Abstract
Racial differences in the prevalence of anemia in patients with heart failure have been noted. The diagnosis of anemia in heart failure patients can be confounded by many factors. Plasma volume expansion is one of the most prominent confounders. We will study the difference of anemia prevalence using two different diagnostic techniques: peripheral hemoglobin recommended by the World Health Organization (WHO criteria) and blood volume (BV) analysis. We will also compare racial disparities in the prevalence of anemia using both measures. 60 patients with heart failure and preserved ejection fraction (HFPEF) underwent measurement of BV by a radio-labeled albumin technique. Anemia was defined by both WHO criteria and by measured RBCV>10% below ideal. Anemia was found in 67% of patients by the peripheral hemoglobin technique with no racial disparity. Only 35% of the patients had anemia by the BV analysis with a two fold higher prevalence among Hispanics compared to Whites and Blacks. In patients with HFPEF, the diagnosis of anemia based on hemoglobin is confounded by plasma volume derangements resulting in significant over-diagnosis in this cohort. Racial differences in the rate of anemia were found. Such data could have important implications for the diagnosis and management of anemia in ethnic minorities with HFPEF.
Keywords: Heart Failure, anemia, ejection fraction, blood volume, overdiagnosis
Introduction
Anemia is common in subjects with heart failure, associated with increased morbidity including hospitalizations, mortality, and a reduced quality of life 1. These associations are present in heart failure, regardless of LVEF 2–3. These data have led to several randomized clinical trials using erythropoietin stimulating agents4–5, iron6 and their combination7–8, predominately in subjects with systolic heart failure, to determine safety and efficacy. These trials have demonstrated reduction in hospitalizations, improvement in functional capacity and ventricular function but are limited by their small sample size and short duration 9.
The diagnosis of anemia is usually made by measurement of hemoglobin values from standard peripheral blood. However, in patients with volume overload states such as systolic heart failure, hemodilution has been shown to be a common cause for low hemoglobin10 and has been suggested to be the most potent factor for the low hemoglobin observed in subjects with heart failure and a reduced ejection fraction11. Such data raise concerns that for many patients with systolic heart failure, treating anemia with agents to stimulate red cell production may not be justified. Additionally, alterations in plasma volume (PV) occur as a compensation for the contracted red blood cell volume (RBCV) in order to maintain the overall blood volume (BV) at a constant level 12, and could also confound the diagnosis of anemia. Finally, the chronic use of medications such as diuretics that act by contracting plasma volume could result in an under-diagnosis of anemia based on standard hemoglobin measures.
In the general population 13–14 and among patients with heart failure either in the setting of a reduced or normal/preserved ejection fraction2, the prevalence of anemia is higher among Blacks than Whites. Despite the fact that Hispanics are the largest and fastest-growing ethnic minority in the United States15, data on the prevalence of anemia in this cohort in comparison to other racial groups in subjects with heart failure is lacking. We hypothesized that analysis of blood volume in subjects with HFPEF could provide insights into racial differences among subjects affected by this heterogeneous clinical syndrome.
Methods
Study Subjects
Subjects were outpatients referred for evaluation and treatment to the Columbia University Medical Center Heart Failure Center. Subjects aged >21 years diagnosed with HF with a preserved ejection fraction (e.g. ≥ 45%) were studied. The diagnosis of heart failure was based on the National Health and Nutrition Examination Survey congestive heart failure criteria with a score >=3.16 Subjects with acute decompensated HF, severe renal dysfunction (serum creatinine >3.0 mg/dl or history of nephrotic syndrome), and severe hepatic dysfunction (serum liver enzymes >3 times the upper limits of normal or history of cirrhosis) were excluded. Cardiac medications included diuretics, digoxin, renin-angiotensin system inhibitors, and/or beta-adrenergic receptor antagonists that were stable before the measurement of blood volume. Sixty ambulatory patients with HFPEF were studied: 33% white, 40% Latino, 27% Black. The Institutional Review Board at Columbia University Medical Center approved the protocol. All subjects gave written informed consent before participation.
Hemoglobin measures
Hemoglobin was measured as part of a routine complete blood count from the hospital core laboratory (Sysmex XE 2100; Sysmex Corporation, Kobe, Japan). Anemia was defined according to WHO criteria as a hemoglobin <12 gm/dl in women and <13 gm/dl in men17.
Blood Volume Analysis
Plasma volume was determined after the intravenous administration of iodine-131-labeled albumin, as has been described previously18–19. Blood volume and red blood cell volume were calculated from the plasma volume measurement, the measured hematocrit corrected for trapped plasma, and mean body hematocrit. Blood volume components (plasma, red cell and total volume) were determined and compared to normal values adjusted for age, gender and weight on the basis of the ideal weight system to yield % deviations from normal. Thus, in addition to reporting absolute values, we report percentage deviation from expected values on the basis of the ideal weight system. Anemia, based on blood volume analysis, was defined by RBCV<10% below ideal. To determine if PV compensation in patients with RBCV deficits was appropriate, the absolute PV compensation in response to RBCV deficit was calculated as deviation from ideal PV – RBCV deficit, and the % compensation was calculated as (deviation from ideal PV - RBCV deficit)/ideal PV.
Statistical Analysis
Data are expressed as mean ± SD, unless otherwise noted. Dichotomous variables were compared using chi-square analysis with Fisher’s exact test when appropriate, and continuous variables were compared using ANOVA with a Bonferroni’s post hoc test to control for multiple comparisons for those variables that were normally distributed. For the blood volume data, a non-parametric test (Kruskal Wallis) was employed given the non-normality of the data. A pvalue ≤0.05 was considered significant. SAS version 9.1 (SAS Institute Inc., Cary, North Carolina) was used for all analyses.
Results
The patients studied were older adults, predominately women with an average NYHA Class of 2.5 ± 0.6 and a normal ejection fraction (54±6%). They had underlying chronic renal insufficiency similar to other large series of hospitalized HFPEF patients 20–21 with average hemoglobin of 11.6±2.1 gm/dl. Racial subgroups differed with regards to gender, body weight and size and the use of beta blocker and aldosterone antagonists but not with regard to blood pressure, echocardiographic or laboratory assessments (Table 1).
Table 1.
Demographic and Clinical Characteristics
| Overall (n=60) | Whites (n=20) | Blacks (n=16) | Hispanics (n=24) | P value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years) | 70 ± 12.9 | 75 ± 13 | 68 ± 15 | 67 ± 11 | NS |
| Gender (% female) | 63 | 40 | 81 | 71 | 0.02 |
| Body Size | |||||
| Weight (kg) | 80 ± 14 | 87 ± 18 | 81 ± 15 | 74 ± 7* | 0.01 |
| BMI | 30 ± 4.3 | 31 ± 4 | 31 ± 5.8 | 29 ± 3 | NS |
| BSA (m2) | 1.89 ± 0.2 | 1.99 ± 0.3 | 1.9 ± 0.2 | 1.8± 0.1* | 0.01 |
| Anemic (%) | 67 | 65 | 50 | 79 | NS |
| Medications (% taking) | |||||
| Loop diuretics | 68 | 65 | 69 | 70 | NS |
| Thiazide Diuretics | 21 | 20 | 19 | 25 | NS |
| ACE inhibitors | 66 | 65 | 81 | 58 | NS |
| Beta Blockers | 66 | 45 | 56 | 92 | <0.001 |
| Aldosterone Antagonist | 20 | 40 | 19 | 4 | <0.01 |
| Calcium channel blockers | 43 | 25 | 50 | 54 | NS |
| Blood Pressure (mm Hg) | |||||
| Systolic blood pressure | 142 ± 16.5 | 137 ± 18 | 145 ± 16 | 143 ± 15 | NS |
| Diastolic blood pressure | 73 ± 11.4 | 73 ± 14 | 75 ± 11 | 73 ± 10 | NS |
| Three dimensional Echocardiography | |||||
| EDVI (ml/m2) | 58 ± 12 | 60 ± 10 | 62 ± 16 | 55 ± 8 | NS |
| SVI (ml/m2) | 32 ± 6 | 32 ± 6 | 34 ± 8 | 30 ± 5 | NS |
| EF (%) | 54 ± 6 | 54 ± 5 | 54 ± 6 | 55 ± 6 | NS |
| LV mass (grams/m2) | 78 ± 22 | 78 ± 16 | 85 ± 22 | 73 ± 26 | NS |
| EDV/Mass ratio | 0.80 ± 0.24 | 0.80 ± 0.2 | 0.77 ± 0.24 | 0.83 ± 0.26 | NS |
| Laboratory Results | |||||
| Hemoglobin (gm/dl) | 11.6 ± 2.1 | 12 ± 2.2 | 12 ± 2 | 11± 2 | NS |
| Hematocrit (%) | 36 ± 4.9 | 36 ± 5.5 | 37 ± 5 | 36± 4 | NS |
| BUN (mg/dl) | 32 ± 17 | 35 ± 16 | 31 ± 22 | 30 ± 15 | NS |
| Creatinine (mg/dl) | 1.5 ± 0.6 | 1.5 ± 0.5 | 1.6 ± 0.7 | 1.4 ± 0.6 | NS |
| eGFR (ml/min) | 52 ± 20 | 49 ± 15 | 53 ± 24 | 53 ± 22 | NS |
p < 0.05 versus Whites by ANOVA with post hoc Bonferonni correction
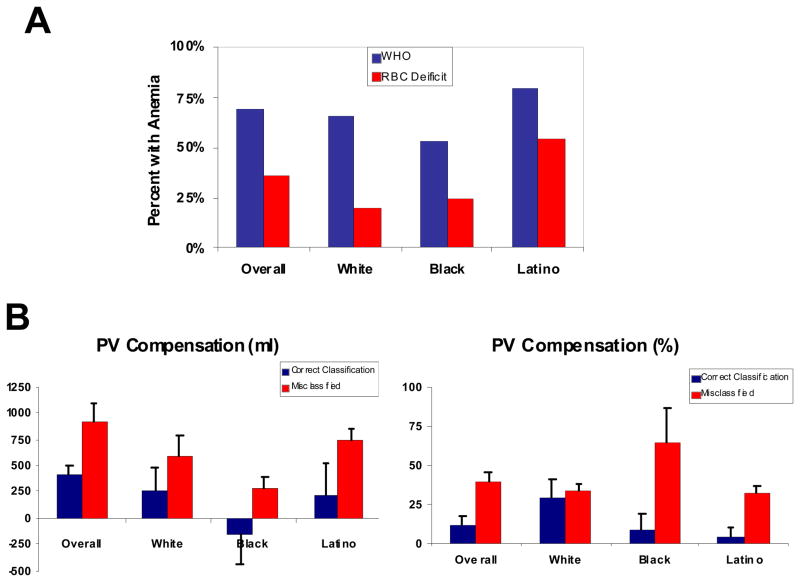
Anemia, as defined by WHO criteria, was present in 40 (67%) subjects, similar to other series22 and did not differ significantly by gender or race. However, by RBCV deficit, anemia was present in only 21 of those subjects (35% overall) (Figure 1). In all groups studied - White, Black and Latino - there was a significant difference in the rate of anemia diagnosed by WHO criteria as compared to measured RBCV deficit. None of the subjects first classified as non-anemic by WHO criteria were then re-classified as anemic by blood volume analysis. Overall in 45% of Whites, 25% of Hispanics and 25% of Blacks the diagnosis of anemia was reclassified when using RBCV deficit compared to hemoglobin.
Figure 1.
Panel A: The prevalence of anemia in the overall cohort and in each racial subgroup by WHO criteria (blue bars) and by red blood cell deficit (red bars). Panel B. Differences in plasma volume compensation (by volume and percentage) between patients diagnosed correctly by WHO and RBCV criteria (real anemia) and those misclassified as anemic by WHO criteria stratified by race.
Blood volume indices overall and by racial group are shown in Table 2. On average, those subjects with HFPEF demonstrated an expanded blood volume, which was primarily attributable to an increase in plasma volume. While overall blood volumes, red cell volumes and plasma volumes differed by race, these differences were no longer significant when controlling for differences in gender and body size between cohorts (e.g. % deviation did not differ). The difference in the prevalence of anemia as determined by hemoglobin and RBCV was in part attributable to greater absolute PV compensation in response to RBCV deficit. Specifically, among subjects who were correctly classified by WHO criteria, plasma volume compensation for the red cell deficit was an appropriate physiologic response, while for subjects who were misclassified by WHO criteria in comparison to red cell volume measures, the plasma volume compensation was excessive (See Figure 1, Panel B). The plasma volume overcompensation was higher in misclassified subjects compared to correctly classified subjects in all racial subgroups, but this was particularly true for Blacks and Latinos compared to Whites.
Table 2.
Blood Volume in HFPEF stratified by Racial Groups
| Overall (n=60) | Whites (n=20) | Blacks (n=16) | Hispanics (n=24) | P value* | |
|---|---|---|---|---|---|
| Blood Volume (ml) | 4696 ± 1137 (3328 to 6946) | 5301 ± 1282 (3492 to 7808) | 4214 ± 783 (3355 to 6620) | 4514 ± 1021 (3402 to 6751) | 0.0126 |
| Excess/Deficit Blood volume (ml) | 423 ± 603 (−525 to 1527) | 594 ± 682 (−5 to 2099) | 373 ± 621 (−813 to 1459) | 313 ± 508 (−443 to 1332) | NS |
| BV Deviation (%) | 9 ± 13 (−11 to 29) | 12 ± 14 (0 to 45) | 8 ± 13 (−17 to 28) | 7 ± 11 (−9 to 25) | NS |
| Red Cell Volume (ml) | 1474 ± 479 (948 to 2428) | 1754 ± 612 (967 to 3042) | 1298 ± 218 (1068 to 1797) | 1357 ± 380 (876 to 2048) | 0.0174 |
| Excess/Deficit Red Cell volume (ml) | 65 ± 465 (−631 to 708) | 178 ± 424 (−387 to 1044) | 198 ± 558 (−762 to 1469) | −117 ± 383 (−576 to 413) | NS |
| RBC Deviation (%) | 4 ± 27 (−36 to 39) | 9 ± 27 (−25 to 63) | 11 ± 30 (−39 to 70) | −6 ± 23 (−34 to 27) | NS |
| Plasma Volume (ml) | 3223 ± 755 (2274 to 4825) | 3547 ± 761 (2495 to 5094) | 2915 ± 686 (2126 to 4983) | 3157 ± 718 (2269 to 4623) | 0.0145 |
| Excess/Deficit Plasma volume (ml) | 504 ± 456 (−10 to 1582) | 622 ± 489 (84 to 1670) | 370 ± 474 (−118 to 1914) | 495 ± 406 (44 to 1311) | NS |
| Plasma Deviation (%) | 17 ± 15 (0 to 47) | 21 ± 15 (3 to 49) | 13 ± 16 (−4 to 62) | 17 ± 13 (2 to 40) | NS |
Mean ± SD (95% CI)
by Kruskal-Wallis Test
Discussion
The principal findings in this study are that among subjects with HFPEF the prevalence of anemia differs between WHO criteria that employ hemoglobin measurement as compared to blood volume analysis, with an overestimate of the prevalence of anemia by the WHO criteria in part due to an excess expansion of plasma volume in response to the RBC deficit. This error was present in all racial subgroups. Finally, the rate of a true anemia (e.g. RBC deficit) was highest among Hispanics as compared to Whites and Blacks.
Anemia in Multi-ethnic groups
Although the American Hispanic population is fastest growing ethnic minority in the United States, there is a paucity of data on the prevalence of anemia in this group compared to other ethnicities and no data that we are aware of regarding ethnic differences specifically in the population with HFPEF. Available data does suggest important racial/ethnic differences in the prevalence of anemia in the general population and the underlying cause. In the Third National Health and Nutrition Examination Survey (NHANES III), the prevalence of anemia in persons older than 65 years varied significantly by race with an overall prevalence of 10.6%, that was lowest for Non-Hispanic whites (9.0%), slightly higher in Mexican Americans (10.4%) but substantially higher in non-Hispanic blacks (27.8%)13. Similarly, a study of the prevalence and incidence of anemia in patients with diabetes mellitus showed a higher prevalence of anemia among Blacks and mixed ethnicities than Latinos, Whites or Asians 23. In that study, a multivariate model of prevalent anemia that adjusted for gender, age, clinic site, utilization, proximate estimated GFR, albuminuria, diabetes treatment and EPO use, found that the odds of anemia were higher among Blacks [odds ratio (OR) 2.26, 95% confidence interval (CI) 2.12–2.41], those of mixed ethnicity (OR 1.37, 95% CI 1.31–1.44) or Latino (OR 1.15, 95% CI 1.07–1.24), compared with whites. Finally, in a population based sample that evaluated anemia prevalence and iron status among older Hispanics of Caribbean origin compared to a cohort of Whites, the prevalence of anemia was higher in older Hispanics than in Non-Hispanic older Whites, after controlling for relevant confounders. This was in part attributable to significant ethnic differences in nutrient intake and intake of specific food items that resulted in different iron stores 24. We found a higher percentage of Hispanics with HFPEF were anemic by both hemoglobin and by blood volume analysis than Whites or Blacks.
Plasma volume in anemia and heart failure
Anemia in HF patients may be a result of iron deficiency, hemodilution19, anemia of chronic disease 25, malnutrition 26, renal insufficiency 26, or medication induced anemia 25. Among the population with advanced systolic heart failure hemodilution is common 27. Similarly, our data suggest that a significant percentage of subjects with HFPEF have expansions of plasma volume that are beyond physiologic compensation for the red blood cell deficit and are in part hemodilutional in nature. Indeed, chronic anemia in the absence of heart failure is associated with plasma volume expansion and edema because of decreased blood viscosity and the resulting effects of nitric oxide-mediated vasodilatation25. As a result, there is a decline in systemic vascular resistance and because of arterial under-filling an activation of the renin-angiotensin system and concomitant PV expansion 25. Accordingly, the plasma volume expansion in anemic patients replaces decreased RBC mass 12 in order to maintain a normal blood volume. In heart failure patients, alterations in plasma volume that occur in response to the underlying cardiac and or renal dysfunction and subsequent neurohormonal activation further add to the physiologically expanded plasma volume in anemic subjects -- and confound the diagnosis as shown by these data. Additionally, HFPEF subjects often have concomitant co-morbid conditions including obesity and renal insufficiency, which affect plasma volume regulation and could be an explanation for the observed findings.
Clinical implications
Appropriate treatment of anemia associated with heart failure may improve the outcomes of patients by enhancing physical function and quality of life 26, but treatment requires an accurate diagnosis and identification of the underlying etiology in order to select effective interventions. Commonly used treatments for non-dilutional anemia when used in cases of hemodilutional anemia can put the patient under unnecessary risk secondary to adverse effects of medication and may contribute to adverse outcomes28–29. The current data raise the possibility that further definition of the low hemoglobin phenotype in subjects with heart failure using blood volume analysis could provide important physiologic insights regarding therapeutic interventions. Of course, such recommendations must await the formal performance of clinical trials that are testing such hypotheses 30, and the recognition that plasma volume is dynamically regulated and fluctuates widely in response to multiple physiologic signals.
Limitations
The study is limited by the small sample size with limited power to detect differences between groups and the potential for a type II (beta) error. The use of self reports to classify race/ethnicity, which is in part a clustering of common genetic characteristics, but they are also influenced by social, environmental, and lifestyle factors that may have a huge effect on cardiovascular health. Given the cross sectional nature of our study we cannot exclude the effect of residual unmeasured confounders. However, factors known to affect blood volume including body size and gender were accounted for in the current analyses and the use of medications that could alter blood volume did not differ between the groups studied.
Conclusions
In patients with HFPEF, the diagnosis of anemia by hemoglobin is confounded by alterations in blood volume components. These differences are present in all racial subgroups. Such data could have important implications for the diagnosis and management of anemia in ethnic populations with HFPEF.
Citations
- 1.Groenveld HF, Januzzi JL, Damman K, et al. Anemia and mortality in heart failure patients a systematic review and meta-analysis. J Am Coll Cardiol. 2008;52:818–27. doi: 10.1016/j.jacc.2008.04.061. [DOI] [PubMed] [Google Scholar]
- 2.O’Meara E, Clayton T, McEntegart MB, et al. Clinical correlates and consequences of anemia in a broad spectrum of patients with heart failure: results of the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Program. Circulation. 2006;113:986–94. doi: 10.1161/CIRCULATIONAHA.105.582577. [DOI] [PubMed] [Google Scholar]
- 3.Cohen RS, Mubashir A, Wajahat R, Mani S, Hummel S, Maurer MS. The cardio-renal-anemia syndrome in elderly subjects with heart failure and a normal ejection fraction: a comparison with heart failure and low ejection fraction. Congest Heart Fail. 2006;12:186–91. doi: 10.1111/j.1527-5299.2006.05412.x. [DOI] [PubMed] [Google Scholar]
- 4.Jin B, Luo X, Lin H, Li J, Shi H. A meta-analysis of erythropoiesis-stimulating agents in anaemic patients with chronic heart failure. Eur J Heart Fail. 2010;12:249–53. doi: 10.1093/eurjhf/hfp182. [DOI] [PubMed] [Google Scholar]
- 5.van der Meer P, Groenveld HF, Januzzi JL, Jr, van Veldhuisen DJ. Erythropoietin treatment in patients with chronic heart failure: a meta-analysis. Heart. 2009;95:1309–14. doi: 10.1136/hrt.2008.161091. [DOI] [PubMed] [Google Scholar]
- 6.Anker SD, Comin Colet J, Filippatos G, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med. 2009;361:2436–48. doi: 10.1056/NEJMoa0908355. [DOI] [PubMed] [Google Scholar]
- 7.Silverberg DS, Blum M, Agbaria Z, et al. The effect of i.v. iron alone or in combination with low-dose erythropoietin in the rapid correction of anemia of chronic renal failure in the predialysis period. Clin Nephrol. 2001;55:212–9. [PubMed] [Google Scholar]
- 8.Silverberg DS, Wexler D, Sheps D, et al. The effect of correction of mild anemia in severe, resistant congestive heart failure using subcutaneous erythropoietin and intravenous iron: a randomized controlled study. J Am Coll Cardiol. 2001;37:1775–80. doi: 10.1016/s0735-1097(01)01248-7. [DOI] [PubMed] [Google Scholar]
- 9.Ngo K, Kotecha D, Walters JA, et al. Erythropoiesis-stimulating agents for anaemia in chronic heart failure patients. Cochrane Database Syst Rev. 2010:CD007613. doi: 10.1002/14651858.CD007613.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Mancini DM, Katz SD, Lang CC, LaManca J, Hudaihed A, Androne AS. Effect of erythropoietin on exercise capacity in patients with moderate to severe chronic heart failure. Circulation. 2003;107:294–9. doi: 10.1161/01.cir.0000044914.42696.6a. [DOI] [PubMed] [Google Scholar]
- 11.Adlbrecht C, Kommata S, Hulsmann M, et al. Chronic heart failure leads to an expanded plasma volume and pseudoanaemia, but does not lead to a reduction in the body’s red cell volume. Eur Heart J. 2008;29:2343–50. doi: 10.1093/eurheartj/ehn359. [DOI] [PubMed] [Google Scholar]
- 12.Wintrobe L. Wintrobe’s Clinical Hematology. 9. Williams & Wilkins; 1992. [Google Scholar]
- 13.Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–8. doi: 10.1182/blood-2004-05-1812. [DOI] [PubMed] [Google Scholar]
- 14.Patel KV, Harris TB, Faulhaber M, et al. Racial variation in the relationship of anemia with mortality and mobility disability among older adults. Blood. 2007;109:4663–70. doi: 10.1182/blood-2006-10-055384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davidson JA, Kannel WB, Lopez-Candales A, et al. Avoiding the looming Latino/Hispanic cardiovascular health crisis: a call to action. Ethn Dis. 2007;17:568–73. [PubMed] [Google Scholar]
- 16.Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol. 1992;20:301–6. doi: 10.1016/0735-1097(92)90094-4. [DOI] [PubMed] [Google Scholar]
- 17.Nutritional anaemias. Report of a WHO scientific group. World Health Organ Tech Rep Ser. 1968;405:5–37. [PubMed] [Google Scholar]
- 18.Abramov D, Cohen RS, Katz SD, Mancini D, Maurer MS. Comparison of blood volume characteristics in anemic patients with low versus preserved left ventricular ejection fractions. Am J Cardiol. 2008;102:1069–72. doi: 10.1016/j.amjcard.2008.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Androne AS, Katz SD, Lund L, et al. Hemodilution is common in patients with advanced heart failure. Circulation. 2003;107:226–9. doi: 10.1161/01.cir.0000052623.16194.80. [DOI] [PubMed] [Google Scholar]
- 20.Sweitzer NK, Lopatin M, Yancy CW, Mills RM, Stevenson LW. Comparison of clinical features and outcomes of patients hospitalized with heart failure and normal ejection fraction (> or =55%) versus those with mildly reduced (40% to 55%) and moderately to severely reduced (<40%) fractions. Am J Cardiol. 2008;101:1151–6. doi: 10.1016/j.amjcard.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klapholz M, Maurer M, Lowe AM, et al. Hospitalization for heart failure in the presence of a normal left ventricular ejection fraction: results of the New York Heart Failure Registry. J Am Coll Cardiol. 2004;43:1432–8. doi: 10.1016/j.jacc.2003.11.040. [DOI] [PubMed] [Google Scholar]
- 22.Dunlay SM, Weston SA, Redfield MM, Killian JM, Roger VL. Anemia and heart failure: a community study. Am J Med. 2008;121:726–32. doi: 10.1016/j.amjmed.2008.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmed AT, Go AS, Warton EM, Parker MM, Karter AJ. Ethnic differences in anemia among patients with diabetes mellitus: the Diabetes Study of Northern California (DISTANCE) Am J Hematol. 2010;85:57–61. doi: 10.1002/ajh.21577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seaverson EL, Buell JS, Fleming DJ, et al. Poor iron status is more prevalent in Hispanic than in non-Hispanic white older adults in Massachusetts. J Nutr. 2007;137:414–20. doi: 10.1093/jn/137.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anand IS. Heart failure and anemia: mechanisms and pathophysiology. Heart Fail Rev. 2008;13:379–86. doi: 10.1007/s10741-008-9088-8. [DOI] [PubMed] [Google Scholar]
- 26.Felker GM, Adams KF, Jr, Gattis WA, O’Connor CM. Anemia as a risk factor and therapeutic target in heart failure. J Am Coll Cardiol. 2004;44:959–66. doi: 10.1016/j.jacc.2004.05.070. [DOI] [PubMed] [Google Scholar]
- 27.Androne AS, Hryniewicz K, Hudaihed A, Mancini D, Lamanca J, Katz SD. Relation of unrecognized hypervolemia in chronic heart failure to clinical status, hemodynamics, and patient outcomes. Am J Cardiol. 2004;93:1254–9. doi: 10.1016/j.amjcard.2004.01.070. [DOI] [PubMed] [Google Scholar]
- 28.Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019–32. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 29.Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–98. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 30.Clinicaltrails.gov. Safety and Efficacy of Direct Blood Volume Measurement in the Treatment of Heart Failure (TEAM-HF) 2009: NCT01001312. [Google Scholar]