in sepsis. J Immunol. 2010;184:1401–9. doi: 10.4049/jimmunol.0902307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hotchkiss RS, McConnell KW, Bullok K, Davis CG, Chang KC, Schwulst SJ, et al. TAT-BH4 and TAT-Bcl-xL peptides protect against sepsis-induced lymphocyte apoptosis in vivo. J Immunol. 2006;176:5471–7. doi: 10.4049/jimmunol.176.9.5471. [DOI] [PubMed] [Google Scholar]
- 56.Hotchkiss RS, Coopersmith CM, Karl IE. Prevention of lymphocyte apoptosis: A potential treatment of sepsis? Clin Infect Dis. 2005;41(Suppl 7):S465–9. doi: 10.1086/431998. [DOI] [PubMed] [Google Scholar]
- 57.Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306:2594–605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kalechman Y, Gafter U, Gal R, Rushkin G, Yan D, Albeck M, et al. Anti-IL-10 therapeutic strategy using the immunomodulator AS101 in protecting mice from sepsis-induced death: Dependence on timing of immunomodulating intervention. J Immunol. 2002;169:384–92. doi: 10.4049/jimmunol.169.1.384. [DOI] [PubMed] [Google Scholar]
- 59.Muenzer JT, Davis CG, Chang K, Schmidt RE, Dunne WM, Coopersmith CM, et al. Characterization and modulation of the immunosuppressive phase of sepsis. Infect Immun. 2010;78:1582–92. doi: 10.1128/IAI.01213-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wisnoski N, Chung CS, Chen Y, Huang X, Ayala A. The contribution of CD4+CD25+T-regulatory-cells to immune suppression in sepsis. Shock. 2007;27:251–7. doi: 10.1097/01.shk.0000239780.33398.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Inoue S, Bo L, Bian J, Unsinger J, Chang K, Hotchkiss RS. Dose-dependent effect of anti-CTLA-4 on survival in sepsis. Shock. 2011;36:38–44. doi: 10.1097/SHK.0b013e3182168cce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brahmamdam P, Inoue S, Unsinger J, Chang KC, McDunn JE, Hotchkiss RS. Delayed administration of anti-PD-1 antibody reverses immune dysfunction and improves survival during sepsis. J Leukoc Biol. 2010;88:233–40. doi: 10.1189/jlb.0110037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marshall JC. Clinical trials of mediator-directed therapy in sepsis: What have we learned? Intensive Care Med. 2000;26(Suppl 1):S75–83. doi: 10.1007/s001340051122. [DOI] [PubMed] [Google Scholar]
- 64.Caldwell CC, Hotchkiss RS. The first step in utilizing immune-modulating therapies: Immune status determination. Crit Care. 2011;15:108. doi: 10.1186/cc9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bo L, Wang F, Zhu J, Li J, Deng X. Granulocyte-colony stimulating factor (G-CSF) and granulocyte-macrophage colony stimulating factor (GM-CSF) for sepsis: A meta-analysis. Crit Care. 2011;15:R58. doi: 10.1186/cc10031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mohammad RA. Use of granulocyte colony-stimulating factor in patients with severe sepsis or septic shock. Am J Health Syst Pharm. 2010;67:1238–45. doi: 10.2146/ajhp090325. [DOI] [PubMed] [Google Scholar]
- 67.Leentjens J, Kox M, Koch RM, Preijers F, Joosten LA, van der Hoeven JG, et al. Reversal of Immunoparalysis in Humans in vivo: A Double-blind Placebo-controlled Randomized Pilot-Study. Am J Respir Crit Care Med. 2012 doi: 10.1164/rccm.201204-0645OC. [DOI] [PubMed] [Google Scholar]
- 68.Mera S, Tatulescu D, Cismaru C, Bondor C, Slavcovici A, Zanc V, et al. Multiplex cytokine profiling in patients with sepsis. APMIS. 2011;119:155–63. doi: 10.1111/j.1600-0463.2010.02705.x. [DOI] [PubMed] [Google Scholar]
- 69.Flohé S, Lendemans S, Selbach C, Waydhas C, Ackermann M, Schade FU, et al. Effect of granulocyte-macrophage colony-stimulating factor on the immune response of circulating monocytes after severe trauma. Crit Care Med. 2003;31:2462–9. doi: 10.1097/01.CCM.0000089640.17523.57. [DOI] [PubMed] [Google Scholar]
- 70.Otto GP, Sossdorf M, Claus RA, Rödel J, Menge K, Reinhart K, et al. The late phase of sepsis is characterized by an increased microbiological burden and death rate. Crit Care. 2011;15:R183. doi: 10.1186/cc10332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nelson JE, Cox CE, Hope AA, Carson SS. Chronic critical illness. Am J Respir Crit Care Med. 2010;182:446–54. doi: 10.1164/rccm.201002-0210CI. [DOI] [PMC free article] [PubMed] [Google Scholar]


