Abstract
Aims
We recently reported that acute exposure to nicotine vasodilates the renal vasculature of male rats via facilitation of endothelial nitric oxide synthase (eNOS). In this study, we investigated whether this effect of nicotine is sexually dimorphic and the role of estrogen in modulating the nicotine effect.
Main methods
Nicotine-evoked vasodilation was evaluated in phenylephrine-preconstricted perfused kidneys obtained from male, proestrus female, ovariectomized (OVX) and estrogen-replaced OVX (OVXE2) rats.
Key findings
Nicotine infusion (5×10−5, 1×10−4, and 5×10−4 M) produced greater concentration-dependent reductions in the renal perfusion pressure (RPP) in isolated kidney from proestrus females than from males. Inhibition of NOS by NG-nitro-L-arginine abolished the nicotine-evoked reduction in RPP and abolished the gender difference in the nicotine effect. Nicotine vasodilation was also attenuated in kidneys isolated from OVX and diestrus rats, models characterized by reduced estrogen levels. Further, estrogen or L-arginine supplementation in OVX rats largely restored the renal vasodilatory response to nicotine. Estrogen receptor blockade by tamoxifen abrogated the enhanced nicotine-evoked vasodilation elicited by E2 in OVX rats. The nitrite/nitrate levels and protein expressions of eNOS and α7 nicotinic cholinergic receptor (α7 nAChRs) were significantly higher in renal tissues of OVXE2 compared with OVX rats, suggesting a facilitatory effect for E2 on α7 nAChRs/eNOS signaling.
Significance
Estrogen-dependent facilitation of NOS signaling mediates the enhanced vasodilator capacity of nicotine in the renal vasculature of female rats. Preliminary evidence also suggests a potential role for α7 nAChRs in this estrogen-dependent phenomenon.
Keywords: Rat, gender, nicotine, estrogen, nitric oxide synthase type III, nicotinic acetylcholine receptor alpha7, renal vasodilation
Introduction
Reported findings have shown that acute exposure to nicotine causes vasodilation in several vascular preparations. The relaxant effect of nicotine in the dorsal aorta of the giant shovelnose ray has been attributed to facilitation of endothelial cyclooxygenase pathway (Donald et al., 2004). In the cerebral artery, nicotine causes neurogenic vasodilation that involves the release of nitric oxide from perivascular nitrergic nerve terminals (Si and Lee, 2002). In other vascular preparations, a neuronally released calcitonin gene-related peptide has been implicated in the vasodilatory effect of nicotine (Eguchi et al., 2007). It should be noted, however, that the net vascular effect of nicotine is influenced by several factors including animal species, vascular beds, dose and duration of nicotine regimen. Nicotine has also been shown to cause vasoconstriction probably via sympathetic neural stimulation (Marano et al., 1999), endothelial dysfunction (Black et al., 2001), or the release of vasopressin (Groudine and Morley, 1996) or endothelin (Haak et al., 1994). In the vascular smooth muscle cells of the rat tail artery, nicotine produced dual effects (increase or decrease) on K+ currents depending on the concentration of the drug (Tang et al., 1999). This effect of nicotine was partly dependent on nicotinic receptors and also involved direct interaction with K+ channels (Tang et al., 1999).
In a recent report (El-Mas et al., 2008a), we established the first experimental evidence that implicated eNOS in nicotine-evoked vasodilations in the rat perfused kidney. Such action was abolished after (i) chemical denudation of the endothelium, and (ii) pharmacologic inhibition of NOS or guanylate cyclase activity (El-Mas et al., 2008a). Notably, because our previous study (El-Mas et al., 2008a) was undertaken in male rats, it remains unclear whether nicotine interacts similarly with the renal vasculature of female rats. The possibility of sexual dimorphism in the nicotine-renovascular interaction is highly likely because mounting evidence suggests considerable roles for gender and hormonal factors in the regulation of vascular tone (Thompson and Khalil, 2003).
Therefore, the first objective of the present study was to compare the renovascular responsiveness to nicotine in proestrus female vs. male rats. The proestrus phase was selected because it exhibits the highest plasma estrogen levels (Marcondes et al., 2001), thereby eliminating the impact of fluctuations in hormonal levels on the measured parameters. Because preliminary results showed significantly greater vasodilatory effects for nicotine in kidneys from female, than from male, rats, a series of studies was performed to investigate the role of estrogen modulation of NOS activity in the sex-related differences in the nicotine-renovascular interaction. In these studies we investigated renovascular responsiveness to nicotine under the following conditions: (i) pharmacologic inhibition of NOS activity in male and proestrus female, (ii) reduced plasma estrogen levels (OVX or diestrus rats), and (iii) OVX rats following treatment with L-arginine, 17β-estradiol, or tamoxifen. Finally, renal nitrite/nitrate level and Western blot analyses were performed to determine whether alterations in the abundance of α7 nAChRs and eNOS proteins contributed to the estrogen-dependent renovascular action of nicotine.
Materials and Methods
Male and female Wistar rats (200–240 g, High Institute of Public Health, Alexandria, Egypt) were used in the present study. Experiments were performed in accordance with the European Community guidelines for the use of experimental animals and were approved by the institutional ethics committee.
The rat isolated perfused kidney
The rat kidney was isolated and perfused according to the method described in our previous studies (El-Mas et al., 2003, 2004). Briefly, rats were anesthetized with thiopental sodium (50 mg/kg, i.p.), the abdomen was opened by a midline incision and the left kidney was exposed. The left renal artery was dissected free from its surrounding tissues. Loose ties were made around the renal artery and the abdominal aorta, proximal and distal to the renal artery. A beveled 18-gauge needle connected to a 5-ml syringe filled with heparinized saline (100 U/ml) was used for cannulation. The aorta was ligated, and the left renal artery was cannulated via an incision made in the aorta. The cannula was immediately secured with ligatures and the kidney was flushed with heparinized saline and rapidly excised from its surrounding tissues.
The kidney was transferred into a jacketed glass chamber maintained at 37°C and continuously perfused with Krebs’ solution (NaCl 120, KCl 5, CaCl2 2.5, MgSO4.7H2O 1.2, KH2PO4 1.2, NaHCO3 25, and glucose 11 mM) maintained at 37°C and gassed with 95% O2 and 5% CO2. Kidney perfusion was adjusted at a constant flow rate of 5 ml/min using a peristaltic pump (Model P3-Pharmacia Fine Chemicals®). The pump delivered a pulsatile flow, and an open circuit was used so that the venous effluent was allowed to drain freely. The kidney perfusion pressure was continuously monitored by means of a Gould-Statham pressure transducer distal to the pump and recorded on a Grass polygraph (model 7D). Inasmuch as the renal flow was kept constant, changes in perfusion pressure were indicative of alterations in renal vascular resistance. An equilibration period of 30 min was allowed at the beginning of the experiment to ensure stabilization of the kidney perfusion pressure. To study the vasodilatory effects of nicotine, the renal vascular tone was elevated by continuous infusion of the α1-adrenoceptor agonist phenylephrine (10 μM) as described in our previous studies (El-Mas et al., 2003, 2004). In some experiments involving perfused kidneys from proestrus female rats, a higher concentration of phenylephrine (20 μM) was used to achieve the same level of preconstriction seen in male preparations.
Ovariectomy
Bilateral ovariectomy was performed as described in our previous studies (El-Mas and Abdel-Rahman, 1998b, 1999). A single 2–3 cm incision was made in the back skin and underlying muscle. The ovaries were isolated, tied-off with sterile suture and removed. The muscle and skin were sutured and the rats were allowed approximately 2 weeks to recover prior to experimentation in perfused kidneys. Sham operation involved exposure of the ovaries without isolation. After ovariectomy or sham operation, each rat received an intramuscular injection of 50,000U/kg of benzathine benzylpenicillin in an aqueous suspension (Retarpen, Sandoz GmbH, Austria). Rats were housed individually in separate cages.
Measurement of plasma estrogen
The levels of plasma estradiol were measured by the radioimmunoassay (COAT-A-COUNT®, Diagnostic Products Corporation, LA, USA) as described in our previous studies (El-Mas and Abdel-Rahman, 1999, 2001).
Western blotting
For the determination of renal eNOS and α7-nAChRs protein levels, kidneys were homogenized on ice in a homogenization buffer [50 mM Tris (pH 7.5), 0.1 mM EGTA, 0.1 mM EDTA, 2 μM leupeptin, 1 mM phenylmethylsulfonyl fluoride, 0.1% (vol/vol) Nonidet P-40, 0.1% SDS, and 0.1% deoxycholate]. After centrifugation (12,000 g for 10 min), protein in the supernatant was quantified (Bio-Rad protein assay system; Bio-Rad, Hercules, CA). Protein extracts (50 μg per lane) were run on a 4 to 12% SDS-polyacrylamide gel electrophoresis gel (Invitrogen, Carlsbad, CA) and electroblotted to nitrocellulose membranes. Blots were blocked for 120 min at room temperature in tris-buffered saline/tween20 (TBS/T) buffer (100 mM Tris pH 7.5, 150 mM NaCl, 0.1% Tween 20) containing 5% non-fat milk. For eNOS measurement, mouse IgG1 (Cat# 610296 BD Transduction Laboratories) was used as 1:2000 and 30 μg protein/lane was loaded. The secondary antibody was anti-mouse antibody (1:2000). For nicotinic acetylcholine receptor α7 subunit, 60 μg protein/lane was loaded and rat monoclonal antibody antibody (1:200; Cat# ab24644, Abcam) was used; secondary antibody was anti-rat (ab6734 Abcam) 1:2000. After 3 washes with TBS/T buffer, the blots were incubated for 60 min at room temperature with the appropriate horseradish peroxidase-linked species-specific anti-IgG (1:2,000, GE Healthcare BIO-Sciences Corp, Piscataway, NJ). After 3 washes with the TBS/T buffer, the blots were detected by enhanced chemiluminescence system and exposed to an X-ray film. Equivalent sample loading was confirmed by stripping membranes with blot restore membrane rejuvenation solution (SignaGen Laboratories, Gaithersburg, MD) and reprobing with anti-actin antibody (Sigma). Bands of eNOS and α7 nAChRs were quantified by measuring the integrated density (mean density × area) using the NIH Image software (version 1.62) (El-Mas et al. 2008b, 2009) and presented as a ratio to actin.
Protocols and experimental groups
Gender-related effect of nicotine on the renal perfusion pressure
A total of 8 groups of rats, 4 males and 4 proestrus females (n=6–9 each), were used in this experiment to investigate the effect of infusion of nicotine (5×10−5, 1×10−4, or 5×10−4 M) or its vehicle (saline) on RPP in phenylephrine-preconstricted isolated kidneys obtained from male and proestrus female rats. Nicotine infusion started when the phenylephrine-induced tone reached steady levels (~30 min) and continued for 20 min. Each concentration of nicotine was tested in a separate group of rats (n=6–9) and changes in RPP from pre-nicotine values were compared to those obtained in control (saline-treated) preparations.
Another two groups of rats, one male (n=8) and one proestrus female (n=9), were used to determine the effect of NOS inhibition by L-NNA (200 μM) on the vasodilatory effect of subsequently infused nicotine (5×10−4 M). After stabilization of the phenylephrine-induced tone, kidneys were infused with L-NNA for 20 min before starting the infusion of nicotine.
Role of estrogen in the nicotine-renovascular interaction
In this experiment we investigated the role of estrogen in the manifestation of the renal vasodilatory effect of nicotine in female rats. The effect of nicotine (5×10−4 M) on RPP was evaluated in kidneys obtained from OVX rats treated daily with sesame oil (0.5 ml/kg s.c., n=7), 17β-estradiol (E2, 50 μg/kg s.c., n=8) (El-Mas and Abdel-Rahman, 1998a; Yamaguchi et al., 2001), tamoxifen (estrogen receptor blocker, 1 mg/kg by gavage, n=7) (Tao et al., 2006), or E2+tamoxifen (n=6). These treatments started on the 10th day after OVX and continued for the following 5 consecutive days. Rats in all groups were sacrificed 15 days after OVX and kidneys were isolated, perfused, and preconstricted with phenylephrine. Thirty min after tone stabilization, nicotine (5×10−4 M) was infused for 20 min and changes in RPP from pre-nicotine levels were computed. One more OVX group (n=5) was used to determine the effect of L-arginine infusion on nicotine-evoked vasodilation. L-arginine (10−4 M) was infused for 20 min in phenylephrine-preconstricted kidneys obtained from OVX rats. This was followed by the infusion of L-aginine plus nicotine (5×10−4 M) for another 20 min period. Right kidneys of OVX and OVXE2 rats were harvested and stored at −80 °C for measurement of renal eNOS and α7-nAChRs protein levels as described earlier. The renal nitrite/nitrate (NOx) levels were measured by the fluorometric assay kit in accordance with manufacturer’s instructions (Cayman Chemical Company, Ann Arbor, MI) and as detailed elsewhere (Misko et al., 1993).
One group of diestrus female rats (n=7), which exhibit lower estrogen levels compared with proestrus rats (Marcondes et al., 2001), was used in this study to lend support to the modulatory role of estrogen in the renal vasodilatory action of nicotine. Kidneys obtained from diestrus female rats were perfused, subjected to continuous infusion with phenylephrine (10 μM), and then infused with nicotine (5×10−4 M) for 20 min. The phase of the cycle (diestrus or proestrus) was identified through microscopic examination of vaginal smears. Blood was collected from proestrus, OVX, OVXE2, diestrus, and male rats at the time of sacrifice for the determination of plasma estrogen. Collected blood samples were centrifuged at 4000 rpm for 15 min. The plasma was aspirated and stored at −20 °C till analyzed.
Drugs
Nicotine (Merck Schuchardt OHG, Hohenbrunn, Germany), phenylephrine hydrochloride, NG-nitro-L-arginine, 17β-estradiol, L-arginine (Sigma Chemical Co., St. Louis, MO, U.S.A.), tamoxifen citrate (Novartis AG, Basel, Switzerland), thiopental sodium (Thiopental, Biochemie GmbH, Vienna, Austria) were purchased from commercial vendors.
Data analysis and statistics
Values are expressed as mean±S.E.M. Changes caused by nicotine in the RPP from baseline (pre-nicotine) values in different experimental groups were computed. Further, to obtain a measure of the cumulative nicotine effect, the area under the curve (AUC) was calculated for individual experiments using trapezoidal integration (Graph pad prism, version 3.02) (El-Mas et al., 2008a). The analysis of variance (ANOVA) followed by a Newman-Keuls post-hoc analysis was used for multiple comparisons with the level of significance set at P<0.05.
Results
Gender-related effect of nicotine on RPP
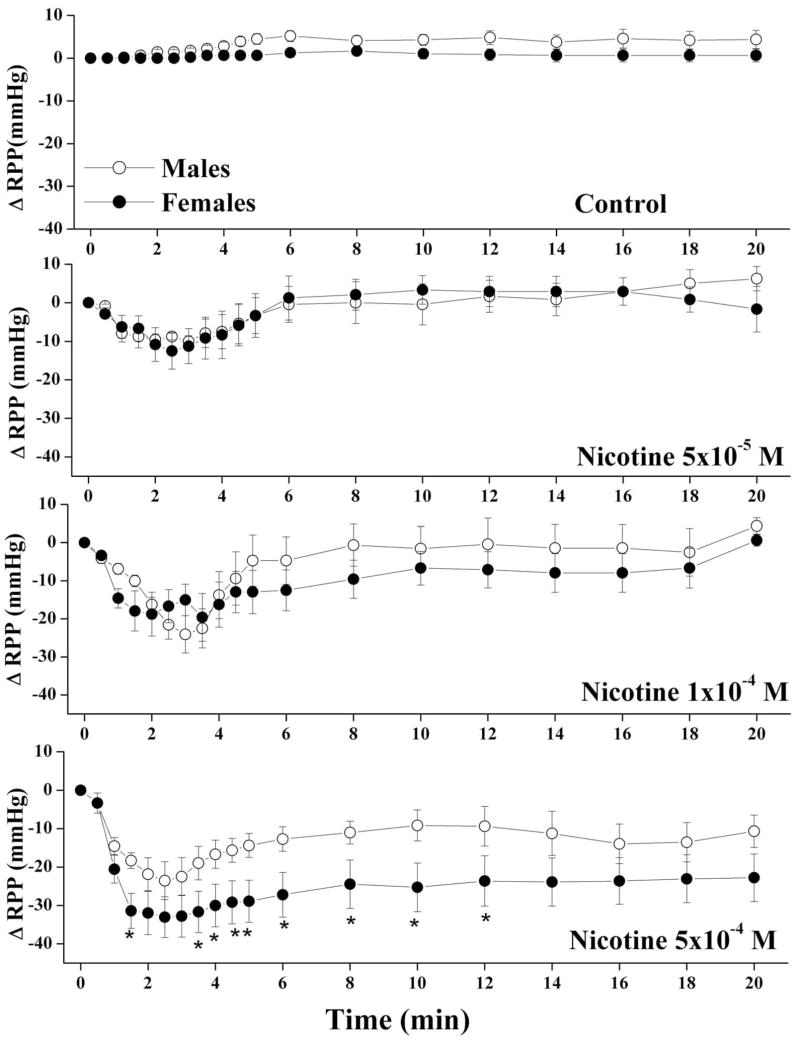
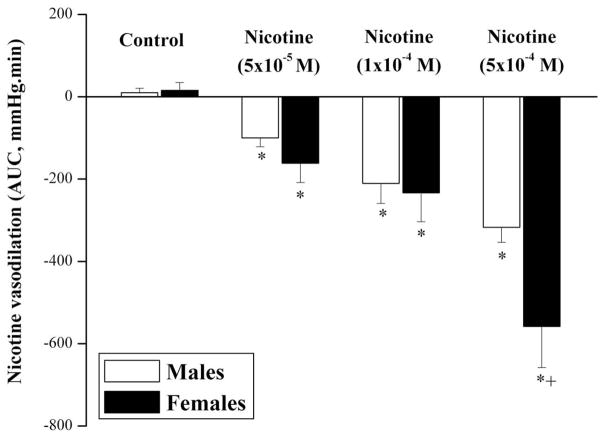
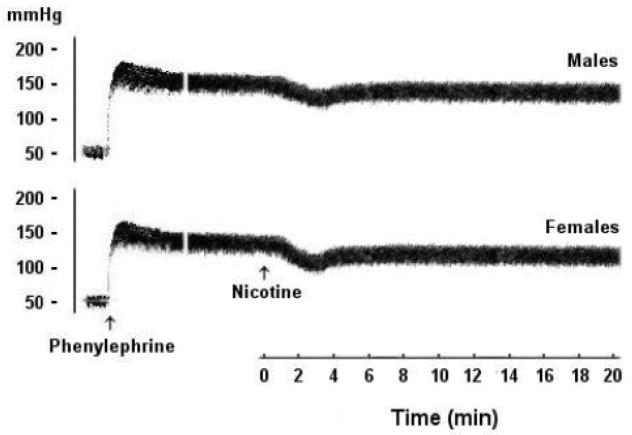
The average basal RPP in male and proestrus female preparations were similar (61±5 and 64±6 mmHg, respectively). Also, the elevations in the RPP caused by continuous infusion of phenylephrine into kidneys obtained from male and proestrus female were not statistically different (126±21 and 98±10 mmHg, respectively). Under conditions of sustained elevations in renovascular tone, the infusion of nicotine (5×10−5, 1×10−4, or 5×10−4 M) produced concentration-dependent reductions in RPP in both male and female kidneys with a nadir observed at approximately 3 min (Fig. 1). Computation of the AUC of the vasodilatory action of nicotine during the entire 20 min experimentation period showed that the vasodilatory responses elicited by nicotine were greater in female than in male preparations (Fig. 2). These male-female differences were statistically significant only in case of the highest concentration of nicotine (5×10−4 M, Figs. 1 and 2). Representative tracings of the vasodilatory response elicited by nicotine (5×10−4 M) in male and female kidneys are depicted in figure 3.
Fig. 1.
Changes in renal perfusion pressure (RPP) evoked by the infusion of nicotine (5×10−5, 1×10−4, or 5×10−4 M) in phenylephrine (10–20 μM)-preconstricted isolated perfused kidneys obtained from male and proestrus female rats. Each concentration of nicotine was infused in a separate group of rats. Values are mean±S.E.M. of 6–9 observations. *P<0.05 compared with corresponding male values.
Fig. 2.
Bar graphs showing the area under the curve (mmHg.min) of the vasodilatory effect of nicotine (5×10−5, 1×10−4, or 5×10−4 M) in phenylephrine (10–20 μM)-preconstricted isolated perfused kidneys obtained from male and proestrus female rats. *P<0.05 vs. corresponding control values, +P<0.05 vs. corresponding male values.
Fig. 3.
Representative tracings showing the decreases in renal perfusion pressure evoked by infusion of nicotine (5×10−4 M) in phenylephrine (10–20 μM)-preconstricted isolated perfused kidneys from male and proestrus female rats.
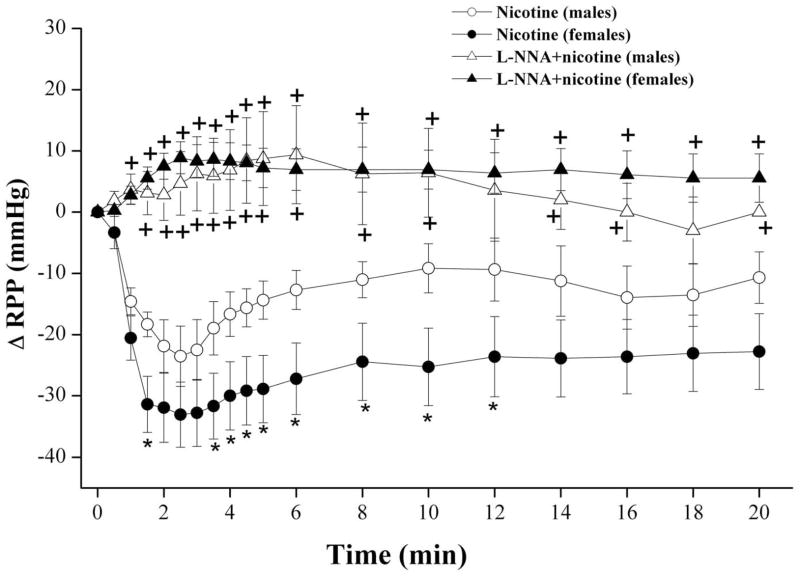
Figure 4 illustrates the effect of NOS inhibition produced by L-NNA (200 μM) on the vasodilatory response to subsequently infused nicotine (5×10−4 M) in phenylephrine-preconstricted perfused kidneys. The infusion of L-NNA virtually abolished the vasodilating effect of nicotine in both male and proestrus female preparations (Fig. 4). Further, the gender-related RPP difference caused by nicotine was not evident in the presence of L-NNA (Fig. 4).
Fig. 4.
Changes in renal perfusion pressure (RPP) evoked by the infusion of nicotine (5×10−4 M) in phenylephrine (10–20 μM)-preconstricted isolated perfused kidneys obtained from male and proestrus female rats in the absence and presence of NG-nitro-L-arginine (L-NNA, 200 μM). Values are mean±S.E.M. of 7–9 observations. *P<0.05 vs. corresponding male values, +P<0.05 vs. corresponding nicotine values in the same gender.
Role of estrogen in the nicotine-renovascular interaction
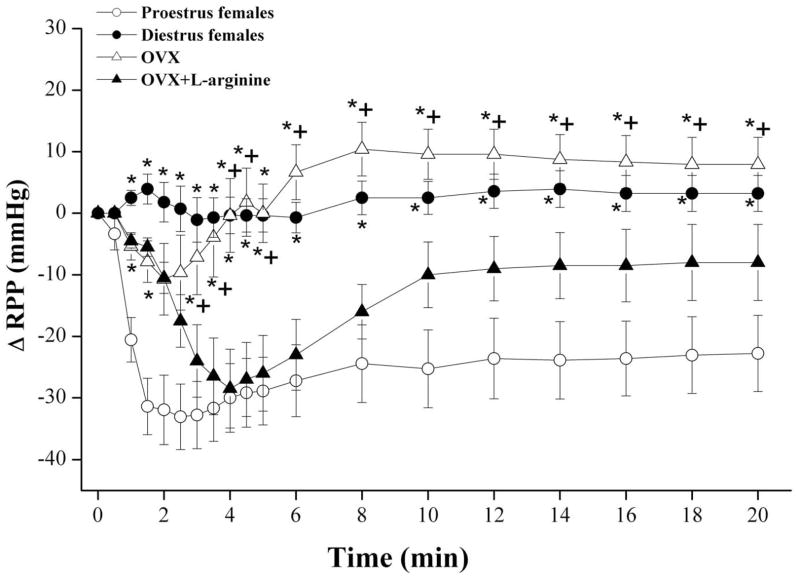
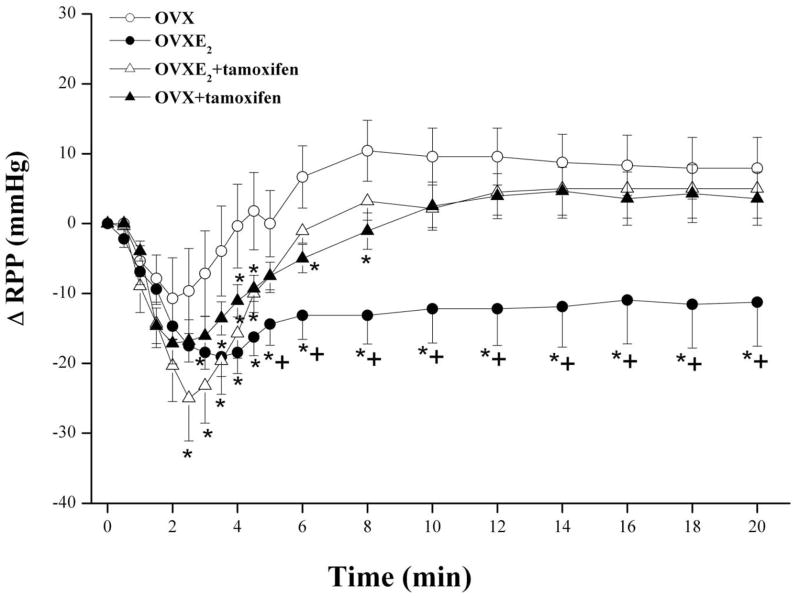
The influence of nicotine on RPP in diestrus females as well as in OVX rats in the absence and presence of L-arginine, estrogen, or tamoxifen are shown in figures 5 and 6. The infusion of nicotine (5×10−4 M) in phenylephrine-preconstricted kidneys obtained from diestrus female rats elicited no changes in RPP (Fig. 5). In OVX preparations, nicotine caused short-lived (<3 min) vasodilation that was followed by an increase in RPP to levels slightly higher than pre-nicotine values (Fig. 5). The vasodilatory response to nicotine was largely restored in OVX preparations infused 20 min earlier with L-arginine (Fig. 5). Similarly, nicotine caused significant decreases in RPP in kidneys obtained from OVX rats supplemented with estrogen (50 μg/kg/day s.c., 5 days; Fig. 6).
Fig. 5.
Changes in renal perfusion pressure (RPP) evoked by the infusion of nicotine (5×10−4 M) in phenylephrine (10–20 μM)-preconstricted isolated perfused kidneys obtained from proestrus, diestrus, and ovariectomized (OVX) female rats. The effect of pretreatment with L-arginine (10−4 M) on nicotine responses in OVX preparations are also shown. Values are mean±S.E.M. of 7–9 observations. *P<0.05 vs. corresponding proestrus values, +P<0.05 vs. corresponding OVX+L-arginine values.
Fig. 6.
Effect of 5-day treatment of ovariectomized (OVX) rats with 17β-estradiol (E2, 50 μg/kg/day), tamoxifen (estrogen receptor blocker, 1 mg/kg/day), or their combinations on changes in renal perfusion pressure (RPP) evoked by the infusion of nicotine (5×10−4 M) in phenylephrine (10–20 μM)-preconstricted isolated perfused kidneys. Values are mean±S.E.M. of 6–8 observations. *P<0.05 vs. corresponding OVX values, +P<0.05 vs. corresponding OVXE2+tamoxifen values.
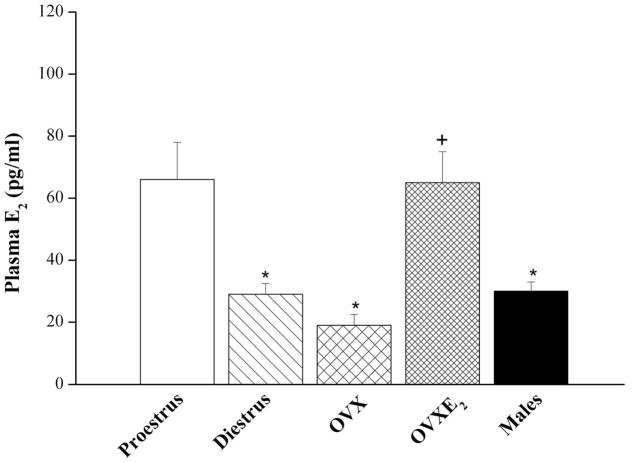
The AUC of nicotine-evoked vasodilation in OVX treated with L-arginine or estrogen was similar (299±72 and 271±73 mmHg.min) but remained significantly lower than corresponding values of proestrus females (558±100 mmHg.min). The nicotine-evoked decreases in RPP demonstrated in OVXE2 were significantly inhibited in preparations treated simultaneously with tamoxifen (AUC, 102±34 mmHg.min). In OVXE2+tamoxifen preparations, the vasodilatory effect of nicotine continued for about 4 min and disappeared afterwards (Fig. 6). Also, OVX preparations treated with tamoxifen alone showed slight decreases in RPP in response to nicotine (Fig. 6). Similar plasma estrogen levels were demonstrated in OVX, diestrus, and male rats that were significantly lower than those of proestrus rats (Fig. 7). Also, estrogen replacement of OVX rats restored plasma estrogen to proestrus levels (Fig. 7).
Fig. 7.
Plasma estrogen levels in male, proestrus, diestrus, and ovariectomized (OVX) female rats with or without estrogen supplementation (E2, 50 μg/kg/day for 5 consecutive days). Values are mean±S.E.M. of 7–9 observations. *P<0.05 vs. corresponding proestrus values, +P<0.05 vs. corresponding OVX values.
Compared with OVX values, estrogen supplementation of OVX rats caused significant increases in renal levels of NOx and protein expressions of α7-nAChRs and eNOS (Fig. 8).
Fig. 8.
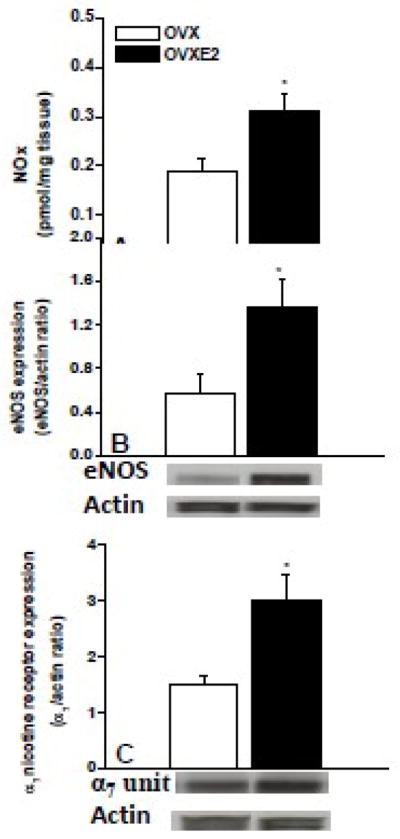
Levels of nitrite/nitrate (NOx, panel A) and protein expression of eNOS (panel B) and α7 nicotine receptor subunit (panel C) in renal tissues of ovariectomized (OVX) and 17β-estradiol (E2, 50 μg/kg/day)-replaced OVX rats (OVXE2). Illustrative gels of targeted proteins are also shown. Values are means±S.E.M of 5–6 observations. *P<0.05 vs. OVX values.
Discussion
The present study addressed two important questions pertinent to the gender-related effect of nicotine on renovascular tone. The first part of the study clearly demonstrated significantly higher reduction in renovascular tone elicited by nicotine in female than in male rats. The study was extended to test the hypothesis that facilitation of estrogen/eNOS signaling contributed to the renal vasodilatory action of nicotine in female rats. The results showed that nicotine produced concentration-dependent renal vasodilations in male and proestrus female kidneys; significantly greater vasodilation was produced by 5×10−4 M nicotine in female than in male preparations. The vasodilatory effect of nicotine together with the male-female difference was abolished in L-NNA-pretreated preparations, suggesting a fundamental role for NOS in the renovascular vasodilation elicited by nicotine in both genders as well as in the sex-related difference in this response. The greater renovascular vasodilation caused by nicotine in female kidney is mediated, at least partly, via facilitation by estrogen of α7-nAChRs/eNOS signaling because (i) the response was substantially attenuated in kidneys of OVX or diestrus female rat, which exhibited reduced estrogen levels, (ii) pretreatment of OVX rats with L-arginine or estrogen largely restored the vasodilatory effect of nicotine, (iii) blockade of estrogen receptors by tamoxifen abrogated the increase in nicotine vasodilation caused by estrogen replacement in OVX rats, and (iv) estrogen replacement in OVX rats increased renal levels of nitrite/nitrate and protein expressions of eNOS and α7-nAChRs.
The current study highlights two important features of the gender-related effect of nicotine in the renal vasculature. First, the demonstration of sexual dimorphism in the renovascular effect of nicotine depends fundamentally on the concentration of nicotine employed. While significantly greater vasodilations were observed in proestrus female than in male kidneys when infused with 5×10−4 M nicotine, the decreases in RPP caused by lower concentrations of nicotine in the two rat preparations were not statistically different. Second, regardless of the rat gender, nicotine-evoked vasodilation was abolished in the presence of L-NNA, indicating the full dependence of the vasodilatory action of nicotine on renal NOS activity. This view is consistent with our recent observation that the vasodilatory effect of nicotine is abolished in renal tissues of male rats subjected to endothelium denudation (El-Mas et al., 2008a). By the same token, the results of the L-NNA experiment may infer that a higher baseline NOS activity and/or expression in female renal tissues might account for the gender-related differences in nicotine vasodilation. This premise agrees with the observations that the endothelium of female arteries generates greater amounts of NO than that of males (Kauser and Rubanyi, 1994; Wellman et al., 1996) and that the attenuation of endothelium-dependent vasorelaxation caused by NOS inhibition is more pronounced in female arterial preparations (Kähönen et al., 1998).
Remarkably, reported clinical and experimental studies highlighted a role for estrogen in the enhanced endothelial NO activity in the female population. For instance, estrogen increases vascular NOS expression or activity (Knot et al., 1999; Geary et al., 2000) and is responsible for the gender-difference in endothelial NO release (Knot et al., 1999). Supplementation with estrogen improves endothelium-dependent flow-mediated vasodilation and enhances NO release in the forearm vasculature (Lieberman et al., 1994). Furthermore, prolonged treatment of human coronary endothelial cells with estrogen increases basal and ATP- and A23187-induced NO release (Yang et al., 2000). These findings together with the established positive correlation between nicotinic receptors and NOS signaling (Zayas et al., 2002; Papadopolou et al., 2004; Smith and Dar, 2007) incited us to postulate that the greater NOS-dependent vasodilatory effect of nicotine in the renal vasculature of female rats might be estrogen-related. To test this hypothesis, several experimental approaches were pursued in the current study. We first investigated the nicotine-renovascular interaction in two models of female rats that exhibit reduced plasma estrogen levels, OVX and diestrus rats. The OVX rat is considered as a model of human surgical menopause because it exhibits a drastic reduction in circulating estrogen levels (Alper and Schmitz, 1996; El-Mas and Abdel-Rahman, 2001). Further, the plasma estrogen level in female rats is reduced by approximately 70% in the diestrus compared with the proestrus phase (Marcondes et al., 2001). These findings were confirmed in the present study, which showed that compared with proestrus females, plasma estrogen levels were substantially reduced in OVX and diestrus females. Interestingly, the infusion of nicotine into phenylephrine-preconstricted perfused kidneys obtained from OVX or diestrus rats elicited little or no changes in the RPP, hence supporting an important role for ovarian hormones in the nicotine-renovascular interaction.
We also utilized another approach to investigate the effect of a 5-day supplementation of OVX rats with 17β-estradiol on nicotine vasodilations, renal NOx content and eNOS protein expression. The protein expression of renal α7-nAChRs was also measured to determine whether it is estrogen sensitive and whether it correlates with changes in the nicotine vasodilatory response and eNOS signaling. The α7-nAChR/NOS cascade has been shown to mediate vasodilations of cholinergic origin in cerebral arteries (Si and Lee, 2002). The α7-nAChR also contributes to nicotine-induced increase in NO generation in isolated rat dorsal root ganglion neurons (Papadopolou et al., 2004). Although the α7-nAChR has been characterized in renal tissues (Yeboah et al., 2008), with a potential crosstalk between α7-nAChR and renal NOS has not been explored. Our results showed that compared with respective OVX values, estrogen replacement in OVX rats restored plasma estrogen to physiological levels as reported elsewhere (Yamaguchi et al., 2001) and substantially increased the vasodilatory effect of nicotine, NO bioavailability, and renal protein expression of α7 -nAChRs and eNOS. These findings, along with the remarkable increase in the renal vasodilatory effect of nicotine in L-arginine-treated OVX, raise the possibility that α7 -nAChR and NOS/NO interdependent signaling mediates the estrogen-dependent vasodilatory effect of nicotine. More studies are obviously needed to ascertain this possibility. Another interesting finding was that despite the significant increase in nicotine-evoked vasodilation caused by estrogen replacement in OVX rats, the nicotine response remained lower than that of proestrus levels especially during the early phase of the response (see fig. 6). This finding infers that other ovarian hormones such as progesterone, which promotes vasodilation (Molinari et al., 2001), might also be important for full expression of nicotine-evoked renal vasodilation. It is also likely that a longer estrogen treatment period (>5 days) might be necessary to fully restore nicotine-evoked vasodilation in OVX renal vasculature.
Tamoxifen is the first generation of a group of structurally diverse compounds called selective estrogen receptor modulators that exhibit both agonistic and antagonistic activities at estrogen receptor sites depending on the target tissue and hormonal milieu (Leung et al., 2007). When used alone, tamoxifen relaxes smooth muscle arterial preparations and this effect is attenuated by the selective estrogen receptor antagonist ICI 182,780, suggesting a role for estrogen receptor activation in the effect of tamoxifen (Figtree et al., 2000; Hutchison et al., 2001). On the other hand, tamoxifen exhibits its estrogen receptor antagonistic activity and attenuates the vasorelaxant effect of estrogen when vascular preparations are simultaneously treated with the two drugs (Nechmad et al., 1998; Gonzales and Kanagy, 1999). In the present investigation, tamoxifen was employed to verify the involvement of estrogen receptors in the nicotine-estrogen renovascular interaction. We examined the renovascular effect of nicotine in OVX rats treated with tamoxifen alone or in combination with estrogen. The results showed that the effect of tamoxifen on nicotine vasodilation depended on hormonal status. In OVX rats that received no estrogen, tamoxifen slightly and transiently potentiated the renal vasodilating effect of nicotine. In contrast, when concurrently administered with estrogen, tamoxifen significantly attenuated the facilitatory effect of estrogen on nicotine-evoked vasodilation. It is conceivable, therefore, to propose that the presence of physiological estrogen level to sustain estrogen receptor signaling is necessary for the mediation of the estrogen-dependent nicotine-induced vasodilations in the renal vasculature.
It is imperative to comment on the apparently greater vasodilatory action of nicotine in male compared with OVX or diestrus preparations (see figures 1 and 5). Contribution of higher blood estrogen levels to the greater response in male rats seems unlikely because in agreement with reported findings (Shaw et al., 2001), plasma estrogen levels in male rats were not statistically different from those of OVX or diestrus rats in the present study. Other factors, therefore, appear to be responsible for the greater vadilatory effect of nicotine in the male, compared with OVX or diestrous rats. One conceivable candidate is the male gonadal hormone testosterone. In a previous study undertaken in isolated perfused kidneys of male rats, we have shown that testosterone, like estrogen, exerts a facilitatory effect on endothelium-dependent vasodilation (El-Mas et al., 2003). In this latter study, cholinergically-mediated vasorelaxations were reduced after castration and were restored to normal levels in testosterone-replaced preparations (El-Mas et al., 2003). A positive modulation by testosterone of endothelial function has also been reported in non-renal vascular beds (Ding and Stallone, 2001; Tatchum-Talom et al., 2002).
In conclusion, the present study provides insights into cellular mechanisms involved in the gender- and estrogen-dependent vasodilatory responses elicited by acute exposure to nicotine in the rat renal vasculature. The renal vasodilatory effect of nicotine was entirely dependent on NOS activity and was of greater magnitude in proestrus female compared with male preparations apparently due to the higher NOS activity in the former. Further, the facilitation of α7-nAChRs/eNOS signaling probably contribute to the estrogen-dependent renal vasodilatory action of nicotine in the female population because: (i) nicotine-evoked vasodilation was substantially attenuated under conditions of reduced estrogen levels such as those seen in rats with bilateral OVX or during the diestrus phase of the cycle, (ii) treatment of with L-arginine or 17β-estradiol restored in part nicotine-evoked vasodilation in kidneys of OVX rats, (iii) the enhancement of nicotine-evoked vasodilation caused by estrogen replacement was abrogated when OVX rats were concurrently treated with the estrogen receptor antagonist tamoxifen, and (iv) estrogen replacement in OVX rats dramatically increased the renal levels of nitrite/nitrate and protein expressions of eNOS and α7-nAChRs.
Acknowledgments
Supported by the Faculty of Pharmacy, University of Alexandria, Egypt and by Grant R01 AA014441 from the National Institute on Alcohol Abuse and Alcoholism. The authors thank Ms. Kui Sun for her technical assistance.
Footnotes
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alper RH, Schmitz TM. Estrogen increases the bradycardia elicited by central administration of the serotonin1A agonist 8-OH-DPAT in conscious rats. Brain Research. 1996;716 (1–2):224–228. doi: 10.1016/0006-8993(96)00069-8. [DOI] [PubMed] [Google Scholar]
- Black CE, Huang N, Neligan PC, Levine RH, Lipa JE, Lintlop S, Forrest CR, Pang CY. Effect of nicotine on vasoconstrictor and vasodilator responses in human skin vasculature. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2001;281 (4):R1097–1104. doi: 10.1152/ajpregu.2001.281.4.R1097. [DOI] [PubMed] [Google Scholar]
- Ding AQ, Stallone JN. Testosterone-induced relaxation of rat aorta is androgen structure specific and involves K+ channel activation. Journal of Applied Physiology. 2001;91 (6):2742–2750. doi: 10.1152/jappl.2001.91.6.2742. [DOI] [PubMed] [Google Scholar]
- Donald JA, Broughton BR, Bennett MB. Vasodilator mechanisms in the dorsal aorta of the giant shovelnose ray, Rhinobatus typus (Rajiformes; Rhinobatidae) Comparative Biochemistry and Physiology Part A, Molecular & Integrative Physiology. 2004;137 (1):21–31. doi: 10.1016/s1095-6433(03)00260-5. [DOI] [PubMed] [Google Scholar]
- Eguchi S, Miyashita S, Kitamura Y, Kawasaki H. α3β4-Nicotinic receptors mediate adrenergic nerve- and peptidergic (CGRP) nerve-dependent vasodilation induced by nicotine in rat mesenteric arteries. British Journal of Pharmacology. 2007;151 (8):1216–1223. doi: 10.1038/sj.bjp.0707331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. An association between the estrogen-dependent hypotensive effect of ethanol and an elevated brainstem c-jun mRNA in female rats. Brain Research. 2001;912 (1):79–88. doi: 10.1016/s0006-8993(01)02727-5. [DOI] [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Estrogen enhances baroreflex control of heart rate in conscious ovariectomized rats. Canadian Journal of Physiology and Pharmacology. 1998a;76 (4):381–386. [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Estrogen-dependent hypotensive effects of ethanol in conscious female rats. Alcoholism, Clinical and Experimental Research. 1999;23 (4):624–632. [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Ovariectomy abolishes ethanol-induced impairment of baroreflex control of heart rate in conscious rats. European Journal of Pharmacology. 1998b;349 (2–3):253–261. doi: 10.1016/s0014-2999(98)00202-7. [DOI] [PubMed] [Google Scholar]
- El-Mas MM, Afify EA, Omar AG, Mohy El-Din MM, Sharabi FM. Testosterone depletion contributes to cyclosporine-induced chronic impairment of acetylcholine renovascular relaxations. European Journal of Pharmacology. 2003;468 (3):217–224. doi: 10.1016/s0014-2999(03)01720-5. [DOI] [PubMed] [Google Scholar]
- El-Mas MM, El-gowilly SM, Gohar EY, Ghazal AM. Pharmacological characterization of cellular mechanisms of the renal vasodilatory effect of nicotine in rats. European Journal of Pharmacology. 2008a;588 (2–3):294–300. doi: 10.1016/j.ejphar.2008.04.048. [DOI] [PubMed] [Google Scholar]
- El-Mas MM, Fan M, Abdel-Rahman AA. Endotoxemia-mediated induction of cardiac inducible nitric-oxide synthase expression accounts for the hypotensive effect of ethanol in female rats. Journal of Pharmacology and Experimental Therapeutics. 2008b;324 (1):368–375. doi: 10.1124/jpet.107.127498. [DOI] [PubMed] [Google Scholar]
- El-Mas MM, Fan M, Abdel-Rahman AA. Facilitation of myocardial PI3K/Akt/nNOS signaling contributes to ethanol-evoked hypotension in female rats. Alcoholism, Clinical and Experimental Research. 2009;33(7):1158–1168. doi: 10.1111/j.1530-0277.2009.00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mas MM, Mohy El-Din MM, El-gowilly SM, Sharabi FM. Regional and endothelial differences in the cyclosporine attenuation of adenosine receptor-mediated vasorelaxations. Journal of Cardiovascular Pharmacology. 2004;43 (4):562–573. doi: 10.1097/00005344-200404000-00012. [DOI] [PubMed] [Google Scholar]
- Figtree GA, Webb CM, Collins P. Tamoxifen acutely relaxes coronary arteries by an endothelium-, nitric oxide-, and estrogen receptor-dependent mechanism. Journal of Pharmacology and Experimental Therapeutics. 2000;295 (2):519–523. [PubMed] [Google Scholar]
- Geary GG, Krause DN, Duckles SP. Estrogen reduces mouse cerebral artery tone through endothelial NOS- and cyclooxygenase-dependent mechanisms. American Journal of Physiology, Heart and Circulatory Physiology. 2000;279 (2):H511–519. doi: 10.1152/ajpheart.2000.279.2.H511. [DOI] [PubMed] [Google Scholar]
- Gonzales RJ, Kanagy NL. Endothelium-Independent Relaxation of Vascular Smooth Muscle by 17beta-Estradiol. Journal of Cardiovascular Pharmacology and Therapeutics. 1999;4 (4):227–234. doi: 10.1177/107424849900400404. [DOI] [PubMed] [Google Scholar]
- Groudine SB, Morley JN. Recent problems with paracervical vasopressin: a possible synergistic reaction with nicotine. Medical Hypotheses. 1996;47 (1):19–21. doi: 10.1016/s0306-9877(96)90036-5. [DOI] [PubMed] [Google Scholar]
- Haak T, Jungmann E, Raab C, Usadel KH. Elevated endothelin-1 levels after cigarette smoking. Metabolism. 1994;43 (3):267–269. doi: 10.1016/0026-0495(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Hutchison SJ, Chou TM, Chatterjee K, Sudhir K. Tamoxifen is an acute, estrogen-like, coronary vasodilator of porcine coronary arteries in vitro. Journal of Cardiovascular Pharmacology. 2001;38 (5):657–665. doi: 10.1097/00005344-200111000-00002. [DOI] [PubMed] [Google Scholar]
- Kähönen M, Tolvanen JP, Sallinen K, Wu X, Pörsti I. Influence of gender on control of arterial tone in experimental hypertension. American Journal of Physiology. 1998;275 (1 Pt 2):H15–22. doi: 10.1152/ajpheart.1998.275.1.H15. [DOI] [PubMed] [Google Scholar]
- Kauser K, Rubanyi GM. Gender difference in bioassayable endothelium-derived nitric oxide from isolated rat aortae. American Journal of Physiology. 1994;267 (6 Pt 2):H2311–2317. doi: 10.1152/ajpheart.1994.267.6.H2311. [DOI] [PubMed] [Google Scholar]
- Knot HJ, Lounsbury KM, Brayden JE, Nelson MT. Gender differences in coronary artery diameter reflect changes in both endothelial Ca2+ and ecNOS activity. American Journal of Physiology. 1999;276 (3 Pt 2):H961–969. doi: 10.1152/ajpheart.1999.276.3.H961. [DOI] [PubMed] [Google Scholar]
- Leung FP, Tsang SY, Wong CM, Yung LM, Chan YC, Leung HS, Yao X, Huang Y. Raloxifene, tamoxifen and vascular tone. Clinical and Experimental Pharmacology and Physiology. 2007;34 (8):809–813. doi: 10.1111/j.1440-1681.2007.04684.x. [DOI] [PubMed] [Google Scholar]
- Lieberman EH, Gerhard MD, Uehata A, Walsh BW, Selwyn AP, Ganz P, Yeung AC, Creager MA. Estrogen improves endothelium-dependent, flow-mediated vasodilation in postmenopausal women. Annals of Internal Medicine. 1994;121 (12):936–941. doi: 10.7326/0003-4819-121-12-199412150-00005. [DOI] [PubMed] [Google Scholar]
- Marano G, Ramirez A, Mori I, Ferrari AU. Sympathectomy inhibits the vasoactive effects of nicotine in conscious rats. Cardiovascular Research. 1999;42 (1):201–205. doi: 10.1016/s0008-6363(98)00326-5. [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Miguel KJ, Melo LL, Spadari-Bratfisch RC. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiology & Behavior. 2001;74 (4–5):435–440. doi: 10.1016/s0031-9384(01)00593-5. [DOI] [PubMed] [Google Scholar]
- Misko TP, Schilling RJ, Salvemini D, Moore WM, Currie MG. A fluorometric assay for the measurement of nitrite in biological samples. Analytical Biochemistry. 1993;214 (1):11–16. doi: 10.1006/abio.1993.1449. [DOI] [PubMed] [Google Scholar]
- Molinari C, Battaglia A, Grossini E, Mary DA, Stoker JB, Surico N, Vacca G. The effect of progesterone on coronary blood flow in anaesthesized pigs. Experimental Physiology. 2001;86 (1):101–108. doi: 10.1113/eph8602076. [DOI] [PubMed] [Google Scholar]
- Nechmad A, Merin G, Schwalb H, Shimon DV, Borman JB, Milgalter E, Mosseri M. Estrogen induces nitric oxide-mediated vasodilation of human mammary arteries in vitro. Nitric Oxide. 1998;2 (6):460–466. doi: 10.1006/niox.1998.0202. [DOI] [PubMed] [Google Scholar]
- Papadopolou S, Hartmann P, Lips KS, Kummer W, Haberberger RV. Nicotinic receptor mediated stimulation of NO-generation in neurons of rat thoracic dorsal root ganglia. Neuroscience Letters. 2004;361 (1):32–35. doi: 10.1016/j.neulet.2003.08.064. [DOI] [PubMed] [Google Scholar]
- Shaw L, Taggart M, Austin C. Effects of the oestrous cycle and gender on acute vasodilatory responses of isolated pressurized rat mesenteric arteries to 17 beta-oestradiol. British Journal of Pharmacology. 2001;132 (5):1055–1062. doi: 10.1038/sj.bjp.0703908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si ML, Lee TJ. Alpha7-nicotinic acetylcholine receptors on cerebral perivascular sympathetic nerves mediate choline-induced nitrergic neurogenic vasodilation. Circulation Research. 2002;91 (1):62–69. doi: 10.1161/01.res.0000024417.79275.23. [DOI] [PubMed] [Google Scholar]
- Smith AD, Dar MS. Involvement of the alpha4beta2 nicotinic receptor subtype in nicotine-induced attenuation of delta9-THC cerebellar ataxia: role of cerebellar nitric oxide. Pharmacology, Biochemistry, and Behavior. 2007;86 (1):103–112. doi: 10.1016/j.pbb.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Tang G, Hanna ST, Wang R. Effects of nicotine on K+ channel currents in vascular smooth muscle cells from rat tail arteries. European Journal of Pharmacology. 1999;364 (2–3):247–254. doi: 10.1016/s0014-2999(98)00833-4. [DOI] [PubMed] [Google Scholar]
- Tao X, Brodie AM, Nnane IP. The effect of tamoxifen on the pharmacokinetics of letrozole in female rats. Biopharmaceutics & Drug Disposition. 2006;27 (7):335–344. doi: 10.1002/bdd.514. [DOI] [PubMed] [Google Scholar]
- Tatchum-Talom R, Martel C, Marette A. Effects of ethinyl estradiol, estradiol, and testosterone on hindlimb endothelial function in vivo. Journal of Cardiovascular Pharmacology. 2002;39 (4):496–502. doi: 10.1097/00005344-200204000-00005. [DOI] [PubMed] [Google Scholar]
- Thompson J, Khalil RA. Gender differences in the regulation of vascular tone. Clinical and Experimental Pharmacology and Physiology. 2003;30 (1–2):1–15. doi: 10.1046/j.1440-1681.2003.03790.x. [DOI] [PubMed] [Google Scholar]
- Wellman GC, Bonev AD, Nelson MT, Brayden JE. Gender differences in coronary artery diameter involve estrogen, nitric oxide, and Ca(2+)-dependent K+ channels. Circulation Research. 1996;79 (5):1024–1030. doi: 10.1161/01.res.79.5.1024. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Honda H, Tamura K, Kogo H. Possible mechanisms for the suppressing action of 17β-estradiol on β-adrenoceptor-mediated vasorelaxation in rat aorta. European Journal of Pharmacology. 2001;427 (1):61–67. doi: 10.1016/s0014-2999(01)01190-6. [DOI] [PubMed] [Google Scholar]
- Yang S, Bae L, Zhang L. Estrogen increases eNOS and NOx release in human coronary artery endothelium. Journal of Cardiovascular Pharmacology. 2000;36 (2):242–247. doi: 10.1097/00005344-200008000-00015. [DOI] [PubMed] [Google Scholar]
- Yeboah MM, Xue X, Javdan M, Susin M, Metz CN. Nicotinic acetylcholine receptor expression and regulation in the rat kidney after ischemia-reperfusion injury. American Journal of Physiology Renal Physiology. 2008;295 (3):F654–661. doi: 10.1152/ajprenal.90255.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayas RM, Qazi S, Morton DB, Trimmer BA. Nicotinic-acetylcholine receptors are functionally coupled to the nitric oxide/cGMP-pathway in insect neurons. Journal of Neurochemistry. 2002;83 (2):421–431. doi: 10.1046/j.1471-4159.2002.01147.x. [DOI] [PubMed] [Google Scholar]