Abstract
To determine whether the myocardial response to ischemia/reperfusion (I/R) injury varies depending on genetic background, gender, age, body temperature, and arterial blood pH, we studied 1074 mice from 19 strains (including 129S6/SvEvTac (129S6), B6/129P2-Ptgs2tm1Unc, B6/129SvF2/J, B6/129/D2, B6/CBAF1, B6/DBA/1JNcr, BALB/c, BPH2/J, C57BL/6/J (B6/J), C3H/DBA, C3H/FB/FF, C3H/HeJ-Pde6brd1, FVB/N/J [FVB/N], FVB/B6, FVB/ICR and Crl:ICR/H [ICR]) and distributed them into 69 groups depending on strain and: (i) two phases of ischemic preconditioning (PC); (ii) coronary artery occlusion (O) time; (iii) gender; (iv) age; (v) blood transfusion; (vi) core body temperature; and (vii) arterial blood pH. Mice underwent O either without (non-preconditioned [naïve]) or with prior cyclic O/reperfusion (R) (PC stimulus) consisting of six 4-min O/4-min R cycles 10 min (early PC, EPC) or 24 h (late PC, LPC) prior to 30 or 45-min O and 24 h R. In B6/J and B6/129/D2 mice, almost the entire risk region was infarcted after a 60-min O. Of the naïve mouse hearts, B6/ecSODWT and FVB/N mice had infarct sizes significantly smaller than those of the other mice. All strains except FVB/N benefited from the cardioprotection afforded by the early phase of PC; in contrast, development of LPC was inconsistent amongst groups and was strain-dependent. Female gender (i) was associated with reduced infarct size in ICR mice, (ii) determined whether LPC developed in ICR mice, and (iii) limited the protection afforded by EPC in 129S6 mice. Importantly, mild hypothermia (1 °C decrease in core temperature) and mild acidosis (0.18 decrease in blood pH) resulted in a striking cardioprotective effect in ICR mice: 67.5% and 43.0% decrease in infarct size, respectively. Replacing blood losses with crystalloid fluids (instead of blood) during surgery also reduced infarct size. To our knowledge, this is the largest analysis of the determinants of infarct size in mice ever published. The results demonstrate that genetic background, gender, age (but not in ICR), body temperature and arterial blood pH have a major impact on infarct size, and thus need to be carefully measured and/or taken into account when designing a study of myocardial infarction in mice; failure to do so makes results uninterpretable. For example, core temperature and blood pH need to be measured, respiratory acidosis (or alkalosis) and hypothermia (or hyperthermia) must be avoided, and comparisons cannot be made between mouse strains or genders that exhibit different susceptibility to I/R injury (e.g., FVB/N male mice and ICR female mice are inherently protected against I/R injury).
Keywords: myocardial infarction, cardioprotection, ischemia/reperfusion injury, strain, gender, age, blood transfusion, core body temperature, pH
INTRODUCTION
Endogenous protective mechanisms exist within the heart that limit the extent of myocardial tissue insult following ischemia/reperfusion (I/R) injury [6, 8]. Various animal models such as dogs, swine and large rodents have been utilized extensively to investigate these protective effects, specifically those afforded by two very powerful, yet, fundamentally different mechanisms, direct cardioprotection and ischemic preconditioning (PC). Direct cardioprotection is confined to the non-preconditioned (naïve) myocardium and utilizes preexistent intracellular biomolecular mechanisms that, once provoked, directly limit myocellular death. Ischemic PC is an adaptive phenomenon capable of producing a highly defensive cardiac phenotype via brief repetitive episodes of myocardial ischemia. These episodes render the heart resistant to the lethal consequences of subsequent acute I/R injury; two distinct phases exist: an early phase (EPC) that develops rapidly after the stimulus but dissipates within 2–3 h, and a late phase (LPC) that becomes apparent 12–24 h later and persists for approximately 72 h. Prior to the established use of murine models for the study of myocardial I/R injury, our understanding of the molecular mechanisms underlying cardioprotection remained somewhat rudimentary [40, 56]. The decade following a study published by our group validating the physiologic relevance for the study of EPC and LPC in the mouse model [30] coincided with a surge in the literature produced by the basic science community in the various fields of cardioprotection.
Indeed, the murine model has greatly accelerated our understanding of the biochemical and cellular bases surrounding both direct cardioprotection and ischemic PC. However, this model is not without shortcomings. First, new evidence has surfaced from other laboratories indicating that genotype also contributes to observed phenotypic differences in both naïve and preconditioned mouse hearts [1, 20, 57]. At present, however, these data only exist for a few strains of mouse [1, 20, 57], and whether strain-related differences exist for the favorable effects afforded by PC is not currently known. Secondly, although not yet realized in the mouse, gender-specific variability among myocardial infarct-related injuries has been documented [17]. Finally, and equally important is the close consideration that must be given to the technical challenges associated with experimentally inducing myocardial infarctions in mice. It is apparent that fundamental physiologic variables (e.g., body temperature, oxygenation, acid-base balance, and hemodynamic characteristics affecting myocardial perfusion pressure), which are largely controllable, can significantly alter in a direct manner the extent of tissue injury in response to I/R if not closely monitored and kept within normal limits [10, 30, 32].
Thus, as strain-related and physiologic differences amongst mouse strains can potentially alter the murine heart’s response to ischemia, it becomes crucial, for the preservation of scientific method, to illumine these identifiable variables in order to remove confounding factors that may erroneously account for the phenotypic and functional differences observed experimentally. By diligently addressing these issues, methods and strategies can be developed that may help to standardize our approach to the investigation of cardioprotection in the mouse model. Accordingly, the present study was undertaken to establish whether and to what extent strain, gender, age and modulation of temperature and pH affect the susceptibility of the heart to I/R injury, both with and without early and late PC stimuli. To this end, we enrolled 1074 mice from 19 established and widely utilized commercially available strains and used the end point of infarct size (expressed as a percent of the myocardial region at risk) to determine tissue injury using an in vivo model of I/R. To our knowledge, this is the largest analysis of the determinants of infarct size in mice ever published.
METHODS
The investigation conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 86-23, Revised 1996). The experimental procedures and protocols were reviewed and approved by the Animal Care and Use Committee of the University of Louisville School of Medicine (Louisville, KY).
Animals
This study was performed in male and female mice ages 6–101 wk. The analysis was conducted in 1074 mice (including those excluded, Table 1), most of which were used in previously published studies [11–14, 16, 21, 23–25, 27, 28, 30, 37, 41, 43, 48, 50, 51, 53, 60, 61, 64, 68, 69, 71]. A total of 19 different mouse strains were utilized: 129/SvEvTac (129S6, Taconic Labs) [3], B6;129P2-Ptgs2tm1Unc (B6/129P2; C57BL/6 [B6], 129P2 mixed background from Taconic Labs) [65], B6/129SvF2/J (B6/129F2; B6 and 129/Sv mixed background from Jackson Labs) [21, 24], B6/129Sv/D2 (B6/129/D2, Merck Research Laboratories, West Point, PA) [23], B6/CBAF1 (B6/CBA, University of Düsseldorf, Germany) [36], B6/DBA/1JNcr (B6/DBA, Kyoto, Japan) [58], BALB/c (Jackson Labs), BPH2/J (BPH, the hypertensive mice from Jackson Labs), C57BL/6/J (B6/J, Jackson Labs) [25], C57BL/6/AR1 WT (B6/ARWT, wild-types control mice with B6 background for the aldose reductase-1 deficient mice) [39], C57BL/6/ecSOD (B6/ecSODWT, wildtype control mice with B6 background for extracellular superoxide dismutase deficient mice) [42], C57BL/6/p55 WT (B6/p55WT, wildtype control mice for tumor necrosis-α receptor type-1 deficient mice engineered on the C57BL/6 background) [38], C3H/DBA (Jackson Labs), C3H/FB/FF (Jackson Labs), C3H/HeJ-Pde6brd1 (C3H/He, Jackson Labs), FVB/N/J (FVB/N, Jackson Labs), FVB/B6 (FVB/N and B6 mixed background from University of Louisville), FVB/ICR (FVB/N and ICR mixed background from University of Louisville) and Crl:ICR/H (ICR, Harlan Labs) [14, 22, 27, 30, 31, 49, 51–53, 64, 67, 68]. Mice were maintained in microisolator cages under specific pathogen-free conditions in a room with a temperature of 24 °C, 55–65% relative humidity, and a 12 h light-dark cycle.
Table 1.
Reasons for Excluding Mice from Studies
|
|
|||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Groups | I | II | III | IV | V | VI | VII | VIII | IX | X | XI | XII | XIII | XIV | XV | XVI | XVII | XVIII | XIX | ||||||||
|
|
|||||||||||||||||||||||||||
| Bleeding | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | ||||||||
| Death | 3 | 2 | 1 | 3 | 3 | 1 | 0 | 1 | 2 | 0 | 1 | 1 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | ||||||||
| Technical problems | 1 | 0 | 2 | 2 | 3 | 0 | 0 | 1 | 2 | 0 | 0 | 3 | 2 | 0 | 1 | 0 | 1 | 0 | 6 | ||||||||
| Poor postmortem staining | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||
| Mice instrumented | 21 | 15 | 19 | 24 | 20 | 18 | 5 | 8 | 25 | 8 | 13 | 22 | 17 | 6 | 7 | 28 | 28 | 5 | 62 | ||||||||
|
|
|||||||||||||||||||||||||||
| Mice excluded | 5 | 2 | 4 | 6 | 6 | 1 | 0 | 2 | 4 | 0 | 1 | 4 | 6 | 0 | 2 | 2 | 4 | 0 | 10 | ||||||||
| Mice included in study | 16 | 13 | 15 | 18 | 14 | 17 | 5 | 6 | 21 | 8 | 12 | 18 | 11 | 6 | 5 | 26 | 24 | 5 | 52 | ||||||||
| Mice included in study, % | 76.2 | 86.7 | 78.9 | 75.0 | 70.0 | 94.4 | 100.0 | 75.0 | 84.0 | 100.0 | 92.3 | 81.8 | 64.7 | 100.0 | 71.4 | 92.9 | 85.7 | 100.0 | 83.9 | ||||||||
|
|
|||||||||||||||||||||||||||
| Groups | XX | XXI | XXII | XXIII | XXIV | XXV | XXVI | XXVII | XXVIII | XXIX | XXX | XXXI | XXXII | XXXIII | XXXIV | XXXV | XXXVI | XXXVII | XXXVIII | XXXIX | XL | XLI | XLII | XLIII | |||
|
|
|||||||||||||||||||||||||||
| Bleeding | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | |||
| Death | 1 | 0 | 0 | 0 | 1 | 2 | 2 | 4 | 0 | 1 | 0 | 1 | 6 | 2 | 5 | 0 | 7 | 3 | 4 | 5 | 2 | 0 | 6 | 4 | |||
| Technical problems | 0 | 1 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 1 | 0 | 0 | 1 | 2 | 2 | 1 | 2 | 0 | 3 | 2 | 2 | |||
| Poor postmortem staining | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 2 | 1 | |||
| Mice instrumented | 5 | 12 | 15 | 4 | 19 | 13 | 15 | 14 | 6 | 16 | 21 | 16 | 23 | 16 | 15 | 6 | 23 | 14 | 10 | 25 | 14 | 19 | 55 | 58 | |||
|
|
|||||||||||||||||||||||||||
| Mice excluded | 1 | 1 | 2 | 0 | 2 | 2 | 2 | 4 | 0 | 2 | 1 | 5 | 7 | 2 | 5 | 1 | 11 | 5 | 5 | 8 | 2 | 6 | 12 | 7 | |||
| Mice included in study | 4 | 11 | 13 | 4 | 17 | 11 | 13 | 10 | 6 | 14 | 20 | 11 | 16 | 14 | 10 | 5 | 12 | 9 | 5 | 17 | 12 | 13 | 43 | 51 | |||
| Mice included in study, % | 80.0 | 91.7 | 86.7 | 100.0 | 89.5 | 84.6 | 86.7 | 71.4 | 100.0 | 87.5 | 95.2 | 68.8 | 69.6 | 87.5 | 66.7 | 83.3 | 52.2 | 64.3 | 50.0 | 68.0 | 85.7 | 68.4 | 78.2 | 87.9 | |||
|
| |||||||||||||||||||||||||||
| Groups | XLIV | XLV | XLVI | XLVII | XLVIII | XLIX | L | LI | LII | LIII | LIV | LV | LVI | LVII | LVIII | LIX | LX | LXI | LXII | LXIII | LXIV | LXV | LXVI | LXVII | LXVIII | LXVIX | TOTAL |
|
| |||||||||||||||||||||||||||
| Bleeding | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 12 |
| Death | 0 | 0 | 2 | 0 | 0 | 2 | 1 | 1 | 2 | 1 | 1 | 3 | 0 | 2 | 0 | 1 | 1 | 2 | 2 | 1 | 6 | 1 | 0 | 2 | 0 | 115 | |
| Technical problems | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 52 |
| Poor postmortem staining | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 14 |
| Mice instrumented | 5 | 4 | 17 | 8 | 12 | 15 | 8 | 8 | 14 | 8 | 16 | 8 | 10 | 6 | 6 | 9 | 21 | 11 | 18 | 8 | 17 | 28 | 8 | 8 | 9 | 7 | 1074 |
|
|
|||||||||||||||||||||||||||
| Mice excluded | 1 | 0 | 2 | 0 | 0 | 3 | 2 | 1 | 2 | 1 | 1 | 0 | 3 | 0 | 2 | 0 | 2 | 2 | 5 | 2 | 1 | 7 | 2 | 0 | 2 | 0 | 193 |
| Mice included in study | 4 | 4 | 15 | 8 | 12 | 12 | 6 | 7 | 12 | 7 | 15 | 8 | 7 | 6 | 4 | 9 | 19 | 9 | 13 | 6 | 16 | 21 | 6 | 8 | 7 | 7 | 881 |
| Mice included in study, % | 80.0 | 100.0 | 88.2 | 100.0 | 100.0 | 80.0 | 75.0 | 87.5 | 85.7 | 87.5 | 93.8 | 100.0 | 70.0 | 100.0 | 100.0 | 66.7 | 90.5 | 81.8 | 72.2 | 75.0 | 94.1 | 75.0 | 75.0 | 100.0 | 77.8 | 100.0 | 82.0 |
I, 129S6, MI; II, B6/129P2, MI; III, B6/129F2, MI; IV, B6/129/D2, MI; V, B6/CBA, MI; VI, B6/DBA, MI; VII, BALB/c, MI; VIII, BPH, MI; IX, B6/J, MI; X, B6/ARWT, MI; XI, B6/ecSODWT, MI; XII, B6/p55WT, MI; XIII, C3H/DBA, MI; XIV, C3H/FB/FF, MI; XV, C3H/He, MI; XVI, FVB/N, MI; XVII, FVB/B6, MI; XVIII, FVB/ICR, MI; XIX, ICR, MI; XX, 129S6, EPC; XXI, B6/129F2, EPC; XXII, B6/129/D2, EPC; XXIII, B6/DBA, EPC; XXIV, B6/J, EPC; XXV, B6/p55WT, EPC; XXVI, FVB/N, MI O 45′; XXVII, FVB/N, EPC O 45′; XXVIII, FVB/B6, EPC; XXIX, ICR, EPC sham; XXXI, 129S6, LPC; XXXII, B6/129P2, LPC; XXXIII, B6/129F2, LPC sham; XXXIV, B6/129F2, LPC; XXXV, B6/129/D2, LPC; XXXVI, B6/DBA, LPC; XXXVII, BALB/c, LPC; XXXVIII, BPH, LPC; XXXIX, B6/J, LPC; XL, FVB/N, LPC; XLI, FVB/B6, LPC; XLII, ICR, LPC sham; XLIII, ICR, LPC; XLIV, B6/129/D2, MI, O 60′; XLV, B6/J, MI, O 60′; XLVI, FVB/N, MI, O 60′; XLVII, ICR, MI, O 10′; XLVIII, ICR, MI, O 20′; XLIX, 129S6, female, MI; L, 129S6, female, EPC; LI, C3H/He, female, MI; LII, FVB/N, female, MI; LIII, FVB/B6, female, MI; LIV, ICR, female, MI; LV, ICR, female, LPC; LVI, B6/CBA, old, MI; LVII, B6/J, 6 wks, MI; LVIII, B6/J, old, MI; LIX, C3H/FB/FF, old, MI; LX, ICR, 9 wks, MI R4h; LXI, ICR, middle, MI, R4h; LXII, ICR, old, MI, R4h; LXIII, ICR, middle, MI; LXIV, ICR, MI, blood, T 37.0°C, Ph = 7.40 (standard setting); LXV, ICR, MI, no blood; LXVI, ICR, MI, T 38.0 °C; LXVII, ICR, MI, T 36.0 °C; LXVIII, ICR, MI, pH = 7.52; LXIX, ICR, MI, pH = 7.17.
Experimental Protocol
The experimental protocol has been described in depth previously [24, 30] and the overall experimental design is detailed in (Fig. 1). Mice were assigned to 69 different groups depending on strain and: (i) two phases of ischemic preconditioning (PC); (ii) occlusion (O) time; (iii) gender; (iv) age; (v) performance of blood transfusion; (vi) body temperature; and (vii) body pH. Acute myocardial infarction (MI) was produced in male groups I–XIX by 30-min coronary O followed by 24 h R (Fig. 1A) and the average infarct sizes of these non-preconditioned (naïve) hearts were compared. Groups XX–XXX (early phase of PC, EPC) underwent six 4-min coronary O/4-min R cycles 10 min prior to 30-min O followed by 24 h R, with the exception of naïve group XXV, EPC group XXVI (both underwent 45-min O) and EPC sham group XXIX (open-chest 1 h without PC); average infarct sizes of EPC groups were compared to that of their respective naïve and/or EPC sham infarct groups (Fig. 1B). Mice in groups XXXI–XLIII (late phase of PC, LPC) were preconditioned with six 4-min O/4-min R cycles 24 h prior 30-min O, with the exception of LPC group XL (45-min O) and LPC sham groups (groups XXXIII and XLII); average infarct sizes of LPC groups were compared to that of their respective naïve and/or LPC sham infarct groups (Fig. 1C). Mice from groups XLIV–XLVIII (IV, IX, XVI, XIX and XXV) underwent MI with varying periods of coronary O (including 10, 20, 30, 45, and 60-min) followed by 24 h R; average infarct sizes were compared based on O times (Fig. 1D). Average infarct sizes in male strains of mice from groups II, XV, XVI–XX and XLII–XLIII were compared to that of their respective female strains in groups XLIX–LV; mice underwent MI consisting of 30-min O followed by 24 h R either with or without EPC or LPC stimulus (Fig. 1E). In addition, male mice ranging in ages from 6–101 wk underwent MI (30-min O followed by 4 h or 24 h R, Fig. 1F) and the average infarct sizes were compared (groups V, IX, XIV, XIX and LVI–LXIII). Finally, male ICR mice in groups LXIV–LXIX underwent MI consisting of 30-min O followed by 24 h R, and were compared based on with or without blood infusion (groups LXIV–LXV), or different body core temperature (groups LXVI–LXVII), and pH differences (obtained by different ventilator settings, groups LXVIII–LXIX) during surgery (Fig. 1G).
Figure 1. Experimental Protocols.
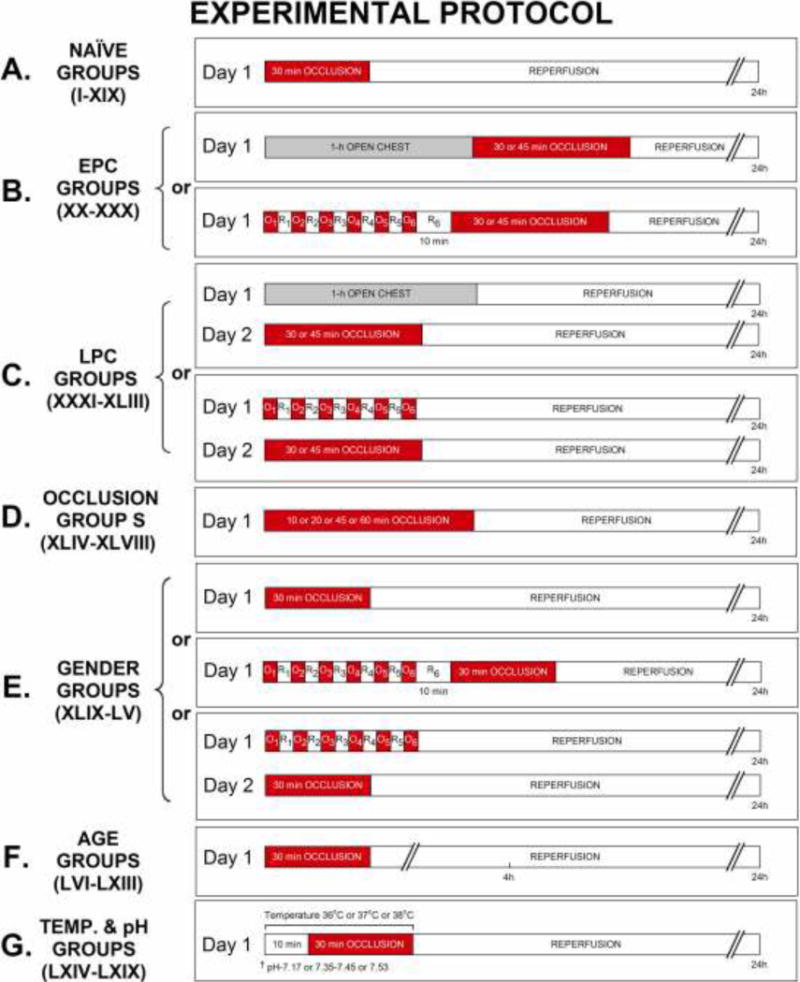
Sixty-nine groups of nine-teen strains mice were used. (A) Mice in groups I–XIX underwent acute myocardial infarction (MI) consisting of 30-min coronary occlusion (O) and followed by 24 h of reperfusion (R). (B) On day 1, mice in the early PC sham (EPC sham) group XXIX were subjected to 60-min open chest 10 min prior to 30-min O and followed by 24 h of R. Mice in the early preconditioned (PC, EPC) groups XX–XXV and XXVII–XXX underwent a sequence of six cycles of 4-min coronary O/4-min R cycles 10 min prior to 30-min O and followed by 24 h of R. (C) On day 1, mice in the late PC sham (LPC sham) groups XXXIII and XLII were subjected to 60-min open chest and underwent 30-min O 24 h later on day 2. Mice in the late PC (LPC) groups XXXI–XXXII, XXXIV–XL and XLIII were preconditioned with six 4-min O/4-min R cycles and underwent 30-min O 24 h later on day 2. (D) Mice in groups XLIV–XLVIII underwent O times ranging from 10–60-min followed by 24 h R. (E) Female mice in groups XLIX–LV underwent MI consisting of 30-min O followed by 24 h R either with or without EPC or LPC stimulus. (F) Mice, ranging in ages from 6–101 wk, in groups LVI–LXIII underwent MI consisting of 30-min O followed by either 4 or 24 h R. (G) ICR mice in groups LXIV–LXIX underwent MI consisting of 30-min O followed by 24 h R, and were compared based on body core temperature and pH differences during surgery. On day 2 or 3, hearts from all groups (I–LXIX) were harvested and subjected to postmortem tissue analysis including triphenyltetrazolium chloride, Phthalo blue dye staining to determine infarct size.
Animal Surgery
The mice were anesthetized with pentobarbital sodium (60 mg/kg i.p), intubated, and ventilated with room air supplemented with oxygen at a rate of 105 strokes/min and with a tidal volume of 0.25–0.33 ml with the use of a small rodent ventilator. These respiratory settings were found to result in optimal values of arterial pH (7.39 ± 0.01) and PCO2 (31 ± 2 mmHg) [34, 46, 70]; except in groups LXVIII and LXIX where arterial blood pH was altered experimentally (pH of 7.52 ± 0.01 and 7.17 ± 0.01, respectively) by increasing or decreasing minute ventilation, the product of the respiratory rate and tidal volume. Body temperature was carefully monitored with a rectal probe and maintained using heating pads and heat lamps as close as possible to 37.0 ± 0.2 degrees Celsius (°C); except in groups LXVI and LXVII where body temperature was deliberately either increased or decreased by about 1.0 ± 0.2 °C. Since hypothermia is associated with bradycardia, which has been suggested to limit infarct size [19], we closely monitored heart rate (HR) in all groups (Tables 2 to 9). To prevent hypotension (which may due to the artificial respiration and blood loss) during surgical procedures, blood from a donor mouse was given intravenously at 40 ml/kg divided into three equal boluses [24, 30]; except in groups LXV where blood loss was replaced with crystalloid alone at the same rate and boluses. After the administration of antibiotics, the chest was opened through a midline sternotomy and a nontraumatic balloon occluder was implanted around the mid-portion of the left anterior descending coronary artery using an 8-0 nylon suture. After the coronary O/R protocol, the chest was closed in layers and the mice were allowed to recover.
Table 2.
Rectal Temperature on the Day of the Coronary Occlusion (Naïve Acute MI Groups)
| GROUPS | Pre-occlusion | Occlusion | Reperfusion | ||||
|---|---|---|---|---|---|---|---|
| 5 min | 15 min | 30 min | 5 min | 10 min | 15 min | ||
| Temperature (°C) | |||||||
| I, 129S6, MI | 36.9 ± 0.0 | 37.0 ± 0.1 | 37.0 ± 0.0 | 37.0 ± 0.0 | 37.0 ± 0.0 | 37.1 ± 0.0 | 37.0 ± 0.0 |
| II, B6/129P2, MI | 36.9 ± 0.1 | 37.1 ± 0.1 | 37.0 ± 0.1 | 37.0 ± 0.1 | 36.9 ± 0.1 | 36.9 ± 0.1 | 36.9 ± 0.1 |
| III, B6/129F2, MI | 36.9 ± 0.1 | 37.1 ± 0.1 | 37.0 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.0 | 37.0 ± 0.1 | 37.1 ± 0.1 |
| IV, B6/129/D2, MI | 37.0 ± 0.1 | 37.3 ± 0.1 | 37.0 ± 0.0 | 37.1 ± 0.0 | 37.0 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.1 |
| V, B6/CBA, MI | 37.0 ± 0.0 | 37.1 ± 0.1 | 37.1 ± 0.0 | 37.1 ± 0.0 | 37.1 ± 0.1 | 36.9 ± 0.1 | 37.0 ± 0.1 |
| VI, B6/DBAF1, MI | 36.9 ± 0.1 | 37.0 ± 0.1 | 36.9 ± 0.1 | 37.0 ± 0.1 | 37.0 ± 0.1 | 36.9 ± 0.1 | 37.0 ± 0.1 |
| VII, BALB/c, MI | 36.9 ± 0.0 | 37.1 ± 0.1 | 37.0 ± 0.1 | 37.0 ± 0.0 | 37.1 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.1 |
| VIII, BPH, MI | 36.9 ± 0.0 | 37.0 ± 0.1 | 36.9 ± 0.1 | 36.9 ± 0.1 | 36.9 ± 0.1 | 36.9 ± 0.1 | 36.8 ± 0.0 |
| IX, B6/J, MI | 37.0 ± 0.1 | 37.2 ± 0.1 | 37.0 ± 0.0 | 37.1 ± 0.0 | 37.0 ± 0.1 | 37.0 ± 0.1 | 37.1 ± 0.1 |
| X, B6/ARWT, MI | 37.0 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.1 | 37.0 ± 0.1 | 36.9 ± 0.1 | 36.9 ± 0.1 | 36.9 ± 0.0 |
| XI, B6/ecSODWT, MI | 37.1 ± 0.1 | 37.1 ± 0.1 | 37.0 ± 0.0 | 37.1 ± 0.1 | 37.0 ± 0.1 | 36.9 ± 0.1 | 37.0 ± 0.1 |
| XII, B6/p55WT, MI | 37.0 ± 0.1 | 37.0 ± 0.1 | 37.0 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.1 | 36.8 ± 0.1 | 37.2 ± 0.1 |
| XIII, C3H/DBA, MI | 36.8 ± 0.1 | 37.1 ± 0.0 | 36.9 ± 0.1 | 37.0 ± 0.1 | 37.0 ± 0.1 | 37.0 ± 0.1 | 37.0 ± 0.0 |
| XIV, C3H/FB/FF, MI | 37.2 ± 0.1 | 37.1 ± 0.1 | 36.9 ± 0.1 | 37.1 ± 0.1 | 37.0 ± 0.1 | 36.9 ± 0.1 | 37.1 ± 0.1 |
| XV, C3H/He, MI | 36.9 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.1 | 37.0 ± 0.1 | 37.1 ± 0.1 | 37.0 ± 0.1 | 36.9 ± 0.1 |
| XVI, FVB/N, MI | 37.0 ± 0.1 | 37.0 ± 0.1 | 37.1 ± 0.0 | 37.0 ± 0.1 | 37.0 ± 0.1 | 37.0 ± 0.1 | 37.0 ± 0.1 |
| XVII, FVB/B6, MI | 37.1 ± 0.1 | 37.1 ± 0.0 | 37.1 ± 0.1 | 37.1 ± 0.0 | 37.1 ± 0.0 | 37.1 ± 0.0 | 37.1 ± 0.0 |
| XVIII, FVB/ICR, MI | 36.8 ± 0.2 | 37.2 ± 0.1 | 37.0 ± 0.2 | 37.1 ± 0.2 | 37.1 ± 0.1 | 36.9 ± 0.1 | 36.9 ± 0.1 |
| XIX, ICR, MI | 36.9 ± 0.1 | 37.1 ± 0.1 | 37.0 ± 0.1 | 36.9 ± 0.1 | 36.9 ± 0.0 | 36.9 ± 0.1 | 36.8 ± 0.1 |
Data are means ± SEM. Measurements of rectal temperature and heart rate were taken before the 30-min coronary occlusion (pre-occlusion), at 5, 15 and 30 min into the 30-min occlusion, and at 5, 15 and 30 min after reperfusion. Rectal temperature was continuously monitored and carefully controlled throughout the experiment, as detailed in the text.
Table 9.
Rectal Temperature and Heart Rate on the Day of the 30-min Coronary Occlusion (Temperature & pH Groups)
| GROUPS | Pre-occlusion | Occlusion | Reperfusion | ||||
|---|---|---|---|---|---|---|---|
| 5 min | 15 min | 30 min | 5 min | 10 min | 15 min | ||
| Temperature (°C) | |||||||
| LXIV, ICR, standard | 37.0 ± 0.0 | 37.1 ± 0.0 | 37.0 ± 0.0 | 36.9 ± 0.0 | 37.0 ± 0.0 | 36.9 ± 0.0 | 36.9 ± 0.1 |
| LXV, ICR, no blood | 36.9 ± 0.1 | 37.0 ± 0.0 | 37.0 ± 0.0 | 37.0 ± 0.0 | 37.0 ± 0.1 | 37.0 ± 0.0 | 37.0 ± 0.1 |
| LXVI, ICR, T 38.0 °C | 38.0 ± 0.2 | 37.9 ± 0.2 | 38.1 ± 0.3 | 38.1 ± 0.3 | 38.1 ± 0.1 | 37.9 ± 0.1 | 37.9 ± 0.1 |
| LXVII, ICR, T 36.0 °C | 35.7 ± 0.1 | 36.1 ± 0.2 | 35.8 ± 0.1 | 35.8 ± 0.0 | 35.8 ± 0.0 | 35.8 ± 0.1 | 35.8 ± 0.0 |
| LXVIII, ICR, pH = 7.52 | 36.9 ± 0.1 | 37.0 ± 0.0 | 37.0 ± 0.1 | 36.9 ± 0.1 | 37.1 ± 0.1 | 37.0 ± 0.1 | 37.0 ± 0.1 |
| LXIX, ICR, pH = 7.17 | 36.9 ± 0.1 | 37.0 ± 0.1 | 37.1 ± 0.1 | 36.9 ± 0.1 | 36.9 ± 0.1 | 36.8 ± 0.1 | 36.9 ± 0.1 |
| Heart rate (beats/min) | |||||||
| LXIV, ICR, standard | 557 ± 14 | 571 ± 12 | 570 ± 9 | 566 ± 11 | 567 ± 11 | 556 ± 14 | 571 ± 14 |
| LXV, ICR, no blood | 541 ± 10 | 556 ± 11 | 549 ± 13 | 533 ± 14 | 548 ± 15 | 564 ± 16 | 571 ± 17 |
| LXVI, ICR, T 38.0 °C | 582 ± 27 | 591 ± 41 | 580 ± 44 | 587 ± 54 | 597 ± 55 | 584 ± 55 | 578 ± 47 |
| LXVII, ICR, T 36.0 °C | 545 ± 26 | 585 ± 35 | 566 ± 42 | 576 ± 44 | 585 ± 48 | 589 ± 48 | 577 ± 41 |
| LXVIII, ICR, pH = 7.52 | 483 ± 29 | 501 ± 36 | 496 ± 37 | 518 ± 45 | 513 ± 40 | 486 ± 37 | 504 ± 27 |
| LXIX, ICR, pH = 7.17 | 580 ± 29 | 613 ± 27 | 607 ± 16 | 627 ± 22 | 605 ± 33 | 581 ± 21 | 574 ± 20 |
Data are means ± SEM. Measurements of rectal temperature and heart rate were taken before the 30-min coronary occlusion (pre-occlusion), at 5, 15 and 30 min into the 30-min occlusion, and at 5, 15 and 30 min after reperfusion. Rectal temperature was continuously monitored and carefully controlled throughout the experiment, as detailed in the text.
Postmortem Tissue Staining and Infarct Size Measurement
At the conclusion of the study, infarct size was calculated as a percentage of the region at risk, as previously described [24, 30]. Briefly, hearts were excised and perfused with Krebs-Henseleit solution through an aortic cannula. To delineate infarcted myocardium from viable myocardium, the heart was perfused with 1% triphenyltetrazolium chloride in phosphate buffer. To delineate the occluded/reperfused bed, the coronary artery was tied at the site of the previous O and the aortic root was perfused with 10% Phthalo blue dye [16, 23–30]. As a result of this procedure, the region at risk was identified by the absence of blue dye, whereas the rest of the left ventricle was stained dark blue. The left ventricle was cut into five to seven transverse slices, which were fixed in 10% neutral buffered formaldehyde, weighed, and photographed under a microscope. The corresponding areas were measured by computerized videoplanimetry, and from these measurements the infarct size was calculated as a percentage of the region at risk [16, 23–30].
Statistical Analysis
Data are expressed as mean ± SEM. Differences were analyzed with a one-way ANOVA. Following ANOVA, comparisons were performed using unpaired Student’s t-test with the Bonferroni correction. In addition, weighted arithmetic means (WAM)[2] were calculated from the average infarct sizes of the naïve hearts with either O 30-min (groups I–XIX, Table 10 and Fig. 2) or O 60-min (groups XLIV–XLVI, Table 131 and Fig. 5) and used as the surrogate control value. Because (1) ICR mice are the most widely used outbred mouse strain and (2) the average infarct sizes in the naïve hearts from this group was not different from WAM, this group was chosen as the control group when individual comparisons of average infarct sizes were made amongst the naïve hearts. P < 0.05 was considered statistically significant. All statistical analyses were performed using either SPSS (version 8.0, Chicago, IL) or SigmaStat (version 3.5, Ashburn, VA) statistical softwares.
Table 10.
Size of Left Ventricle, Risk Region, and Infarct (30-min Occlusion, Male Naïve Groups)
| Strain | Age | Body (g) |
Heart (mg) |
H/B wt (%) |
LV (mg) |
Risk Region (mg) |
Infarct (mg) |
Risk Region (% of LV) |
Infarct (% of risk region) |
WAM (% of risk region) |
Infarct (% of LV) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I, n = 16 | 129/S6 | 26 ± 3 | 28.8 ± 0.8 | 167 ± 5 | 0.58 ± 0.01 | 121 ± 4 | 39 ± 2 | 23 ± 2 | 32.4 ± 1.5 | 58.4 ± 2.8 | 50.0 ± 2.7 | 21.1 ± 1.4 |
| II, n = 13 | B6/129P2 | 31 ± 4 | 31.0 ± 0.6 | 175 ± 6 | 0.57 ± 0.01 | 137 ± 5 | 54 ± 4 | 32 ± 3 | 38.6 ± 2.6 | 59.5 ± 2.8 | 49.9 ± 2.7 | 23.7 ± 2.0 |
| III, n = 15 | B6/129F2 | 14 ± 1 | 29.2 ± 1.0 | 161 ± 8 | 0.55 ± 0.01 | 119 ± 6 | 43 ± 3 | 26 ± 2 | 36.8 ± 2.0 | 58.6 ± 1.8 | 50.0 ± 2.7 | 21.7 ± 1.5 |
| IV, n = 18 | B6129/D2 | 17 ± 1 | 32.4 ± 1.1 | 172 ± 5 | 0.53 ± 0.02 | 129 ± 4 | 43 ± 2 | 24 ± 2 | 33.9 ± 1.6 | 55.5 ± 2.8 | 50.1 ± 2.7 | 18.9 ± 1.4 |
| V, n = 14 | B6/CBA | 18 ± 2 | 31.0 ± 2.1 | 141 ± 8 | 0.46 ± 0.02 | 107 ± 7 | 39 ± 2 | 22 ± 1 | 38.6 ± 2.6 | 55.7 ± 3.7 | 50.1 ± 2.7 | 21.4 ± 1.8 |
| VI, n = 17 | B6/DBA | 23 ± 2 | 30.2 ± 0.9 | 155 ± 7 | 0.51 ± 0.01 | 117 ± 6 | 39 ± 3 | 20 ± 2 | 33.5 ± 2.1 | 50.7 ± 2.7 | 50.4 ± 2.7 | 17.0 ± 1.4 |
| VII, n = 5 | BALB/c | 23 ± 1 | 28.1 ± 0.7 | 158 ± 9 | 0.56 ± 0.02 | 110 ± 11 | 40 ± 6 | 25 ± 4 | 36.1 ± 3.0 | 61.7 ± 1.5 | 50.2 ± 2.6 | 22.4 ± 2.3 |
| VIII, n = 6 | BPH | 15 ± 0 | 22.8 ± 0.4 | 168 ± 6 | 0.73 ± 0.02 | 130 ± 4 | 41 ± 5 | 23 ± 3 | 31.6 ± 3.4 | 55.6 ± 1.9 | 50.3 ± 2.7 | 17.7 ± 2.2 |
| IX, n = 21 | B6/J | 15 ± 2 | 27.0 ± 1.4 | 138 ± 8 | 0.51 ± 0.02 | 104 ± 6 | 40 ± 4 | 25 ± 2 | 37.7 ± 2.2 | 64.5 ± 2.1§‡ | 49.3 ± 2.6 | 24.0 ± 1.4 |
| X, n = 8 | B6/ARWT | 15 ± 0 | 28.5 ± 0.7 | 117 ± 6 | 0.41 ± 0.02 | 87 ± 5 | 30 ± 2 | 16 ± 2 | 34.5 ± 2.0 | 53.1 ± 5.2 | 50.3 ± 2.7 | 18.8 ± 2.7 |
| XI, n = 12 | B6/ecSODWT | 19 ± 1 | 29.8 ± 0.6 | 148 ± 4 | 0.50 ± 0.01 | 109 ± 3 | 39 ± 3 | 12 ± 2 | 35.5 ± 2.8 | 32.7 ± 3.8*§‡ | 51.2 ± 2.5 | 11.5 ± 1.8*§‡ |
| XII, n = 18 | B6/p55WT | 19 ± 0 | 31.8 ± 1.0 | 150 ± 6 | 0.47 ± 0.01 | 112 ± 4 | 46 ± 3 | 27 ± 2 | 41.6 ± 2.0 | 59.7 ± 2.7 | 49.8 ± 2.7 | 24.9 ± 1.7 |
| XIII, n = 11 | C3H/DBA | 17 ± 1 | 28.9 ± 0.8 | 160 ± 6 | 0.55 ± 0.02 | 119 ± 6 | 43 ± 3 | 24 ± 3 | 35.9 ± 2.2 | 55.9 ± 3.7 | 50.2 ± 2.7 | 20.4 ± 2.2 |
| XIV, n = 6 | C3H/FB/FF | 12 ± 0 | 30.6 ± 0.9 | 133 ± 7 | 0.44 ± 0.03 | 99 ± 4 | 38 ± 3 | 16 ± 2 | 37.9 ± 2.2 | 43.4 ± 4.5 | 50.6 ± 2.7 | 16.6 ± 2.2 |
| XV, n = 5 | C3H/He | 37 ± 2 | 31.1 ± 0.7 | 163 ± 6 | 0.52 ± 0.02 | 108 ± 8 | 46 ± 6 | 21 ± 5 | 42.7 ± 3.1 | 43.6 ± 6.0 | 50.5 ± 2.7 | 19.0 ± 3.5 |
| XVI, n = 26 | FVB/N | 18 ± 2 | 29.6 ± 0.7 | 146 ± 4 | 0.50 ± 0.01 | 107 ± 3 | 39 ± 2 | 7 ± 1 | 36.4 ± 1.5 | 17.9 ± 2.4*§‡ | 53.6 ± 1.9 | 4.6 ± 0.8*§‡ |
| XVII, n = 24 | FVB/B6 | 17 ± 1 | 28.2 ± 1.2 | 133 ± 6 | 0.48 ± 0.01 | 99 ± 5 | 42 ± 2 | 20 ± 1 | 43.0 ± 1.7 | 49.3 ± 2.9 | 50.5 ± 2.7 | 21.4 ± 1.6 |
| XVIII, n = 5 | FVB/ICR | 15 ± 0 | 27.0 ± 0.9 | 117 ± 8 | 0.43 ± 0.03 | 80 ± 4 | 30 ± 4 | 13 ± 2 | 37.0 ± 3.8 | 45.4 ± 6.4 | 50.5 ± 2.7 | 15.9 ± 1.6 |
| XIX, n = 52 | ICR | 9 ± 0 | 34.2 ± 0.6 | 170 ± 4 | 0.48 ± 0.02 | 122 ± 3 | 47 ± 2 | 24 ± 1 | 38.9 ± 1.5 | 50.8 ± 1.6 | 50.3 ± 2.7 | 19.8 ± 1.0 |
The experimental protocols for the all groups of mice are specified in the legend to Fig. 1. LV, left ventricle; Body, body weight; Heart, total heart weight (ventricles and atria); WAM, weighted arithmetic mean of the groups with 30 min O. Data are means ± SEM.
P < 0.05 vs. correlated strain groups.
P < 0.05 vs. group XIX (naïve heart control).
P < 0.05 vs. remaining groups WAM (Fig. 2).
Figure 2. MI in Nineteen Strains.
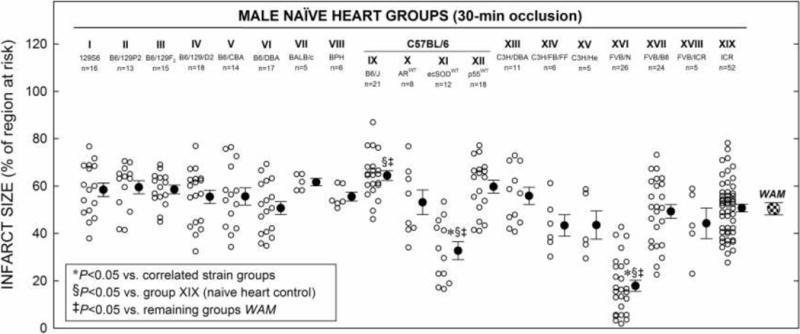
Myocardial infarct size in non-preconditioned (naïve) hearts from nineteen strains of male mice (groups I–XIX) that received 30-min O followed by 24 h R (Fig. 1 A). Infarct size is expressed as percentage of region at risk. Individual mice (○) and Mean ± SEM (●) for respective groups (n, number of mice); weighted arithmetic mean (WAM, 50.4 ± 2.6% of region at risk) for all infarcted naïve groups (checkered circle).
Figure 5. Naïve Infarct Size vs. Occlusion Time in Four Strains.
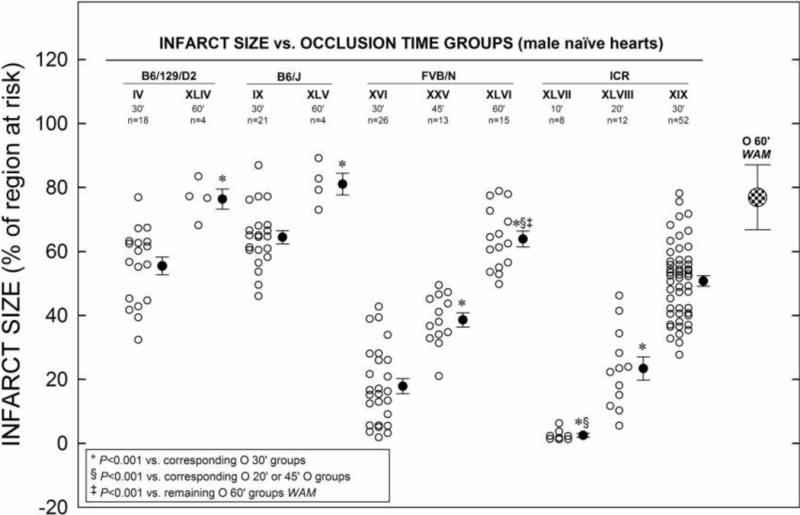
Myocardial infarct size in non-preconditioned (naïve) hearts from four different strains of mice in groups XLIV–XLIII (including young adult groups IV, IX, XVI, XIX and XXVI) that received 10–60-min O followed by 24 h R (Fig. 1 D). Infarct size is expressed as percentage of region at risk. Individual mice (○) and Mean ± SEM (●), for respective groups (n, number of mice); weighted arithmetic mean (WAM, 76.9 ± 10.2% of region at risk) for all infarcted with 60-min O naïve groups (checkered circle).
RESULTS
Animals Excluded
After exclusions, a total of 881 mice from 19 different strains were analyzed in the study. Of these, 193 mice were excluded for the reasons specified in Table 1.
Studies of Myocardial Infarction
Hemodynamic variables
By experimental design, rectal temperature remained within a narrow physiological range (36.8° to 37.02°C) in all but two groups (groups LXVI and LXVII) in which body temperature was intentionally either increased or lowered 1 °C for experimental purpose, respectively (Tables 2–9). HR was measured 5 min before the 30-min O, during the O, and after R (Tables 2–9). The average HR in groups I–LXIX ranged from 448 to 693 beats/min at pre-occlusion beats/min (Tables 2–9).
Region at risk and infarct size
1. Naïve hearts
There were no significant differences among the correlated groups with respect to body weight or heart weight or weight of the region at risk or % of region at risk (Tables 10–16). The susceptibility of the 19 different strains of mice to ischemia following a 30-min O and 24 h of R was evaluated (Figs. 1A and 2). Amongst the infarcted naïve mouse hearts, an overall analysis of variance followed by between-group comparisons was performed to determine whether particular strains displayed infarct sizes different from those of the remaining groups. In addition, since the weighted arithmetic mean (WAMtotal) infarct size of all 19 groups (50.4 ± 2.6% of risk region) corresponded most closely to the ICR strain (group XIX), the most widely utilized outbred mouse strain (http://www.harlan.com/), individual comparisons were made between groups using the ICR average infarct size (50.8 ± 1.6% of the risk region) as a naïve control (Table 10 and Fig. 2). Using both of these methods of comparison, only two strains, B6/ecSODWT (group XI) and especially FVB/N (group XVI) mice, demonstrated average infarct sizes smaller than those of the remaining groups and ICR naïve control mouse infarcts (32.7 ± 3.8% and 17.9 ± 2.4% vs. 50.8 ± 1.6% of the risk region, respectively; P < 0.05, Fig. 2). Thus, B6/ecSODWT (on B6 background) and FVB/N naïve hearts are remarkably less susceptible to I/R injury than other strains, indicating that these genotypes confer significant, innate cardioprotection. Interestingly, the B6/J (group IX) mice demonstrated average infarct sizes that were significantly larger than the ICR control mice infarcts and the remaining WAM (64.5 ± 2.1 vs. 50.8 ± 1.6% and 49.3 ± 2.6 % of the risk region, respectively; P < 0.05, Table 10 and Fig. 2), indicating that these genotypes confer significantly less innate resistance to I/R injury.
Table 16.
Size of Left Ventricle, Risk Region, and Infarct (Male, Blood Infusion, Temperature & pH, 30-min occlusion)
| Strain | Age | Body (g) |
Heart (mg) |
H/B wt (%) |
LV (mg) |
Risk Region (mg) |
Infarct (mg) |
Risk Region (% of LV) |
Infarct (% of risk region) |
Infarct (% of LV) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| LXIV, n = 16, standard | ICR | 12 ± 0 | 35.1 ± 0.7 | 168 ± 6 | 0.48 ± 0.01 | 121 ± 5 | 43 ± 3 | 20 ± 1 | 35.4 ± 1.9 | 48.4 ± 2.5 | 16.9 ± 1.0 |
| LXV, n = 21, no blood | ICR | 10 ± 0 | 37.1 ± 0.6 | 161 ± 4 | 0.43 ± 0.01 | 114 ± 3 | 42 ± 2 | 12 ± 1 | 37.1 ± 1.7 | 28.5 ± 3.0* | 10.4 ± 1.1* |
| LXVI, n = 6, T 38.0°C | ICR | 11 ± 0 | 41.7 ± 0.6 | 196 ± 4 | 0.47 ± 0.01 | 136 ± 4 | 63 ± 6 | 35 ± 6 | 45.4 ± 4.4 | 56.4 ± 6.1 | 26.1 ± 4.8 |
| LXVII, n = 8, T 36.0°C | ICR | 13 ± 1 | 39.0 ± 0.3 | 178 ± 5 | 0.46 ± 0.01 | 125 ± 3 | 47 ± 4 | 7 ± 1 | 37.4 ± 2.4 | 15.7 ± 4.1*§ | 5.4 ± 1.2*§ |
| LXVIII, n = 7, pH = 7.52 | ICR | 13 ± 1 | 40.5 ± 1.0 | 196 ± 8 | 0.48 ± 0.01 | 139 ± 7 | 56 ± 6 | 33 ± 5 | 40.9 ± 4.5 | 57.1 ± 3.8 | 23.9 ± 3.7 |
| LXIX, n = 7, pH = 7.17 | ICR | 13 ± 0 | 41.0 ± 0.8 | 209 ± 10 | 0.51 ± 0.02 | 154 ± 8 | 56 ± 5 | 15 ± 3 | 36.4 ± 3.0 | 27.7 ± 6.5*‡ | 10.1 ± 2.5‡ |
The experimental protocols for the all groups of mice are specified in the legend to Fig. 1. LV, left ventricle; Body, body weight; Heart, total heart weight (ventricles and atria). Data are means ± SEM.
P < 0.05 vs. Group LXIV.
P < 0.05 vs. Group LXVI.
2. EPC
Mice from various strains also underwent an EPC protocol consisting of six 4-min coronary O/4-min R cycles 10 min prior to the 30 min (and 45-min, naïve group XXVI and EPC group XXVII) of coronary O and followed by 24 h R (Fig. 1C). Among the groups studied, compared to each respective corresponding naïve infarct group, only FVB/N (group XXVII) mice were subjected to 45-min of coronary O with or with EPC stimuli (Fig. 3). We did not study EPC with 30 min O in FVB/N mice, because the infarct size in this strain was dramatically smaller than any other strain of mice with a 30 min O (Fig. 2). These data indicate that, with the exception of FVB/N mice, the early phase of PC exists in all strains of mice tested and the magnitude of its cardioprotective effects is similar amongst the strains. In group XXIX (ICR, EPC sham group), keeping the chest open for 60 min before the 30-min coronary O had no effect on infarct size (55.0 ± 2.2% of the region at risk vs. 50.8 ± 1.6% in group XIX) (Fig. 3), indicating that our standard surgical procedure does not affect infarct size in our model. As shown in Tables 10 and 11 and Fig. 3, infarct size in groups XXIX was similar to that observed in groups XIX, indicating that a sternotomy with a 60-min period of open-chest state without PC stimuli did not affect the extent of cell death induced by a subsequent 30-min coronary O. Therefore, in this mouse model of MI, naïve groups can be used as controls.
Figure 3. EPC Effect in Eight Strains.
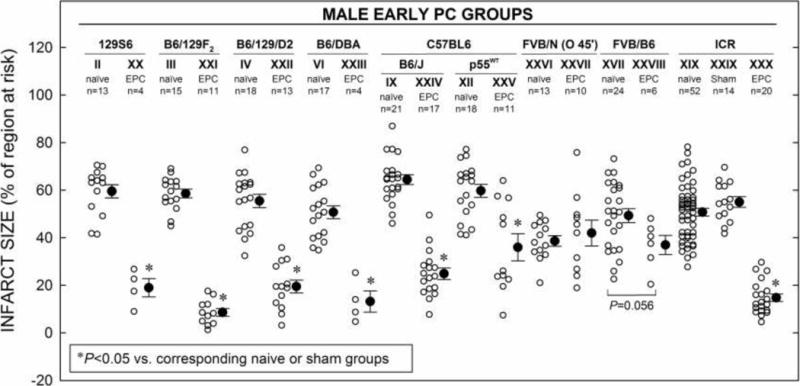
Myocardial infarct size in hearts from eight different strains of male mice in the early preconditioning (PC, EPC) groups (XX–XXVIII and XXX) that received an EPC stimulus consisting of a sequence of six cycles of 4-min coronary O/4-min R cycles 10 min prior to 30-min O (with the exception of groups XXV and XXVI where 45-min O was used), followed by 24 h R (Fig. 1 B). Infarct sizes in EPC groups are compared to that of their respective naïve or EPC sham groups. Infarct size is expressed as percentage of region at risk. Individual mice (○) and Mean ± SEM (●) for respective groups (n, number of mice).
Table 11.
Size of Left Ventricle, Risk Region, and Infarct (Male Early PC groups)
| Strain | Age | Body (g) |
Heart (mg) |
H/B wt (%) |
LV (mg) |
Risk Region (mg) |
Infarct (mg) |
Risk Region (% of LV) |
Infarct (% of risk region) |
Infarct (% of LV) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| XX, n = 4 | 129/S6 | 17 ± 3 | 30.0 ± 1.3 | 160 ± 7 | 0.54 ± 0.03 | 125 ± 6 | 35 ± 7 | 7 ± 2 | 28.6 ± 6.3 | 19.0 ± 3.8* | 6.1 ± 2.1* |
| XXI, n = 11 | B6129F2 | 30 ± 1 | 32.1 ± 1.5 | 154 ± 10 | 0.48 ± 0.02 | 114 ± 10 | 40 ± 4 | 4 ± 1 | 35.3 ± 2.3 | 8.6 ± 1.6* | 3.2 ± 0.7* |
| XXII, n = 13 | B6129/D2 | 11 ± 0 | 27.8 ± 0.7 | 149 ± 7 | 0.54 ± 0.02 | 111 ± 6 | 37 ± 2 | 7 ± 1 | 34.4 ± 1.9 | 19.5 ± 2.7* | 6.6 ± 0.9* |
| XXIII, n = 4 | B6/DBA | 26 ± 2 | 33.9 ± 1.9 | 146 ± 7 | 0.44 ± 0.03 | 106 ± 4 | 35 ± 5 | 4 ± 1 | 32.9 ± 3.6 | 13.2 ± 4.5* | 4.0 ± 1.2* |
| XXIV, n = 17 | B6/J | 16 ± 1 | 28.5 ± 0.8 | 144 ± 5 | 0.51 ± 0.01 | 111 ± 4 | 41 ± 2 | 10 ± 1 | 37.3 ± 1.6 | 24.9 ± 2.4* | 9.3 ± 0.9* |
| XXV, n = 11 | B6/p55WT | 16 ± 1 | 30.2 ± 1.1 | 156 ± 4 | 0.52 ± 0.02 | 121 ± 4 | 44 ± 3 | 16 ± 3 | 36.5 ± 2.4 | 36.0 ± 5.7* | 13.8 ± 2.5* |
| XXVI, n = 13, MI O 45′ | FVB/N | 14 ± 1 | 28.3 ± 0.8 | 139 ± 3 | 0.50 ± 0.02 | 104 ± 3 | 36 ± 2 | 13 ± 1 | 35.6 ± 1.7 | 38.6 ± 2.2 | 12.6 ± 1.0 |
| XXVII, n = 10, EPC O 45′ | FVB/N | 17 ± 2 | 28.0 ± 0.6 | 136 ± 6 | 0.49 ± 0.03 | 96 ± 4 | 34 ± 2 | 14 ± 2 | 35.4 ± 1.3 | 42.0 ± 5.5 | 14.8 ± 2.0 |
| XXVIII, n = 6 | FVB/B6 | 8 ± 0 | 24.0 ± 1.7 | 120 ± 10 | 0.50 ± 0.03 | 91 ± 9 | 31 ± 3 | 11 ± 1 | 34.5 ± 3.0 | 37.0 ± 4.0 | 12.7 ± 1.8 |
| XXIX, n = 14, EPC sham | ICR | 8 ± 0 | 33.6 ± 1.2 | 164 ± 8 | 0.49 ± 0.02 | 121 ± 7 | 50 ± 4 | 27 ± 2 | 41.9 ± 2.8 | 55.0 ± 2.2 | 22.9 ± 1.9 |
| XXX, n = 20 | ICR | 9 ± 0 | 33.6 ± 1.3 | 158 ± 5 | 0.48 ± 0.01 | 114 ± 3 | 51 ± 2 | 8 ± 1 | 45.5 ± 2.7 | 14.8 ± 1.6* | 6.9 ± 1.0* |
3. LPC
When mice in the LPC groups underwent six 4-min coronary O/4-min R cycles 24 h prior to the 30 min (and 45-min, LPC group XXXVII; Fig. 1C) of coronary O, infarct size was markedly reduced in B6/129P2 (group XXXII), B6129F2 (group XXXIV), B6/DBA (group XXXVI), Balb/c (group XXXVII), BPH (group XXXVIII), B6/J (group XXXIX), and ICR (group XLIII) strains, compared with their corresponding naïve or sham control groups, indicating the development of a LPC effect (Fig. 4). In contrast, infarct size was not decreased in 129S6 (group XXXI), B6/129/D2 (group XXXV), FVB/N (group XL, infarct size actually increased, P < 0.05), and FVB/B6 (group XLI) mice, indicating that LPC failed to develop in these four strains (Fig. 4). These results demonstrate that, unlike the well-preserved protective effects afforded by EPC among the mouse strains studied, the development of LPC is quite variable. Again, as shown in Tables 10 and 12 and Fig. 4, infarct size in groups XXXIII and XLII was similar to that observed in groups III and XIX, indicating that a sternotomy with a 60-min period of open-chest state without PC stimuli did not affect the extent of cell death induced by a 30-min coronary occlusion 24 h later. Therefore, naïve groups can be used as control groups in these studies.
Figure 4. LPC Effect in Eleven Strains.

Myocardial infarct size in hearts from eleven different strains of male mice in the late preconditioning (PC, LPC) groups (XXXI–XXXII, XXXIV–XLI, and XLIII) that received a sequence of six cycles of 4-min coronary O/4-min R cycles 24 h prior to 30-min O (with the exception of group XL where 45-min O was used), followed by 24 h R (Fig. 1 C). Infarct size is expressed as percentage of region at risk. Individual mice (○) and Mean ± SEM (●) for respective groups (n, number of mice).
Table 12.
Size of Left Ventricle, Risk Region, and Infarct (Male Late PC Groups)
| Strain | Age | Body (g) |
Heart (mg) |
H/B wt (%) |
LV (mg) |
Risk Region (mg) |
Infarct (mg) |
Risk Region (% of LV) |
Infarct (% of risk region) |
Infarct (% of LV) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| XXXI, n = 11 | 129/S6 | 15 ± 1 | 30.7 ± 1.1 | 178 ± 6 | 0.58 ± 0.02 | 133 ± 5 | 46 ± 4 | 28 ± 3 | 34.5 ± 2.6 | 61.2 ± 2.9 | 21.0 ± 1.7 |
| XXXII, n = 16 | B6/129P2 | 43 ± 7 | 31.6 ± 0.7 | 196 ± 10 | 0.62 ± 0.03 | 150 ± 9 | 62 ± 4 | 18 ± 1 | 41.6 ± 2.0 | 29.1 ± 3.0* | 12.1 ± 1.5* |
| XXXIII, n = 14, LPC sham | B6/129F2 | 11 ± 0 | 29.0 ± 0.8 | 165 ± 7 | 0.57 ± 0.03 | 118 ± 6 | 41 ± 2 | 25 ± 1 | 34.4 ± 1.3 | 60.9 ± 3.6 | 20.9 ± 1.4 |
| XXXIV, n = 10 | B6/129F2 | 18 ± 2 | 31.7 ± 1.5 | 145 ± 9 | 0.46 ± 0.02 | 109 ± 6 | 38 ± 4 | 6 ± 1 | 34.2 ± 2.8 | 19.3 ± 3.4* | 6.2 ± 1.0* |
| XXXV, n = 5 | B6/129/D2 | 12 ± 0 | 27.7 ± 1.4 | 141 ± 7 | 0.51 ± 0.02 | 104 ± 8 | 45 ± 5 | 28 ± 4 | 43.2 ± 2.9 | 62.1 ± 3.3 | 27.2 ± 2.9 |
| XXXVI, n = 12 | B6/DBA | 22 ± 3 | 29.0 ± 0.7 | 131 ± 6 | 0.45 ± 0.01 | 98 ± 5 | 38 ± 3 | 15 ± 1 | 39.5 ± 2.4 | 38.2 ± 2.5* | 14.8 ± 1.1 |
| XXXVII, n = 9 | BALB/c | 24 ± 3 | 31.9 ± 1.0 | 162 ± 5 | 0.51 ± 0.02 | 120 ± 4 | 42 ± 4 | 18 ± 4 | 34.5 ± 2.6 | 39.2 ± 6.2* | 14.5 ± 2.9 |
| XXXVIII, n = 5 | BPH | 40 ± 5 | 27.4 ± 1.0 | 198 ± 6 | 0.73 ± 0.02 | 155 ± 5 | 13 ± 4 | 47 ± 4 | 30.8 ± 2.8 | 26.4 ± 6.5* | 8.3 ± 2.5* |
| XXXIX, n = 17 | B6/J | 18 ± 1 | 29.4 ± 0.9 | 156 ± 6 | 0.54 ± 0.03 | 114 ± 4 | 42 ± 2 | 14 ± 1 | 37.4 ± 1.9 | 32.9 ± 2.3* | 12.3 ± 1.0* |
| XL, n = 12, O45′ | FVB/N | 11 ± 1 | 27.4 ± 0.4 | 134 ± 4 | 0.49 ± 0.02 | 96 ± 4 | 38 ± 3 | 27 ± 2 | 39.7 ± 2.6 | 69.7 ± 3.7§ | 28.0 ± 2.5§ |
| XLI, n = 13 | FVB/B6 | 19 ± 1 | 29.1 ± 0.7 | 126 ± 4 | 0.43 ± 0.01 | 93 ± 3 | 35 ± 3 | 30 ± 2 | 43.4 ± 1.6 | 59.7 ± 3.4 | 25.8 ± 1.6 |
| XLII, n = 43, LPC sham | ICR | 11 ± 1 | 38.9 ± 0.7 | 175 ± 4 | 0.45 ± 0.01 | 120 ± 3 | 49 ± 2 | 27 ± 2 | 40.8 ± 1.6 | 53.7 ± 1.7 | 22.2 ± 1.1 |
| XLIII, n = 51 | ICR | 8 ± 0 | 33.9 ± 0.5 | 170 ± 4 | 0.50 ± 0.01 | 121 ± 2 | 50 ± 2 | 12 ± 1 | 41.3 ± 1.7 | 24.3 ± 1.6* | 9.7 ± 0.7* |
The experimental protocols for the all groups of mice are specified in the legend to Fig. 1. LV, left ventricle; Body, body weight; Heart, total heart weight (ventricles and atria). Data are means ± SEM.
P < 0.05 vs. corresponding naïve or sham groups.
4. Occlusion Time
Figure 5 shows average infarct sizes in naïve hearts of various strains of mice exposed to different coronary O times (ranging from 10–60-min). Of the strains studied, all showed progressively larger average infarct sizes as the coronary O time increased. In B6/129/D2 and B6/J mice, almost all of the risk region (~80%) was infarcted after a 60-min O. Interestingly, only after 60-min O did infarct size in naïve hearts of FVB/N mice (group XLVI, 63.9 ± 2.5%) become significantly smaller than the WAM in group XLV (B6/J, 81.1 ± 3.4%) and remaining groups with 60-min O (78.7 ± 2.3%). Moreover, infarct size in FVB/N mice with 60 min MI became similar to that of the other strains undergoing a 30-min O (FVB/N 63.9 ± 2.5% vs. B6/129/D2 55.5 ± 2.8%, B6/J 64.5 ± 2.1% and ICR 50.8 ± 1.6% of the risk region, respectively; Tables 10 and 13, and Fig. 5) indicating a notable degree of innate cardioprotection in this strain.
Table 13.
Size of Left Ventricle, Risk Region, and Infarct (Occlusion Time Groups in Male Mice)
| Strain | Age | Body (g) |
Heart (mg) |
H/B wt (%) |
LV (mg) |
Risk Region (mg) |
Infarct (mg) |
Risk Region (% of LV) |
Infarct (% of risk region) |
WAM (% of risk region) |
Infarct (% of LV) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| XLIV, n = 4, O 60′ | B6/129/D2 | 13 ± 0 | 28.4 ± 1.6 | 134 ± 9 | 0.47 ± 0.03 | 107 ± 9 | 45 ± 3 | 34 ± 4 | 42.0 ± 0.8 | 76.4 ± 3.1* | 77.3 ± 7.0 | 32.0 ± 1.1* |
| XLV, n = 4, O 60′ | B6/J | 18 ± 0 | 28.9 ± 0.6 | 156 ± 4 | 0.54 ± 0.02 | 117 ± 4 | 43 ± 3 | 35 ± 1 | 37.0 ± 1.5 | 81.1 ± 3.4* | 73.4 ± 5.1 | 29.9 ± 0.1* |
| XLVI, n = 15, O 60′ | FVB/N | 14 ± 2 | 25.9 ± 1.3 | 118 ± 7 | 0.46 ± 0.01 | 88 ± 4 | 36 ± 2 | 23 ± 2 | 40.9 ± 1.6 | 63.9 ± 2.5*§‡ | 78.9 ± 1.9 | 26.2 ± 1.6*§ |
| XLVII, n = 8, O 10′ | ICR | 13 ± 0 | 43.4 ± 0.7 | 177 ± 8 | 0.41 ± 0.02 | 123 ± 7 | 36 ± 2 | 1 ± 0 | 33.3 ± 1.6 | 2.5 ± 0.6*§ | – | 0.9 ± 0.3*§ |
| XLVIII, n = 12, O 20′ | ICR | 11 ± 1 | 40.1 ± 0.9 | 165 ± 6 | 0.41 ± 0.01 | 120 ± 4 | 54 ± 4 | 13 ± 3 | 45.9 ± 3.9 | 23.4 ± 3.6* | – | 11.0 ± 2.1* |
The experimental protocols for the all groups of mice are specified in the legend to Fig. 1. LV, left ventricle; Body, body weight; Heart, total heart weight (ventricles and atria); WAM, weighted arithmetic mean of the groups with 60 min O. Data are means ± SEM.
P < 0.05 vs. corresponding O 30′ groups.
P < 0.05 vs. corresponding O 20′ or O 45′ groups.
5. Gender
Average infarct sizes were also evaluated with regard to gender and strain (Fig. 6). Infarct sizes were similar in the naïve hearts of both male and female mice studied, save those in ICR mice, where the infarct size in females (group LIV) was significantly smaller than that in males (group XIX; 50.8 ± 1.6% vs. 40.8 ± 3.9% of the risk region in male and female, respectively; P < 0.001). Moreover, the development of late PC was gender-specific in ICR mice, being absent in the females. However, in 129S6 mice, when the EPC stimuli were applied 10 min prior to 30-min coronary O, males (group XX) experienced significantly more cardioprotection than females (group XLV) (19.0 3.8% vs. 41.1 ± 4.9% of the risk region, respectively; Fig. 6, P < 0.05); indicating a gender-specific difference in susceptibility to the cardioprotective effects afforded by EPC in the 129S6 strain.
Figure 6. Infarct Size vs. Gender in Five Strains.
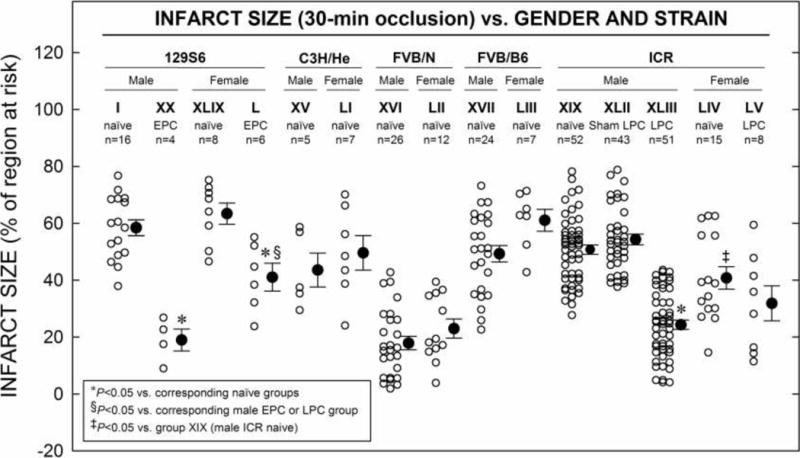
Myocardial infarct size in hearts from five different strains of female mice (groups XLIV–XLVIII and LI–LII) and male mice (groups II, XV, XVI, XVII, XX, and XLIX) that underwent MI consisting of 30-min O followed by 24 h R, either with or without early preconditioning (PC, EPC) or late PC (LPC) stimulus (Fig. 1 E). Infarct is expressed as percentage of region at risk of infarction. Individual mice (○) and Mean ± SEM (●), for respective groups (n, number of mice).
6. Age
Interestingly, age-related differences in infarct size were observed in B6/CBA and B6/J mice (Fig. 7). Infarct size was smaller in B6/CBA, 78 wk old mice (group LVI) compared with adult (18 wk old, group V) mice (42.8 ± 3.5% vs. 55.7 ± 3.7%; Fig. 7, P < 0.05). The infarct size was also smaller in B6/J, 52 wk old mice (group LVIII) compared with either young (6 wk, group LVII) or adult (18 wk old, group IX) mice (46.5 ± 3.0% vs. 67.2 ± 5.0% and 64.5 ± 2.1%, respectively; Tables 10 and 15; Fig. 7, P < 0.05). But, no age- and reperfusion duration-related differences in infarct size were observed in ICR mice (Tables 10 and 15; Fig. 7).
Figure 7. Infarct Size vs. Age in Four Strains.
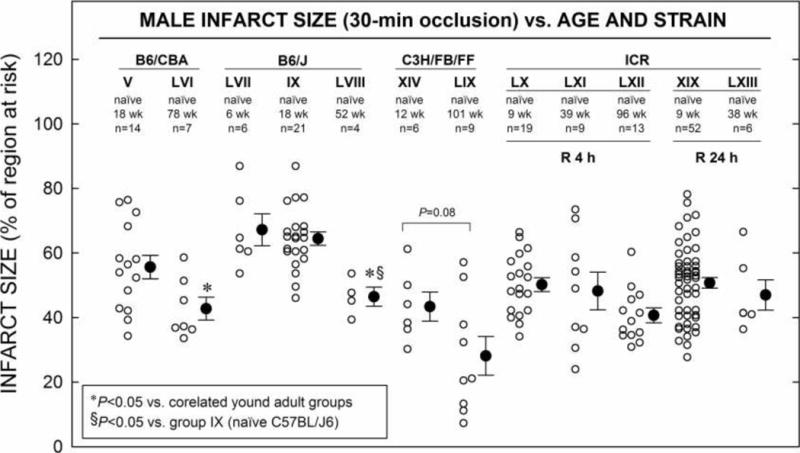
Myocardial infarct size in non-preconditioned (naïve) hearts from mice ranging in ages from 6–101 wk V, IX, XIV, XIX and LIII–LIX underwent MI consisting of 30-min O, followed by 24 h R (Fig. 1 F). Infarct is expressed as percentage of region at risk of infarction. Individual mice (○) and Mean ± SEM (●), for respective groups (n, number of mice).
Table 15.
Size of Left Ventricle, Risk Region, and Infarct (Male, Age and Strain Groups, 30-min occlusion)
| Strain | Age | Body (g) |
Heart (mg) |
H/B wt (%) |
LV (mg) |
Risk Region (mg) |
Infarct (mg) |
Risk Region (% of LV) |
Infarct (% of risk region) |
Infarct (% of LV) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| LVI, n = 7 | B6/CBA | 78 ± 0 | 39.1 ± 2.8 | 157 ± 10 | 0.41 ± 0.02 | 118 ± 8 | 37 ± 4 | 16 ± 2 | 32.0 ± 3.4 | 42.8 ± 3.5* | 13.4 ± 1.2* |
| LVII, n = 6, | B6/J | 6 ± 0 | 17.8 ± 0.6 | 91 ± 5 | 0.52 ± 0.03 | 75 ± 3 | 23 ± 2 | 16 ± 2 | 32.3 ± 2.3 | 67.2 ± 5.0 | 21.6 ± 2.1 |
| LVIII, n = 4 | B6/J | 52 ± 10 | 35.1 ± 1.3 | 157 ± 14 | 0.45 ± 0.06 | 119 ± 11 | 45 ± 3 | 21 ± 3 | 38.8 ± 5.9 | 46.5 ± 3.0*§ | 18.4 ± 4.0 |
| LIX, n = 9 | C3H/FB/FF | 101 ± 0 | 34.6 ± 1.2 | 167 ± 9 | 0.48 ± 0.02 | 116 ± 7 | 40 ± 2 | 12 ± 3 | 35.0 ± 2.1 | 28.6 ± 6.0 | 9.7 ± 2.1 |
| LX, R 4h, n = 19 | ICR | 9 ± 1 | 35.1 ± 0.9 | 179 ± 8 | 0.46 ± 0.04 | 128 ± 6 | 45 ± 3 | 23 ± 2 | 35.6 ± 2.1 | 50.2 ± 2.1 | 17.9 ± 1.3 |
| LXI, R 4h, n = 9 | ICR | 39 ± 1 | 40.0 ± 1.7 | 206 ± 12 | 0.52 ± 0.03 | 148 ± 10 | 51 ± 5 | 26 ± 5 | 35.1 ± 3.4 | 48.2 ± 5.8 | 18.2 ± 3.5 |
| LXII, R 4h, n = 13 | ICR | 96 ± 3 | 46.8 ± 1.2 | 258 ± 11 | 0.55 ± 0.03 | 182 ± 8 | 73 ± 4 | 30 ± 2 | 40.7 ± 2.3 | 40.7 ± 2.3 | 16.2 ± 0.8 |
| LXIII, n = 6 | ICR | 38 ± 1 | 40.1 ± 1.3 | 193 ± 7 | 0.48 ± 0.01 | 151 ± 6 | 67 ± 4 | 32 ± 3 | 44.5 ± 1.3 | 47.0 ± 4.7 | 20.8 ± 1.9 |
The experimental protocols for the all groups of mice are specified in the legend to Fig. 1. LV, left ventricle; Body, body weight; Heart, total heart weight (ventricles and atria). Data are means ± SEM.
P < 0.05 vs. corresponding young naïve groups.
7. Blood transfusion, body temperature and pH
In ICR mice that underwent 30-min of coronary O and 24 h R, a significantly smaller infarct size was achieved when blood transfusion was replaced with crystalloid infusion (group LXV 28.5 ± 3.0% vs. group LXIV 48.4% ± 2.5% and group XIX 50.8 ± 1.6% of risk region, respectively; Tables 10 and 16; Fig. 8). Moreover, increasing body core temperature by only 1.0 ± 0.2 °C in naïve hearts subjected to 30-min of coronary O and 24 h R resulted in a slightly bigger average infarct size (group LXIV, 48.4 ± 2.5% vs. group LXVII, 56.4 ± 6.1% of risk region; Tables 10 and 16; Fig. 8). Conversely, lowering core body temperature by only 1.0 ± 0.2 °C in naïve hearts subjected to 30-min of coronary O and 24 h R resulted in a dramatically smaller average infarct size (group LXIV, 48.4 ± 2.5% vs. group LXVII, 15.7 ± 4.1% of risk region; Tables 10 and 16; Fig. 8), indicating that mild hypothermia, as defined here (−1.0 ± 0.2 °C from normal physiologic temperature [normothermia]), results in a striking direct cardioprotective effect in ICR mice that is independent of HR (Table 9). On other hand, increasing body core temperature to 38 ± 0.2 °C in naïve hearts subjected to a 30-min of coronary O and 24 h R protocol resulted in bigger infarct size (group LXVI 56.4 ± 6.1% vs. group LXIV 48.2 ± 2.5% of risk region; Fig. 8), although this was not a statistically significant difference. Also, alteration of minutes ventilation (specifically by decreasing both respiratory rate and tidal volume) and resultant respiratory acidosis (pH = 7.17 ± 0.01) in ICR mice led to a cardioprotective effect (48.4 ± 2.5% vs. 27.7 ± 6.5% of risk region, respectively; Tables 10 and 16; Fig. 8).
Figure 8. Infarct Size vs. Blood, Temperature and pH in ICR Mice.
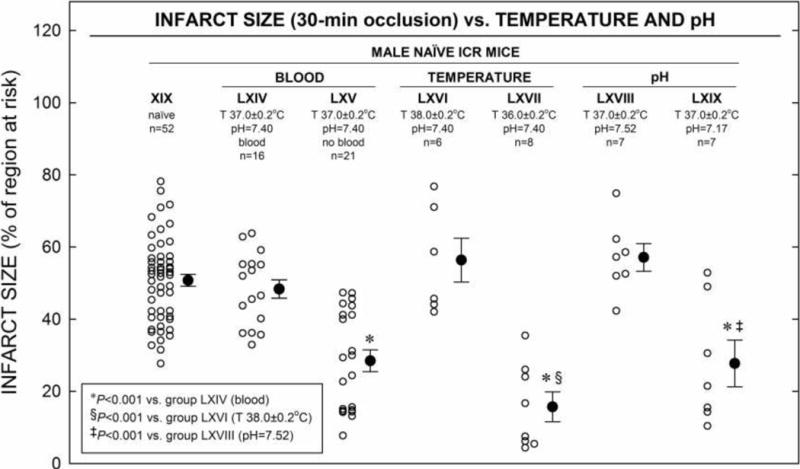
Myocardial infarct size in non-preconditioned (naïve) hearts from ICR mice (LXIV–LXIX) that received 30-min O followed by 24 h R (Fig. 1 G). Infarct size is expressed as percentage of region at risk. Individual mice (○) and Mean ± SEM (●), for respective groups (n, number of mice).
DISCUSSION
This study identifies genetic and experimental variables that alter the myocardial response to I/R tissue injury in a wide array of established mouse strains. The findings have far-reaching implications. We provide a number of novel observations that can be summarized as follows: (i) when comparing infarct size among 19 of the most widely utilized mouse strains, we found that certain wild-type (WT) mice engineered from the C57BL/6 (B6) background (B6/ecSODWT) display a significant level of innate cardioprotection, an effect that is even more striking in FVB/N mice, even after 45-min of coronary O; (ii) although the early phase of PC (EPC) existed in virtually all of the strains studied (except FVB/N) and the magnitude of its cardioprotective effects was similar amongst the strains, this was not true for the late phase of PC (LPC), which was inconsistently observed among strains; (iii) there exists gender-specific susceptibility to the cardioprotection afforded by EPC in 129S6 mice and to I/R injury in the naïve heart of ICR mice; and finally, (iv) core temperature, the choice of vehicle for blood loss replacement, and the acid-base status during surgery have a striking influence on infarct size in ICR mice. The obvious implication is that body temperature and arterial blood pH need to be routinely measured and carefully controlled in studies of myocardial infarction in mice, and that blood losses cannot be replaced with crystalloids but need to be replaced with blood.
Strain-related differences in the susceptibility of the heart to I/R injury were first suggested in 1999 by Miller and colleagues using an experimental I/R and EPC model in FVB/N and B6/J mice [57]. For instance, they observed approximately 20% smaller infarcts in FVB/N mice as compared to B6/J (36.7 ± 4.5% vs. 56.4 ± 8.3% region at risk, respectively) following 60 min coronary O, and here, we observed the same (63.9 ± 2.5% vs. 81.1 ± 3.4% region at risk; Tables 10 and 13; Fig. 5). Most notable, however, is the fact that when we compared FVB/N mice to the other 19 strains, they demonstrated a marked direct cardioprotective effect (34% smaller infarct size) following a 30-min O, an effect similar to that afforded by EPC in other strains (Fig. 3). Explanation for this striking difference in FVB/N mice is beyond the scope of the present study. However, others have made similar observations and it has been suggested that resistance to (1) vascular thrombosis, [54] (2) dystrophic cardiac calcinosis, [44] and (3) LV myocardial rupture following MI is also genetically conferred to the FVB/N strain [17].
In contrast to the degree of innate cardioprotection observed in FVB/N mice, we found persistent resistance to the effects of EPC in this strain only, even following a 30, 45 or 60-min coronary O (Fig. 3; data not shown for 30 and 60 min). These findings are discordant with those of others who report a protective effect in FVB/N mice afforded by EPC [57]. Interestingly, since these authors used an EPC stimulus consisting of three cycles of 5-min coronary O/5-min R cycles followed by 2.5 h of R prior to 60-min coronary O, it is unlikely that these differences can be explained by the dissimilarities in the delivery of the EPC stimulus; as a lesser stimulus elicited a protective effect [57]. It would be more plausible to postulate perhaps that other less strictly controlled physiologic variables (described below) contributed to this observed cardioprotection; however, this is conjectural.
The marked degree of resistance to I/R tissue injury observed in our wild-type B6/ecSODWT mice engineered on the B6 background has also been observed in other wild-type mice engineered on this same background [20]. Specifically, Gorog et al. observed strain-dependent genotypic influences on myocardial susceptibility to I/R injury between wild-types created from mitogen-activated protein kinase kinase 3 knockout mice [B6/MKK2WT] and B6/J. Although it may be tempting to infer an association between these observed effects and presumed incomplete perturbation of the targeted gene, it is most likely that this cardioprotection can be best explained by so-called background effects. These effects originate from retained genetic remnants derived from the background of the embryonic stem cell mouse donor used to generate the original chimeric knockout mice (e.g., 129/S6) and are manifested as some phenotype in the subsequent offspring. Since the ablated gene of interest was originally flanked by alleles derived from a different strain, these genes are retained in subsequent generations, even following the reestablishment of the WT genotype via the breeding of heterozygotes. As such, the linkage disequilibrium created by these flanking genes can potentially influence the phenotype [18]. Although a noteworthy finding here, the precise explanation for this phenotype in this particular strain is, nevertheless, beyond the scope of this report.
While others have reported gender-specific variability in the susceptibility to the pathologic sequelae of myocardial tissue injury following MI in mice [17], we demonstrate, for the first time, a difference in the degree and the type of cardioprotection that is both gender-dependent and strain-related in mice (Fig. 6). That these differences are obviously multifactorial and therefore not subject to scrutiny in this particular study, does not completely prevent speculation as to the underlying mechanisms. As the most likely explanation for this variability lies in gender-related hormonal differences, our observations could in fact be the result of estrogen influences on processes such as myocardial inflammation and remodeling following MI [9]. However, the influence of testosterone levels on processes such as these must be considered as well.
Interestingly, we found that no age-dependent response to I/R injury was observed in ICR strains studied, not even in mice older than 100 weeks of age. However, we found that infarct size was smaller in old B6/CBA mice (group V [18 wk old] 55.7 ± 3.7% vs. group LVI [78 wk] 42.8 ± 3.5% of region at risk, P = 0.037; Tables 10 and 15, Fig. 7) and B6/J mice (group LVII [6 wk] 67.2.7 ± 5.0% or group IX [18 wk] 64.2 ± 2.1% vs. group LVIII [52 wk] 46.5 ± 3.0% of region at risk, respectively, P = 0.008 and P = 0.007; Tables 10 and 15, Fig. 7). Notably, this effect is not shared in rat models of I/R injury. In fact, one study, using rats of comparable age (96 wk) to our eldest group (Fig. 7), showed significantly greater myocardial tissue injury as compared to their 24 wk-old counterparts of the same genetic background [47]. Whether this effect is observed clinically in patients of advanced age is still somewhat a matter of debate [5].
Previous reports performed in swine, rabbit and canine models of experimental MI have implicated core body temperature as a major determinant of infarct size [10, 15, 33, 62]. The authors of these studies clearly demonstrated that even mild hypothermia (defined as at least 35.0°C) confers a substantial infarct-limiting effect to the myocardium. Others have expounded on this premise by illustrating a direct relationship between the degree of hypothermia and the degree of cardioprotection that is independent of heart rate or the severity of the ischemic insult (i.e., the coronary artery O time) [32, 66]. However, these findings have yet to be validated in the mouse. To our knowledge, no previous study has examined the impact of body temperature on myocardial infarct size in mice. Here, we demonstrate that even mild hypothermia (a decrease from a normothermic body temperature [37.0 ± 0.2] of approximately 1.0°C to 36 ± 0.2°C) results in a striking cardioprotective effect (Fig. 8). These data provide a cogent argument for implementation of new standards for core body temperature control during coronary artery O/R in mice. Furthermore, this finding underscores the necessity for strict maintenance of normothermia, as defined here, during surgery if the inferences regarding I/R tissue injury made in murine models are to be reliable. It is sobering to note that many studies in mice do not even report body temperature. Our data imply that almost any infarct size (from very large to very small) can be obtained in mice solely on the basis of variations in temperature. As for the mechanisms responsible for this protective hypothermic effect, it has been suggested that hypothermic perfusion results in increased myocardial ATP preservation, favorable myocardial metabolic substrate utilization and a reduction in the energy demands placed on the heart during both ischemia and R [59].
Among the many other fundamental physiological variables that must be tightly regulated during surgery, blood pressure is perhaps one of the most important. Maintenance of an acceptable aortic pressure sufficient to adequately preserve coronary perfusion during surgery is accomplished, in large part, by intravascular expansion with either crystalloid or whole blood. Models of I/R have been established using both crystalloid-perfused and blood-perfused models and, until now, neither appeared to significantly impact the infarct size [55]. Our results demonstrate that, in the mouse model, blood is required to prevent hypotension and replace blood losses and that replacing blood with crystalloid solutions results in an artifactual cardioprotective effect (Fig. 8, Table 16).
Finally, we demonstrate that even a minor deviation from physiologic arterial blood pH (7.39) towards an acidic pH (7.17) confers a significant cardioprotective effect to ICR mice, while a basic pH had no effect. These findings are similar to previous reports describing this acidosis-related cardioprotective phenomenon [46]. However, whereas we observed cardioprotection at a pH of 7.17, others required significantly lower pHs (< 7) to achieve this effect [34], indicating that blood pH must be meticulously controlled in the mouse. The phenomenon whereby rapid restoration of physiologic pH during the R phase of I/R injury actually contributes to, rather than attenuating, the lethal effects of R has been well described [34, 46, 70]. Though many mechanisms for this pH-dependent protective effect have been proposed, perhaps the most convincing evidence stems from work describing the mitochondrial permeability transition pore (mPTP). Recent data have shown that assembly of this mPTP within the inner mitochondrial membrane in response to acidic reperfusate leads directly to cell death by apoptosis if the insult is mild or to necrosis if the insult is profound [4]. The intricate molecular basis of this protective effect is beyond the scope of the present study.
Conclusions
We report herein the largest analysis of the determinants of infarct size in mice performed heretofore. Genetically engineered mice have proven to be indispensable as scientific research tools, particularly to our understanding of the molecular and cellular mechanisms underlying cardioprotection. Here, we have discovered not only that these biological instruments display genetically-dependent differences in their susceptibility to I/R injury, but also that perturbation of fundamental physiological variables during surgery alters greatly the severity of myocardial injury during ischemia. Unfortunately, these important differences have been largely ignored in the literature; as a consequence, it is likely that many erroneous interpretations, either propitious or unfavorable, have been made when murine models of acute I/R injury have been utilized. For example, many mouse studies did not even measure body temperature or arterial blood pH.
Our results demonstrate that genetic background, gender, age, body temperature, and arterial blood pH have a major impact on infarct size, and thus need to be carefully measured and/or taken into account when performing studies of MI in mice; failure to do so will make results uninterpretable. For example, core temperature and blood pH must be measured routinely, respiratory acidosis and hypothermia (or hyperthermia) need to be carefully avoided, and comparisons cannot be made between mouse strains or genders that exhibit different susceptibility to I/R injury (e.g., FVB/N male mice and ICR female mice are inherently protected against I/R injury).
This report provides the groundwork required to standardize experimental methods used in MI models in mice. We hope that the present results will alert the community to the need to recognize that both the susceptibility of mice to ischemic injury and their resistance or proclivity to cardioprotection exhibit major strain- and/or gender-related differences, and are also affected to a major extent by physiological variables such as body temperature and arterial blood pH. It is quite plausible that many or most previous differences in results obtained by various laboratories were caused by the fact that the confounding influences of these determinants of infarct size were ignored.
Because a standardized approach has been lacking in the preclinical investigation of cardioprotective strategies, many inconsistencies have been observed when attempting translation into the clinical setting [7, 35, 45, 63]. Accordingly, a collaborative network of research laboratories is required to test novel cardioprotective strategies in a range of animal MI models to establish a standard by which consistent cardioprotection can be observed reproducibly [7, 35, 45, 63]. We hope that the data provided herein will offer a metric by which cardioprotective strategies can be scrutinized in murine models of MI.
Table 3.
Heart Rate on the Day of the Coronary Occlusion (Naïve Acute MI Groups)
| GROUPS | Pre-occlusion | Occlusion | Reperfusion | ||||
|---|---|---|---|---|---|---|---|
| 5 min | 15 min | 30 min | 5 min | 10 min | 15 min | ||
| Heart rate (beats/min) | |||||||
| I, 129S6, MI | 565 ± 9 | 586 ± 8 | 595 ± 11 | 591 ± 15 | 579 ± 11 | 581 ± 11 | 582 ± 15 |
| II, B6/129P2, MI | 536 ± 15 | 564 ± 14 | 565 ± 15 | 577 ± 17 | 562 ± 18 | 563 ± 18 | 580 ± 22 |
| III, B6/129F2, MI | 523 ± 21 | 546 ± 16 | 541 ± 15 | 555 ± 16 | 554 ± 18 | 556 ± 18 | 560 ± 24 |
| IV, B6/129/D2, MI | 497 ± 8 | 531 ± 9 | 537 ± 9 | 527 ± 9 | 532 ± 10 | 543 ± 9 | 543 ± 10 |
| V, B6/CBA, MI | 515 ± 22 | 529 ± 25 | 547 ± 27 | 546 ± 25 | 541 ± 24 | 537 ± 24 | 538 ± 24 |
| VI, B6/DBAF1, MI | 541 ± 23 | 584 ± 21 | 580 ± 22 | 608 ± 21 | 609 ± 27 | 601 ± 31 | 610 ± 25 |
| VII, BALB/c, MI | 501 ± 20 | 534 ± 13 | 530 ± 14 | 522 ± 14 | 536 ± 11 | 540 ± 17 | 553 ± 24 |
| VIII, BPH, MI | 592 ± 13 | 649 ± 20 | 605 ± 24 | 629 ± 24 | 617 ± 24 | 623 ± 29 | 626 ± 28 |
| IX, B6/J, MI | 518 ± 17 | 540 ± 15 | 537 ± 18 | 558 ± 15 | 563 ± 17 | 558 ± 15 | 573 ± 18 |
| X, B6/ARWT, MI | 467 ± 20 | 485 ± 22 | 494 ± 22 | 513 ± 24 | 511 ± 23 | 501 ± 26 | 531 ± 9 |
| XI, B6/ecSODWT, MI | 522 ± 33 | 513 ± 25 | 498 ± 28 | 517 ± 28 | 516 ± 26 | 529 ± 33 | 533 ± 37 |
| XII, B6/p55WT, MI | 490 ± 22 | 474 ± 23 | 477 ± 23 | 483 ± 24 | 490 ± 23 | 475 ± 27 | 496 ± 22 |
| XIII, C3H/DBA, MI | 515 ± 17 | 568 ± 25 | 559 ± 26 | 561 ± 28 | 591 ± 33 | 567 ± 28 | 557 ± 33 |
| XIV, C3H/FB/FF, MI | 533 ± 23 | 533 ± 15 | 520 ± 19 | 531 ± 18 | 536 ± 12 | 505 ± 16 | 533 ± 15 |
| XV, C3H/He, MI | 556 ± 25 | 590 ± 20 | 590 ± 27 | 564 ± 28 | 551 ± 32 | 578 ± 29 | 539 ± 28 |
| XVI, FVB/N, MI | 540 ± 24 | 564 ± 19 | 567 ± 21 | 571 ± 23 | 583 ± 26 | 578 ± 23 | 594 ± 25 |
| XVII, FVB/B6, MI | 538 ± 15 | 548 ± 13 | 555 ± 14 | 578 ± 17 | 607 ± 18 | 592 ± 22 | 606 ± 18 |
| XVIII, FVB/ICR, MI | 556 ± 25 | 568 ± 30 | 564 ± 35 | 572 ± 43 | 564 ± 34 | 558 ± 27 | 582 ± 33 |
| XIX, ICR, MI | 634 ± 36 | 678 ± 21 | 660 ± 30 | 687 ± 14 | 673 ± 14 | 669 ± 13 | 677 ± 17 |
Data are means ± SEM. Measurements of rectal temperature and heart rate were taken before the 30-min coronary occlusion (pre-occlusion), at 5, 15 and 30 min into the 30-min occlusion, and at 5, 15 and 30 min after reperfusion. Rectal temperature was continuously monitored and carefully controlled throughout the experiment, as detailed in the text.
Table 4.
Rectal Temperature and Heart Rate on the Day of Occlusion (Early PC Groups)
| GROUPS | Pre-occlusion | Occlusion | Reperfusion | ||||
|---|---|---|---|---|---|---|---|
| 5 min | 15 min | 30 min | 5 min | 10 min | 15 min | ||
| Temperature (°C) | |||||||
| XX, 129S6, EPC | 37.2 ± 0.1 | 37.2 ± 0.1 | 37.0 ± 0.1 | 37.1 ± 0.1 | 37.2 ± 0.1 | 37.1 ± 0.0 | 37.1 ± 0.1 |
| XXI, B6/129F2, EPC | 37.2 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.1 | 37.0 ± 0.1 | 36.9 ± 0.1 | 37.1 ± 0.1 | 37.2 ± 0.2 |
| XXII, B6/129/D2, EPC | 37.1 ± 0.1 | 37.0 ± 0.1 | 37.0 ± 0.1 | 37.1 ± 0.0 | 37.1 ± 0.1 | 37.0 ± 0.1 | 37.2 ± 0.1 |
| XXIII, B6/DBA, EPC | 36.9 ± 0.0 | 37.0 ± 0.1 | 37.0 ± 0.1 | 36.9 ± 0.0 | 37.0 ± 0.1 | 36.9 ± 0.1 | 36.9 ± 0.1 |
| XXIV, B6/J, EPC | 37.0 ± 0.1 | 37.0 ± 0.1 | 36.9 ± 0.1 | 36.9 ± 0.1 | 37.0 ± 0.1 | 37.0 ± 0.1 | 36.9 ± 0.1 |
| XXV, B6/p55WT, EPC | 36.9 ± 0.0 | 37.0 ± 0.1 | 36.9 ± 0.1 | 36.9 ± 0.0 | 37.0 ± 0.1 | 36.9 ± 0.1 | 36.9 ± 0.1 |
| XXVI, FVB/N, MI O 45′ | 37.1 ± 0.2 | 37.1 ± 0.0 | 37.0 ± 0.1 | 37.0 ± 0.2 | 36.9 ± 0.0 | 37.1 ± 0.1 | 37.1 ± 0.1 |
| XXVII, FVB/N, EPC O 45′ | 37.0 ± 0.1 | 37.1 ± 0.1 | 36.9 ± 0.0 | 37.0 ± 0.1 | 37.0 ± 0.1 | 37.0 ± 0.1 | 36.9 ± 0.1 |
| XXVIII, FVB/B6, EPC | 37.0 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.2 | 36.9 ± 0.1 | 36.9 ± 0.1 | 36.9 ± 0.0 |
| XXIX, ICR, EPC sham | 37.1 ± 0.1 | 37.2 ± 0.1 | 37.3 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.1 |
| XXX, ICR, EPC | 37.3 ± 0.2 | 37.0 ± 0.1 | 37.0 ± 0.1 | 37.2 ± 0.1 | 37.1 ± 0.1 | 36.7 ± 0.1 | 36.7 ± 0.1 |
| Heart rate (beats/min) | |||||||
| XX, 129S6, EPC | 578 ± 21 | 575 ± 21 | 570 ± 22 | 578 ± 25 | 587 ± 23 | 581 ± 32 | 574 ± 34 |
| XXI, B6/129F2, EPC | 529 ± 16 | 520 ± 15 | 516 ± 15 | 523 ± 14 | 540 ± 13 | 474 ± 15 | 473 ± 17 |
| XXII, B6/129/D2, EPC | 505 ± 16 | 517 ± 18 | 511 ± 15 | 516 ± 11 | 527 ± 8 | 536 ± 13 | 536 ± 11 |
| XXIII, B6/DBA, EPC | 587 ± 18 | 579 ± 19 | 561 ± 18 | 568 ± 16 | 596 ± 16 | 585 ± 18 | 612 ± 16 |
| XXIV, B6/J, EPC | 533 ± 9 | 542 ± 13 | 534 ± 10 | 535 ± 8 | 543 ± 13 | 544 ± 14 | 556 ± 21 |
| XXV, B6/p55WT, EPC | 589 ± 17 | 583 ± 18 | 562 ± 17 | 572 ± 16 | 602 ± 13 | 592 ± 15 | 621 ± 12 |
| XXVI, FVB/N, MI O 45′ | 484 ± 17 | 540 ± 31 | 558 ± 26 | 556 ± 28 | 570 ± 33 | 568 ± 31 | 576 ± 46 |
| XXVII, FVB/N, EPC O 45′ | 542 ± 17 | 544 ± 19 | 555 ± 28 | 539 ± 25 | 559 ± 25 | 531 ± 22 | 540 ± 22 |
| XXVIII, FVB/B6, EPC | 515 ± 26 | 513 ± 35 | 491 ± 7 | 509 ± 21 | 473 ± 13 | 463 ± 10 | 478 ± 19 |
| XXIX, ICR, EPC sham | 637 ± 15 | 666 ± 14 | 697 ± 15 | 686 ± 16 | 690 ± 17 | 674 ± 14 | 674 ± 16 |
| XXX, ICR, EPC | 549 ± 20 | 551 ± 23 | 540 ± 28 | 543 ± 18 | 536 ± 16 | 532 ± 15 | 562 ± 20 |
Data are means ± SEM. Measurements of rectal temperature and heart rate were taken before the 30-min coronary occlusion (pre-occlusion), at 5, 15 and 30 min into the 30-min occlusion, and at 5, 15 and 30 min after reperfusion. Rectal temperature was continuously monitored and carefully controlled throughout the experiment, as detailed in the text.
Table 5.
Rectal Temperature and Heart Rate on the Day of Occlusion (Late PC Groups)
| GROUPS | Pre-occlusion | Occlusion | Reperfusion | ||||
|---|---|---|---|---|---|---|---|
| 5 min | 15 min | 30 min | 5 min | 10 min | 15 min | ||
| Temperature (°C) | |||||||
| XXXI, 129S6, LPC | 36.9 ± 0.1 | 37.1 ± 0.1 | 37.0 ± 0.1 | 37.2 ± 0.1 | 37.0 ± 0.1 | 36.9 ± 0.1 | 36.9 ± 0.1 |
| XXXII, B6/129P2, LPC | 36.9 ± 0.0 | 37.0 ± 0.0 | 37.0 ± 0.0 | 37.0 ± 0.0 | 37.0 ± 0.0 | 37.0 ± 0.0 | 37.0 ± 0.1 |
| XXXIII, B6/129F2, LPC sham | 37.2 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.1 | 37.0 ± 0.1 | 36.9 ± 0.1 | 36.9 ± 0.2 |
| XXXIV, B6/129F2, LPC | 37.1 ± 0.1 | 37.2 ± 0.1 | 37.1 ± 0.1 | 37.0 ± 0.1 | 36.8 ± 0.2 | 37.0 ± 0.2 | 37.1 ± 0.1 |
| XXXV, B6/129/D2, LPC | 37.0 ± 0.1 | 37.1 ± 0.0 | 37.0 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.1 | 37.2 ± 0.1 | 37.2 ± 0.2 |
| XXXVI, B6/DBA, LPC | 36.8 ± 0.2 | 37.0 ± 0.1 | 37.0 ± 0.1 | 37.0 ± 0.1 | 36.9 ± 0.9 | 37.0 ± 0.1 | 37.0 ± 0.1 |
| XXXVII, BALB/c, LPC | 37.2 ± 0.1 | 37.4 ± 0.1 | 37.3 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.2 | 37.3 ± 0.2 |
| XXXVIII, BPH, LPC | 36.8 ± 0.0 | 37.2 ± 0.1 | 37.0 ± 0.1 | 37.2 ± 0.1 | 36.9 ± 0.1 | 37.0 ± 0.1 | 37.1 ± 0.1 |
| XXXIX, B6/J, LPC | 37.0 ± 0.1 | 37.2 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.1 | 36.9 ± 0.1 | 37.1 ± 0.2 | 37.2 ± 0.1 |
| XL, FVB/N, LPC | 36.9 ± 0.1 | 37.1 ± 0.1 | 37.2 ± 0.1 | 37.1 ± 0.1 | 37.0 ± 0.1 | 37.0 ± 0.1 | 37.0 ± 0.1 |
| XLI, FVB/B6, LPC | 37.0 ± 0.1 | 37.1 ± 0.0 | 37.0 ± 0.1 | 37.0 ± 0.1 | 37.0 ± 0.1 | 37.1 ± 0.2 | 37.1 ± 0.1 |
| XLII, ICR, LPC sham | 37.1 ± 0.1 | 37.2 ± 0.1 | 37.3 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.1 |
| XLIII, ICR, LPC | 36.9 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.1 | 36.9 ± 0.1 | 36.9 ± 0.0 | 36.9 ± 0.1 | 36.8 ± 0.1 |
| Heart rate (beats/min) | |||||||
| XXXI, 129S6, LPC | 608 ± 21 | 624 ± 22 | 637 ± 21 | 652 ± 22 | 640 ± 23 | 634 ± 25 | 645 ± 25 |
| XXXII, B6/129P2, LPC | 590 ± 14 | 619 ± 11 | 611 ± 11 | 608 ± 10 | 618 ± 10 | 612 ± 12 | 620 ± 10 |
| XXXIII, B6/129F2, LPC sham | 577 ± 29 | 567 ± 24 | 565 ± 24 | 585 ± 33 | 601 ± 34 | 583 ± 33 | 607 ± 28 |
| XXXIV, B6/129F2, LPC | 552 ± 25 | 559 ± 22 | 565 ± 22 | 560 ± 24 | 558 ± 26 | 569 ± 24 | 578 ± 27 |
| XXXV, B6/129/D2, LPC | 563 ± 26 | 595 ± 32 | 586 ± 36 | 588 ± 32 | 590 ± 27 | 596 ± 23 | 616 ± 38 |
| XXXVI, B6/DBA, LPC | 632 ± 19 | 663 ± 25 | 666 ± 34 | 671 ± 42 | 663 ± 41 | 675 ± 41 | 657 ± 47 |
| XXXVII, BALB/c, LPC | 609 ± 35 | 609 ± 20 | 616 ± 27 | 607 ± 33 | 621 ± 29 | 627 ± 19 | 654 ± 62 |
| XXXVIII, BPH, LPC | 693 ± 15 | 720 ± 12 | 701 ± 15 | 715 ± 8 | 707 ± 17 | 684 ± 28 | 701 ± 12 |
| XXXIX, B6/J, LPC | 569 ± 28 | 613 ± 40 | 597 ± 39 | 606 ± 34 | 613 ± 32 | 633 ± 29 | 644 ± 28 |
| XL, FVB/N, LPC | 606 ± 9 | 661 ± 19 | 670 ± 13 | 678 ± 20 | 699 ± 19 | 707 ± 13 | 717 ± 35 |
| XLI, FVB/B6, LPC | 562 ± 26 | 591 ± 20 | 586 ± 20 | 608 ± 16 | 610 ± 29 | 593 ± 34 | 597 ± 19 |
| XLII, ICR, LPC sham | 637 ± 15 | 666 ± 14 | 697 ± 15 | 686 ± 16 | 690 ± 17 | 674 ± 14 | 674 ± 16 |
| XLIII, ICR, LPC | 566 ± 9 | 618 ± 17 | 620 ± 16 | 636 ± 15 | 640 ± 16 | 634 ± 21 | 635 ± 21 |
Data are means ± SEM. Measurements of rectal temperature and heart rate were taken before the 30-min coronary occlusion (pre-occlusion), at 5, 15 and 30 min into the 30-min occlusion, and at 5, 15 and 30 min after reperfusion. Rectal temperature was continuously monitored and carefully controlled throughout the experiment, as detailed in the text.
Table 6.
Rectal Temperature and Heart Rate on the Day of the Coronary Occlusion (Occlusion Time Groups)
| GROUPS | Pre-occlusion | Occlusion | Reperfusion | ||||
|---|---|---|---|---|---|---|---|
| 5 min | 15 min | 30 min | 5 min | 10 min | 15 min | ||
| Temperature (°C) | |||||||
| XLIV, B6/129/D2, MI, O 60′ | 37.2 ± 0.1 | 37.3 ± 0.0 | 37.1 ± 0.2 | 37.0 ± 0.1 | 37.2 ± 0.2 | 37.0 ± 0.2 | 37.1 ± 0.1 |
| XLV, B6/J, MI, O 60′ | 36.9 ± 0.1 | 36.9 ± 0.0 | 37.0 ± 0.1 | 36.9 ± 0.1 | 37.1 ± 0.1 | 37.2 ± 0.0 | 37.2 ± 0.1 |
| XLVI, FVB/N, MI, O 60′ | 36.9 ± 0.2 | 37.0 ± 0.2 | 37.2 ± 0.1 | 37.1 ± 0.1 | 36.9 ± 0.2 | 37.0 ± 0.1 | 36.9 ± 0.1 |
| XLVII, ICR, MI, O 10′ | 37.0 ± 0.1 | 37.1 ± 0.1 | 37.0 ± 0.1 | 37.0 ± 0.1 | 37.0 ± 0.1 | ||
| XLVIII, ICR, MI, O 20′ | 37.0 ± 0.1 | 37.1 ± 0.1 | 36.9 ± 0.1 | 37.2 ± 0.0 | 37.0 ± 0.1 | 37.0 ± 0.1 | |
| Heart rate (beats/min) | |||||||
| XLIV, B6/129/D2, MI, O 60′ | 448 ± 29 | 496 ± 24 | 507 ± 32 | 544 ± 13 | 592 ± 23 | 584 ± 9 | 587 ± 10 |
| XLV, B6/J, MI, O 60′ | 461 ± 28 | 475 ± 18 | 482 ± 8 | 492 ± 17 | 476 ± 34 | 494 ± 26 | 468 ± 30 |
| XLVI, FVB/N, MI, O 60′ | 499 ± 25 | 549 ± 25 | 579 ± 32 | 563 ± 16 | 568 ± 19 | 570 ± 15 | 570 ± 16 |
| XLVII, ICR, MI, O 10′ | 558 ± 11 | 594 ± 12 | 575 ± 22 | 575 ± 18 | 562 ± 21 | ||
| XLVIII, ICR, MI, O 20′ | 508 ± 24 | 573 ± 24 | 557 ± 21 | 541 ± 19 | 513 ± 28 | 519 ± 20 | |
Data are means ± SEM. Measurements of rectal temperature and heart rate were taken before the 30-min coronary occlusion (pre-occlusion), at 5, 15, 30, 45 and 60 min into the 60-min occlusion, and at 5, 15 and 30 min after reperfusion. Rectal temperature was continuously monitored and carefully controlled throughout the experiment, as detailed in the text.
Table 7.
Rectal Temperature and Heart Rate on the Day of the 30-min Coronary Occlusion (Female Groups)
| GROUPS | Pre-occlusion | Occlusion | Reperfusion | ||||
|---|---|---|---|---|---|---|---|
| 5 min | 15 min | 30 min | 5 min | 10 min | 15 min | ||
| Temperature (°C) | |||||||
| XLIX, 129S6, female, MI | 36.9 ± 0.1 | 37.2 ± 0.0 | 36.9 ± 0.1 | 37.2 ± 0.0 | 37.1 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.1 |
| L, 129S6, female, EPC | 37.2 ± 0.1 | 37.2 ± 0.2 | 37.0 ± 0.1 | 37.0 ± 0.1 | 37.1 ± 0.2 | 37.2 ± 0.2 | 37.0 ± 0.1 |
| LI, C3H/He, female, MI | 36.8 ± 0.1 | 37.0 ± 0.2 | 37.1 ± 0.1 | 37.0 ± 0.2 | 37.0 ± 0.1 | 37.0 ± 0.1 | 36.9 ± 0.2 |
| LII, FVB/N, female, MI | 36.9 ± 0.1 | 37.0 ± 0.0 | 37.0 ± 0.0 | 37.1 ± 0.1 | 37.1 ± 0.0 | 36.9 ± 0.1 | 36.9 ± 0.1 |
| LIII, FVB/B6, female, MI | 36.9 ± 0.2 | 37.1 ± 0.1 | 37.1 ± 0.1 | 37.0 ± 0.1 | 37.0 ± 0.1 | 37.0 ± 0.1 | 37.0 ± 0.1 |
| LIV, ICR, female, MI | 37.0 ± 0.1 | 37.2 ± 0.1 | 37.0 ± 0.1 | 36.8 ± 0.0 | 37.0 ± 0.1 | 37.1 ± 0.1 | 36.9 ± 0.1 |
| LV, ICR, female, LPC | 37.1 ± 0.0 | 37.0 ± 0.1 | 37.1 ± 0.1 | 36.9 ± 0.0 | 36.9 ± 0.1 | 36.9 ± 0.1 | 37.1 ± 0.1 |
| Heart rate (beats/min) | |||||||
| XLIX, 129S6, female, MI | 528 ± 22 | 552 ± 19 | 544 ± 32 | 556 ± 22 | 561 ± 24 | 547 ± 21 | 556 ± 31 |
| L, 129S6, female, EPC | 572 ± 22 | 586 ± 23 | 562 ± 29 | 580 ± 35 | 589 ± 34 | 563 ± 57 | 539 ± 22 |
| LI, C3H/He, female, MI | 558 ± 23 | 610 ± 14 | 620 ± 29 | 582 ± 24 | 571 ± 32 | 605 ± 32 | 546 ± 36 |
| LII, FVB/N, female, MI | 507 ± 18 | 558 ± 11 | 579 ± 10 | 566 ± 15 | 575 ± 22 | 575 ± 18 | 562 ± 21 |
| LIII, FVB/B6, female, MI | 551 ± 29 | 562 ± 25 | 567 ± 29 | 573 ± 34 | 560 ± 41 | 574 ± 38 | 583 ± 45 |
| LIV, ICR, female, MI | 536 ± 23 | 559 ± 61 | 548 ± 20 | 534 ± 21 | 539 ± 27 | 517 ± 25 | 536 ± 29 |
| LV, ICR, female, LPC | 585 ± 18 | 639 ± 24 | 641 ± 21 | 636 ± 28 | 651 ± 24 | 640 ± 17 | 655 ± 23 |
Data are means ± SEM. Measurements of rectal temperature and heart rate were taken before the 30-min coronary occlusion (pre-occlusion), at 5, 15 and 30 min into the 30-min occlusion, and at 5, 15 and 30 min after reperfusion. Rectal temperature was continuously monitored and carefully controlled throughout the experiment, as detailed in the text.
Table 8.
Rectal Temperature and Heart Rate on the Day of the 30-min Coronary Occlusion (Age and Strain Groups)
| GROUPS | Pre-occlusion | Occlusion | Reperfusion | ||||
|---|---|---|---|---|---|---|---|
| 5 min | 15 min | 30 min | 5 min | 10 min | 15 min | ||
| Temperature (°C) | |||||||
| LVI, B6/CBA, old, MI | 36.9 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.1 | 37.0 ± 0.1 | 36.8 ± 0.1 | 37.1 ± 0.1 | 37.0 ± 0.1 |
| LVII, B6/J, 6 wks, MI | 36.9 ± 0.1 | 37.1 ± 0.1 | 37.0 ± 0.1 | 36.9 ± 0.1 | 37.0 ± 0.1 | 37.1 ± 0.1 | 37.0 ± 0.1 |
| LVIII, B6/J, old, MI | 37.1 ± 0.1 | 37.2 ± 0.0 | 37.1 ± 0.1 | 37.0 ± 0.1 | 37.1 ± 0.1 | 37.2 ± 0.1 | 37.0 ± 0.1 |
| LIX, C3H/FB/FF, old, MI | 37.0 ± 0.1 | 37.1 ± 0.1 | 37.0 ± 0.1 | 36.9 ± 0.1 | 37.0 ± 0.1 | 37.1 ± 0.1 | 37.0 ± 0.1 |
| LX, ICR, 9 wks, MI R 4h | 36.9 ± 0.1 | 36.9 ± 0.1 | 37.0 ± 0.1 | 36.9 ± 0.0 | 36.8 ± 0.1 | 36.9 ± 0.1 | 36.9 ± 0.0 |
| LXI, ICR, middle, MI, R 4h | 37.1 ± 0.1 | 37.2 ± 0.0 | 37.1 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.1 | 37.0 ± 0.1 | 37.1 ± 0.1 |
| LXII, ICR, old, MI, R 4h | 36.9 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.1 | 37.0 ± 0.1 | 37.0 ± 0.1 | 36.9 ± 0.1 | 36.9 ± 0.1 |
| LXIII, ICR, middle, MI | 37.2 ± 0.0 | 37.2 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.1 | 37.1 ± 0.1 | 37.0 ± 0.1 | 37.1 ± 0.0 |
| Heart rate (beats/min) | |||||||
| LVI, B6/CBA, old, MI | 532 ± 25 | 568 ± 25 | 573 ± 23 | 556 ± 27 | 562 ± 26 | 549 ± 21 | 538 ± 27 |
| LVII, B6/J, 6 wks, MI | 492 ± 17 | 495 ± 18 | 468 ± 15 | 469 ± 18 | 481 ± 21 | 490 ± 22 | 483 ± 16 |
| LVII, B6/J, old, MI | 583 ± 23 | 576 ± 10 | 556 ± 26 | 592 ± 36 | 602 ± 41 | 616 ± 41 | 621 ± 22 |
| LIX, C3H/FB/FF, old, MI | 540 ± 33 | 562 ± 34 | 555 ± 37 | 522 ± 42 | 525 ± 34 | 529 ± 33 | 556 ± 26 |
| LX, ICR, 9 wks, MI R 4h | 534 ± 26 | 578 ± 19 | 560 ± 15 | 587 ± 12 | 573 ± 11 | 569 ± 15 | 577 ± 12 |
| LXI, ICR, middle, MI, R 4h | 561 ± 22 | 578 ± 22 | 570 ± 16 | 561 ± 19 | 565 ± 19 | 555 ± 25 | 569 ± 25 |
| LXII, ICR, old, MI, R 4h | 579 ± 16 | 602 ± 20 | 588 ± 22 | 598 ± 33 | 597 ± 33 | 595 ± 31 | 608 ± 34 |
| LXIII, ICR, middle, MI | 648 ± 24 | 659 ± 26 | 633 ± 26 | 625 ± 24 | 621 ± 19 | 615 ± 16 | 623 ± 20 |
Data are means ± SEM. Measurements of rectal temperature and heart rate were taken before the 30-min coronary occlusion (pre-occlusion), at 5, 15 and 30 min into the 30-min occlusion, and at 5, 15 and 30 min after reperfusion. Rectal temperature was continuously monitored and carefully controlled throughout the experiment, as detailed in the text.
Table 14.
Size of Left Ventricle, Risk Region, and Infarct (Female Gender Groups)
| Strain | Age | Body (g) |
Heart (mg) |
H/B wt (%) |
LV (mg) |
Risk Region (mg) |
Infarct (mg) |
Risk Region (% of LV) |
Infarct (% of risk region) |
Infarct (% of LV) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| XLIX, n = 8, MI | 129S6 | 27 ± 2 | 24.1 ± 0.7 | 139 ± 5 | 0.58 ± 0.02 | 102 ± 3 | 30 ± 2 | 19 ± 2 | 29.6 ± 1.6 | 63.4 ± 3.7 | 18.8 ± 1.5 |
| L, n = 6, EPC | 129S6 | 19 ± 1 | 22.9 ± 1.1 | 126 ± 7 | 0.55 ± 0.03 | 101 ± 5 | 30 ± 2 | 12 ± 2 | 30.3 ± 1.1 | 41.1 ± 4.9*§ | 12.4 ± 1.5*§ |
| LI, n = 7, MI | C3H/He | 36 ± 2 | 24.4 ± 0.6 | 119 ± 5 | 0.49 ± 0.02 | 85 ± 4 | 28 ± 2 | 14 ± 2 | 32.6 ± 2.4 | 49.6 ± 6.1 | 16.5 ± 2.4 |
| LII, n = 12, MI 30′ | FVB/N | 33 ± 1 | 26.4 ± 1.0 | 134 ± 6 | 0.51 ± 0.02 | 97 ± 4 | 37 ± 3 | 8 ± 1 | 37.7 ± 2.3 | 23.0 ± 3.3 | 8.6 ± 1.4 |
| LIII, n = 7, MI 30′ | FVB/B6 | 16 ± 1 | 22.9 ± 0.7 | 130 ± 5 | 0.57 ± 0.02 | 98 ± 4 | 40 ± 2 | 25 ± 2 | 41.4 ± 1.9 | 61.1 ± 3.8 | 25.3 ± 2.0 |
| LIV, n = 15, MI | ICR | 12 ± 0 | 26.4 ± 0.6 | 137 ± 5 | 0.51 ± 0.01 | 98 ± 4 | 40 ± 2 | 16 ± 2 | 41.0 ± 1.8 | 40.8 ± 3.9‡ | 17.0 ± 1.8‡ |
| LV, n = 8, LPC | ICR | 13 ± 1 | 30.1 ± 0.5 | 147 ± 9 | 0.49 ± 0.03 | 104 ± 6 | 40 ± 3 | 12 ± 2 | 39.2 ± 1.8 | 31.9 ± 6.1 | 11.6 ± 2.0 |
The experimental protocols for the all groups of mice are specified in the legend to Fig. 1. LV, left ventricle; Body, body weight; Heart, total heart weight (ventricles and atria). Data are means ± SEM.
P < 0.05 vs. corresponding naïve or sham groups.
P < 0.05 vs. corresponding male EPC or LPC groups.
Acknowledgments
This study was supported in part by NIH grants R01 HL55757, HL-70897, HL-76794, and P01HL78825. We would like to thank Xian-Liang Tang and Michael Book for all of their help with statistical analysis in this manuscript.
References
- 1.Abdullah I, Lepore JJ, Epstein JA, Parmacek MS, Gruber PJ. MRL mice fail to heal the heart in response to ischemia-reperfusion injury. Wound Repair Regen. 2005;13:205–208. doi: 10.1111/j.1067-1927.2005.130212.x. [DOI] [PubMed] [Google Scholar]
- 2.Akdemir B, Kara S, Polat K, Guven A, Gunes S. Ensemble adaptive network-based fuzzy inference system with weighted arithmetical mean and application to diagnosis of optic nerve disease from visual-evoked potential signals. Artif Intell Med. 2008;43:141–149. doi: 10.1016/j.artmed.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin IJ, Guo Y, Srinivasan S, Boudina S, Taylor RP, Rajasekaran NS, Gottlieb R, Wawrousek EF, Abel ED, Bolli R. CRYAB and HSPB2 deficiency alters cardiac metabolism and paradoxically confers protection against myocardial ischemia in aging mice. Am J Physiol Heart Circ Physiol. 2007;293:H3201–3209. doi: 10.1152/ajpheart.01363.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boengler K, Hilfiker-Kleiner D, Heusch G, Schulz R. Inhibition of permeability transition pore opening by mitochondrial STAT3 and its role in myocardial ischemia/reperfusion. Basic Res Cardiol. 2010;105:771–785. doi: 10.1007/s00395-010-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boengler K, Schulz R, Heusch G. Loss of cardioprotection with ageing. Cardiovasc Res. 2009;83:247–261. doi: 10.1093/cvr/cvp033. [DOI] [PubMed] [Google Scholar]
- 6.Bolli R. Preconditioning: a paradigm shift in the biology of myocardial ischemia. Am J Physiol Heart Circ Physiol. 2007;292:H19–27. doi: 10.1152/ajpheart.00712.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolli R, Becker L, Gross G, Mentzer R, Jr, Balshaw D, Lathrop DA, Ischemia NWGotToTfPtHf Myocardial protection at a crossroads: the need for translation into clinical therapy. Circ Res. 2004;95:125–134. doi: 10.1161/01.RES.0000137171.97172.d7. [DOI] [PubMed] [Google Scholar]
- 8.Bolli R, Marban E. Molecular and cellular mechanisms of myocardial stunning. Physiol Rev. 1999;79:609–634. doi: 10.1152/physrev.1999.79.2.609. [DOI] [PubMed] [Google Scholar]
- 9.Cavasin MA, Tao ZY, Yu AL, Yang XP. Testosterone enhances early cardiac remodeling after myocardial infarction, causing rupture and degrading cardiac function. Am J Physiol Heart Circ Physiol. 2006;290:H2043–2050. doi: 10.1152/ajpheart.01121.2005. [DOI] [PubMed] [Google Scholar]
- 10.Chien GL, Wolff RA, Davis RF, van Winkle DM. “Normothermic range” temperature affects myocardial infarct size. Cardiovasc Res. 1994;28:1014–1017. doi: 10.1093/cvr/28.7.1014. [DOI] [PubMed] [Google Scholar]
- 11.Dawn B, Guo Y, Rezazadeh A, Huang Y, Stein AB, Hunt G, Tiwari S, Varma J, Gu Y, Prabhu SD, Kajstura J, Anversa P, Ildstad ST, Bolli R. Postinfarct cytokine therapy regenerates cardiac tissue and improves left ventricular function. Circ Res. 2006;98:1098–1105. doi: 10.1161/01.RES.0000218454.76784.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dawn B, Guo Y, Rezazadeh A, Wang OL, Stein AB, Hunt G, Varma J, Xuan YT, Wu WJ, Tan W, Zhu X, Bolli R. Tumor necrosis factor-alpha does not modulate ischemia/reperfusion injury in naive myocardium but is essential for the development of lateb preconditioning. J Mol Cell Cardiol. 2004;37:51–61. doi: 10.1016/j.yjmcc.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Dawn B, Tiwari S, Kucia MJ, Zuba-Surma EK, Guo Y, Sanganalmath SK, Abdel-Latif A, Hunt G, Vincent RJ, Taher H, Reed NJ, Ratajczak MZ, Bolli R. Transplantation of bone marrow-derived very small embryonic-like stem cells attenuates left ventricular dysfunction and remodeling after myocardial infarction. Stem Cells. 2008;26:1646–1655. doi: 10.1634/stemcells.2007-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dawn B, Xuan YT, Guo Y, Rezazadeh A, Stein AB, Hunt G, Wu WJ, Tan W, Bolli R. IL-6 plays an obligatory role in late preconditioning via JAK-STAT signaling and upregulation of iNOS and COX-2. Cardiovasc Res. 2004;64:61–71. doi: 10.1016/j.cardiores.2004.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duncker DJ, Klassen CL, Ishibashi Y, Herrlinger SH, Pavek TJ, Bache RJ. Effect of temperature on myocardial infarction in swine. Am J Physiol. 1996;270:H1189–1199. doi: 10.1152/ajpheart.1996.270.4.H1189. [DOI] [PubMed] [Google Scholar]
- 16.Flaherty MP, Guo Y, Tiwari S, Rezazadeh A, Hunt G, Sanganalmath SK, Tang XL, Bolli R, Dawn B. The role of TNF-alpha receptors p55 and p75 in acute myocardial ischemia/reperfusion injury and late preconditioning. J Mol Cell Cardiol. 2008;45:735–741. doi: 10.1016/j.yjmcc.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao XM, Xu Q, Kiriazis H, Dart AM, Du XJ. Mouse model of post-infarct ventricular rupture: time course, strain- and gender-dependency, tensile strength, and histopathology. Cardiovasc Res. 2005;65:469–477. doi: 10.1016/j.cardiores.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 18.Gerlai R. Gene-targeting studies of mammalian behavior: is it the mutation or the background genotype? Trends Neurosci. 1996;19:177–181. doi: 10.1016/s0166-2236(96)20020-7. [DOI] [PubMed] [Google Scholar]
- 19.Gho BC, Schoemaker RG, van den Doel MA, Duncker DJ, Verdouw PD. Myocardial protection by brief ischemia in noncardiac tissue. Circulation. 1996;94:2193–2200. doi: 10.1161/01.cir.94.9.2193. [DOI] [PubMed] [Google Scholar]
- 20.Gorog DA, Tanno M, Kabir AM, Kanaganayagam GS, Bassi R, Fisher SG, Marber MS. Varying susceptibility to myocardial infarction among C57BL/6 mice of different genetic background. J Mol Cell Cardiol. 2003;35:705–708. doi: 10.1016/s0022-2828(03)00082-8. [DOI] [PubMed] [Google Scholar]
- 21.Guo Y, Bao W, Wu WJ, Shinmura K, Tang XL, Bolli R. Evidence for an essential role of cyclooxygenase-2 as a mediator of the late phase of ischemic preconditioning in mice. Basic Res Cardiol. 2000;95:479–484. doi: 10.1007/s003950070024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo Y, Bao W, Wu WJ, Tang XL, Bolli R. Nitric oxide donors induce late preconditioning against myocardial infarction in mice. J Mol Cell Cardiol. 1999;31:A11, A-19. [Google Scholar]
- 23.Guo Y, Bolli R, Bao W, Wu WJ, Black RG, Murphree SS, Salvatore CA, Jacobson MA, Auchampach JA. Targeted deletion of the A3 adenosine receptor confers resistance to myocardial ischemic injury and does not prevent early preconditioning. J Mol Cell Cardiol. 2001;33:825–830. doi: 10.1006/jmcc.2001.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo Y, Jones WK, Xuan YT, Tang XL, Bao W, Wu WJ, Han H, Laubach VE, Ping P, Yang Z, Qiu Y, Bolli R. The late phase of ischemic preconditioning is abrogated by targeted disruption of the inducible NO synthase gene. Proc Natl Acad Sci USA. 1999;96:11507–11512. doi: 10.1073/pnas.96.20.11507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo Y, Li Q, Wu WJ, Tan W, Zhu X, Mu J, Bolli R. Endothelial nitric oxide synthase is not necessary for the early phase of ischemic preconditioning in the mouse. J Mol Cell Cardiol. 2008;44:496–501. doi: 10.1016/j.yjmcc.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo Y, Sanganalmath SK, Wu W, Zhu X, Huang Y, Tan W, Ildstad ST, Li Q, Bolli R. Identification of inducible nitric oxide synthase in peripheral blood cells as a mediator of myocardial ischemia/reperfusion injury. Basic Res Cardiol. 2012;107:253. doi: 10.1007/s00395-012-0253-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo Y, Stein AB, Wu WJ, Tan W, Zhu X, Li QH, Dawn B, Motterlini R, Bolli R. Administration of a CO-releasing molecule at the time of reperfusion reduces infarct size in vivo. Am J Physiol Heart Circ Physiol. 2004;286:H1649–1653. doi: 10.1152/ajpheart.00971.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo Y, Stein AB, Wu WJ, Zhu X, Tan W, Li Q, Bolli R. Late preconditioning induced by NO donors, adenosine A1 receptor agonists, and delta1-opioid receptor agonists is mediated by iNOS. Am J Physiol Heart Circ Physiol. 2005;289:H2251–2257. doi: 10.1152/ajpheart.00341.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo Y, Tang XL, Bao W, Bolli R. How to produce infarction in the mouse in vivo. Downey JM, editor. The ISHR Handbook of Experimental of Laboratory Procedures (HELP) 2000 http://www.ishrworld.org/
- 30.Guo Y, Wu WJ, Qiu Y, Tang XL, Yang Z, Bolli R. Demonstration of an early and a late phase of ischemic preconditioning in mice. Am J Physiol. 1998;275:H1375–1387. doi: 10.1152/ajpheart.1998.275.4.H1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo Y, Wu WJ, Zhu X, Tan W, Bolli R. The development of the early phase of ischemic preconditioning in mice is not strain dependent. J Mol Cell Cardiol. 2003;35:A47(P110). [Google Scholar]
- 32.Hale SL, Kloner RA. Ischemic preconditioning and myocardial hypothermia in rabbits with prolonged coronary artery occlusion. Am J Physiol. 1999;276:H2029–2034. doi: 10.1152/ajpheart.1999.276.6.H2029. [DOI] [PubMed] [Google Scholar]
- 33.Hale SL, Kloner RA. Myocardial temperature in acute myocardial infarction: protection with mild regional hypothermia. Am J Physiol. 1997;273:H220–227. doi: 10.1152/ajpheart.1997.273.1.H220. [DOI] [PubMed] [Google Scholar]
- 34.Halestrap A. Biochemistry: a pore way to die. Nature. 2005;434:578–579. doi: 10.1038/434578a. [DOI] [PubMed] [Google Scholar]
- 35.Hausenloy DJ, Baxter G, Bell R, Botker HE, Davidson SM, Downey J, Heusch G, Kitakaze M, Lecour S, Mentzer R, Mocanu MM, Ovize M, Schulz R, Shannon R, Walker M, Walkinshaw G, Yellon DM. Translating novel strategies for cardioprotection: the Hatter Workshop Recommendations. Basic Res Cardiol. 2010;105:677–686. doi: 10.1007/s00395-010-0121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heger J, Godecke A, Flogel U, Merx MW, Molojavyi A, Kuhn-Velten WN, Schrader J. Cardiac-specific overexpression of inducible nitric oxide synthase does not result in severe cardiac dysfunction. Circ Res. 2002;90:93–99. doi: 10.1161/hh0102.102757. [DOI] [PubMed] [Google Scholar]
- 37.Heusch G, Buchert A, Feldhaus S, Schulz R. No loss of cardioprotection by postconditioning in connexin 43-deficient mice. Basic Res Cardiol. 2006;101:354–356. doi: 10.1007/s00395-006-0589-0. [DOI] [PubMed] [Google Scholar]
- 38.Higuchi Y, McTiernan CF, Frye CB, McGowan BS, Chan TO, Feldman AM. Tumor necrosis factor receptors 1 and 2 differentially regulate survival, cardiac dysfunction, and remodeling in transgenic mice with tumor necrosis factor-alpha-induced cardiomyopathy. Circulation. 2004;109:1892–1897. doi: 10.1161/01.CIR.0000124227.00670.AB. [DOI] [PubMed] [Google Scholar]
- 39.Ho HT, Chung SK, Law JW, Ko BC, Tam SC, Brooks HL, Knepper MA, Chung SS. Aldose reductase-deficient mice develop nephrogenic diabetes insipidus. Mol Cell Biol. 2000;20:5840–5846. doi: 10.1128/mcb.20.16.5840-5846.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hutter JJ, Mestril R, Tam EK, Sievers RE, Dillmann WH, Wolfe CL. Overexpression of heat shock protein 72 in transgenic mice decreases infarct size in vivo. Circulation. 1996;94:1408–1411. doi: 10.1161/01.cir.94.6.1408. [DOI] [PubMed] [Google Scholar]
- 41.Jones WK, Flaherty MP, Tang XL, Takano H, Qiu Y, Banerjee S, Smith T, Bolli R. Ischemic preconditioning increases iNOS transcript levels in conscious rabbits via a nitric oxide-dependent mechanism. J Mol Cell Cardiol. 1999;31:1469–1481. doi: 10.1006/jmcc.1999.0983. [DOI] [PubMed] [Google Scholar]
- 42.Jung O, Marklund SL, Geiger H, Pedrazzini T, Busse R, Brandes RP. Extracellular superoxide dismutase is a major determinant of nitric oxide bioavailability: in vivo and ex vivo evidence from ecSOD-deficient mice. Circ Res. 2003;93:622–629. doi: 10.1161/01.RES.0000092140.81594.A8. [DOI] [PubMed] [Google Scholar]
- 43.Kleinbongard P, Heusch G, Schulz R. TNFalpha in atherosclerosis, myocardial ischemia/reperfusion and heart failure. Pharmacol Ther. 2010;127:295–314. doi: 10.1016/j.pharmthera.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 44.Korff S, Riechert N, Schoensiegel F, Weichenhan D, Autschbach F, Katus HA, Ivandic BT. Calcification of myocardial necrosis is common in mice. Virchows Arch. 2006;448:630–638. doi: 10.1007/s00428-005-0071-7. [DOI] [PubMed] [Google Scholar]
- 45.Lefer DJ, Bolli R. Development of an NIH consortium for preclinicAl AssESsment of CARdioprotective therapies (CAESAR): a paradigm shift in studies of infarct size limitation. J Cardiovasc Pharmacol Ther. 2011;16:332–339. doi: 10.1177/1074248411414155. [DOI] [PubMed] [Google Scholar]
- 46.Lemasters JJ, Bond JM, Chacon E, Harper IS, Kaplan SH, Ohata H, Trollinger DR, Herman B, Cascio WE. The pH paradox in ischemia-reperfusion injury to cardiac myocytes. EXS. 1996;76:99–114. doi: 10.1007/978-3-0348-8988-9_7. [DOI] [PubMed] [Google Scholar]
- 47.Lesnefsky EJ, Gallo DS, Ye J, Whittingham TS, Lust WD. Aging increases ischemia-reperfusion injury in the isolated, buffer-perfused heart. J Lab Clin Med. 1994;124:843–851. [PubMed] [Google Scholar]
- 48.Li Q, Guo Y, Ou Q, Cui C, Wu WJ, Tan W, Zhu X, Lanceta LB, Sanganalmath SK, Dawn B, Shinmura K, Rokosh GD, Wang S, Bolli R. Gene transfer of inducible nitric oxide synthase affords cardioprotection by upregulating heme oxygenase-1 via a nuclear factor-{kappa}B-dependent pathway. Circulation. 2009;120:1222–1230. doi: 10.1161/CIRCULATIONAHA.108.778688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Q, Guo Y, Ou Q, Wu WJ, Chen N, Zhu X, Tan W, Yuan F, Dawn B, Luo L, Hunt GN, Bolli R. Gene transfer as a strategy to achieve permanent cardioprotection II: rAAV-mediated gene therapy with heme oxygenase-1 limits infarct size 1 year later without adverse functional consequences. Basic Res Cardiol. 2011;106:1367–1377. doi: 10.1007/s00395-011-0208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Q, Guo Y, Tan W, Ou Q, Wu WJ, Sturza D, Dawn B, Hunt G, Cui C, Bolli R. Cardioprotection afforded by inducible nitric oxide synthase gene therapy is mediated by cyclooxygenase-2 via a nuclear factor-kappaB dependent pathway. Circulation. 2007;116:1577–1584. doi: 10.1161/CIRCULATIONAHA.107.689810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Q, Guo Y, Tan W, Stein AB, Dawn B, Wu WJ, Zhu X, Lu X, Xu X, Siddiqui T, Tiwari S, Bolli R. Gene therapy with iNOS provides long-term protection against myocardial infarction without adverse functional consequences. Am J Physiol Heart Circ Physiol. 2006;290:H584–589. doi: 10.1152/ajpheart.00855.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Q, Guo Y, Wu WJ, Ou Q, Zhu X, Tan W, Yuan F, Chen N, Dawn B, Luo L, O’Brien E, Bolli R. Gene transfer as a strategy to achieve permanent cardioprotection I: rAAV-mediated gene therapy with inducible nitric oxide synthase limits infarct size 1 year later without adverse functional consequences. Basic Res Cardiol. 2011;106:1355–1366. doi: 10.1007/s00395-011-0207-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Q, Guo Y, Xuan YT, Lowenstein CJ, Stevenson SC, Prabhu SD, Wu WJ, Zhu Y, Bolli R. Gene therapy with inducible nitric oxide synthase protects against myocardial infarction via a cyclooxygenase-2-dependent mechanism. Circ Res. 2003;92:741–748. doi: 10.1161/01.RES.0000065441.72685.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li TT, Larrucea S, Souza S, Leal SM, Lopez JA, Rubin EM, Nieswandt B, Bray PF. Genetic variation responsible for mouse strain differences in integrin alpha 2 expression is associated with altered platelet responses to collagen. Blood. 2004;103:3396–3402. doi: 10.1182/blood-2003-10-3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meldrum DR, Cleveland JC, Jr, Cain BS, Meng X, Harken AH. Increased myocardial tumor necrosis factor-alpha in a crystalloid-perfused model of cardiac ischemia-reperfusion injury. Ann Thorac Surg. 1998;65:439–443. doi: 10.1016/s0003-4975(97)01297-6. [DOI] [PubMed] [Google Scholar]
- 56.Michael LH, Entman ML, Hartley CJ, Youker KA, Zhu J, Hall SR, Hawkins HK, Berens K, Ballantyne CM. Myocardial ischemia and reperfusion: a murine model. Am J Physiol. 1995;269:H2147–2154. doi: 10.1152/ajpheart.1995.269.6.H2147. [DOI] [PubMed] [Google Scholar]
- 57.Miller DL, Van Winkle DM. Ischemic preconditioning limits infarct size following regional ischemia-reperfusion in in situ mouse hearts. Cardiovasc Res. 1999;42:680–684. doi: 10.1016/s0008-6363(99)00005-x. [DOI] [PubMed] [Google Scholar]
- 58.Murata T, Ushikubi F, Matsuoka T, Hirata M, Yamasaki A, Sugimoto Y, Ichikawa A, Aze Y, Tanaka T, Yoshida N, Ueno A, Oh-ishi S, Narumiya S. Altered pain perception and inflammatory response in mice lacking prostacyclin receptor. Nature. 1997;388:678–682. doi: 10.1038/41780. [DOI] [PubMed] [Google Scholar]
- 59.Ning XH, Xu CS, Song YC, Xiao Y, Hu YJ, Lupinetti FM, Portman MA. Hypothermia preserves function and signaling for mitochondrial biogenesis during subsequent ischemia. Am J Physiol. 1998;274:H786–793. doi: 10.1152/ajpheart.1998.274.3.H786. [DOI] [PubMed] [Google Scholar]
- 60.Sanganalmath SK, Abdel-Latif A, Bolli R, Xuan YT, Dawn B. Hematopoietic cytokines for cardiac repair: mobilization of bone marrow cells and beyond. Basic Res Cardiol. 2011;106:709–733. doi: 10.1007/s00395-011-0183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sanganalmath SK, Stein AB, Guo Y, Tiwari S, Hunt G, Vincent RJ, Huang Y, Rezazadeh A, Ildstad ST, Dawn B, Bolli R. The beneficial effects of postinfarct cytokine combination therapy are sustained during long-term follow-up. J Mol Cell Cardiol. 2009;47:528–535. doi: 10.1016/j.yjmcc.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schwartz LM, Verbinski SG, Vander Heide RS, Reimer KA. Epicardial temperature is a major predictor of myocardial infarct size in dogs. J Mol Cell Cardiol. 1997;29:1577–1583. doi: 10.1006/jmcc.1997.0391. [DOI] [PubMed] [Google Scholar]
- 63.Schwartz Longacre L, Kloner RA, Arai AE, Baines CP, Bolli R, Braunwald E, Downey J, Gibbons RJ, Gottlieb RA, Heusch G, Jennings RB, Lefer DJ, Mentzer RM, Murphy E, Ovize M, Ping P, Przyklenk K, Sack MN, Vander Heide RS, Vinten-Johansen J, Yellon DM, National Heart L, Blood Institute NIoH New horizons in cardioprotection: recommendations from the 2010 National Heart, Lung, and Blood Institute Workshop. Circulation. 2011;124:1172–1179. doi: 10.1161/CIRCULATIONAHA.111.032698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stein AB, Guo Y, Tan W, Wu WJ, Zhu X, Li Q, Luo C, Dawn B, Johnson TR, Motterlini R, Bolli R. Administration of a CO-releasing molecule induces late preconditioning against myocardial infarction. J Mol Cell Cardiol. 2005;38:127–134. doi: 10.1016/j.yjmcc.2004.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tiano HF, Loftin CD, Akunda J, Lee CA, Spalding J, Sessoms A, Dunson DB, Rogan EG, Morham SG, Smart RC, Langenbach R. Deficiency of either cyclooxygenase (COX)-1 or COX-2 alters epidermal differentiation and reduces mouse skin tumorigenesis. Cancer Res. 2002;62:3395–3401. [PubMed] [Google Scholar]
- 66.van den Doel MA, Gho BC, Duval SY, Schoemaker RG, Duncker DJ, Verdouw PD. Hypothermia extends the cardioprotection by ischaemic preconditioning to coronary artery occlusions of longer duration. Cardiovasc Res. 1998;37:76–81. doi: 10.1016/s0008-6363(97)00222-8. [DOI] [PubMed] [Google Scholar]
- 67.Wang GW, Guo Y, Vondriska TM, Zhang J, Zhang S, Tsai LL, Zong NC, Bolli R, Bhatnagar A, Prabhu SD. Acrolein consumption exacerbates myocardial ischemic injury and blocks nitric oxide-induced PKCepsilon signaling and cardioprotection. J Mol Cell Cardiol. 2008;44:1016–1022. doi: 10.1016/j.yjmcc.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 68.Xuan YT, Guo Y, Han H, Zhu Y, Bolli R. An essential role of the JAK-STAT pathway in ischemic preconditioning. Proc Natl Acad Sci U S A. 2001;98:9050–9055. doi: 10.1073/pnas.161283798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xuan YT, Guo Y, Zhu Y, Wang OL, Rokosh G, Bolli R. Endothelial nitric oxide synthase plays an obligatory role in the late phase of ischemic preconditioning by activating the protein kinase C epsilon p44/42 mitogen-activated protein kinase pSer-signal transducers and activators of transcription 1/3 pathway. Circulation. 2007;116:535–544. doi: 10.1161/CIRCULATIONAHA.107.689471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 71.Zuba-Surma EK, Kucia M, Dawn B, Guo Y, Ratajczak MZ, Bolli R. Bone marrow-derived pluripotent very small embryonic-like stem cells (VSELs) are mobilized after acute myocardial infarction. J Mol Cell Cardiol. 2008;44:865–873. doi: 10.1016/j.yjmcc.2008.02.279. [DOI] [PMC free article] [PubMed] [Google Scholar]


