Abstract
Context:
Procalcitonin is a biomarker of bacterial sepsis. It is unclear if scrub typhus, a rickettsial illness, is associated with elevated procalcitonin levels.
Aim:
To assess if scrub typhus infection is associated with high procalcitonin levels and whether high levels portend a poorer prognosis.
Setting and Design:
Retrospective study of patients with severe scrub typhus infection, admitted to the medical intensive care unit of a tertiary care university affiliated teaching hospital.
Materials and Methods:
Eighty-four patients with severe scrub typhus infection that also had procalcitonin levels were assessed.
Statistical Analysis:
Relationship between procalcitonin and mortality explored using univariate and multivariate analyses.
Results:
The mean (±standard deviation) age was 40.0 ± 15.5 years. Patients were symptomatic for 8.3 ± 4.3 days prior to presentation. The median admission procalcitonin level was 4.0 (interquartile range 1.8 to 8.5) ng/ml; 59 (70.2%) patients had levels >2 ng/ml. Invasive mechanical ventilation was required in 65 patients; 20 patients died. On univariate analysis, admission procalcitonin was associated with increased odds of death [odds ratio (OR) 1.09, 95% confidence interval (CI) 1.03 to 1.18]. On multivariate logistic regression analysis including procalcitonin and APACHE-II score, the APACHE-II score was significantly associated with mortality (OR 1.16, 95% CI 1.06 to 1.30, P = 0.004) while a trend was observed with procalcitonin (OR 1.05, 95%CI 1.01 to 1.13, P = 0.09). The area under the receiver operating characteristic (ROC) curve, AUC, for mortality was 0.77 for procalcitonin and 0.78 for APACHE-II.
Conclusions:
Procalcitonin is elevated in severe scrub typhus infection and may be associated with higher mortality.
Keywords: Biomarker, intensive care unit, outcome, procalcitonin, scrub typhus
Introduction
Scrub typhus, a rickettsial illness caused by Orientia tsutsugamushi, is endemic to East and Southeast Asia and Northern Australia.[1,2] It is a common cause of undifferentiated acute febrile illness (AFI) in India[3] and can be associated with multi-organ dysfunction.[4] Procalcitonin is a biomarker frequently used in bacterial sepsis.[5] It is unclear if this biomarker is elevated in rickettsial illnesses, particularly in scrub typhus. The aim of the study was to assess if procalcitonin is elevated in severe scrub typhus infection and whether higher levels portend a poorer prognosis.
Materials and Methods
In this retrospective study, consecutive patients admitted to the medical intensive care unit (ICU) with a diagnosis of scrub typhus infection with organ dysfunction[6] between August 2008 and April 2010 who also had procalcitonin levels assessed were studied. Patients were identified from the ICU database. A diagnosis of scrub typhus was made when a patient with an AFI had an eschar and a positive IgM enzyme-linked immunosorbent assay (ELISA) for scrub typhus and other causes of fever excluded.[3] An individual was considered scrub typhus seropositive if a value of ≥16 units was obtained by the Rickettsia Scrub Typhus Group ELISA (Panbio Ltd, Brisbane, Australia) for those individuals tested before August 18, 2009. For those tested thereafter, an optical density of ≥0.5 was considered as diagnostic using Scrub Typhus Detect IgM ELISA (InBios International Inc., Seattle, USA). Our institution changed ELISA brands as the former test became unavailable. Patient demographics, laboratory data, and outcome parameters were obtained from in-patient case-records and hospital electronic database.
Severity of illness scores [Acute Physiology and Chronic Health Evaluation II (APACHE-II) and Sequential Organ Failure Assessment (SOFA) scores] were calculated at admission. Procalcitonin immunoassays were performed using the TRACE (Time Resolved Amplified Cryptate Emission) method on a BRAMHS KRYPTOR analyzer (B*R*A*M*H*S GmbH). The test is routinely done in our clinical biochemistry department as and when samples are received. In the event of the kit being unavailable, the sample is stored at −20°C. The lower limit of detection was 0.02 ng/ml. Only the procalcitonin level obtained within 48 h of admission was included in the study. Consistent with several studies that used a cut-off of >2 ng/ml for diagnosis of sepsis,[5] we assessed whether levels >2 ng/ml were associated with a worse outcome. The outcome of interest was mortality. The relationship between procalcitonin and mortality was explored using univariate and multivariate logistic regression analyses and Fisher's exact tests. Receiver operating characteristic (ROC) curves were generated to assess the relationship between procalcitonin and severity of illness scores on mortality.[7] R version 2.12.2 statistical software was used. The study was approved by the Institutional Review Board. Waiver of informed consent was obtained in view of the retrospective nature of the study.
Results
During the study period, 2263 patients were admitted to the ICU; 116 patients (5.1%) were diagnosed as scrub typhus based on presentation as AFI, a positive IgM ELISA to scrub typhus, the exclusion of other diagnoses, and a therapeutic response to doxycycline. Of the 116 patients, 86 also had procalcitonin levels determined. All 86 patients had a blood culture at admission. Two of these cultures (2.3%) grew pathogenic organisms (Escherichia coli, Acinetobacter baumannii). These patients were excluded. Seven cultures grew contaminants; repeat cultures were negative in these 7 patients. The study cohort thus comprised of 84 patients aged 40.0 ± 15.5 years, presenting with symptoms for 8.3 ± 4.3 days. Other causes of undifferentiated fever such as malaria, dengue, leptospirosis, and enteric fever were excluded by appropriate tests. The admission APACHE-II (n = 73, 20.1 ± 7.7) and SOFA scores (n = 74, 10.4 ± 3.6) indicated a cohort of very sick patients. The median (interquartile range) admission procalcitonin level was 4.0 ng/ml (1.8 to 8.5). Procalcitonin level >2 ng/ml was observed in 59 (70.2%) patients. Ventilation, either invasive or non-invasive, was required in 65/82 (79.3%) patients. Nine patients (11.1%) required dialysis. The duration of hospitalization was 11.4 ± 10.1 days. Twenty patients (23.8%) died.
There was a significant association between mortality and a procalcitonin cut-off of >2 ng/ml (P = 0.005); mortality was higher in those with an admission procalcitonin of >2 ng/ml (19/59 vs. 1/25). Admission procalcitonin (without dichotomization) was associated with an increased odds of death on univariate analysis [odds ratio (OR) 1.09, 95% confidence interval (CI) 1.03 to 1.18, P = 0.011]. On univariate logistic regression analysis, several other factors were also associated with mortality [Table 1]. This included APACHE and SOFA scores, shock at presentation, admission creatinine level, and need for dialysis. The duration of symptoms, altered mental status at admission, and need for mechanical ventilation were associated with a trend to increased mortality. On multivariate logistic regression analysis including procalcitonin and APACHE-II score, the APACHE-II score was significantly associated with mortality (OR 1.16, 95%CI 1.06 to 1.30, P = 0.004), while a trend was observed with procalcitonin (OR 1.05, 95%CI 1.01 to 1.13, P = 0.09). In multivariate logistic models when included with procalcitonin, need for dialysis during admission remained significantly associated with increased mortality risk (OR 5.67, 95%CI 1.16 to 28.53, P = 0.029), although the interval was very wide while procalcitonin retained an almost identical relationship to that described univariately (OR 1.09, 95%CI 1.03 to 1.19, P = 0.022). No other variables from Table 1 remained significantly associated with mortality when included with either procalcitonin, APACHE-II score or SOFA score (P > 0.21, results not presented). The ROC area under the curve for mortality was similar for procalcitonin (0.77), APACHE-II (0.78) and SOFA (0.77) scores [Figure 1].
Table 1.
Baseline characteristics and outcomes of patients admitted to ICU with severe scrub typhus and procalcitonin level assessed
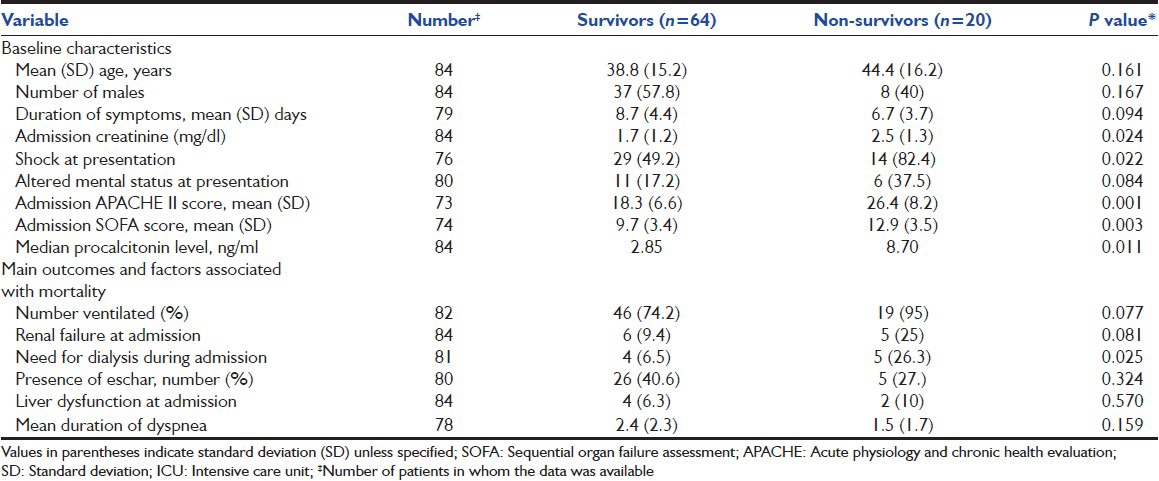
Figure 1.
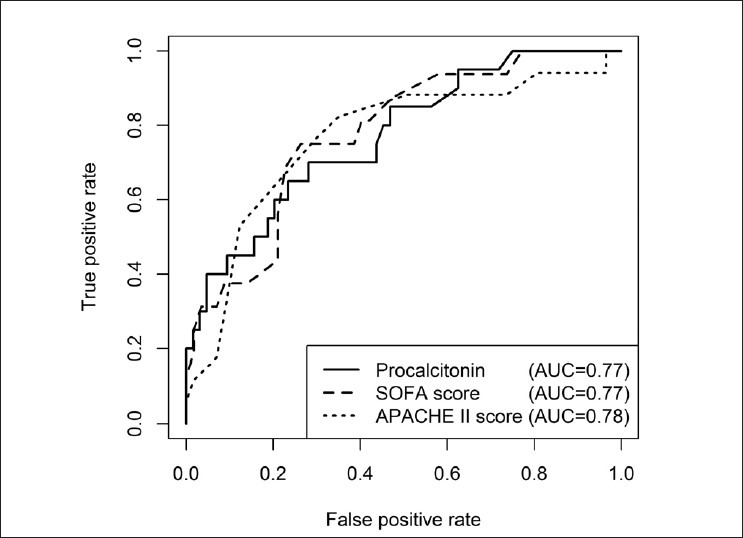
Receiver operating characteristic curves demonstrating the association between mortality and procalcitonin level, Acute physiology and chronic health evaluation II (APACHE-II) score, and sequential organ failure assessment (SOFA) score in patients admitted to the intensive care unit with severe scrub typhus infection. The area under the curve (AUC) for procalcitonin was 0.77. The AUC for APACHE-II and SOFA scores were 0.78 and 0.77, respectively
Discussion
Although procalcitonin was initially described as a biomarker for bacterial infections, it can be elevated in other settings such as malaria,[8] trauma,[9] surgery,[10] pancreatitis,[11] and burns.[12] Procalcitonin has also been used to discriminate between bacterial pneumonia, tuberculosis, and Pneumocystis jirovecii in the setting of human immunodeficiency virus infection presenting with community acquired pneumonia.[13] To our knowledge, ours is the first study to evaluate procalcitonin in a rickettsial illness, namely scrub typhus. We observed that procalcitonin was elevated in a significant proportion of patients with severe scrub typhus infection and that higher levels were probably associated with a worse prognosis. Patients with bacteremia at presentation were excluded, suggesting that high procalcitonin levels in scrub typhus occurs independent of co-existent bacterial sepsis. Although all our patients with AFI with organ-dysfunction were treated with empiric antibiotics (usually pipericillin-tazobactam along with enteral doxycycline and intravenous azithromycin) pending culture and serology reports, in those with the typical eschar and multi-organ dysfunction suggestive of scrub typhus (acute respiratory distress syndrome with or without shock, renal failure, elevated transaminase levels, leukocytosis, and thrombocytopenia), only doxycycline and azithromycin were administered. Combination therapy with doxycycline and azithromycin was used due to non-availability of intravenous doxycycline in our country and concerns regarding absorption of doxycycline in critically ill patients. Intravenous azithromycin (500 mg) was administered once daily for 5 days and enteral doxycycline (100 mg twice daily) for 7 days in all patients.
We observed that procalcitonin may identify a subset of patients who may have an unfavourable outcome. On univariate analysis, procalcitonin levels were significantly associated with mortality. However, on multivariate analysis that included procalcitonin and APACHE-II score, only a trend was observed. Although a procalcitonin level of >2 ng/ml was associated with mortality using Fisher's exact test, the predictive accuracy (defined as the total number of patients correctly identified as having survived or died out of the total number of patients) was highest (82.6%) at a threshold of 17.7 ng/ml. The area under the ROC curve (AUC) for procalcitonin in our study of 0.77 was similar to that of APACHE-II and SOFA scores, tools that are conventionally used to predict outcome in critically ill patients. The correlation between clinical severity-of-illness scores or prognostic scores and serum procalcitonin has been previously reported.[14] The AUC for procalcitonin for severe scrub typhus infection was also marginally better than the AUC of 0.72 for procalcitonin in severe malaria in children.[8]
The following limitations need to be kept in mind while interpreting the results. Several factors are known to impact procalcitonin estimation, including age, gender, body mass index, smoking,[15] presence of renal failure,[16] and previous sepsis.[17] These could have potentially impacted procalcitonin levels. The study was also retrospective in nature, and procalcitonin levels were not available in 28% of the 116 scrub typhus patients admitted to our ICU during the study period. The choice of the serological test to diagnosis scrub typhus also merits mention. Although the indirect immunofluorescent antibody (IFA) assay is considered the gold standard assay, recent studies have shown that the scrub ELISA test has a good sensitivity and specificity as compared to IFA and that the indirect immunoperoxidase tests and could serve as gold standard assays for the diagnosis of scrub typhus.[18]
Despite these limitations, this study provides new information that procalcitonin is elevated in critically ill patients with scrub typhus infection and may be associated with a poorer prognosis. Further studies exploring the role of serial procalcitonin assays and combinations of biomarkers may help prognosticate outcomes better in patients with severe scrub typhus infection.
Footnotes
Source of Support: The project was funded by Fluid Research Grant, Christian Medical College Hospital
Conflict of Interest: None declared.
References
- 1.Koh GC, Maude RJ, Paris DH, Newton PN, Blacksell SD. Diagnosis of scrub typhus. Am J Trop Med Hyg. 2010;82:368–70. doi: 10.4269/ajtmh.2010.09-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graves S, Stenos J. Rickettsioses in Australia. Ann N Y Acad Sci. 2009;1166:151–5. doi: 10.1111/j.1749-6632.2009.04530.x. [DOI] [PubMed] [Google Scholar]
- 3.Chrispal A, Boorugu H, Gopinath KG, Prakash JA, Chandy S, Abraham OC, et al. Scrub typhus: An unrecognized threat in South India-clinical profile and predictors of mortality. Trop Doct. 2010;40:129–33. doi: 10.1258/td.2010.090452. [DOI] [PubMed] [Google Scholar]
- 4.Thap LC, Supanaranond W, Treeprasertsuk S, Kitvatanachai S, Chinprasatsak S, Phonrat B. Septic shock secondary to scrub typhus: Characteristics and complications. Southeast Asian J Trop Med Public Health. 2002;33:780–6. [PubMed] [Google Scholar]
- 5.Uzzan B, Cohen R, Nicolas P, Cucherat M, Perret GY. Procalcitonin as a diagnostic test for sepsis in critically ill adults and after surgery or trauma: A systematic review and meta-analysis. Crit Care Med. 2006;34:1996–2003. doi: 10.1097/01.CCM.0000226413.54364.36. [DOI] [PubMed] [Google Scholar]
- 6.van Gestel A, Bakker J, Veraart CP, van Hout BA. Prevalence and incidence of severe sepsis in Dutch intensive care units. Crit Care. 2004;8:R153–62. doi: 10.1186/cc2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 8.Erdman LK, Dhabangi A, Musoke C, Conroy AL, Hawkes M, Higgins S, et al. Combinations of host biomarkers predict mortality among Ugandan children with severe malaria: A retrospective case-control study. PLoS One. 2011;6:e17440. doi: 10.1371/journal.pone.0017440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mimoz O, Benoist JF, Edouard AR, Assicot M, Bohuon C, Samii K. Procalcitonin and C-reactive protein during the early posttraumatic systemic inflammatory response syndrome. Intensive Care Med. 1998;24:185–8. doi: 10.1007/s001340050543. [DOI] [PubMed] [Google Scholar]
- 10.Meisner M, Tschaikowsky K, Hutzler A, Schick C, Schüttler J. Postoperative plasma concentrations of procalcitonin after different types of surgery. Intensive Care Med. 1998;24:680–4. doi: 10.1007/s001340050644. [DOI] [PubMed] [Google Scholar]
- 11.Ammori BJ, Becker KL, Kite P, Snider RH, Nylén ES, White JC, et al. Calcitonin precursors in the prediction of severity of acute pancreatitis on the day of admission. Br J Surg. 2003;90:197–204. doi: 10.1002/bjs.4036. [DOI] [PubMed] [Google Scholar]
- 12.Von Heimburg D, Stieghorst W, Khorram-Sefat R, Pallua N. Procalcitonin–a sepsis parameter in severe burn injuries. Burns. 1998;24:745–50. doi: 10.1016/s0305-4179(98)00109-0. [DOI] [PubMed] [Google Scholar]
- 13.Nyamande K, Lalloo UG. Serum procalcitonin distinguishes CAP due to bacteria, Mycobacterium tuberculosis and PJP. Int J Tuberc Lung Dis. 2006;10:510–5. [PubMed] [Google Scholar]
- 14.Becker KL, Snider R, Nylen ES. Procalcitonin in sepsis and systemic inflammation: A harmful biomarker and a therapeutic target. Br J Pharmacol. 2010;159:253–64. doi: 10.1111/j.1476-5381.2009.00433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.d’Herbomez M, Caron P, Bauters C, Do Cao C, Schlienger JL, Sapin R, et al. Reference range of serum calcitonin levels in humans: Influence of calcitonin assays, sex, age, and cigarette smoking. Eur J Endocrinol. 2007;157:749–55. doi: 10.1530/EJE-07-0566. [DOI] [PubMed] [Google Scholar]
- 16.Amour J, Birenbaum A, Langeron O, Le Manach Y, Bertrand M, Coriat P, et al. Influence of renal dysfunction on the accuracy of procalcitonin for the diagnosis of postoperative infection after vascular surgery. Crit Care Med. 2008;36:1147–54. doi: 10.1097/CCM.0b013e3181692966. [DOI] [PubMed] [Google Scholar]
- 17.Charles PE, Ladoire S, Snauwaert A, Prin S, Aho S, Pechinot A, et al. Impact of previous sepsis on the accuracy of procalcitonin for the early diagnosis of blood stream infection in critically ill patients. BMC Infect Dis. 2008;8:163. doi: 10.1186/1471-2334-8-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coleman RE, Sangkasuwan V, Suwanabun N, Eamsila C, Mungviriya S, Devine P, et al. Comparative evaluation of selected diagnostic assays for the detection of IgG and IgM antibody to Orientia tsutsugamushi in Thailand. Am J Trop Med Hyg. 2002;67:497–503. doi: 10.4269/ajtmh.2002.67.497. [DOI] [PubMed] [Google Scholar]


