Abstract
The calcium-sensing receptor (CaR) is a G-protein-coupled receptor (GPCR) that binds and signals in response to extracellular calcium and other polycations. It is highly expressed on parathyroid and kidney cells, where it participates in the regulation of systemic calcium homeostasis. It is also expressed on many other cell types and is involved in a wide array of biological functions such as cell growth and differentiation, ion transport and hormone secretion. It has been described to couple to several different G-proteins including Gαi/0, Gαq/11 and Gα12/13. Recently, it has also been shown to stimulate cAMP production by coupling to Gαs in immortalized or malignant breast cells. The CaR is expressed on cells in the anterior pituitary and had previously been described to stimulate cAMP production in these cells. In this report, we examined signaling from the CaR in murine pituitary corticotroph-derived, AtT-20 cells. We found that CaR activation led to the stimulation of cAMP production, and PTHrP and ACTH secretion from these cells. Furthermore, manipulation of cAMP levels was able to modulate PTHrP and ACTH secretion independent of changes in extracellular calcium. Finally, we demonstrated that the CaR couples to Gαs in AtT-20 cells. Therefore, in pituitary corticotroph-like cells, as in breast cancer cells, the CaR utilizes Gαs and activates cAMP production to stimulate hormone secretion.
Keywords: G-protein coupled receptor, calcimimetic, intracellular signaling, cyclic-AMP
INTRODUCTION
The extracellular calcium-sensing receptor (CaR) is a 7-transmembrane-spanning, G protein-coupled, cell-surface receptor (GPCR) that binds and signals in response to ionized calcium and other cations or cationic compounds such as magnesium, gadolinium or neomycin (Brown and MacLeod, 2001; Tfelt-Hansen and Brown, 2005; Brown, 2007). It was initially discovered as a parathyroid calcium sensor and subsequently was shown to be required for the normal regulation of calcium metabolism (Brown et al., 1993; Chattopadhyay and Brown, 2006). The CaR is prominently expressed in the parathyroid glands and the kidney, where it functions in the regulation of parathyroid hormone (PTH) secretion and renal calcium handling respectively (Quarles, 2003; Chattopadhyay and Brown, 2006). However, the CaR is also expressed in many other organs and participates in the regulation of a variety of processes such as fluid and ion transport, cellular proliferation, differentiation and apoptosis, and hormone secretion (Brown and MacLeod, 2001; Tfelt-Hansen and Brown, 2005; Brown, 2007).
As with other GPCR’s, signaling from the CaR is initiated by activation of heterotrimeric G proteins. Most literature has suggested that the CaR couples primarily to Gαi/0 and Gαq/11 (Brown and MacLeod, 2001; Hofer and Brown, 2003; Ward, 2004). Signaling pathways downstream of the receptor appear to vary somewhat in different cell types. In HEK cells transfected with the CaR and in parathyroid cells, activation of the receptor stimulates phospholipase C (PLC) activity and generates inositol tris-phosphate (IP3) and intracellular calcium transients (Brown and MacLeod, 2001; Hofer and Brown, 2003; Ward, 2004). This, in turn, has been described to result in activation of mitogen-activated protein kinase (MAPK) cascades involving the extracellular signal-regulated kinases (ERK) 1 and 2, p38 and the stress-activated, cJun N-kinase (JNK) (Kifor et al., 2001; Corbetta et al., 2002; Ward, 2004). Activation of this pathway has been shown to be important for the ability of CaR signaling to inhibit PTH secretion from dispersed parathyroid cells (Kifor et al., 2001; Corbetta et al., 2002). Stimulation of the CaR also acts via Gαi to inhibit adenylyl cyclase and reduce cAMP levels in several cell types, although this has not been thought to be a major contributor to the regulation of PTH secretion (Hofer and Brown, 2003; Ward, 2004). We have recently shown that inhibition of cAMP is important for the ability of calcium to inhibit parathyroid hormone-related protein (PTHrP) secretion from normal mouse mammary epithelial cells (Mamillapalli et al., 2008). Furthermore, we also demonstrated that in immortalized mammary epithelial cells or in human breast cancer cells, the CaR switches from coupling to Gαi to coupling to Gαs (Mamillapalli et al., 2008). This causes a reversal of the biological effects of calcium such that activation of the CaR stimulates, instead of inhibits, PTHrP secretion. Thus, the CaR activates distinct signaling events in different cells, at least in part, by utilizing different G-proteins, including coupling to either Gαi or Gαs, depending on the setting.
PTHrP is a peptide growth factor originally described to cause humoral hypercalcemia of malignancy (Strewler, 2000; Wysolmerski, 2008). It is produced by many different normal cells and has important local functions in diverse sites such as the skeleton, vasculature, teeth and mammary gland (Strewler, 2000; Wysolmerski, 2008). In normal mammary epithelial cells and in breast cancer cell lines, CaR signaling regulates PTHrP production (Sanders et al., 2000; VanHouten et al., 2004; Mamillapalli et al., 2008). The CaR has also been shown to modulate PTHrP production in astrocytes, osteoblasts, cytotrophoblasts, ovarian surface epithelial cells and HEK 293 cells transfected with the CaR (Chattopadhyay, 2006). Therefore, PTHrP is responsive to changes in extracellular calcium in many sites. Both PTHrP and the CaR are expressed in the normal pituitary gland, pituitary tumors and pituitary cell lines, although their biological function(s) in pituitary cells is not clear (Ikeda et al., 1988; Asa et al., 1990; Fraser et al., 1991; Ito et al., 1993; Holt et al., 1994; Emanuel et al., 1996; Danks et al., 1997; Ferry et al., 1997; Romoli et al., 1999; Loretz, 2008). Previous studies have shown that the stimulation of corticotroph, somatotroph and melanotroph cell lines with extracellular calcium increased, rather than decreased, intracellular cAMP levels (Emanuel et al., 1996; Romoli et al., 1999; Zivadinovic et al., 2002; van den Hurk et al., 2008). Given the recent data showing that the CaR couples to Gαs in breast cancer cells (Mamillapalli et al., 2008), we asked if the CaR might also couple to Gαs in anterior pituitary cells and stimulate PTHrP production by increasing intracellular cAMP. In this report we demonstrate this to be the case. Activation of the CaR in murine pituitary corticotroph-derived, AtT-20, cells activated Gαs, increased cAMP production and stimulated PTHrP and adrenocorticotropic hormone (ACTH) secretion.
MATERIALS AND METHODS
Materials
Ham’s F12 medium, calcium-free DMEM, gentamycin, dispase, NuPAGE LDS sample buffer, CaR siRNAs, and TRIzol were purchased from Invitrogen Life Technologies (Carlsbad, CA). Forskolin, H-89, N6,2-O-dibutyryl adenosine 3′,5′-cyclic monophosphate sodium salt (Bt2cAMP), 3-isobutyl-1-methylxanthine (IBMX), guanosine 5′-diphosphate sodium salt (GDP), carbachol and guanosine 5′-triphosphate sodium salt hydrate (GTP) were purchased from Sigma-Aldrich (St. Louis, MO). Fetal bovine serum (FBS) was obtained from Atlanta Biologicals (Atlanta, GA). [35S]GTPγS was from Perkin-Elmer Life Sciences (Boston, MA) and ACTH radioimmunoassay kits were obtained from MP Biomedicals, LLC (Orangeburg, NY). PTHrP immunoradiometric assay (IRMA) kits were from Diagnostic Systems Laboratories (Webster, TX) and Correlate-EIA direct cAMP assay kits were from Assay Designs (Ann Arbor, MI). Polyvinylidene difluoride membrane came from Fisher Scientific (Atlanta, GA). Antibodies recognizing Gαs (anti-Gαs) and both Gαq and Gα11 (anti-Gαq/11) were purchased from Upstate Biotechnology (Temecula, CA) while the anti-β-actin antibody was obtained from Cell Signaling Technologies (Danvers, MA). Goat anti-mouse secondary antibody was obtained from Jackson ImmunoResearch (West Grove, PA). The Message Clean system was obtained from Gen Hunter (Nashville, TN), and Brilliant SYBR-Green QRT-PCR Master Mix kit was purchased from Stratagene (La Jolla, CA). Protease inhibitors and the enhanced chemiluminescence kit, employing the SuperSignal West Pico substrate were purchased from Pierce Chemical (Rockford, IL). The phospholipase C (PLC) inhibitor U-73122 and the protein kinase C (PKC) inhibitor, Go6976, were purchased from Sigma-Aldrich (St. Louis, MO).
Cell Culture
AtT-20/LAF1 (AtT-20) cells (American Type Culture Collection) were grown in Ham’s F12 or DMEM medium supplemented with 15% FBS, 2.5 μg/ml amphotericin B and 50 μg/ml gentamycin in a 5% CO2 atmosphere at 37°C. In preparation for experiments, the media was switched to DMEM containing 0.1 mM calcium, 0.2% bovine serum albumin (BSA) and antibiotics, and the final calcium content was adjusted to the desired concentration with the addition of calcium chloride.
cAMP Assay
AtT-20 cells were seeded into 6-well plates. After reaching 70–80% confluence, cells were switched to media containing 2.5 mM calcium, 5 mM calcium or the polycationic agonist neomycin (300 μM). Cells were incubated at 37°C for 30 min and washed with cold phosphate-buffered saline (PBS). Cell lysates were prepared with 0.1 M HCl and cAMP was measured in cell lysates by enzyme immunoassay (EIA) as per the manufacturer’s instructions. In additional experiments, cells were preincubated with forskolin at varying doses for 30 min before additional calcium or neomycin was added. The amount of cAMP was corrected for the total protein content of each individual lysate.
ACTH Secretion
AtT-20 cells were seeded into 24-well plates and treated with 2.5 mM calcium, 5 mM calcium or 300 μM neomycin for 90 min at 37°C. ACTH was measured in samples of conditioned media immediately or after storage at −80°C using a commercial radioimmunoassay (MP Biomedicals, LLC, Orangeburg, NY). In other experiments, cells were preincubated with varying doses of forskolin (0.1 – 10 μM), Bt2cAMP (1 mM), the PKA inhibitor, H89 (10 μM), the PLC inhibitor U73122 (10μM) or the PKC inhibitor, Go6976 (10nM), for 1–2 h before the addition of CaCl2 (5 mM) or neomycin for 90 min. ACTH levels were normalized to the total cellular protein from each well.
Measurement of PTHrP secretion
AtT-20 cells were seeded into 12-well plates at a density of 106 cells per well. After 48 h, the cells were treated with 2.5 mM calcium, 5 mM calcium or 300 μM neomycin. Conditioned medium was removed after 16 h and PTHrP (1–86) was measured using a two-site immunoradiometric assay (IRMA) following the manufacturer’s instructions (Diagnostic Systems Laboratories, Webster, TX). PTHrP levels were normalized to the total cellular protein from each well. In other experiments, cells were preincubated with varying doses of forskolin (0.1 – 10 μM), Bt2cAMP (1 mM), the PKA inhibitor H89 (10 μM), the PLC inhibitor, U73122 (10μM), or the PKC inhibitor, Go6976 (10nM), for 1–2 h before the addition of CaCl2 (5 mM), or neomycin.
Western blot analysis
Cells were washed with ice-cold phosphate-buffered saline, and then lysed in 400 μl of Nonidet-40 lysis buffer (20mM Tris base, pH 8.0, 150 mM NaCl, 1% Nonidet P-40 with protease inhibitors). Cell lysates were centrifuged at 14,000g to pellet debris and protein concentrations were measured using Bio-Rad protein assay reagent. Protein samples (25 μg) were resolved on 12% SDS-PAGE minigels and electroblotted onto polyvinylidene difluoride membranes. The blots were blocked for 1 h with 5% nonfat dry milk in PBS-0.05% Tween, washed, and incubated overnight at 4°C with polyclonal anti-Gαs antibody (1:500). The membranes were washed and then incubated with goat anti-rabbit IgG conjugated to horseradish peroxidase (1:5,000), and signals were detected using a SuperSignal West Pico detection kit. The same membrane was stripped and blotted again for β-actin protein with anti-β-actin antibody.
Quantitative Real Time RT-PCR
Total RNA was isolated from cells 48 hrs. post transfection using TRIzol as per the manufacturer’s instructions. Any contaminating DNA was removed using the Message Clean system. The Brilliant SYBR-Green QRT-PCR Master Mix kit was used to perform one-step qRT-PCRs, in triplicate, using 20 ng of RNA with a reaction volume of 20μl on the Opticon 2 DNA Engine (MJR, Waltham, MA). Reverse transcription was carried out for 30 min at 50°C, followed by 40 cycles consisting of 30 s at 95°C and 1 min at 60°C. The glyceraldehyde-3-phosphate dehydrogenase primers were as follows: forward, 5′-CGTCCCGTAGACAAAATGGT-3′, and reverse, 5′-TCAATGAAGGGGTCGTTGAT-3′, and the CaR primers were forward, 5′-ATGTTTGGCTACTGTTTGG-3′, and reverse, 5′-CAGAGCCTTGGAGAC GGTGT-3′. For each experimental sample, a control without reverse transcriptase was run to verify that the amplification product arose from cDNA and not from genomic DNA. The relative expression levels, normalized to glyceraldehyde-3phosphate dehydrogenase, were determined using the comparative CT method (also known as the 2−ΔΔCT method) (Applied-Biosystems, 1997; Mamillapalli et al., 2008). CaR mRNA levels were expressed as the fold suppression in CaR siRNA-transfected cells relative to basal expression in non-transfected cells.
[35S]GTPγS binding assay
Coupling of Gαs or Gαq/11 to the CaR was assessed using the [35S]GTPγS binding assay as described previously (Mamillapalli et al., 2008). Briefly, cell membranes were prepared from actively growing AtT-20 cells. The cells were washed with 10mM Tris-maleate buffer, pH 7.4, scraped into a 6 ml aliquot of Tris-maleate buffer and passed 15 times through an 18-gauge needle. The resultant cell lysate was centrifuged at 5000g for 10 minutes and the supernatant was then centrifuged at 100,000g for 30 minutes to pellet the membranes. The membrane pellet was resuspended in 10mM Tris-maleate buffer and passed through a 22-gauge needle. Membrane protein concentrations were assessed using the Bio-Rad protein assay reagent. Membrane preparations were used immediately or were frozen at −80°C for later use. The assay reaction mixture contained membrane protein (850 μg) and 360 nM [35S]GTPγS in 50 μl of 50 mM Tris-HCl, pH 7.4, 2.8 mM MgCl2, 100 mM NaCl, and 1μM GDP. The reaction was initiated by adding 5 mM CaCl2, 300 μM of neomycin or 100 μM carbachol for 30 min at 30°C, and the reaction was terminated by adding 600 μl of ice-cold immunoprecipitation buffer (50 mM Tris-HCl, pH 7.5, 20 mM MgCl2, 150 mM NaCl, 0.5% Nonidet P-40, 20 μg/ml aprotinin, 100 μM GDP, and 100 μM GTP), followed by a 30-min incubation on an orbital shaker at 4°C. After incubation, the reaction contents were centrifuged at 14,000 g, and the supernatant was transferred to 110 μl of a previously prepared anti-Gαs or anti-Gαq/11 antibody-protein A-Sepharose mixture. The resulting mixtures were incubated for 1h at 4°C on an orbital shaker, and immunoprecipitates were pelleted by centrifugation at 4,000 g for 2 min at 4 °C. The pellet was washed three times, boiled for 1 min in 500 μl of 0.5% SDS, and transferred to scintillation vials containing 4 ml of scintillation liquid. Samples were analyzed in triplicate on a scintillation counter (Beckman Coulter, LS5000 TA and TD liquid scintillation system, Fullerton, CA). Non-immune serum was used to control for nonspecific binding of [35S]GTPγS.
Silencing of CaR expression
AtT-20 cells were grown to 70% confluence and then transfected with Casr Stealth Select RNAi (MSS202652 or MSS202653, 260 pmol/well) (Invitrogen) using Lipofectamine™-2000 (7.8 μl/well) according to the manufacturer’s protocol. The Stealth RNAi Negative Control Med GC (Invitrogen) was transfected in parallel as a control in all experiments. To verify knockdown of CaR mRNA, total cellular RNA was isolated from cells 48-hours post-transfection and subjected to RT-PCR as described above. cAMP, PTHrP and ACTH levels were assayed at 48-hours post transfection as described above.
Statistical Analysis
Each experiment was performed at least three times and each individual experiment was performed in triplicate. However, although there were 9 or more replicates, statistical analysis was performed using the number of completely independent experiments. Data are expressed as means ± the standard error of the mean (S.E.). Statistical significance was determined using one-way analysis of variance (ANOVA) with the Newman-Keuls multiple comparisons test. All statistical analyses were carried out using Graph Pad Prism 4.00 for Macintosh (Graph-Pad Software for Science Inc., San Diego).
RESULTS
CaR Activation Increases cAMP Levels and Stimulates PTHrP and ACTH Secretion in AtT-20 cells
We first treated pituitary, AtT-20 cells with increasing doses of calcium and the Type I calcimimetic agent, neomycin, and measured cAMP production and PTHrP secretion. AtT-20 cells are murine corticotroph-derived cells previously shown to express the CaR (Emanuel et al., 1996). As shown in Fig. 1A, exposure of these cells to increasing doses of extracellular calcium (from 0.1mM to 5.0 mM) for 30 minutes caused a dose-dependant rise in intracellular cAMP. Treatment with 300 μM neomycin at 0.1 mM calcium also increased cAMP levels, suggesting that the CaR mediated the effects of calcium on cAMP concentrations. The pituitary is known to produce PTHrP (Ikeda et al., 1988; Asa et al., 1990; Ito et al., 1993; Holt et al., 1994; Danks et al., 1997), so we next measured PTHrP secretion by AtT-20 cells treated with extracellular calcium or neomycin. PTHrP levels in conditioned media mirrored the changes in cAMP. As shown in Fig. 1B, increasing doses of extracellular calcium led to a progressive increase in PTHrP secretion by these cells as did treatment with neomycin. Finally, previous reports had demonstrated that CaR activation increases ACTH secretion by pituitary cells (Emanuel et al., 1996; Ferry et al., 1997). Therefore, we also measured ACTH secretion by these cells and confirmed that increasing doses of calcium and treatment with a calcimimetic agent triggered ACTH secretion by AtT-20 cells in a manner identical to the changes we observed for cAMP and PTHrP (Fig. 1C).
Figure 1. Activation of the CaR stimulates cAMP accumulation and PTHrP and ACTH Secretion.
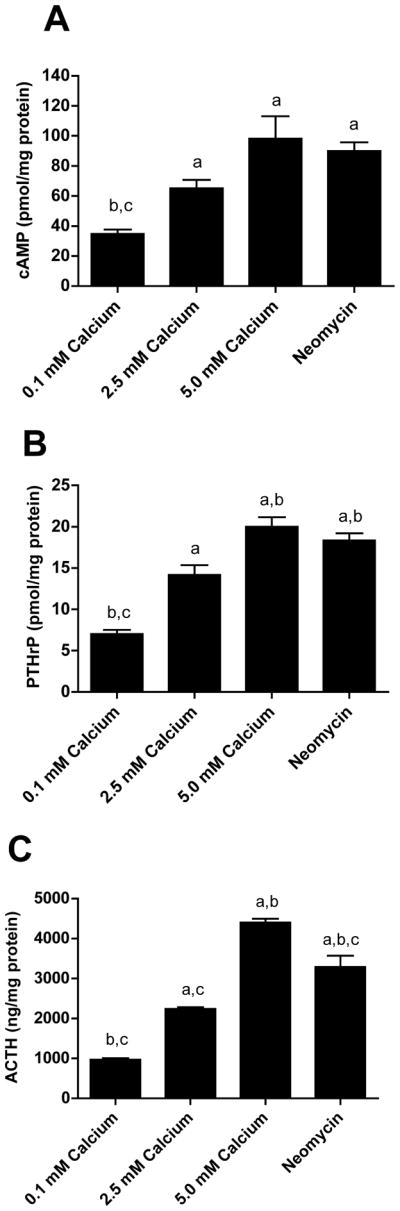
A) Intracellular cAMP levels in AtT-20 cells treated with 0.1 mM, 2.5 mM or 5 mM extracellular calcium and neomycin (300 μM). B) PTHrP levels were increased in conditioned media from cells treated with 2.5 mM or 5 mM calcium or neomycin. C) ACTH levels were increased in conditioned media from cells treated with extracellular calcium or neomycin. Bars represent the means ± S.E. of three independent experiments. Statistical significance (p<0.05) is noted on the graphs: a denotes a significant difference compared with 0.1mM calcium, b denotes a significant difference compared with 2.5mM calcium and c denotes a significant difference as compared with 5mM calcium.
Elevations in cAMP stimulate PTHrP and ACTH secretion from AtT-20 cells
In order to determine if CaR activation stimulated PTHrP and ACTH secretion by causing an increase in cAMP, we next asked if elevated cAMP levels would stimulate PTHrP and ACTH secretion independent of changes in extracellular calcium. We used forskolin, a pharmacologic activator of adenylyl cyclase to increase cAMP levels and also treated cells with dibutyryl-cAMP (dBcAMP), a cell-permeable synthetic analog of cAMP that is resistant to degradation by phosphodiesterases (Ryan and Heidrick, 1974; Insel and Ostrom, 2003; Mamillapalli et al., 2008). As expected, forskolin led to a dose-dependant increase in cAMP levels in AtT-20 cells at 0.1 mM calcium (Fig. 2A). Figure 2B demonstrates that treating AtT-20 cells with increasing doses of forskolin stimulated the secretion of PTHrP into the media, mimicking the effects of treatment with high calcium or calcimimetics. There was not a one-to-one correlation between cAMP and PTHrP levels and at higher doses of forskolin, the relative increase in PTHrP secretion leveled off. Nonetheless, increasing cAMP concentrations led to a progressive increase in PTHrP secretion. Treatment with forskolin had similar effects on ACTH release from these cells (Fig. 2C). Treating cells with dBcAMP at 0.1mM calcium also stimulated PTHrP and ACTH secretion (Supplemental Fig. 1). These data suggest that cAMP may mediate the actions of the CaR on PTHrP and ACTH secretion within these cells.
Figure 2. Manipulation of cAMP levels alters PTHrP and ACTH secretion in AtT-20 cells.
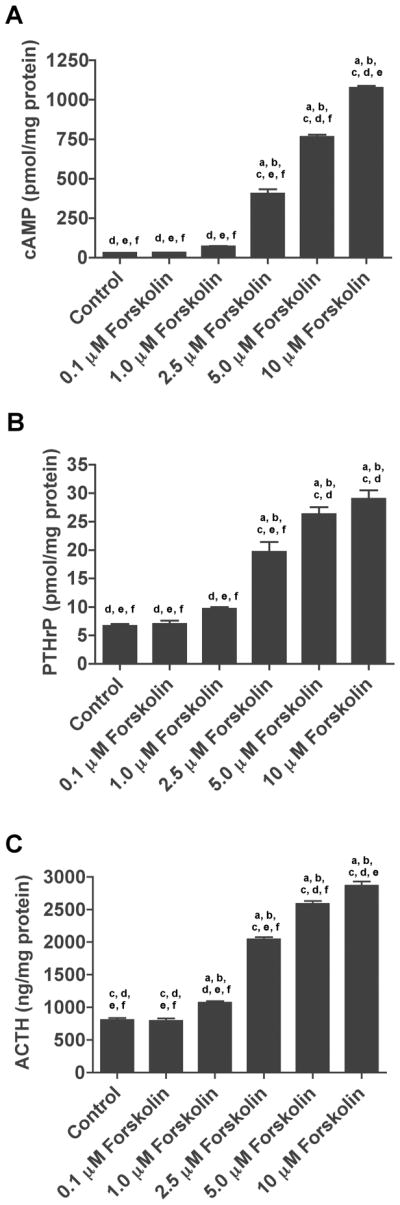
A) Intracellular levels of cAMP from AtT-20 cells treated with varying doses of forskolin (0.1 – 10μM) in the presence of 0.1 mM calcium. B) & C) Secretion of PTHrP (B) and ACTH (C) into conditioned media in response to varying doses of forskolin at 0.1mM calcium. Control denotes 0.1mM calcium without forskolin. Raising cAMP levels independent of changes in extracellular calcium stimulated both PTHrP and ACTH secretion by AtT-20 cells in a dose-dependant fashion. In all panels bars represent the mean ± S.E. of three individual experiments. Statistical significance (p<0.01) is denoted on the graphs: a denotes a significant difference compared with control, b denotes a significant difference as compared to 0.1 μM forskolin, c denotes a significant difference compared with 1.0 μM forskolin, d denotes a significant difference compared with 2.5 μM forskolin, e denotes a significant difference compared with 5 μM forskolin and f denotes a significant difference compared with 10 μM forskolin.
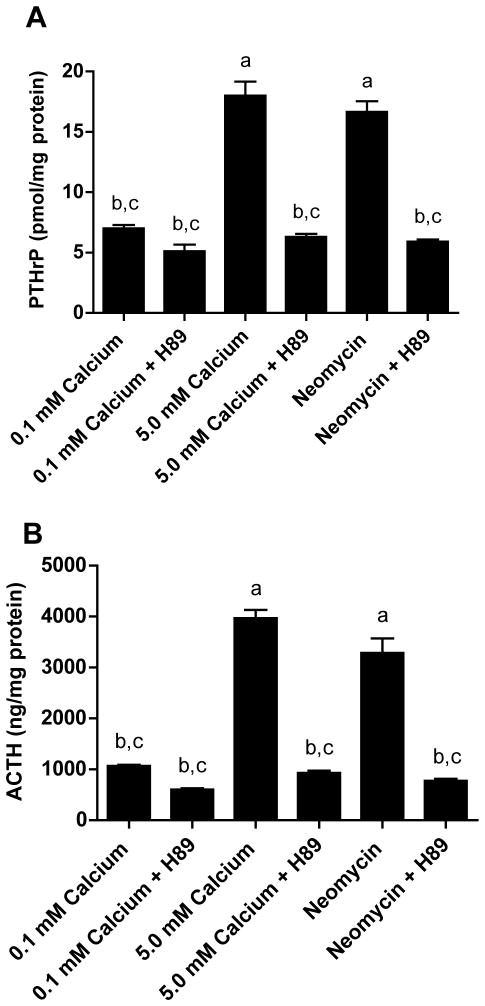
Inhibition of PKA Blocks the Effects of CaR Activation on ACTH and PTHrP Secretion
Many of the effects of cAMP are mediated through activation of protein kinase A (PKA) (Taylor et al., 2005). Therefore, in order to confirm that cAMP contributes to the effects of CaR signaling on PTHrP and ACTH secretion, we asked if inhibition of PKA activity could block PTHrP and/or ACTH release in response to calcium or neomycin. For this purpose, we treated AtT-20 cells with the selective PKA inhibitor, H-89 (Taylor et al., 2005; Mamillapalli et al., 2008). Treatment with H89 lowered basal levels of PTHrP and ACTH secretion only slightly at 0.1 mM calcium. However, H-89 effectively blocked the stimulation of PTHrP (Fig. 3A) and ACTH (Fig. 3B) secretion by high calcium (5.0 mM) or neomycin. Activation of the CaR receptor has previously been shown to trigger phophoinositol accumulation and to trigger increases in intracellular calcium concentrations in these cells (Emanuel et al., 1996). Either of these second messenger systems might contribute to or synergize with cAMP in the regulation of PTHrP and/or ACTH secretion from these cells. Therefore, we also examined the effects of the phospholipase C (PLC) inhibitor, U73122, and the protein kinase C (PKC) inhibitor, Go6976. As shown in Supplemental Fig. 2, neither inhibition of PLC nor inhibition of PKC prevented the secretion of PTHrP or ACTH in response to calcium or neomycin, suggesting that these pathways are not required for the effects of calcium on their secretion. These data demonstrate that the cAMP/PKA pathway regulates ACTH and PTHrP secretion from these cells, and suggest that CaR activation stimulates ACTH and PTHrP production in a cAMP/PKA-dependant fashion.
Figure 3. cAMP/PKA signaling mediates the effects of CaR activation on PTHrP and ACTH secretion.
A) PTHrP levels in conditioned media harvested from AtT-20 cells pretreated with the PKA inhibitor, H89 (10 μM), prior to exposure to high calcium (5 mM) or neomycin. H89 lowered basal rates of PTHrP secretion and blocked the response to high calcium or neomycin. B) ACTH levels in conditioned media from cells pretreated with H89 (10 μM), prior to exposure to high calcium (5 mM) or neomycin. As with PTHrP, inhibition of PKA lowered basal secretion of ACTH and blocked the effects of calcium or neomycin. Data are means ± S.E. from three independent experiments. Statistical significance (p<0.01 or lower) is noted on the graph: a denotes a significant difference as compared with 0.1mM calcium, b denotes a significant difference as compared with 5mM calcium and c denotes a significant difference as compared to neomycin.
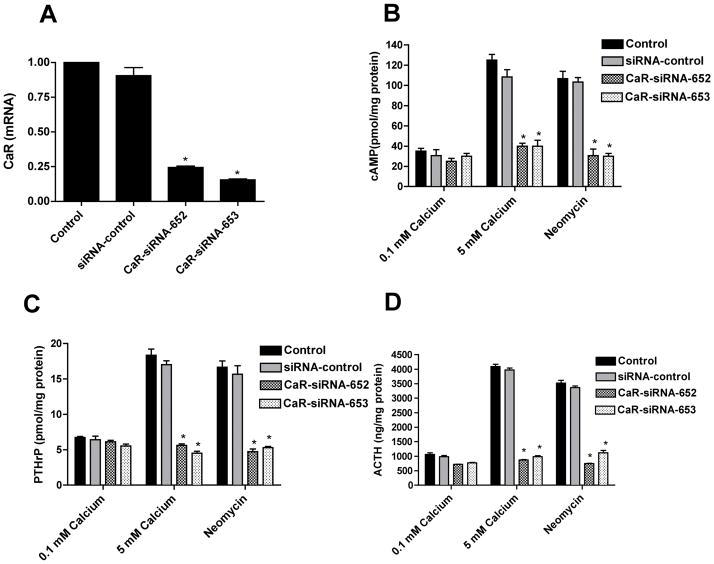
The CaR Mediates the effects of Calcium and Neomycin on cAMP Levels and PTHrP and ACTH Secretion
The fact that neomycin mimicked high extracellular calcium in stimulating cAMP, PTHrP and ACTH levels suggested that the CaR mediates these effects. In order to validate this conclusion, we examined the effects of calcium and neomycin on these parameters after “knocking down” CaR expression in AtT-20 cells using siRNA directed against the CaR (Mamillapalli et al., 2008). As shown in Fig. 4A, transfection of untreated cells with two different siRNAs directed specifically against the CaR reduced the basal levels of CaR mRNA by approximately 75%. In contrast, a non-specific control siRNA resulted in only a 6% decrease in CaR mRNA levels (Fig. 4A). As shown in Fig. 4B, the CaR-specific siRNAs had no effect on baseline cAMP levels but they resulted in a significant inhibition of cAMP accumulation in response to either 5mM calcium or neomycin. This was also associated with the ablation of the effects of 5mM calcium and neomycin on PTHrP (Fig. 4C) and ACTH (Fig. 4D) secretion. In contrast, transfection of the non-specific control siRNA had little effect on cAMP, PTHrP or ACTH production. Since two independent CaR-specific siRNA gave the same result and since the control siRNA did not, it is unlikely that these results are related to any non-specific, off-target effects. Therefore, these data confirm that the CaR mediates the effects of calcium and neomycin on cAMP, PTHrP and ACTH production by AtT-20 cells.
Figure 4. Knockdown of CaR mRNA expression inhibits the effects of high calcium and neomycin on cAMP, PTHrP and ACTH.
A) Measurement of CaR mRNA levels by quantitative, real-time RT-PCR in AtT-20 cells transfected with two different, specific siRNA (CaR-siRNA-652 and CaR-siRNA-653) against the CaR as well as with a control, non-specific siRNA. CaR-specific siRNAs caused approximately a 75% reduction in CaR mRNA levels as compared with the untransfected cells. Bars represent the mean ± S.E. of three individual experiments. * denotes significant differences between CaR-siRNAs (652 and 653) versus untransfected cells (p < 0.001). B), C) & D) show the effects of these CaR siRNAs on intracellular cAMP and PTHrP and ACTH secretion, respectively, in response to CaR activation by high calcium (5 mM) and neomycin. CaR activation failed to increase cAMP, PTHrP or ACTH levels in cells transfected with specific CaR-siRNAs (652 and 653). However, non-transfected cells and cells transfected with non-specific CaR-siRNA (siRNA-control) demonstrated the expected stimulation of cAMP, ACTH and PTHrP levels in response to 5 mM calcium or neomycin. Bars represent the mean ± S.E. of three independent experiments. * denotes significant differences between CaR-siRNAs (652 and 653) versus non-transfected cells, (p < 0.001).
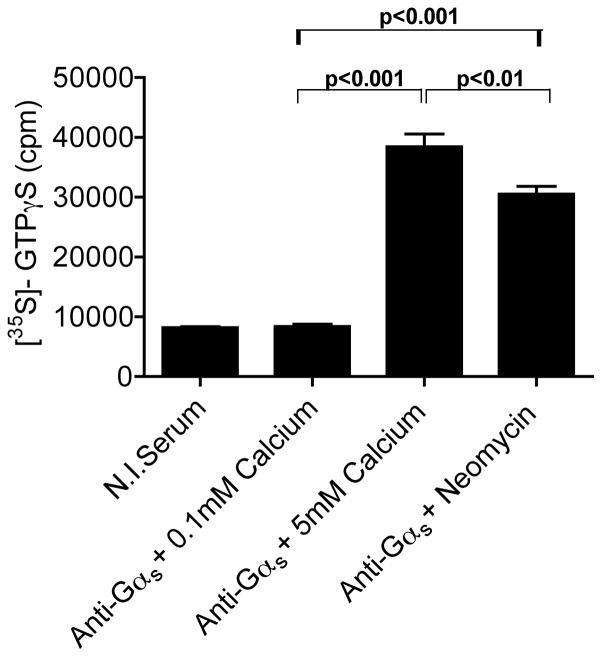
The CaR Couples to Gαs in AtT-20 cells
We had previously demonstrated that the CaR signals through Gαs and stimulates adenylyl cyclase in immortalized mouse mammary epithelial cells and in human breast cancer cells (Mamillapalli et al., 2008). In order to determine if the CaR also couples to Gαs in AtT-20 cells, we first confirmed that these cells express this particular G protein. As expected, Western blot analysis showed that AtT-20 cells express ample Gαs protein (not shown). Next, we performed the [35S]GTPγS binding assay as described previously (Mamillapalli et al., 2008). This assay relies on the activation of the CaR in vitro in the presence of a 35S-labeled, phosphatase-resistant GTP derivative, GTPγS. The G-protein of interest is subsequently immunoprecipitated and the amount of radioactivity within the immunoprecipitate is an index of activation of that particular G-protein in response to receptor activation. Consistent with the lack of effects of the PLC and PKC inhibitors, we found that the CaR did not couple to either Gαq or Gα11 in AtT-20 cells (supplemental Fig. 2). However, as shown in Fig. 5, activation of the CaR led to increased association of [35S]GTPγS with Gαs. Little [35S]GTPγS was incorporated into anti-Gαs immunoprecipitates in the presence of 0.1 mM calcium but incorporation increased approximately 4-fold in response to 5 mM calcium and 3.2-fold in response to neomycin. The incorporation of radioactivity into immunoprecipitates using nonimmune serum served as a control for nonspecific binding of [35S]GTPγS. These results demonstrate that the CaR couples to Gαs in AtT-20 cells and suggest that treatment of these cells with calcium or calcimimetics stimulates adenylyl cyclase resulting in increased cAMP levels, which, in turn, stimulate PTHrP and ACTH secretion.
Figure 5. The CaR couples to G α s in AtT-20 cells.
Results of [35S]GTPγS binding assays from AtT-20 cell membranes using an anti-Gαs specific antibody. As shown in the figure, activation of the CaR with 5 mM calcium or neomycin triggered [35S]GTPγS binding to Gαs. Non-specific binding of [35S]GTPγS was assessed by using non-immune serum (N.I. serum) in control immunoprecipitations. Bars represent the mean ± S.E. of three individual experiments. Statistical significance is denoted on the graphs.
DISCUSSION
Our results confirm that increasing extracellular calcium concentrations stimulate cAMP production in the murine pituitary corticotroph-derived, AtT-20, cell line. Calcium also stimulates PTHrP and ACTH secretion from these cells. The CaR mediates these effects of extracellular calcium because they are mimicked by neomycin and are inhibited by specific, siRNA-induced knockdown of CaR mRNA expression. In addition, we demonstrated that raising cAMP levels, independent from CaR activation, led to the secretion of both PTHrP and ACTH by AtT-20 cells and that blocking PKA activity prevented the secretion of these peptides in response to calcium or neomycin. Therefore, cAMP mediates the effects of CaR activation on the secretion of both PTHrP and ACTH. Finally, we demonstrated that the CaR couples to Gαs in AtT-20 cells, suggesting that the activation of cAMP production by Gαs may be an important pathway through which the CaR modulates neuropeptide release by some pituitary cells.
CaR mRNA and/or protein expression has been detected in the anterior pituitaries of humans, rodents and fish, and in AtT-20 cells (Emanuel et al., 1996; Mitsuma et al., 1999; Zivadinovic et al., 2002; Loretz, 2008). Previous studies on downstream signaling events in pituitary cells have documented that CaR activation stimulates intracellular calcium transients in AtT-20 cells, in cells cultured from rat and Xenopus pituitaries, and in cells cultured from human pituitary adenomas (Emanuel et al., 1996; Ferry et al., 1997; Romoli et al., 1999; Zivadinovic et al., 2002; van den Hurk et al., 2008). In addition to calcium transients, activation of the receptor in AtT-20 cells was reported to cause a pertussis toxin-sensitive accumulation of phosphoinositides, suggesting that at least some signaling pathways are mediated through activation of Gαi (Emanuel et al., 1996). Interestingly, although increases in intracellular calcium in response to CaR activation have been described to be pertussis toxin insensitive in cells from human growth hormone-secreting pituitary adenomas, we could not document coupling of the CaR to Gαq or Gα11 in AtT-20 cells. It is not clear if this represents differences between pituitary cell types or if it suggests differences in the nature of CaR-mediated intracellular calcium mobilization in pituitary cells as compared to parathyroid or other cell types.
Activation of the CaR has also been shown to stimulate cAMP accumulation in pituitary cells (Emanuel et al., 1996; Romoli et al., 1999; Zivadinovic et al., 2002; van den Hurk et al., 2008). Our experiments both confirm and extend these prior data by showing that the CaR couples to Gαs in AtT-20 cells and that the cAMP-PKA pathway is necessary and sufficient to elicit PTHrP and ACTH secretion in response to CaR activation. These results are similar to our recent findings demonstrating that the CaR couples to Gαs in immortalized murine mammary epithelial cells and in human breast cancer cells, and that activation of cAMP/PKA signaling mediates the effects of the CaR on stimulating PTHrP secretion from these cells (Mamillapalli et al., 2008). They are also consistent with the observation that the accumulation of cAMP in response to extracellular calcium was not inhibited by pertussis toxin in AtT-20 cells (Emanuel et al., 1996). While seemingly contradictory from a cAMP standpoint, simultaneous coupling of GPCRs to both Gαs and Gαi has been reported for dopamine D1, opioid and prostacyclin receptors (Sidhu et al., 1991; Cruciani et al., 1993; Lawler et al., 2001). Moreover, when chimeric A(1)/A(2A) adenosine receptors designed to couple to both Gαs and Gαi were studied in HEK-293 cells, Gαs-mediated stimulation of adenylyl cyclase predominated over Gαi-mediated inhibition of adenylyl cyclase (Tucker et al., 2000). Therefore, in AtT-20 cells, it is possible that CaR activation leads to activation of both Gαs and Gαi, and that Gαs stimulates adenylate cyclase, while Gαi activates PLC/calcium transients and/or other downstream events.
Data from this and previous studies demonstrate that extracellular calcium is a potent stimulus for ACTH secretion (Emanuel et al., 1996; Fuleihan et al., 1996; Ferry et al., 1997). The fact that this effect is mimicked by neomycin and other calcimimetics and the fact that it is blocked by siRNA-mediated knockdown of the CaR clearly demonstrate that calcium acts through the CaR to trigger ACTH secretion. Furthermore, inhibition of this effect by H89 suggests that CaR signaling stimulates ACTH release by augmenting intracellular cAMP, a known secretagogue for ACTH (King and Baertschi, 1990). While it is not clear if our results are applicable to normal pituitary corticotrophs, it is interesting to note that elevations in systemic calcium levels have been shown to induce ACTH release in intact human subjects (Fuleihan et al., 1996). This may also be the case in fish, since circulating levels of calcium correlate with circulating levels of corticosteroids (Abbink et al., 2004). The fish pituitary expresses the CaR and calcium-mediated release of cortisol is thought to participate in the regulation of calcium economy (Abbink et al., 2004; Loretz, 2008). The biological significance of calcium-regulated ACTH release is less clear in mammals. It may be a vestige of the ancestral regulation of calcium metabolism by the pituitary gland. Interestingly, some data suggest that polyamines, which are potent CaR agonists, can stimulate ACTH release from the pituitary gland (Yamamori et al., 2001; Cheng et al., 2004). Moreover, there is diurnal variation in polyamine levels in the pituitary, raising the intriguing possibility that signaling through the CaR might be involved in modulating ACTH secretion in a circadian pattern (Yamamori et al., 2001).
Cells in the anterior pituitary have also been noted to produce PTHrP. Its expression has been documented by either in situ hybridization or immunohistochemistry in the pituitaries of mice, rats, frogs, fish and humans (Ikeda et al., 1988; Asa et al., 1990; Ito et al., 1993; Danks et al., 1997). It is also produced by human pituitary adenomas and has been measured in the venous drainage of the human anterior pituitary in a patient undergoing petrosal sinus sampling (Ikeda et al., 1988; Asa et al., 1990; Ito et al., 1993; Hofle et al., 2001). Very little is known about the regulation of PTHrP expression, the specific cell types that normally secrete it, or its function in pituitary cells. In a rat pituitary tumor cell line, PTHrP gene expression was shown to be controlled by estrogen (Holt et al., 1994). Our data demonstrate that PTHrP is also regulated by the CaR through a cAMP/PKA pathway. The PTHrP gene contains a cAMP-response element and previous data have shown that the CaR controls PTHrP production in normal and malignant breast cells through the regulation of cAMP levels (Zajac et al., 1989; Chilco et al., 1998; Mamillapalli et al., 2008). However, the biological function of PTHrP and the reasons for its regulation by extracellular calcium in the pituitary remain obscure. As with ACTH, it has been suggested that PTHrP secretion from the pituitary is involved in the regulation of systemic calcium metabolism in fish (Fraser et al., 1991; Abbink et al., 2004; Abbink et al., 2006; Loretz, 2008). In mammals, data also suggest that PTHrP can affect the growth and vascularity of malignant pituitary tumor cells transplanted into rodents (Akino et al., 1996; Akino et al., 2000). However, more work will be needed to determine the biological function of PTHrP in the pituitary and whether it is regulated by the CaR in the intact gland.
In summary, in this study we demonstrate that the CaR modulates PTHrP and ACTH secretion from mouse pituitary corticotroph-derived AtT-20 cells by regulating cAMP production and coupling to Gαs. We recently demonstrated that the CaR controls cAMP and PTHrP secretion in an opposing fashion in normal versus malignant breast cells by switching its use of Gαi to Gαs (Mamillapalli et al., 2008). Prior to this previous report, the CaR had only been described to couple to Gαi, Gαq/11 and Gα12/13 pathways (Arthur et al., 1997; Hofer and Brown, 2003; Huang et al., 2004; Ward, 2004; Gerbino et al., 2005; Rey et al., 2005). However, AtT-20 cells represent a second example of the receptor coupling to Gαs. Therefore, Gαs should be added to the potential repertoire of G-proteins mediating signaling from the CaR. Furthermore, since AtT-20 cells activate both Gαs and Gαi pathways, these cells may be useful for studying the regulatory mechanisms that mediate G-protein usage by the CaR.
Supplementary Material
Secretion of PTHrP (A) and ACTH (B) into conditioned media in response to dBcAMP (1mM) at 0.1mM calcium. Stimulation of the cAMP/PKA pathway with this cAMP analog led to secretion of PTHrP and ACTH independent of a change in the extracellular calcium concentration. Bars represent the mean ± S.E. of three individual experiments. Statistical significance is noted on the graphs.
A) PTHrP levels in conditioned media harvested from AtT-20 cells pretreated with the PLC inhibitor, U73122 (10μM), or the PKC inhibitor, Go6976 (10nM), prior to exposure to increasing doses of calcium or neomycin. Neither inhibitor blocked the secretion of PTHrP in response to activation of the CaR. B) ACTH levels in conditioned media harvested from AtT-20 cells pretreated with the PLC inhibitor, U73122, or the PKC inhibitor, Go6976, prior to exposure to increasing doses of calcium or neomycin. Neither inhibitor blocked the secretion of ACTH in response to activation of the CaR. Data are means ± S.E. from three independent experiments. Statistical significance is shown on the graph. There were no statistical differences between control, U73122-treated or G06976-treated cells at any level of calcium or neomycin. Therefore, the letters refer to each group of 3 bars: a denotes a significant difference as compared with 0.1mM calcium, b denotes a significant difference as compared with 2.5 mM calcium, c denotes a significant difference as compared with 5.0 mM calcium and d denotes a significant difference as compared with neomycin.
Results of [35S]GTPγS binding assays from AtT-20 cell membranes using an antibody that recognizes both Gαq and Gα11 (anti-Gαq/11). As shown in the figure, stimulation of muscarinc receptors with carbachol (100 μM) led to the association of significant amounts of [35S]GTPγS with immunoprecipitates pulled down with anti-Gαq/11 antibodies. In contrast, stimulation of the CaR with 5mM calcium or neomycin did not trigger [35S]GTPγS binding to either Gαq or Gα11. Non-specific binding of [35S]GTPγS was assessed by using non-immune serum (N.I. serum) in control immunoprecipitations. Bars represent the mean ± S.E. of six individual experiments. Statistical significance is noted on the graph.
Acknowledgments
FUNDING
This work was supported by NIH grant DK069542.
The authors thank Dr. Joshua VanHouten for helpful discussions and for a critical reading of the manuscript.
Footnotes
DECLARATION OF INTERESTS
There are no conflicts of interest that prejudice or could be perceived as prejudicing the impartiality of the research reported in this manuscript.
References
- Abbink W, Bevelander GS, Hang X, Lu W, Guerreiro PM, Spanings T, Canario AV, Flik G. PTHrP regulation and calcium balance in sea bream (Sparus auratus L.) under calcium constraint. J Exp Biol. 2006;209:3550–3557. doi: 10.1242/jeb.02399. [DOI] [PubMed] [Google Scholar]
- Abbink W, Bevelander GS, Rotllant J, Canario AV, Flik G. Calcium handling in Sparus auratus: effects of water and dietary calcium levels on mineral composition, cortisol and PTHrP levels. J Exp Biol. 2004;207:4077–4084. doi: 10.1242/jeb.01254. [DOI] [PubMed] [Google Scholar]
- Akino K, Ohtsuru A, Kanda K, Yasuda A, Yamamoto T, Akino Y, Naito S, Kurokawa M, Iwahori N, Yamashita S. Parathyroid hormone-related peptide is a potent tumor angiogenic factor. Endocrinology. 2000;141:4313–4316. doi: 10.1210/endo.141.11.7875. [DOI] [PubMed] [Google Scholar]
- Akino K, Ohtsuru A, Yano H, Ozeki S, Namba H, Nakashima M, Ito M, Matsumoto T, Yamashita S. Antisense inhibition of parathyroid hormone-related peptide gene expression reduces malignant pituitary tumor progression and metastases in the rat. Cancer Res. 1996;56:77–86. [PubMed] [Google Scholar]
- Applied-Biosystems. User Bulletin #2. 1997. ABI Prism 7700. [Google Scholar]
- Arthur JM, Collinsworth GP, Gettys TW, Quarles LD, Raymond JR. Specific coupling of a cation-sensing receptor to G protein alpha-subunits in MDCK cells. Am J Physiol. 1997;273:F129–135. doi: 10.1152/ajprenal.1997.273.1.F129. [DOI] [PubMed] [Google Scholar]
- Asa SL, Henderson J, Goltzman D, Drucker DJ. Parathyroid hormone-like peptide in normal and neoplastic human endocrine tissues. J Clin Endocrinol Metab. 1990;71:1112–1118. doi: 10.1210/jcem-71-5-1112. [DOI] [PubMed] [Google Scholar]
- Brown EM. The calcium-sensing receptor: physiology, pathophysiology and CaR-based therapeutics. Subcell Biochem. 2007;45:139–167. doi: 10.1007/978-1-4020-6191-2_6. [DOI] [PubMed] [Google Scholar]
- Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature. 1993;366:575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev. 2001;81:239–297. doi: 10.1152/physrev.2001.81.1.239. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay N. Effects of calcium-sensing receptor on the secretion of parathyroid hormone-related peptide and its impact on humoral hypercalcemia of malignancy. Am J Physiol Endocrinol Metab. 2006;290:E761–770. doi: 10.1152/ajpendo.00350.2005. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay N, Brown EM. Role of calcium-sensing receptor in mineral ion metabolism and inherited disorders of calcium-sensing. Mol Genet Metab. 2006;89:189–202. doi: 10.1016/j.ymgme.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Cheng SX, Geibel JP, Hebert SC. Extracellular polyamines regulate fluid secretion in rat colonic crypts via the extracellular calcium-sensing receptor. Gastroenterology. 2004;126:148–158. doi: 10.1053/j.gastro.2003.10.064. [DOI] [PubMed] [Google Scholar]
- Chilco PJ, Leopold V, Zajac JD. Differential regulation of the parathyroid hormone-related protein gene P1 and P3 promoters by cAMP. Mol Cell Endocrinol. 1998;138:173–184. doi: 10.1016/s0303-7207(97)00239-6. [DOI] [PubMed] [Google Scholar]
- Corbetta S, Lania A, Filopanti M, Vicentini L, Ballare E, Spada A. Mitogen-activated protein kinase cascade in human normal and tumoral parathyroid cells. J Clin Endocrinol Metab. 2002;87:2201–2205. doi: 10.1210/jcem.87.5.8492. [DOI] [PubMed] [Google Scholar]
- Cruciani RA, Dvorkin B, Morris SA, Crain SM, Makman MH. Direct coupling of opioid receptors to both stimulatory and inhibitory guanine nucleotide-binding proteins in F-11 neuroblastoma-sensory neuron hybrid cells. Proc Natl Acad Sci U S A. 1993;90:3019–3023. doi: 10.1073/pnas.90.7.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danks JA, McHale JC, Martin TJ, Ingleton PM. Parathyroid hormone-related protein in tissues of the emerging frog (Rana temporaria): immunohistochemistry and in situ hybridisation. J Anat. 1997;190 (Pt 2):229–238. doi: 10.1046/j.1469-7580.1997.19020229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuel RL, Adler GK, Kifor O, Quinn SJ, Fuller F, Krapcho K, Brown EM. Calcium-sensing receptor expression and regulation by extracellular calcium in the AtT-20 pituitary cell line. Mol Endocrinol. 1996;10:555–565. doi: 10.1210/mend.10.5.8732686. [DOI] [PubMed] [Google Scholar]
- Ferry S, Chatel B, Dodd RH, Lair C, Gully D, Maffrand JP, Ruat M. Effects of divalent cations and of a calcimimetic on adrenocorticotropic hormone release in pituitary tumor cells. Biochem Biophys Res Commun. 1997;238:866–873. doi: 10.1006/bbrc.1997.7401. [DOI] [PubMed] [Google Scholar]
- Fraser RA, Kaneko T, Pang PK, Harvey S. Hypo- and hypercalcemic peptides in fish pituitary glands. Am J Physiol. 1991;260:R622–626. doi: 10.1152/ajpregu.1991.260.3.R622. [DOI] [PubMed] [Google Scholar]
- Fuleihan GE, Brown EM, Gleason R, Scott J, Adler GK. Calcium modulation of adrenocorticotropin levels in women--a clinical research center study. J Clin Endocrinol Metab. 1996;81:932–936. doi: 10.1210/jcem.81.3.8772553. [DOI] [PubMed] [Google Scholar]
- Gerbino A, Ruder WC, Curci S, Pozzan T, Zaccolo M, Hofer AM. Termination of cAMP signals by Ca2+ and G(alpha)i via extracellular Ca2+ sensors: a link to intracellular Ca2+ oscillations. J Cell Biol. 2005;171:303–312. doi: 10.1083/jcb.200507054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer AM, Brown EM. Extracellular calcium sensing and signalling. Nat Rev Mol Cell Biol. 2003;4:530–538. doi: 10.1038/nrm1154. [DOI] [PubMed] [Google Scholar]
- Hofle G, Gasser RW, Buchfelder M, Fahlbusch R, Waldenberger P, Finkenstedt G. Elevated inferior petrosal sinus levels of PTHrP in a patient with Cushing’s disease. Clin Endocrinol (Oxf) 2001;54:555–557. doi: 10.1046/j.1365-2265.2001.01101.x. [DOI] [PubMed] [Google Scholar]
- Holt EH, Lu C, Dreyer BE, Dannies PS, Broadus AE. Regulation of parathyroid hormone-related peptide gene expression by estrogen in GH4C1 rat pituitary cells has the pattern of a primary response gene. J Neurochem. 1994;62:1239–1246. doi: 10.1046/j.1471-4159.1994.62041239.x. [DOI] [PubMed] [Google Scholar]
- Huang C, Hujer KM, Wu Z, Miller RT. The Ca2+-sensing receptor couples to Galpha12/13 to activate phospholipase D in Madin-Darby canine kidney cells. Am J Physiol Cell Physiol. 2004;286:C22–30. doi: 10.1152/ajpcell.00229.2003. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Weir EC, Mangin M, Dannies PS, Kinder B, Deftos LJ, Brown EM, Broadus AE. Expression of messenger ribonucleic acids encoding a parathyroid hormone-like peptide in normal human and animal tissues with abnormal expression in human parathyroid adenomas. Mol Endocrinol. 1988;2:1230–1236. doi: 10.1210/mend-2-12-1230. [DOI] [PubMed] [Google Scholar]
- Insel PA, Ostrom RS. Forskolin as a tool for examining adenylyl cyclase expression, regulation, and G protein signaling. Cell Mol Neurobiol. 2003;23:305–314. doi: 10.1023/A:1023684503883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Enomoto H, Usa T, Villadolid MC, Ohtsuru A, Namba H, Sekine I, Yamashita S. Expression of parathyroid hormone related peptide in human pituitary tumours. J Clin Pathol. 1993;46:682–683. doi: 10.1136/jcp.46.7.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kifor O, MacLeod RJ, Diaz R, Bai M, Yamaguchi T, Yao T, Kifor I, Brown EM. Regulation of MAP kinase by calcium-sensing receptor in bovine parathyroid and CaR-transfected HEK293 cells. Am J Physiol Renal Physiol. 2001;280:F291–302. doi: 10.1152/ajprenal.2001.280.2.F291. [DOI] [PubMed] [Google Scholar]
- King MS, Baertschi AJ. The role of intracellular messengers in adrenocorticotropin secretion in vitro. Experientia. 1990;46:26–40. doi: 10.1007/BF01955409. [DOI] [PubMed] [Google Scholar]
- Lawler OA, Miggin SM, Kinsella BT. Protein kinase A-mediated phosphorylation of serine 357 of the mouse prostacyclin receptor regulates its coupling to G(s)-, to G(i)-, and to G(q)-coupled effector signaling. J Biol Chem. 2001;276:33596–33607. doi: 10.1074/jbc.M104434200. [DOI] [PubMed] [Google Scholar]
- Loretz CA. Extracellular calcium-sensing receptors in fishes. Comp Biochem Physiol A Mol Integr Physiol. 2008;149:225–245. doi: 10.1016/j.cbpa.2008.01.037. [DOI] [PubMed] [Google Scholar]
- Mamillapalli R, VanHouten J, Zawalich W, Wysolmerski J. Switching of G-protein usage by the calcium-sensing receptor reverses its effect on parathyroid hormone-related protein secretion in normal versus malignant breast cells. J Biol Chem. 2008;283:24435–24447. doi: 10.1074/jbc.M801738200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuma T, Rhue N, Kayama M, Mori Y, Adachi K, Yokoi Y, Ping J, Nogimori T, Hirooka Y. Distribution of calcium sensing receptor in rats: an immunohistochemical study. Endocr Regul. 1999;33:55–59. [PubMed] [Google Scholar]
- Quarles LD. Extracellular calcium-sensing receptors in the parathyroid gland, kidney, and other tissues. Curr Opin Nephrol Hypertens. 2003;12:349–355. doi: 10.1097/00041552-200307000-00002. [DOI] [PubMed] [Google Scholar]
- Rey O, Young SH, Yuan J, Slice L, Rozengurt E. Amino acid-stimulated Ca2+ oscillations produced by the Ca2+-sensing receptor are mediated by a phospholipase C/inositol 1,4,5-trisphosphate-independent pathway that requires G12, Rho, filamin-A, and the actin cytoskeleton. J Biol Chem. 2005;280:22875–22882. doi: 10.1074/jbc.M503455200. [DOI] [PubMed] [Google Scholar]
- Romoli R, Lania A, Mantovani G, Corbetta S, Persani L, Spada A. Expression of calcium-sensing receptor and characterization of intracellular signaling in human pituitary adenomas. J Clin Endocrinol Metab. 1999;84:2848–2853. doi: 10.1210/jcem.84.8.5922. [DOI] [PubMed] [Google Scholar]
- Ryan WL, Heidrick ML. Role of cyclic nucleotides in cancer. Adv Cyclic Nucleotide Res. 1974;4:81–116. [PubMed] [Google Scholar]
- Sanders JL, Chattopadhyay N, Kifor O, Yamaguchi T, Butters RR, Brown EM. Extracellular calcium-sensing receptor expression and its potential role in regulating parathyroid hormone-related peptide secretion in human breast cancer cell lines. Endocrinology. 2000;141:4357–4364. doi: 10.1210/endo.141.12.7849. [DOI] [PubMed] [Google Scholar]
- Sidhu A, Sullivan M, Kohout T, Balen P, Fishman PH. D1 dopamine receptors can interact with both stimulatory and inhibitory guanine nucleotide binding proteins. J Neurochem. 1991;57:1445–1451. doi: 10.1111/j.1471-4159.1991.tb08312.x. [DOI] [PubMed] [Google Scholar]
- Strewler GJ. The physiology of parathyroid hormone-related protein. N Engl J Med. 2000;342:177–185. doi: 10.1056/NEJM200001203420306. [DOI] [PubMed] [Google Scholar]
- Taylor SS, Kim C, Vigil D, Haste NM, Yang J, Wu J, Anand GS. Dynamics of signaling by PKA. Biochim Biophys Acta. 2005;1754:25–37. doi: 10.1016/j.bbapap.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Tfelt-Hansen J, Brown EM. The calcium-sensing receptor in normal physiology and pathophysiology: a review. Crit Rev Clin Lab Sci. 2005;42:35–70. doi: 10.1080/10408360590886606. [DOI] [PubMed] [Google Scholar]
- Tucker AL, Jia LG, Holeton D, Taylor AJ, Linden J. Dominance of G(s) in doubly G(s)/G(i)-coupled chimaeric A(1)/A(2A) adenosine receptors in HEK-293 cells. Biochem J. 2000;352(Pt 1):203–210. [PMC free article] [PubMed] [Google Scholar]
- van den Hurk MJ, Cruijsen PM, Schoeber JP, Scheenen WJ, Roubos EW, Jenks BG. Intracellular signal transduction by the extracellular calcium-sensing receptor of Xenopus melanotrope cells. Gen Comp Endocrinol. 2008;157:156–164. doi: 10.1016/j.ygcen.2008.04.005. [DOI] [PubMed] [Google Scholar]
- VanHouten J, Dann P, McGeoch G, Brown EM, Krapcho K, Neville M, Wysolmerski JJ. The calcium-sensing receptor regulates mammary gland parathyroid hormone-related protein production and calcium transport. J Clin Invest. 2004;113:598–608. doi: 10.1172/JCI18776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward DT. Calcium receptor-mediated intracellular signalling. Cell Calcium. 2004;35:217–228. doi: 10.1016/j.ceca.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Wysolmerski JJ. Parathyroid hormone-related protein. In: Rosen CJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Washington, D.C: American Society for Bone and Mineral Research; 2008. pp. 127–133. [Google Scholar]
- Yamamori E, Iwasaki Y, Aoki Y, Nomura A, Tachikawa K, Ariyoshi Y, Mutsuga N, Morishita M, Yoshida M, Asai M, Oiso Y, Saito H. Polyamine regulation of the rat pro-opiomelanocortin gene expression in AtT-20 cells. J Neuroendocrinol. 2001;13:774–778. doi: 10.1046/j.1365-2826.2001.00691.x. [DOI] [PubMed] [Google Scholar]
- Zajac JD, Callaghan J, Eldridge C, Diefenbach-Jagger H, Suva LJ, Hudson P, Moseley JM, Michelangeli VP, Pasquini G. Production of parathyroid hormone-related protein by a rat parathyroid cell line. Mol Cell Endocrinol. 1989;67:107–112. doi: 10.1016/0303-7207(89)90236-0. [DOI] [PubMed] [Google Scholar]
- Zivadinovic D, Tomic M, Yuan D, Stojilkovic SS. Cell-type specific messenger functions of extracellular calcium in the anterior pituitary. Endocrinology. 2002;143:445–455. doi: 10.1210/endo.143.2.8637. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Secretion of PTHrP (A) and ACTH (B) into conditioned media in response to dBcAMP (1mM) at 0.1mM calcium. Stimulation of the cAMP/PKA pathway with this cAMP analog led to secretion of PTHrP and ACTH independent of a change in the extracellular calcium concentration. Bars represent the mean ± S.E. of three individual experiments. Statistical significance is noted on the graphs.
A) PTHrP levels in conditioned media harvested from AtT-20 cells pretreated with the PLC inhibitor, U73122 (10μM), or the PKC inhibitor, Go6976 (10nM), prior to exposure to increasing doses of calcium or neomycin. Neither inhibitor blocked the secretion of PTHrP in response to activation of the CaR. B) ACTH levels in conditioned media harvested from AtT-20 cells pretreated with the PLC inhibitor, U73122, or the PKC inhibitor, Go6976, prior to exposure to increasing doses of calcium or neomycin. Neither inhibitor blocked the secretion of ACTH in response to activation of the CaR. Data are means ± S.E. from three independent experiments. Statistical significance is shown on the graph. There were no statistical differences between control, U73122-treated or G06976-treated cells at any level of calcium or neomycin. Therefore, the letters refer to each group of 3 bars: a denotes a significant difference as compared with 0.1mM calcium, b denotes a significant difference as compared with 2.5 mM calcium, c denotes a significant difference as compared with 5.0 mM calcium and d denotes a significant difference as compared with neomycin.
Results of [35S]GTPγS binding assays from AtT-20 cell membranes using an antibody that recognizes both Gαq and Gα11 (anti-Gαq/11). As shown in the figure, stimulation of muscarinc receptors with carbachol (100 μM) led to the association of significant amounts of [35S]GTPγS with immunoprecipitates pulled down with anti-Gαq/11 antibodies. In contrast, stimulation of the CaR with 5mM calcium or neomycin did not trigger [35S]GTPγS binding to either Gαq or Gα11. Non-specific binding of [35S]GTPγS was assessed by using non-immune serum (N.I. serum) in control immunoprecipitations. Bars represent the mean ± S.E. of six individual experiments. Statistical significance is noted on the graph.