Summary
In mammals, dosage compensation between XX and XY individuals occurs through X chromosome inactivation (XCI). The noncoding Xist RNA is expressed and initiates XCI only when more than one X chromosome is present. Current models invoke a dependency on the X-to-autosome ratio (X:A), but molecular factors remain poorly defined. Here, we demonstrate that molecular titration between an X-encoded RNA and an autosomally encoded protein dictates Xist induction. In pre-XCI cells, CTCF protein represses Xist transcription. At the onset of XCI, Jpx RNA is upregulated, binds CTCF, and extricates CTCF from one Xist allele. We demonstrate that CTCF is an RNA-binding protein and is titrated away from the Xist promoter by Jpx RNA. Thus, Jpx activates Xist by evicting CTCF. The functional antagonism via molecular titration reveals a role for long noncoding RNA in epigenetic regulation.
Introduction
Dosage compensation balances X chromosome content between females and males in organisms with the XY mechanism of sex determination. In fruitflies, the single X chromosome in males is transcriptionally upregulated 2-fold; in roundworms, expression from two X chromosomes is halved in hermaphrodites; and in mammals, one of two X's is transcriptionally silenced in females during X chromosome inactivation (XCI) (Cline and Meyer, 1996; Lucchesi et al., 2005; Payer and Lee, 2008; Wutz, 2011). Although dosage compensation is achieved differently in various organisms, all three mechanisms depend on the “X-to-autosome ratio” (X:A), which triggers the epigenetic process only when X:A = 0.5 in fruitflies or X:A ≥ 1.0 in roundworms and in mammals. Failure to achieve dosage compensation results in early embryonic death. In roundworms, X-linked factors (SEX-1, CEH-39) and autosomal signals (SEA-1, SEA-2) antagonize each other at the master regulatory gene, xol-1 (Meyer, 2010). In fruitflies, accumulation of X signal elements (Scute, SisA, Runt, Upd) activates the master regulator Sxl, and autosomal factors, such as Deadpan, together with nuclear volume, oppose the X-linked signals (Barbash and Cline, 1995; Cline and Meyer, 1996; Salz and Erickson, 2010).
In mammals, the X:A ratio currently exists only as a genetic concept. Evidence derives from studies of aneuploids and polyploids indicating that XXX females establish two inactive X's (Xi) when diploid but inactivate only one Xi when tetraploid; furthermore, XXX triploid females establish one or two Xi, consistent with their intermediate X:A ratio (Brown and Chandra, 1973; Gartler et al., 2006). Two conceptual frameworks have been instructive. Early thinking favored the “Blocking Factor Hypothesis,” in which a single complex of autosomal factors binds to and blocks one Xic and protects that X chromosome from silencing (Ohno, 1969; Lyon, 1971; Kay et al., 1994; Starmer and Magnuson, 2009). Remaining Xs are unprotected and become silenced by default. The alternative “Two Factors Model” postulates the existence of not only a blocking factor to protect one X but also a “competence factor” to trigger silencing on additional Xs (Gartler and Riggs, 1983; Lee and Lu, 1999; Lee, 2005). Thus, regardless of details, all current models imply a molecular titration of X-linked and autosomal factors.
Consistent with their separate evolution, fly and worm regulators are not apparently utilized in the mouse. Unlike the invertebrate systems, mammalian dosage compensation is allelically controlled and involves regulatory switches defined by long noncoding RNA (lncRNA) within the master “X-inactivation center” (Xic). Xist RNA emanates from the Xic, coats the future Xi in cis, and deposits silencing complexes along the chromosome (Brockdorff et al., 1992; Brown et al., 1992). Xist is expressed only when X:A ≥1.0, and the number of Xist RNA foci follows the “n-1” rule in diploid cells (n = X chromosome number). Recent work shows that Xist is both negatively and positively regulated. It is repressed by the antisense Tsix RNA and the noncoding Xite locus (Lee and Lu, 1999; Sado et al., 2001; Ogawa and Lee, 2003) but activated by the long noncoding Jpx RNA (Tian et al., 2010) and the E3 ubiquitin ligase, RNF12 (Jonkers et al., 2009) (Figure 1A).
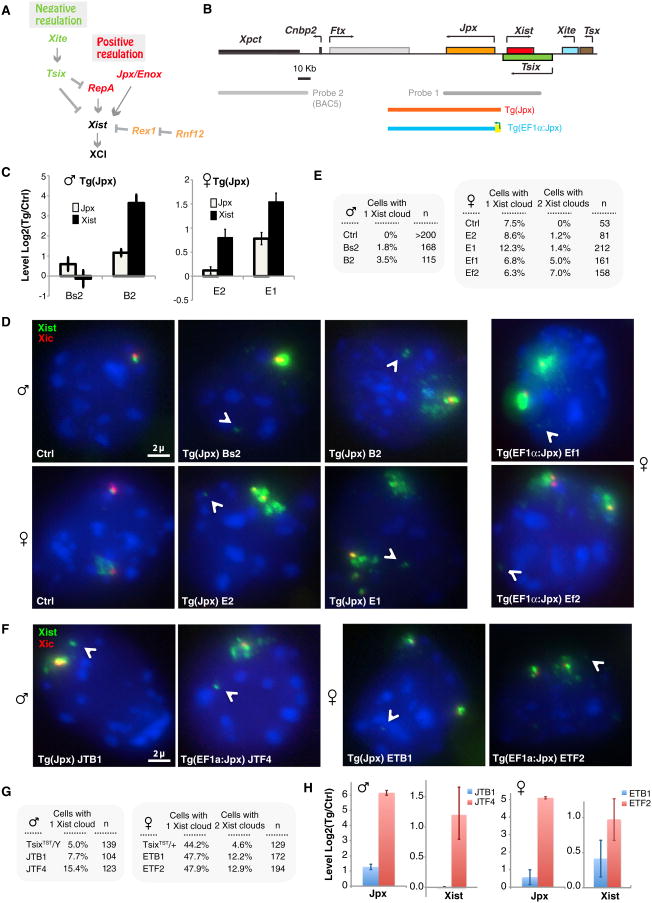
Figure 1. Jpx Overexpression Results in Ectopic Xist Upregulation.
(A) Positive-negative regulation of Xist. Arrows, activation; blunt arrows, repression. (B) Map of Xic, FISH probes, and Jpx transgenes. Tg(Jpx). (C) Quantitation of Jpx and Xist expression on d4 in Tg(Jpx) ES cells by qRT-PCR. Representative independent clones (male Bs2, B2; female E2, E1) are shown. Expression levels normalized to ctrl (control clone). Averages ± SE. See also Figure S1. (D) Xist expression examined by RNA-DNA FISH in d4 ES cells. FITC (green, probe 1), Xist RNA cloud. Cy3 (red), BAC5 probe 2 marks Xic. Arrowheads, Tg insertion site. (E) Quantitation of Xist RNA-DNA FISH results in d4 ES cells. Representative clones shown. n, sample size. See also Figure S1. (F) Disabling Tsix augments Xist's response to Jpx overexpression. RNA-DNA FISH of Tg(EF1a:Jpx) cells in a TsixTST/Y or TsixTST/+ cells on d4 of differentiation. One representative clone shown for each. FITC (green, probe 1), Xist RNA cloud. Cy3 (red), BAC5 probe 2 marks Xic. Arrowheads, Tg insertion site. (G) Quantitation of cells (in F) with the Xist pattern indicated. (H) qRT-PCR of Xist and Jpx RNA levels on d4, normalized to RNA levels in parental TsixTST/Y or TsixTST/+ cells. Averages ± SE. See also Figure S1.
The identities of X-encoded “numerators” and autosomally encoded “denominators” of the X:A ratio have been elusive. In principle, X-linked and autosomal regulators must converge at the Xic—potentially at the Tsix, Xite, or Xist locus. Indeed, integrating extra Xic copies into either a male or female genome mimics the presence of supernumerary Xs and triggers ectopic XCI (reviewed in Starmer and Magnuson, 2009; Lee, 2011). One study implicated the Tsix/Xite loci as binding sites for denominators without identifying specific autosomal factors (Lee, 2005). Another study showed that XCI is sensitive to dosage of autosomal OCT4 protein (Donohoe et al., 2009). The X-encoded E3 ubiquitin ligase, RNF12, was also proposed as a candidate numerator, as excess RNF12 triggers ectopic Xist expression (Jonkers et al., 2009) and RNF12-mediated ubiquitylation of REX1 occurs at the initiation of XCI (Gontan et al., 2012). RNF12's candidacy as numerator may be complicated by its catalytic nature, however. The necessity of precise X:A titration renders catalytic factors conceptually problematic because catalytic factors with rapid enzymatic rates are unlikely to be limited by 2-fold molar differences. Indeed, deleting a single allele of Rnf12—a state that mimics the XY state—delayed but did not abrogate Xist upregulation in mice (Jonkers et al., 2009; Shin et al., 2010; Barakat et al., 2011).
In molecular titration models, numerators are more easily envisioned as stoichiometric than as catalytic factors. Candidate numerators must in theory satisfy several experimental criteria. First, it must be X-linked and escape XCI in order to provide numerical information. Second, it should elicit discrete XCI phenotypes in response to changes in gene copy number. It must also be haploinsufficient, with the +/− state phenocopying the male state; overexpression should simulate supernumerary X's and trigger ectopic XCI. Finally, the numerator must act at the Xic. Intriguingly, the noncoding Jpx/Enox gene resides ∼10 kb upstream of Xist (Chureau et al., 2002; Johnston et al., 2002; Chow et al., 2003), escapes XCI, and is required for Xist activation (Tian et al., 2010). Deleting a single Jpx allele in female cells is sufficient to prevent XCI (Tian et al., 2010). This haploinsufficiency and its escape from XCI raises the intriguing possibility that Jpx RNA may be a numerator. Below, we subject Jpx to the test and define interactions that implicate Jpx in molecular titration of an autosomal factor.
Results
Jpx Induces Xist in a Dose-Dependent Manner
To determine if altering Jpx levels results in quantifiable changes of Xist expression, we introduced a 90 kb Jpx transgene (Tg(Jpx); Figure 1B) into wild-type male and female mouse embryonic stem (ES) cells, a well-established ex vivo model that recapitulates XCI during cell differentiation. Quantitative RT-PCR confirmed overexpression of Jpx RNA between 0.5- to 2-fold in multiple independent clones on day 4 of differentiation (d4; Figure 1C), and RNA-DNA fluorescence in situ hybridization (FISH) showed ectopic Xist clouds in differentiating embryonic stem (ES) cells at d4 (Figures 1D and 1E). Consistent with elevated steady-state levels of Xist shown by qRT-PCR (Figure 1C), 1%–4% of transgenic male cells possessed an Xist cloud, and 1%–2% of diploid female cells exhibited a second Xist cloud. (Note: tetraploid cells were excluded by performing DNA FISH using probe 2; Figure 1D.) Ectopic Xist clouds were neither observed in control male nor female cells carrying vector sequences (ctrl). Thus, whereas reducing Jpx expression by half blocks Xist activation (Tian et al., 2010), 2-fold overexpression of Jpx caused ectopic Xist expression.
The magnitude of overexpression was, however, small. Noting a general correlation between Jpx levels and degree of Xist upregulation (Figures 1C–1E), we asked if increasing Jpx overexpression caused greater Xist induction. By retrofitting the strong Elongation Factor 1α (EF1α) promoter into the Jpx transgene (Tg(EF1α:Jpx); Figure 1B), we further enhanced Jpx activity and observed greater Xist induction. RNA-DNA FISH indicated that up to 7% of cells displayed two Xist clouds (Figures 1D and 1E). Correlation of Jpx and Xist overexpression was also observed in later stages of differentiation (Figure S1A available online; data not shown). Xist expression therefore shows a dose-dependent response to Jpx expression.
Deleting Tsix Augments Jpx's Effect
The transgene experiments showed that, although the number of Xist clouds (10%–14%) was consistently elevated with Jpx overexpression, the percentage of ectopic Xist clouds never exceeded 7%. We recalled that Tsix and Jpx dually regulate Xist expression (Tian et al., 2010), with Xist being difficult to induce when the Tsix allele in cis is intact (Luikenhuis et al., 2001; Stavropoulos et al., 2001). During female differentiation, Tsix's repression on the future Xi enables Xist upregulation, whereas its persistent expression on the future Xa blocks Xist induction. Likewise, during male differentiation, persistent Tsix on the single X chromosome prevents Xist expression. Therefore, could an intact Tsix allele blunt the effect of Jpx overexpression in the Jpx transgenic male and female cells?
To address this, we introduced Tg(EF1α:Jpx) into ES cells carrying a truncated Tsix allele (TsixTST) (Ogawa et al., 2008) and observed that disabling Tsix increased susceptibility to Jpx overexpression (Figures 1F and 1G). In male cells, the number of ectopic Xist clouds increased from 1%–4% in a wild-type background (Figure 1E) to 7.7% in the TsixTST/Y background (JTB1). The percentage further increased to 15.4% when Jpx was overexpressed from the EF1α promoter in the TsixTST/Y background (JTF4) (Figure 1G; χ2 one-tailed, p = 0.01). In female cells, Tsix deficiency increased ectopic Xist clouds from ∼1% in a wild-type background (Figure 1E) to 12.2% in the TsixTST/+ background (ETB1). The percentage further increased to 12.9% when Jpx was overexpressed from the EF1α promoter (ETF2) (Figure 1G; χ2 one-tailed, p < 0.05). (The effect in females was less than in males, likely because Tsix remained intact on one female X; nonetheless, persistent Tsix allele on the future Xa would eventually be downregulated during differentiation, providing an opportunity for Jpx overexpression to act on the newly susceptible Xa, resulting in cells with two Xist clouds.) qRT-PCR showed a further ∼2-fold increase in Xist levels when Jpx is overexpressed from the EF1α promoter after truncating Tsix in female cells; in male cells, an even larger increase in Xist was observed (Figure 1H).
We conclude that the dose-dependent response of Xist to Jpx levels is augmented by deleting Tsix, consistent with the notion that Xist is coregulated by a Jpx-based positive pathway and a Tsix-based negative pathway (Lee and Lu, 1999; Sado et al., 2001; Tian et al., 2010). The presence of intact Tsix may explain why Jpx transgenic overexpression in previous studies did not result in ectopic Xist induction (Augui et al., 2011; Barakat et al., 2011).
CTCF Binds the Xist Promoter and Correlates with Xist Repression
Xist is known to be regulated at the transcriptional level (Sun et al., 2006). Within a 3 kb promoter region of Xist, three binding sites for the zinc finger protein, CTCF (Bell et al., 1999; Ohlsson et al., 2001), have been proposed, including one upstream of mouse Xist (Xist_5′; Navarro et al., 2006), one at human XIST's first promoter (P1; Pugacheva et al., 2005), and one at promoter P2 in mice (Sheardown et al., 1997; Navarro et al., 2006; Essien et al., 2009) (Figure 2A). One study reported a positive correlation between Xist activation and affinity of P1 for CTCF (Pugacheva et al., 2005). Another study proposed CTCF as chromatin insulator for P1 (Navarro et al., 2006). To pinpoint CTCF's role, we asked if CTCF directly binds the three murine sites by performing electrophoretic mobility shift analysis (EMSA). Whereas a GFP control did not shift any probe, purified CTCF strongly shifted Xist_5′ and P2 (Figure 2B). By contrast, CTCF did not shift P1, a site lacking the CTCF consensus motif (Essien et al., 2009). The shift was as robust as that observed for the strong binding site at RS14c, a Tsix-Xist boundary element at Xist's 3′ end (Spencer et al., 2011). Mutating three nucleotides in the consensus motif abolished the shift (Figures 2A and 2B). We conclude that CTCF directly binds Xist_5′ and P2 in vitro.
Figure 2. CTCF Binding to theXistPromoter Is Anticorrelated with Xist Expression.
(A)Xist P1 and P2 promoters, CTCF sites, and EMSA probes. Red, CTCF motif. Green bases, nonconforming CTCF motif. Blue, mutated bases in EMSA probes. (B) DNA EMSA using indicated probes with recombinant CTCF or GFP protein. *, DNA-protein shift. (C) CTCF ChIP quantitative PCR analysis on d0, d3, and d6 ES cells as indicated. Rnf12, negative control; RS14c and H19, positive controls. At least three independent experiments were performed for each time point. Averages ± SE. (D) Allele-specific ChIP for CTCF at Xist P2 site in TsixTST/+ female ES cells. cas, M. castaneus. mus, M. musculus. Two independent experiments in triplicates were performed for each time point. Averages ± SE. (E) CTCF ChIP on d0, d3, and d6 female Jpx+/− ES cells. Three independent experiments were performed for each time point. Averages ± SE. P, one-way t test comparing d0 versus d3 and d6 for each site.
To test binding in vivo, we carried out quantitative chromatin immunoprecipitation (ChIP) for CTCF in male and female ES cells. In undifferentiated (d0) male and female cells, CTCF occupied Xist_5′ and P2 (Figure 2C), consistent with EMSA results. Although CTCF could not bind P1 in the gel shift analysis, occupancy was observed by ChIP, suggesting that CTCF occupancy at P1 could be mediated by other factors in vivo. One possibility is that chromatin looping from P2 brought CTCF in proximity to P1. During differentiation, CTCF enrichment at all three sites remained unchanged in male cells. However, in female cells, binding to P2 was significantly reduced (p = 0.008) by approximately half between d0 and d6 (Figure 2C), the time frame of Xist upregulation. Enrichment at P1 was similarly decreased (but with borderline significance; p = 0.17), consistent with the idea of CTCF looping in from P2. Binding to positive control regions, RS14c and H19, and a negative control, Rnf12, was unaltered during this time frame. These results demonstrate that CTCF binding to P2 is dynamically regulated during XCI.
Given 2-fold changes in P2 binding, we suspected allelic differences. To test the possibility of differential binding to Xa and Xi, we performed allele-specific ChIP quantitative PCR in a genetically marked female ES line, TsixTST/+. In this cell line, a Tsix truncation on the Mus musculus (mus) X chromosome ensures that this chromosome will be Xi and the Mus castaneus (cas)X chromosome will be Xa(Ogawaetal., 2008); a strain-specific polymorphism located 200 bp downstream of P2 (Keane et al., 2011) enabled allelic discrimination by PCR. Indeed, CTCF occupancy was significantly reduced at P2 of the future Xi (Figure 2D), linking the loss of CTCF binding with Xist activation. In the Jpx+/− haploinsufficient ES cell line (Tian et al., 2010), the inability to upregulate Xist during differentiation correlated with persistent binding of CTCF to P2 (Figure 2E). Together, these data show that CTCF binding to Xist P2 is allelically regulated and is anticorrelated with Xist expression.
CTCF Is a Blocking Factor for Xist Upregulation
Anticorrelation of CTCF binding and Xist upregulation suggests that CTCF serves as an Xist repressor. To test this, we overexpressed CTCF in male and female ES cells by creating stable transgenic lines carrying a doxycycline(dox)-inducible bidirectional TRE promoter that, when induced, simultaneously expresses FLAG-tagged CTCF and a GFP marker (Figure 3A). At least two independent transgenic male and female clones were analyzed, and representative results are shown. Induction was tightly regulated by dox and not obviously toxic (Figures 3A and 3B). In male clones (e.g., #11), CTCF overexpression revealed no phenotype between d0 to d11, as embryoid bodies (EB) outgrowth was robust (Figure 3C). By contrast, CTCF overexpression in female clones (e.g., #1, #2) caused severe outgrowth defects after differentiation (Figures 3C and 3D). GFP+ (CTCF+) cells were confined to the EB center, whereas outgrown cells were GFP− (CTCF−), implying viability only when the transgene was silenced. Defects were strictly correlated with CTCF overexpression, as transgenic female clones without overexpression (e.g., #5) showed no growth anomalies (Figure 3D). We conclude that CTCF overexpression is toxic specifically to female cells during differentiation, consistent with an effect on XCI.
Figure 3. CTCF Overexpression Results in Female-Specific EB Outgrowth Defect.
(A) Dox-inducible bidirectional expression of CTCF-3xFLAG and GFP in rtTA-expressing MEF. Normal cell morphology shown by phase-contrast before (Dox—) and after (Dox+) induction of GFP and CTCF. FLAG and CTCF immunofluorescence shown. (B) Western blot of representative transgenic clones shows dox-induction of CTCF-3xFLAG in d0 ES cells (left panel) and d1 female ES cells (right panel). (C) CTCF overexpression results in female-specific defect in outgrowth up to d13. Bright field images show EB outgrowth. GFP indicates transgene (CTCF) overexpression. Note GFP-positive cells in male, but not female, outgrowth in Dox+ condition. After induction, GFP signals are confined to the EB center. (D) Female control, ctrl#5, carries a silenced transgene and grows normally, whereas overexpressers, clones #2 and #1, fail to outgrow. d5 images shown.
We next performed Xist RNA-DNA FISH and found that CTCF-overexpression significantly affected Xist upregulation. Whereas 37%–43% of uninduced cells displayed full Xist clouds by d6, only 7%–8% of CTCF-overexpressing cells showed robust Xist clouds (Figure 4A). Quantitative RT-PCR confirmed a failure to fully upregulate Xist, despite progression through cell differentiation as indicated by normal levels of Oct4 and Bmp4 messenger RNA (mRNA) (Figure 4A). Collectively, these findings demonstrate that CTCF overexpression blunts Xist upregulation and argue that CTCF is a blocking factor for Xist. The proposed repressive effect of CTCF is consistent with the observation that knocking down CTCF results in Xist upregulation (Donohoe et al., 2007).
Figure 4. CTCF Overexpression Inhibits Xist Induction in a Temporally Sensitive Manner.
(A) CTCF overexpression blocks Xist upregulation. Left panel: RNA-DNA FISH on d6 female transgenic cells±Dox. Green, Xist RNA. Red, Xiclocus. Middle panel: Quantitation of Xist clouds ± Dox on d6 for two clones. Only full Xist clouds are scored (not pinpoint signals). Right panel: qRT-PCR on d4 cells for Oct4, Bmp4, and Xist RNA. Averages ± SE. (B) Sensitive time window between d2–d4 of EB differentiation. Dox is applied on the days indicated (red bar) during time course analysis. Phase contrast and corresponding GFP images are shown for d11.
We then asked if CTCF's effects were restricted to the developmental window of Xist upregulation. To perform a time course analysis, we applied dox at various time intervals and observed large differences in relation to specific periods of induction (Figure 4B). As expected, dox induction between d0–d11 caused scant EB outgrowths, whereas untreated EB exhibited robust outgrowth. When dox was applied only between d0–d2, outgrowth was slow to appear but recovered during the dox-free interval. When treatment was extended to d0–d4 or applied between d2–d11, outgrowth was severely restricted and never recovered. On the other hand, dox treatment after d4 had no effect. Thus, female ES cells are sensitive to CTCF overexpression only between d0 and d4, most acutely between d2–d4, the time frame corresponding to the initiation of XCI.
Jpx RNA Titrates CTCF Binding
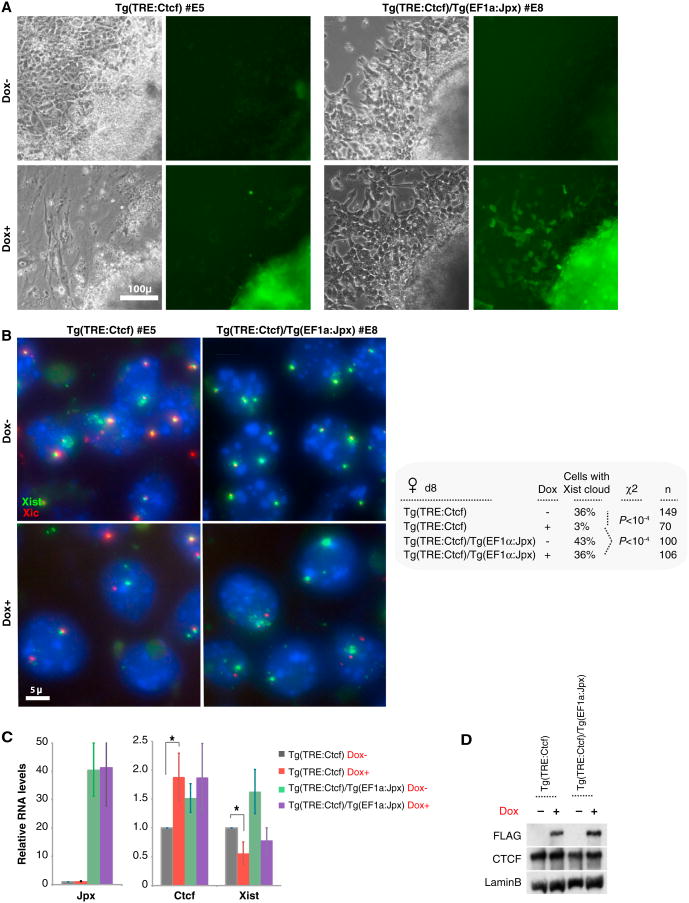
The fact that CTCF persisted at Xist P2 when Jpx is deficient (Figure 2E) hinted that CTCF and Jpx may be antagonistically linked. We asked if overexpressing Jpx could relieve the effect of CTCF overexpression by introducing the EF1α:Jpx transgene into CTCF-overexpressing female cells (Tg(EF1α:Jpx)/Tg(TRE:Ctcf)). Indeed, the outgrowth defect was reversed (Figure 5A, note GFP+ outgrowth), and normal numbers of full Xist clouds were restored, from 3% to 36% (Figure 5B). qRT-PCR and western analysis confirmed Jpx and CTCF overexpression (Figures 5C and 5D). An antagonistic relationship between Jpx and CTCF was also evident from ∼2-fold decreases in Xist levels when CTCF alone was overexpressed (Figure 5C); when Jpx alone was overexpressed, Xist levels increased ∼2-fold; and, finally, when CTCF and Jpx were both overexpressed, Xist was restored to wild-type levels. Therefore, Xist defects due to CTCF overexpression could be overcome by expressing more Jpx. These data indicate that Xist is controlled by a balance of Jpx and CTCF and that Jpx antagonizes CTCF by titrating CTCF's repressive effect on Xist.
Figure 5. Jpx RNA Neutralizes the Outgrowth Defect by Titrating CTCF.
(A) Overexpressing Jpx RNA rescues outgrowth defects caused by CTCF overexpression. Phase contrast and corresponding GFP signals shown on d8 of differentiation. (B) RNA-DNA FISH on d8. Green, Xist RNA; red, Xic locus. FISH counts are shown in the right panel. (C) qRT-PCR of Jpx, Xist, and Ctcf RNA on d8, normalized to Tg(TRE:Ctcf) Dox– (set to 1.0). Averages ± SE of triplicates. *p < 0.05 (one-way t test). (D) CTCF-FLAG induction shown by western blotting.
Jpx RNA Binds CTCF
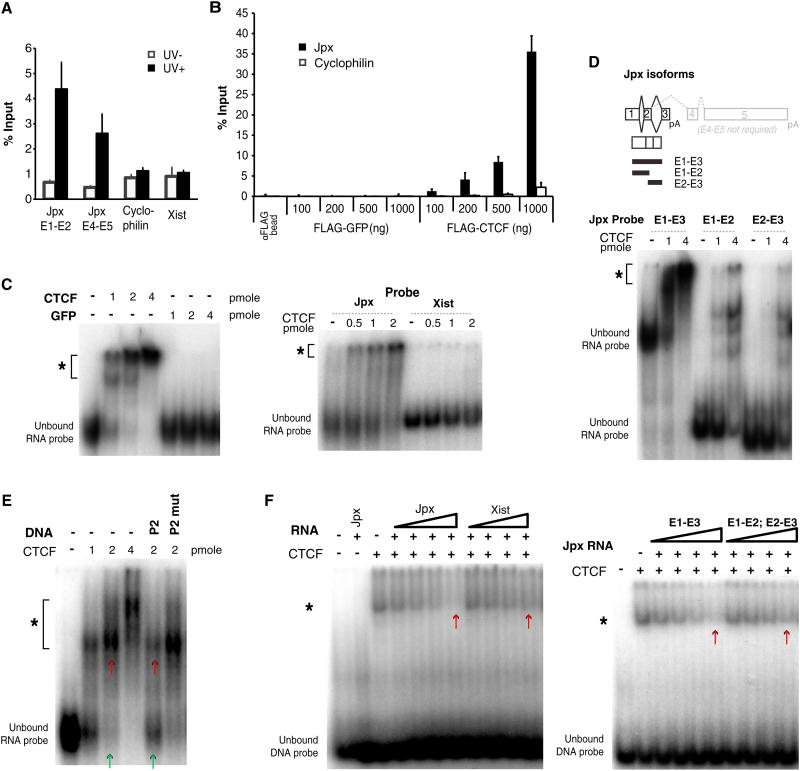
To understand the mechanism of titration, we asked if Jpx RNA and CTCF interact in vivo by performing UV crosslinked RNA immunoprecipitation (UV-RIP) with anti-FLAG antibodies in d12 female ES cells (Figure 6A). Jpx RNA showed significant coimmunoprecipitation with FLAG-CTCF, whereas cyclophilin and Xist RNA did not. When RIP was performed without UV crosslinking, Jpx enrichment was not observed, demonstrating that stringent conditions enabled RNA pull-down only when Jpx and CTCF were crosslinked. Given that UV crosslinks RNA to protein only when in direct contact, these data suggest that Jpx directly interacts with CTCF in vivo.
Figure 6. Jpx RNA Binds to CTCF and Extricates CTCF from the Xist Promoter.
(A) UV-RIP using anti-FLAG antibodies, followed by qRT-PCR in d12 female ES cells. qRT-PCR was performed on Jpx exons 1–2 (E1–E2), exons 4–5 (E4–E5), Cyclophilin control, and Xist. UV-negative controls were processed in parallel. Averages ± SE. (B) In vitro RNA pull-down using total RNA extracted from wild-type female MEFs. Purified FLAG-GFP or -CTCF was incubated with RNA. Pull-downs quantified by qRT-PCR. Averages ± SE. (C) RNA EMSA. *, RNA-protein shift. Left panel: Jpx RNA probe (383 nt) with increasing amounts of purified CTCF or GFP. Right panel: CTCF with Jpx versus 383 nt Xist control probe. (D) RNA EMSA using Jpx isoforms. E1–E3, 383 nt (full length). Truncated E1–E2, 220 nt, or E2–E3, 183 nt. E4–E5 are alternative 3′ ends, which are not required for Xist activation (Lee et al., 1999). (E) Titration EMSA shows that Jpx RNA and P2 DNA compete for binding to CTCF. A 0.5 pmol of Jpx probe, with 2 pmol cold P2 or P2 mut 80 bp DNA competitor. *, RNA-protein shift. Compare lanes marked by green/red arrows. (F) Reciprocal titration: P2 DNA and Jpx RNA compete for binding to CTCF. CTCF and P2 DNA probe were mixed with cold RNA competitor at 0.1, 0.2, 0.4, and 0.8 μg. *, DNA-protein shift. Left panel: With cold Jpx RNA or Xist RNA competitor. Right panel: With full-length Jpx versus truncated Jpx RNA competitor. Compare lanes marked by red arrows.
We then investigated Jpx-CTCF interactions in vitro. RNA pull-down assays using purified FLAG-CTCF showed that CTCF preferentially interacted with Jpx RNA among total cellular RNA extracted from female MEF (Figure 6B). Conversely, Jpx RNA preferentially bound CTCF over control FLAG-GFP. Increasing CTCF levels resulted in greater pull-down of Jpx RNA, demonstrating that the CTCF-Jpx interaction is dose dependent. Jpx RNA has a number of alternative splice forms (Chureau et al., 2002; Johnston et al., 2002; Chow et al., 2003), but genetic analysis indicates that only the region spanning exons 1–3 (E1–E3) is required for function (Lee et al., 1999). In an electrophoretic mobility shift assay (EMSA) using purified recombinant protein, the 383 nt E1-E3 isoform of Jpx was robustly shifted by CTCF, but not GFP (Figure 6C). Increasing CTCF resulted in greater binding to Jpx but not an Xist RNA control of the same size, indicating a specific, direct, and dose-dependent interaction between CTCF and Jpx. The strong shift required full-length E1–E3, as CTCF only weakly shifted E1–E2 and E2–E3 isoforms (Figure 6D). We conclude that CTCF directly binds Jpx RNA.
Jpx Activates the Xist Promoter by Titrating Away CTCF Binding
The physical interaction (Figures 6A–6D) and opposing effects (Figure 5) of Jpx and CTCF raised the possibility that Jpx could titrate CTCF from Xist P2 and, in so doing, activate Xist. Interestingly, EMSA revealed that Jpx RNA and P2 DNA compete for binding to CTCF. In an RNA EMSA, the Jpx-CTCF shift was diminished by adding cold P2 DNA (Figure 6E). This effect was attenuated by mutating the P2 DNA competitor. In the reciprocal DNA EMSA, the P2-CTCF shift was progressively titrated away by increasing amounts of Jpx RNA (Figure 6F, left panel), with intact E1-E3 being required for maximal effect (Figure 6F, right panel). Thus, Jpx RNA and P2 DNA compete for CTCF binding.
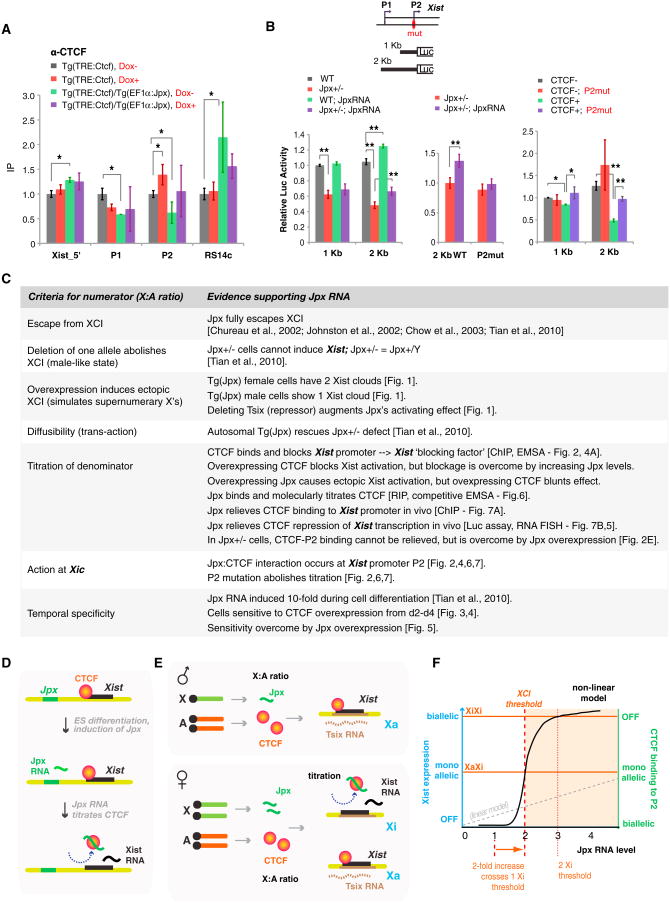
To determine whether the competition results in titration of CTCF-P2 binding in vivo, we performed CTCF ChIP at the Xist promoter and asked how CTCF binding is affected by Jpx or CTCF levels (Figure 7A). When CTCF alone was overexpressed, P2 binding was significantly boosted (red) and Xist upregulation was compromised (Figures 4A and 5B). When Jpx alone was overexpressed, CTCF binding at P2 was reduced approximately by half (green), consistent with titration at one Xist allele and ectopic Xist upregulation (Figure 1). When Jpx and CTCF were overexpressed together (purple), P2 binding was restored. Therefore, Jpx indeed titrates CTCF binding at the Xist P2 promoter.
Figure 7. Jpx RNA and CTCF Titrate Each Other at the Xist Promoter In Vivo.
(A) In vivo titration shown by CTCF ChIP analysis on transgenic female ES cells at d0. Enrichment values were normalized against H19 control and compared to Tg(TRE:Ctcf) Dox– (set to 1.0) for each site using a one-way t test (*p < 0.05). At least three independent experiments were performed for each cell line. Averages ± SE. (B) Titration and modulation of Xist P1+P2 transcription shown by luciferase assays in d4 female ES cells of indicated genotypes. Map of Xist's 5′ end is shown with luciferase constructs. Left panel: Jpx overexpression achieved by Tg(EF1 a:Jpx) in Jpx+/— mutants rescues Xist promoter activity. Middle panel: Rescue is abolished by mutating P2. Right panel: Transcription repression caused by overexpressing CTCF is relieved by mutating P2. CTCF+, CTCF overexpression induced by Dox via Tg(TRE:Ctcf). At least two independent transfections and ≥4 independent luciferase measurements were performed for each experiment. Averages ± SE. *p < 0.05. **p < 0.01. (One-tail t tests in the pairwise comparison indicated.) (C) Summary of data supporting the Jpx-CTCF titration model. (D) Model: Jpx RNA activates Xist by extricating CTCF from the Xist promoter. (E) X:A titration: BF, blocking factor. CF, competence factor. The precise composition of BF and its Xic binding site is currently unknown. CTCF may be a component of BF. Jpx forms CF only when present in 2-fold excess. (F) Nonlinear dynamics would be a necessary property of the titration model, most probably effected by highly cooperative interactions. A sigmoidal binding curve would enable small changes in factor concentration to elicit large biological effects (e.g., all or none binding), in a way not possible with linear dynamics (broken gray line).
To investigate how the CTCF-Jpx titration affects Xist promoter activity, we carried out luciferase (Luc) reporter assays after modulating CTCF and Jpx levels (Figure 7B). Luciferase constructs fused to P1 and/or P2 were introduced into female ES cells and tested during differentiation when Jpx levels normally rise. When introduced into Jpx+/− females, luciferase expression was reduced to half that of wild-type (WT) females (Figure 7B, left panel, black versus red), consistent with Jpx+/− cells being male-like and incapable of supporting Xist upregulation. This defect was overcome by transgenically expressing Jpx RNA (Figure 7B, middle panel). This rescue depended on an intact P2 promoter, as mutating P2 abolished the rescue (P2mut; red versus purple). Finally, Xist promoter activity was repressed by CTCF overexpression (Figure 7B, right panel, black versus green), and the repression could be overcome by mutating P2 (green versus purple). Although the effects were statistically significant with P2 alone (1 kb), the combination of P1+P2 (2 kb) was more robust, suggesting that P1 and P2 together enabled full regulation. We conclude that competitive interactions between CTCF, Jpx RNA, and the Xist promoter regulate Xist transcriptional induction.
Discussion
We have shown that Xist transcription is controlled by adynamic balance of CTCF and Jpx RNA. Our data argue that CTCF is a blocking factor for the Xist promoter, that promoter blockage is relieved by Jpx-mediated eviction of CTCF, and that promoter P2 is the primary site of action. Jpx meets several conceptual criteria for a numerator of the X:A ratio. Jpx resides at the Xic and escapes XCI (Chureau et al., 2002; Johnston et al., 2002; Chow et al., 2003; Tian et al., 2010). Jpx RNA is also diffusible and trans-acting (Tian et al., 2010), two properties that facilitate titration. Moreover, Jpx RNA acts directly at the Xic, specifically at the Xist promoter, where it titrates away CTCF to induce Xist transcription.
The titration model centered on Jpx-CTCF-P2 interactions is supported by experimental observations (summarized in Figure 7C). In wild-type cells, Jpx is expressed at ∼2-fold greater levels in female cells than in male cells (Figure S1B). Deleting a single allele of Jpx in females abolishes Xist upregulation and simulates the XY state (Tian et al., 2010). Conversely, overexpressing Jpx induces ectopic Xist expression, mimicking the presence of additional X chromosomes. Modulating levels of the autosomal factor, CTCF, likewise affects Xist induction. Overexpressing CTCF blocks Xist activation, but this blockage is overcome by increasing Jpx RNA levels. Conversely, ectopic Xist activation caused by Jpx overexpression is blunted by overexpressing CTCF. Furthermore, Xist promoter repression by CTCF cannot be relieved in Jpx+/− cells, but the defect is rescued by exogenous Jpx.
Some curious aspects of our findings are the locus-specific and allelic nature of the Jpx-CTCF-P2 interactions. Because CTCF is ubiquitous, specificity for P2 binding must involve additional factors and proper context. Similarly, Jpx's exclusivity for CTCF bound to P2 must be influenced by proper context. Furthermore, the monoallelic nature of the titration under physiological conditions is likely linked to the X:A ratio. We suggest that Jpx is a component of the competence factor proposed in the Two Factors Model, which postulates that—in addition to an autosomal blocking factor—an X-linked competence factor arises only when X:A ≥1.0 and is essential for inducing Xist (Lee and Lu, 1999; Lee, 2005). Our data suggest that CTCF is a denominator and subunit of the blocking factor. In support of this, overexpression blocks Xist P2 activity, knockdown results in increased Xist expression (Donohoe et al., 2007), and binding to P2 is anticorrelated with Xist expression. In our model, the Jpx:CTCF ratio induces Xist transcription only when a critical threshold is exceeded (Figures 7E and 7F). When expressed from a single X (XY state), Jpx concentrations fail to reach threshold. When expressed from two X's (XX state), Jpx concentrations exceed threshold and result in extrication of CTCF from one Xist allele. Overexpression of Jpx (e.g., in XXX or Tg(Jpx) cells) would result in crossing of a second threshold, leading to eviction of CTCF from both Xist promoters and to biallelic Xist activation.
The all-or-none dynamics of this type of stoichiometric interaction would require a robust “biphasic switch,” as proposed for other bimodal gene expression responses (Cline and Meyer, 1996; Ptashne, 2011; Gutierrez et al., 2012). Nonlinear dynamics, such as those resulting from highly cooperative interactions, would be a necessary property of the Jpx-CTCF-P2 interactions. Nonlinear dynamics enable small changes in factor concentration to elicit large biological effects (e.g., all-or-none binding and transcription induction) in a way that is not possible with linear dynamics (Figure 7F). This concept is exemplified by the classic biphasic switch of lambda phage. The all-or-none effects of cro activation is based on cooperative binding of multiple λ repressors to three operators within the cro promoter (Ptashne, 2011). At the 5′ end of Xist, multiple sites for CTCF and other factors (e.g., YY1) occur, though the potential for coooperativity has not been tested.
Long noted for an abundance of lncRNA, the Xic is now known to regulate XCI through a series of RNA-protein interactions (Lee, 2011). In addition to Xist-Polycomb (Zhao et al., 2008) and Xist-YY1 interactions (Jeon and Lee, 2011) for the initiation of silencing, here we have uncovered Jpx-CTCF interactions for molecular titration. Like YY1, CTCF is a “bivalent” protein, capable of binding both RNA and DNA. Interestingly, CTCF was previously found to be in a larger complex containing SRA (Yao et al., 2010). Perhaps, bivalency of other chromatin factors will also emerge in time as key determinants of epigenetic regulation.
Experimental procedures
Transgenic ES Cell Lines
Tg(Jpx) and Tg(EF1α:Jpx) containing full-length Jpx and Neo marker were made by ET-cloning (Yang and Seed, 2003) from BAC 399K20 and pEF1/ V5-His vector (Invitrogen). The Neo vector was used as control. For bidirectional inducible CTCF and EGFP expression, a Hygro cassette flanked by SV40 promoter and polyA was inserted into pTRE-Tight-Bi (Clontech) using PciI; EGFP was inserted using BglII; 3xFLAG cassette was inserted into NotI-SalI sites; and CTCF complementary DNA (cDNA) was cloned in-frame using MluI and NotI. Wild-type J1 male and 16.7 female ES cells (Lee and Lu, 1999) and TsixTST/Y and TsixTST/+ ES cells (Ogawa and Lee, 2003) have been described previously. Ainv15 male ES cells carrying the tet-activator rtTA were a gift from G. Daley (Kyba et al., 2002). F1-2.1 female ES cells carrying rtTA were a gift from R. Jaenisch (Luikenhuis et al., 2001). Transgenic ES cells were generated by electroporation (Bio-Rad Genepulser) with stable clones picked after 8–11 days under G418 (400 μg/ml), hygromycin B (200 μg/ml), or puromycin (1 μg/ml) selection. Autosomal integration was confirmed by DNA FISH. Doxycycline induction was performed at 1 μg/ml.
RNA/DNA FISH and Immunostaining
Simultaneous RNA-DNA FISH has been described previously (Lee and Lu, 1999). Immuno-FISH was performed as described previously (Zhang et al., 2007). Cells were blocked with PBS, 0.2% Tween20, and 1% BSA. Anti-CTCF (Cell Signaling D31H2) and anti-FLAG M2 (Sigma F1804) were diluted 1:1,000 and incubated at room temperature for 1–2 hr.
qRT-PCR
Total RNA was extracted using TRIzol (Invitrogen), treated with TURBO DNase (Ambion), and reverse transcribed using SuperScript III (Invitrogen) and random primers (Promega). Quantitative PCR was performed using iQ SYBR Green Supermix on a CFX96 (Bio-Rad). PCR primers are as follows.
Jpx: e1-F and e1-R (Tian et al., 2010); mJpx76+(ex1), 5′-TTAGCCA GGCAGCTAGAGGA-3′, and mJpx225-(ex2), AGCCGTATTCCTCCATGGTT. Xist: NS33 and NS66 (Stavropoulos et al., 2001). Oct4: BD151 GGACATGAAAGCCCTGCAGAAGG and BD152 CGAAGCGACAGATGGTGGTCTGGC. Bmp4: BD 172 CTGGACACCTCATCACACGACTACTGG and BD173 GGG CCCAATCTCCACTCCCTTGAGG. Ctcf: BD79 ATCGTAgctagcATGGAAGGT GAGGCGGTTGAAGCC and BD6 tgacatcctggaccacctcaccc. Gapdh: Gapdh F and R (Jeon and Lee, 2011).
FLAG-CTCF and FLAG-GFP Purification
Ctcf or GFP cDNA (pFA6a-GFP(S65T)-kanMX6) with C-terminal 6xHis tag was cloned into pFLAG-2 (Sigma-Aldrich) using EcoRI and XhoI. FLAG-CTCF-6xHis and FLAG-GFP-6xHis proteins were purified from Rosetta-Gami B cells (Novagen). Briefly, cells were induced with 0.2 mM IPTG at 18°C (CTCF) or 1 mM IPTG (GFP) at 30°C and lysed at 4°C with 50 mM sodium phosphate (pH 8.0), 300 mM NaCl, 20 mM imidazole, 0.5% Sarkosyl, and protease inhibitors. Triton X-100 was added to 2% final [v/v]; debris was removed by centrifugation; and proteins were eluted from Ni-NTA resin (QIAGEN) with 50 mM sodium phosphate (pH 8.0), 300 mM NaCl, and 250 mM imidazole. Eluates were dialyzed against 50 mM Tris-HCl (pH 7.5), 2.5 mM MgCl2, 150 mM NaCl, 0.1 mM ZnSO4, 1 mM DTT, 0.1% Tween-20, and 10% glycerol.
DNA and RNA EMSA
For DNA EMASA, these double-stranded 80-mer DNA oligonucleotides were used:
Xist_5′: TTTGGCTTCAGATGCCTTGGAAAGATTCATGCTGCCACCTGCTG GTTTATTCAGTCTGTGTGCATCATTTATTTACTTGT; Xist_5′mut:
TTTGGCTTCAGATGCCTTGGAAAGATTCATGCTGCTACTTGCTAGTTTAT TCAGTCTGTGTGCATCATTTATTTACTTGT; P1:
TTGTGGCCACTCCTCTTCTGGTCTCTCCGCCTTCAGCGCCGCGGATCA GTTAAAGGCGTGCAACGGCTTGCTCCAGCCAT; P2:
GAATGTGTCTAAGATGGCGGAAGTCATGTGACCTGCCCTCTAGTGGT TT CTTTCAGTGATTTTTTTTTTGGCGGGCT TTA; P2 mut:
GAATGTGTCTAAGATGGCGGAAGTCATGTGACCTGTCCTATAGTTGTTTC TTTCAGTGAT TTTTTTTTTGGCGGGCTT TA; and
RS14C: CTATGCATTAGGTTTGGGTGTTATACCCGTGTAGGCCAGCAGA GGGTGTCGGATCCCAAGGAAACCAAGTTACAGACGCC.
To anneal forward and reverse strands, oligos were heated to 95°C and slow-cooled to room temperature in 10 mM Tris-HCl (pH 8.3), 1.5 mM MgCl2, and 50 mM KCl. Double-stranded DNA probes were end-labeled with ATP[γ-32P] and unincorporated ATP removed with Microspin G-50 columns (GE Healthcare). A 2 pmol of recombinant proteins were incubated at room temperature (RT) with 1 pmol of probes in 1 mg poly dI-dC, 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 5 mM MgCl2, 0.1 mM ZnSO4, 1 mM DTT, 10% glycerol, and 0.1% Tween-20. Samples were resolved at RT by TBE-6% or 7% PAGE gel.
RNA EMSA was performed as described previously (Cifuentes-Rojas et al., 2011). RNA probe was in vitro transcribed with T7 RNA polymerase (Ambion) off PCR-amplified cDNA templates created using
T7_Jpx_ex1F, TAATACGACTCACTATAGACGGCACCACCAGGCTTCT; Jpx_ex3R, GAGT TTATTTGGGCTTACAGTTC (Johnston et al., 2002); Jpx_ex2R, AGCCGTATTCCTCCATGGTT; T7_Jpx_ex2F, TAATACGACTCAC TATAGAACCATGGAGGAATACGGCT; T7_Xist_F, TAATACGACTCACTATA GCTACTTACACCTTGGCCTC; and Xist_R, AAGTAATTTGAGTATCAT CTGCC.
In-vitro-transcribed RNA was DNased (TURBO), TRIzol-purified, phosphatased (Alkaline Phosphatase, New England Biolabs), end-labeled with ATP [γ-32P], and purified through Microspin G-50 columns. RNA probe was denatured at 95°C for 2 min and incubated at 50°C for 5 min, 37°C for 15 min, and RT for 15 min, cooled, and maintained in folding buffer (50 mM NaCl, 2 mM MgCl2) on ice prior to the binding reaction. Recombinant proteins were incubated at room temperature with 0.5 pmol RNA probes for 30 min in 10 ml binding buffer containing 1 mg poly dI-dC, 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 5 mM MgCl2, 0.1 mM ZnSO4, 1 mM DTT, 10% glycerol, 0.1% Tween-20, 0.1 mM polyamine, 8 U Rnase Inhibitor (Roche), and 1 mg Yeast transfer RNA (tRNA). Samples were resolved at 4°C by TBE- 5% or 6% PAGE and imaged on BioMaxfilm (Kodak). In DNA-RNA titration EMSA, protein was incubated with DNA competitors prior to adding RNA probes. For RNA-DNA titration experiments, RNA competitors were mixed with protein and DNA probes in RNA EMSA binding buffer, including RNase inhibitor and yeast tRNA.
Chromatin Immunoprecipitation
ChIP was performed as previously described (Blais et al., 2005), with modifications. Approximately 1.5 million ES cells were crosslinked in 1% formaldehyde at RT for 10 min and quenched with glycine (125 mM final concentration) for 5 min at RT. Chromatin was sonicated using Bioruptor XL (Diagenode), treated with RNase A (0.2 μg/ml) at 37°C for 30 min, and 3 mg of anti-CTCF (Cell Signaling 2899) or IgG (Cell Signaling 2729) were incubated with chromatin at 4°C overnight. Protein-antibody complexes were recovered with Dynalbeads Protein G (Invitrogen), DNA was extracted with phenol/chloroform, and quantitative PCR was performed using these primers:
Rnf12_F, CCCCAGGTGAAAGTACTGAGG; Rnf12_R, CTCTCCAGCTC TATTTTCATCG; Xist_5′F, CCCTACCTGAACCACCTCAATAGT; Xist_5′R, AGTTCCCTTTAGGCGTCCCAT (Navarro et al., 2006); P1_F, ACGCGTCATGTCACTGAGC; P1_R, AAAACGTCAAAAATCTCGTGG; P2_F, CTCGACAGCC CAATCTTTGTT; P2_R, ACCAACACTTCCACTTAGCC (Jeon and Lee, 2011); RS14c_F, GGCTACACACAAGATGGCGTCTGTAACTTG; RS14c_R, TAGGTTTGGGTGTTATACCCGTGTAGGC (Spencer et al., 2011); H19_F, GTCACTCAGGCATAGCATTC; and H19_R, GTCTGCCGAGCAATATGTAG.
Allele-specific quantitative PCR at P2 was performed using primers 231_F, TGTGCATTATTGCTTGGTGGCCAG and 371cas_R, TGCGCTCTCCCGA CACTTC or 129_R, CATGCGCTCTCCCGACCTG.
RNA Immunoprecipitation
Crosslink RNA immunoprecipitation (RIP) was performed as previously described (Jeon and Lee, 2011) on 107 Tg(TRE:Ctcf) ES cells differentiated in the presence of 1 μg/ml doxycycline for 12 days. Cells were UV crosslinked at 254 nm(200 J/cm2) in PBS, collected by scraping, and lysed in PBS supplemented with 0.5% NP-40, 0.5% sodium-deoxycholate, RNase inhibitor, and proteinase inhibitor cocktail at 4°C for 30 min. After 15 min at 37°C with 30 U of TURBO DNase, cell debris was removed, and supernatant was used for immunoprecipitation using Flag-agarose beads overnight at 4°C. Beads were washed three times with PBS containing 1% NP-40,0.5% sodium-deoxycholate, and 300 mM NaCl and were resuspended in TURBO DNase buffer with 10 U DNase, RNase inhibitor, and proteinase inhibitor cocktail for 30 min at 37°C. Beads were further washed twice in the abovementioned wash buffer containing 10 mM EDTA. RNA was eluted by incubating in proteinase K buffer (100 mM Tris-HCl [pH 7.5], 50 mM NaCl, 10 mM EDTA, 4 mg/ml proteinase K, and RNase inhibitor) for 20 min at 37°C. Elution was continued for another 20 min after adding 7 M urea. RNA was extracted using TRIzol LS and analyzed by qRT-PCR.
RNA Pull-Down Assay
Purified Flag-tagged CTCF and GFP proteins (200 ng or as indicated) were incubated with 10 μg total RNA from immortalized female MEFs in binding buffer (20 mM HEPES[pH7.5], 150mM NaCl, 0.1% NP-40,1 mM AEBSF, protease inhibitor cocktail, and RNase inhibitor). After 30 min at 4°C, Flag-agarose beads were added for another 30 min. Beads were captured, washed twice with binding buffer, and washed twice with wash buffer (20 mM HEPES [pH7.5],350mM NaCl, 0.1% NP-40,1 mM AEBSF, protease inhibitor cocktail, and RNase inhibitor). RNA was extracted with TRIzol, and quantitative PCR was performed using SYBRGreen or Taqman primers. Relative abundance of Jpx and a housekeeping mRNA, cyclophilin A, was normalized to input. Primers are as follows: mJpx76+(ex1), mJpx225-(ex2), and mJpx339+(ex4), GGGCTCAACAAAAGAGCAAA; mJpx607-(ex5), TGGAGTTATGGCCTCG AAGT; CycophilinA_F, CGATGACGAGCCCTTGG; CycophilinA_R, TCTG CTGTCTTTGGAACTTTGTC; CycophilinA_probe, FAM-CGCGTCTCCTTTGAGCTGTTTGCA-TAMRA.
Luciferase Assay
A 1 kb NheI-PmlI fragment containing Xist P2 was cloned into pGL4.14 vector (Promega) to make 1 Kb-Luc, and a 2 kb MluI-PmlI fragment, including Xist P1 and P2, was cloned to make 2 Kb-Luc. P2 mutation was introduced using QuikChange Multi Site-Directed Mutagenesis Kit (Agilent). Promoterless vector plasmid pGL4.14 served as control. Dual-Glo luciferase assay (Promega) was used with Renilla pGL4.74 as control reporter. Transient transfections were performed using Lipofectamin 2000 (Invitrogen), with plasmids linearized by PstI or PvuI; a 2.5 μg of luciferase construct of 1 Kb-Luc, or 2 Kb-Luc, or promoterless vector, was cotransfected with 0.15μg Renilla pGL4.74 using 8μl Lipofectamin into 106 female ES cells. For overexpressing Jpx, Jpx exons 1–3 were fused to the EF1 α promoter in pEF1/V5-His and cotransfected into d3 female ES cells. pEF1/V5-His transfection was used as control. Jpx overexpression and Luciferase activity were analyzed 24–36 hr posttransfection. For overexpressing CTCF, Tg(TRE:Ctcf) female ES cells were transfected at d2 with Doxycycline (1 mg/ml) applied at d0. Luciferase activities were analyzed 36 hr posttransfection. Firefly and Renilla luminescence was measured on a TopCount NXT Luminescence Counter. Firefly/Renilla luminescence ratio was normalized against the promoterless control.
Supplementary Material
Acknowledgments
We are grateful to R. Jaenisch for the gift of F1-2.1 ES cells and G. Daley for Ainv15. We thank S. Pinter for sharing unpublished data, J. Ahn for sharing ChIP primers, B. Payer and D. Lessing for critically reading the manuscript, and all lab members for engaging discussions. This work is supported by a grant from the National Institutes of Health (RO1-GM58839 to J.T.L.). J.T.L. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Supplemental information: Supplemental Information includes one figure and can be found with this article online at http://dx.doi.org/10.1016/j.cell.2013.05.028.
References
- Augui S, Nora EP, Heard E. Regulation of X-chromosome inactivation by the X-inactivation centre. Nat Rev Genet. 2011;12:429–442. doi: 10.1038/nrg2987. [DOI] [PubMed] [Google Scholar]
- Barakat TS, Gunhanlar N, Pardo CG, Achame EM, Ghazvini M, Boers R, Kenter A, Rentmeester E, Grootegoed JA, Gribnau J. RNF12 activates Xist and is essential for X chromosome inactivation. PLoS Genet. 2011;7:e1002001. doi: 10.1371/journal.pgen.1002001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbash DA, Cline TW. Genetic and molecular analysis of the autosomal component of the primary sex determination signal of Drosophila melanogaster. Genetics. 1995;141:1451–1471. doi: 10.1093/genetics/141.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AC, West AG, Felsenfeld G. The protein CTCF is required for the enhancer blocking activity of vertebrate insulators. Cell. 1999;98:387–396. doi: 10.1016/s0092-8674(00)81967-4. [DOI] [PubMed] [Google Scholar]
- Blais A, Tsikitis M, Acosta-Alvear D, Sharan R, Kluger Y, Dynlacht BD. An initial blueprint for myogenic differentiation. Genes Dev. 2005;19:553–569. doi: 10.1101/gad.1281105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockdorff N, Ashworth A, Kay GF, McCabe VM, Norris DP, Cooper PJ, Swift S, Rastan S. The product of the mouse Xist gene is a 15 kb inactive X-specific transcript containing no conserved ORF and located in the nucleus. Cell. 1992;71:515–526. doi: 10.1016/0092-8674(92)90519-i. [DOI] [PubMed] [Google Scholar]
- Brown SW, Chandra HS. Inactivation system of the mammalian X chromosome. Proc Natl Acad Sci USA. 1973;70:195–199. doi: 10.1073/pnas.70.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CJ, Hendrich BD, Rupert JL, Lafrenière RG, Xing Y, Lawrence J, Willard HF. The human XIST gene: analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell. 1992;71:527–542. doi: 10.1016/0092-8674(92)90520-m. [DOI] [PubMed] [Google Scholar]
- Chow JC, Hall LL, Clemson CM, Lawrence JB, Brown CJ. Characterization of expression at the human XIST locus in somatic, embryonal carcinoma, and transgenic cell lines. Genomics. 2003;82:309–322. doi: 10.1016/s0888-7543(03)00170-8. [DOI] [PubMed] [Google Scholar]
- Chureau C, Prissette M, Bourdet A, Barbe V, Cattolico L, Jones L, Eggen A, Avner P, Duret L. Comparative sequence analysis of the X-inactivation center region in mouse, human, and bovine. Genome Res. 2002;12:894–908. doi: 10.1101/gr.152902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifuentes-Rojas C, Kannan K, Tseng L, Shippen DE. Two RNA subunits and POT1a are components of Arabidopsis telomerase. Proc Natl Acad Sci USA. 2011;108:73–78. doi: 10.1073/pnas.1013021107. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Cline TW, Meyer BJ. Vive la différence: males vs females in flies vs worms. Annu Rev Genet. 1996;30:637–702. doi: 10.1146/annurev.genet.30.1.637. [DOI] [PubMed] [Google Scholar]
- Donohoe ME, Zhang LF, Xu N, Shi Y, Lee JT. Identification of a Ctcf cofactor, Yy1, for the X chromosome binary switch. Mol Cell. 2007;25:43–56. doi: 10.1016/j.molcel.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Donohoe ME, Silva SS, Pinter SF, Xu N, Lee JT. The pluripotency factor Oct4 interacts with Ctcf and also controls X-chromosome pairing and counting. Nature. 2009;460:128–132. doi: 10.1038/nature08098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essien K, Vigneau S, Apreleva S, Singh LN, Bartolomei MS, Hannenhalli S. CTCF binding site classes exhibit distinct evolutionary, genomic, epigenomic and transcriptomic features. Genome Biol. 2009;10:R131. doi: 10.1186/gb-2009-10-11-r131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartler SM, Riggs AD. Mammalian X-chromosome inactivation. Annu Rev Genet. 1983;17:155–190. doi: 10.1146/annurev.ge.17.120183.001103. [DOI] [PubMed] [Google Scholar]
- Gartler SM, Varadarajan KR, Luo P, Norwood TH, Canfield TK, Hansen RS. Abnormal X: autosome ratio, but normal X chromosome inactivation in human triploid cultures. BMC Genet. 2006;7:41. doi: 10.1186/1471-2156-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gontan C, Achame EM, Demmers J, Barakat TS, Rentmeester E, van IJcken W, Grootegoed JA, Gribnau J. RNF12 initiates X-chromosome inactivation by targeting REX1 for degradation. Nature. 2012;485:386–390. doi: 10.1038/nature11070. [DOI] [PubMed] [Google Scholar]
- Gutierrez PS, Monteoliva D, Diambra L. Cooperative binding of transcription factors promotes bimodal gene expression response. PLoS ONE. 2012;7:e44812. doi: 10.1371/journal.pone.0044812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon Y, Lee JT. YY1 tethers Xist RNA to the inactive X nucleation center. Cell. 2011;146:119–133. doi: 10.1016/j.cell.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston CM, Newall AE, Brockdorff N, Nesterova TB. Enox, a novel gene that maps 10 kb upstream of Xist and partially escapes X inactivation. Genomics. 2002;80:236–244. doi: 10.1006/geno.2002.6819. [DOI] [PubMed] [Google Scholar]
- Jonkers I, Barakat TS, Achame EM, Monkhorst K, Kenter A, Rentmeester E, Grosveld F, Grootegoed JA, Gribnau J. RNF12 is an X-encoded dose-dependent activator of X chromosome inactivation. Cell. 2009;139:999–1011. doi: 10.1016/j.cell.2009.10.034. [DOI] [PubMed] [Google Scholar]
- Kay GF, Barton SC, Surani MA, Rastan S. Imprinting and X chromosome counting mechanisms determine Xist expression in early mouse development. Cell. 1994;77:639–650. doi: 10.1016/0092-8674(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Keane TM, Goodstadt L, Danecek P, White MA, Wong K, Yalcin B, Heger A, Agam A, Slater G, Goodson M, et al. Mouse genomic variation and its effect on phenotypes and gene regulation. Nature. 2011;477:289–294. doi: 10.1038/nature10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyba M, Perlingeiro RC, Daley GQ. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002;109:29–37. doi: 10.1016/s0092-8674(02)00680-3. [DOI] [PubMed] [Google Scholar]
- Lee JT. Regulation of X-chromosome counting by Tsix and Xite sequences. Science. 2005;309:768–771. doi: 10.1126/science.1113673. [DOI] [PubMed] [Google Scholar]
- Lee JT. Gracefully ageing at 50, X-chromosome inactivation becomes a paradigm for RNA and chromatin control. Nat Rev Mol Cell Biol. 2011;12:815–826. doi: 10.1038/nrm3231. [DOI] [PubMed] [Google Scholar]
- Lee JT, Lu N. Targeted mutagenesis of Tsix leads to nonrandom X inactivation. Cell. 1999;99:47–57. doi: 10.1016/s0092-8674(00)80061-6. [DOI] [PubMed] [Google Scholar]
- Lee JT, Lu N, Han Y. Genetic analysis of the mouse X inactivation center defines an 80-kb multifunction domain. Proc Natl Acad Sci USA. 1999;96:3836–3841. doi: 10.1073/pnas.96.7.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchesi JC, Kelly WG, Panning B. Chromatin remodeling in dosage compensation. Annu Rev Genet. 2005;39:615–651. doi: 10.1146/annurev.genet.39.073003.094210. [DOI] [PubMed] [Google Scholar]
- Luikenhuis S, Wutz A, Jaenisch R. Antisense transcription through the Xist locus mediates Tsix function in embryonic stem cells. Mol Cell Biol. 2001;21:8512–8520. doi: 10.1128/MCB.21.24.8512-8520.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon MF. Possible mechanisms of X chromosome inactivation. Nat New Biol. 1971;232:229–232. doi: 10.1038/newbio232229a0. [DOI] [PubMed] [Google Scholar]
- Meyer BJ. Targeting X chromosomes for repression. Curr Opin Genet Dev. 2010;20:179–189. doi: 10.1016/j.gde.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro P, Page DR, Avner P, Rougeulle C. Tsix-mediated epigenetic switch of a CTCF-flanked region of the Xist promoter determines the Xist transcription program. Genes Dev. 2006;20:2787–2792. doi: 10.1101/gad.389006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y, Lee JT. Xite, X-inactivation intergenic transcription elements that regulate the probability of choice. Mol Cell. 2003;11:731–743. doi: 10.1016/s1097-2765(03)00063-7. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Sun BK, Lee JT. Intersection of the RNA interference and X-inactivation pathways. Science. 2008;320:1336–1341. doi: 10.1126/science.1157676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson R, Renkawitz R, Lobanenkov VV. CTCF is a uniquely versatile transcription regulator linked to epigenetics and disease. Trends Genet. 2001;17:520–527. doi: 10.1016/s0168-9525(01)02366-6. [DOI] [PubMed] [Google Scholar]
- Ohno S. Evolution of sex chromosomes in mammals. Annu Rev Genet. 1969;3:495–524. [Google Scholar]
- Payer B, Lee JT. X chromosome dosage compensation: how mammals keep the balance. Annu Rev Genet. 2008;42:733–772. doi: 10.1146/annurev.genet.42.110807.091711. [DOI] [PubMed] [Google Scholar]
- Ptashne M. Principles of a switch. Nat Chem Biol. 2011;7:484–487. doi: 10.1038/nchembio.611. [DOI] [PubMed] [Google Scholar]
- Pugacheva EM, Tiwari VK, Abdullaev Z, Vostrov AA, Flanagan PT, Quitschke WW, Loukinov DI, Ohlsson R, Lobanenkov VV. Familial cases of point mutations in the XIST promoter reveal a correlation between CTCF binding and pre-emptive choices of X chromosome inactivation. Hum Mol Genet. 2005;14:953–965. doi: 10.1093/hmg/ddi089. [DOI] [PubMed] [Google Scholar]
- Sado T, Wang Z, Sasaki H, Li E. Regulation of imprinted X-chromosome inactivation in mice by Tsix. Development. 2001;128:1275–1286. doi: 10.1242/dev.128.8.1275. [DOI] [PubMed] [Google Scholar]
- Salz HK, Erickson JW. Sex determination in Drosophila: the view from the top. Fly (Austin) 2010;4:60–70. doi: 10.4161/fly.4.1.11277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheardown SA, Duthie SM, Johnston CM, Newall AE, Formstone EJ, Arkell RM, Nesterova TB, Alghisi GC, Rastan S, Brockdorff N. Stabilization of Xist RNA mediates initiation of X chromosome inactivation. Cell. 1997;91:99–107. doi: 10.1016/s0092-8674(01)80012-x. [DOI] [PubMed] [Google Scholar]
- Shin J, Bossenz M, Chung Y, Ma H, Byron M, Taniguchi-Ishigaki N, Zhu X, Jiao B, Hall LL, Green MR, et al. Maternal Rnf12/RLIM is required for imprinted X-chromosome inactivation in mice. Nature. 2010;467:977–981. doi: 10.1038/nature09457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer RJ, del Rosario BC, Pinter SF, Lessing D, Sadreyev RI, Lee JT. A boundary element between Tsix and Xist binds the chromatin insulator Ctcf and contributes to initiation of X-chromosome inactivation. Genetics. 2011;189:441–454. doi: 10.1534/genetics.111.132662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starmer J, Magnuson T. A new model for random X chromosome inactivation. Development. 2009;136:1–10. doi: 10.1242/dev.025908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavropoulos N, Lu N, Lee JT. A functional role for Tsix transcription in blocking Xist RNA accumulation but not in X-chromosome choice. Proc Natl Acad Sci USA. 2001;98:10232–10237. doi: 10.1073/pnas.171243598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun BK, Deaton AM, Lee JT. A transient heterochromatic state in Xist preempts X inactivation choice without RNA stabilization. Mol Cell. 2006;21:617–628. doi: 10.1016/j.molcel.2006.01.028. [DOI] [PubMed] [Google Scholar]
- Tian D, Sun S, Lee JT. The long noncoding RNA, Jpx, is a molecular switch for X chromosome inactivation. Cell. 2010;143:390–403. doi: 10.1016/j.cell.2010.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wutz A. Gene silencing in X-chromosome inactivation: advances in understanding facultative heterochromatin formation. Nat Rev Genet. 2011;12:542–553. doi: 10.1038/nrg3035. [DOI] [PubMed] [Google Scholar]
- Yang Y, Seed B. Site-specific gene targeting in mouse embryonic stem cells with intact bacterial artificial chromosomes. Nat Biotechnol. 2003;21:447–451. doi: 10.1038/nbt803. [DOI] [PubMed] [Google Scholar]
- Yao H, Brick K, Evrard Y, Xiao T, Camerini-Otero RD, Felsenfeld G. Mediation of CTCF transcriptional insulation by DEAD-box RNA-binding protein p68 and steroid receptor RNA activator SRA. Genes Dev. 2010;24:2543–2555. doi: 10.1101/gad.1967810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LF, Huynh KD, Lee JT. Perinucleolar targeting of the inactive X during S phase: evidence for a role in the maintenance of silencing. Cell. 2007;129:693–706. doi: 10.1016/j.cell.2007.03.036. [DOI] [PubMed] [Google Scholar]
- Zhao J, Sun BK, Erwin JA, Song JJ, Lee JT. Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science. 2008;322:750–756. doi: 10.1126/science.1163045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.