Abstract
Delphinidin (Del), [3,5,7,3′-,4′-,5′-hexahydroxyflavylium], an anthocyanidin and a potent antioxidant abundantly found in pigmented fruits and vegetables exhibits proapoptotic effects in many cancer cells. Here, we determined the effect of Del on growth, apoptosis and differentiation of normal human epidermal keratinocytes (NHEKs) in vitro in submerged cultures and examined its effects in a three-dimensional (3D) epidermal equivalent (EE) model that permits complete differentiation reminiscent of in vivo skin. Treatment of NHEKs with Del (10–40 μm; 24–48 h) significantly enhanced keratinocyte differentiation. In Del-treated cells, there was marked increase in human involucrin (hINV) promoter activity with simultaneous increase in the mRNA and protein expressions of involucrin and other epidermal differentiation markers including procaspase-14 and transglutaminase-1 (TGM1), but without any effect on TGM2. Del treatment of NHEKs was associated with minimal decrease in cell viability, which was not associated with apoptosis as evident by lack of modulation of caspases, apoptosis-related proteins including Bcl-2 family of proteins and poly(ADP-ribose) polymerase cleavage. To establish the in vivo relevance of our observations in submerged cultures, we then validated these effects in a 3D EE model, where Del was found to significantly enhance cornification and increase the protein expression of cornification markers including caspase-14 and keratin 1. For the first time, we show that Del induces epidermal differentiation using an experimental system that closely mimics in vivo human skin. These observations suggest that Del could be a useful agent for dermatoses associated with epidermal barrier defects including aberrant keratinization, hyperproliferation or inflammation observed in skin diseases like psoriasis and ichthyoses.
Keywords: caspase-14, delphinidin, epidermis, involucrin, keratinocyte differentiation, transglutaminase
Introduction
The human skin, predominantly the epidermis, a multilayered stratified squamous epithelium, incessantly exposed to numerous stressors, primarily functions as a protective barrier and regulates body temperature and transepidermal water loss (1–5). The epidermis, typically composed of keratinocytes, progressively undergoes terminal differentiation accompanied with morphological and biochemical changes that result in the densely packed cross-linked impenetrable lipoprotein matrix and corneocytes of the stratum corneum (4).
Epidermal keratinocyte differentiation is a specific type of cell death known as cornification that differs from classical apoptosis, as it does not feature the characteristic cell fragmentation into apoptotic bodies, after which follows phagocytosis. However, cornification requires the expression of a specific isoform of transglutaminase, type I (TGM1), which upon aggregation of keratin filaments by filaggrin (FLG), builds the insoluble keratin-matrix scaffolding for the cornified envelope (4,6,7). Several other cornified envelope precursor proteins, including involucrin, loricrin etc, are substrates for the transglutaminase (8–11). Caspase-14, is a non-apoptotic protease specifically expressed in differentiating epidermal layers of the skin with a known role in the maintenance of epidermal barrier function (12,13).
Because primary normal human epidermal keratinocytes (NHEKs) are a major object of diverse challenges including oxidative stressors, inflammatory mediators, environmental carcinogens and aberrant keratinization, and protect the body against them; it is important to identify and study agents that could modulate the biology and ultimately enhance the function of this crucial barrier. For instance, a natural phytochemical antioxidant that inhibits oxidative stress, hyperproliferation and induces differentiation without triggering apoptosis of normal or even preneoplastic keratinocytes could be useful for treating dermatoses associated with aberrant keratinization, hyperproliferation and inflammation. Previous research efforts identified several botanical compounds including green tea polyphenol, EGCG, sulphoraphane, curcumin, genistein and resveratrol as regulators of keratinocyte biology (14–17). Such agents exert their effects by selectively enhancing NHEK differentiation via several distinct mechanisms with or without triggering apoptosis and inducing keratinocyte proliferation (18). Identifying agents with a more unique mechanism of action, specifically those that induce differentiation without inducing apoptosis would be a welcome addition to the armamentarium of natural products that are regulators of keratinocyte biology.
Del (Fig. 1a; inset), a major dietary antioxidant abundantly found in pigmented fruits and vegetables such as pomegranates, berries, red grapes, purple sweet potatoes, red cabbages, is a reactive oxygen species scavenger and chemopreventive agent in the treatment of oxidative stress and a variety of cancers (19–22). Similar to many other botanical chemopreventive agents, Del exhibits anti-oxidant, anti-inflammatory and antiproliferative properties in cancer cell lines (23–25). We earlier reported that Del treatment of murine skin and HaCaT keratinocytes resulted in protection against UV-induced oxidative stress and apoptosis (19). We hypothesized that Del at low doses may possess a prodifferentiation effect in NHEKs. Here, we provide evidence that Del promotes NHEK differentiation without inducing apoptosis in submerged cultures. To bear relevance to in vivo situation, we reconstituted three-dimensional (3D) epidermal equivalents (EEs) that closely simulate cornification in the human epidermis and made similar observations demonstrating increased differentiation and expression of differentiation markers. To the best of our knowledge, no study has yet reported the prodifferentiation effect of Del on NHEKs or demonstrated its effect in a 3D skin model. Our findings provide a rationale for further investigation of Del in the treatment of aberrant keratinizing skin diseases.
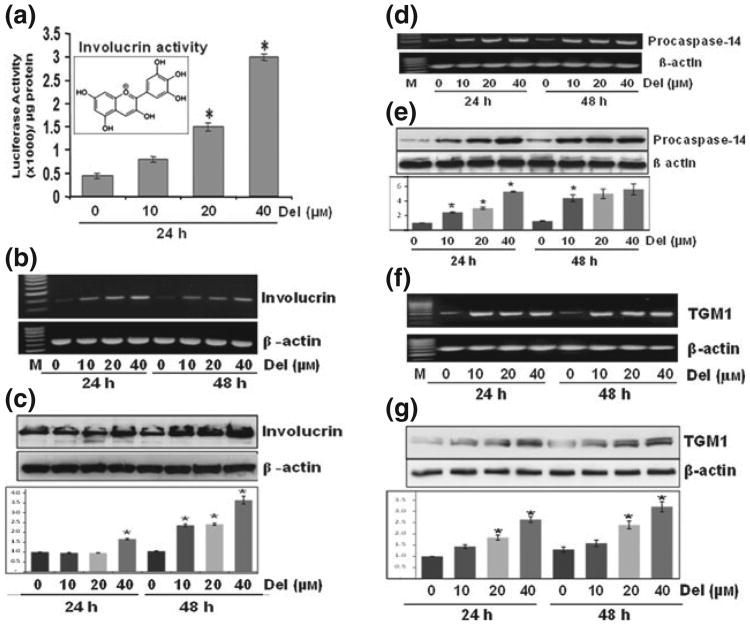
Figure 1.
Del treatment of normal human epidermal keratinocyte (NHEK) induces the gene and protein expression of epidermal differentiation-related proteins Normal human epidermal keratinocytes prepared from neonatal foreskin were treated with Del (0–40 μm) for 24–48 h, and mRNA and protein extracts were prepared as described. (a) Histograms represent the human involucrin promoter activity normalized to Renilla activity in cells treated with Del for 24 h, inset represents the molecular structure of Del (2-(3,4,5-trihydroxyphenyl) chromenylium-3,5,7-triol). (b) Involucrin mRNA expression by reverse transcription-polymerase chain reaction (RT-PCR). (c) Involucrin protein expression by immunoblotting. (d–e) Procaspase-14 mRNA expression by RT-PCR and protein expression by western blotting (f–g) TGM1 mRNA expression by RT-PCR and protein expression by western blotting. Blots are representative of three independent experiments for each treatment group. The blots were stripped and reprobed for β-actin to determine equal protein loading, and the histograms are semiquantitative densitometric results and represent the ratio between target protein and β-actin and is the mean ± SD (n = 3). Each film was scanned on an Epson Perfection V350 photo Scanner (Epson America Inc, Long Beach, CA, USA), and the intensities were quantified using ImageJ (NIH). All assays were performed in duplicate, and each experiment was repeated a minimum of three times. Statistica analysis were performed using ANOVA (Bonferroni's multiple comparison test; *P < 0.05).
Materials and methods
Antibodies and reagents
All antibodies used here and their sources are listed in Table S1. Del chloride (>98% purity) was purchased from Extrasynthese (Genay-Cedex, France), all-trans retinoic acid (atRA), 1, 25(OH)2 vitamin D3 (vitD3), bovine serum albumin (BSA) and dimethyl sulphoxide (DMSO) were purchased from Sigma (St. Louis, MO, USA). The BCA™ protein assay kit and SuperSignal® West Pico chemiluminescent substrate kit (Pierce; Thermo Scientific, Rockford, IL, USA), the mini-protean precast Tris-Glycine gels (BioRad, Hercules, CA, USA) and, except otherwise stated, all other chemicals were obtained from Sigma.
Cell isolation and culture
Normal human epidermal keratinocytes were isolated from deidentified neonatal foreskins obtained from routine circumcisions at Meriter Hospital (Madison); following institutional guidelines under a UW-Madison approved IRB protocol and experiments were conducted with adherence to the Declaration of Helsinki Principles. Normal human epidermal keratinocyte cultures were established from extensively washed and trimmed foreskin tissues as described previously (26). Submerged NHEKs were maintained in low calcium serum-free Epi-Life growth medium supplemented with Human Keratinocytes Growth Supplement HKGS (Invitrogen, Carlsbad, CA, USA) and antibiotic–antimycotic solution. Second-to-sixth passage NHEKs were used in these experiments, and all cultures were maintained at 37°C in a humidified atmosphere containing 5% CO2.
Determination of cell growth, viability, proliferation, apoptosis and cell cycle
The effect of Del (0–40 μm) on cell growth, viability, morphology, cell cycle, apoptosis and proliferation was determined by MTT assay, trypan blue dye exclusion assay, flow cytometry, Annexin-V-FLUOS staining and thymidine analogue, 5-bromo-2-deoxyuridine (BrdU) labelling kits as described in the Appendix S1.
Human involucrin promoter–luciferase activity
Normal human epidermal keratinocytes were seeded at 1.5 × 105 cells per well in 24-well plates and cotransfected with 500 ng of endotoxin-free hINV promoter–luciferase reporter plasmid (pINV-2473; kindly provided by Dr Richard L. Eckert) and 25 ng Renilla plasmid using Lipofectamine 2000 (Invitrogen). After 18 h, the cells were treated with Del (0–40 μm) for 24–48 h, and luciferase activity was measured using the Reporter luciferase assay system and normalized with respect to Renilla activity (Promega, Madison, WI, USA). All assays were performed in duplicate, and each experiment was repeated a minimum of three times.
Generation of 3D reconstituted epidermal equivalents
In vitro reconstructed EEs were generated as described previously (27,28) with slight modifications. Briefly, second-to-third passage NHEKs established in low calcium Epi-Life medium were seeded at 20% confluence and were synchronically switched to and maintained in Progenitor Cell targeted Culture Media (CnT-02-07; CELLnTEC, Bern, Switzerland) prior to harvest and generation of 3D cultures.
After trypsinization, 3 × 105 cells/insert in 250 μl of CnT-02-07 were seeded into 0.4 μm Millicell-PCF inserts (Millipore Corporation, Billerica, MA, USA) each placed in wells of a six well plate containing 2.5 ml of CnT-02-07 medium. After 24–48 h of submerged culture, CnT-02-07 was switched to CnT-02-3DP5 differentiation medium (2 ml outside the insert and 0.4 ml inside the inserts) and further grown for 24 h to initiate differentiation. After initiating differentiation, medium was then removed outside and inside the insert, and the medium outside the insert was then replenished with 1.2 ml of CnT-02-3DP5 complete medium supplemented with or without different concentrations of test compounds, and the cultures were kept at the air–liquid interface from this time point onwards. After every alternate day, fresh prewarmed CnT-02- 3DP5 medium with or without test drugs was replenished and cultures were continued for 4–12 days. At indicated harvest days, (3–4 mmφ) punch biopsy from each insert was obtained, formalin-fixed and processed for either H&E or immunostaining.
Del, vitD3 and atRA treatment of keratinocytes
Del, atRA and vitD3 were dissolved in DMSO and stored as 80 mm, 10−2 m and 0.1 mm aliquots, respectively, at −20°C protected from light. At the time of use, test compounds, Del (0–40 μm), vitD3 (0.1 μm) or atRA (1 μm), were diluted in culture medium containing 0.1% BSA to stabilize the drugs and treatment was started at 80% confluence or starting from when EEs were exposed to air–liquid interface. For the 3D EE studies, the atRA dose (1 μm) selected is known to impair cornification and is used as a negative control (29), whereas vitD3 (1 × 10−7 μm) reportedly enhances cornification and is used as a positive control (30).
Dimethyl sulphoxide at 0.01–0.05% (v/v) served as vehicle-control, and NHEKs were incubated with the drugs for 24–48 h in submerged cultures or for 4–12 days in EE cultures until harvest.
Immunocytochemistry, histology, morphometry and immunostaining of the EEs
Normal human epidermal keratinocytes cultured on chamber slides were immunocytochemically stained by fixating with 4% paraformaldehyde for 30 min at room temperature (rt), washed thrice in phosphate-buffered saline (PBS) and blocked with 2% BSA for 20 min at rt. The slides were incubated with 0.3% H2O2 followed by various primary antibodies overnight at 4°C and were further processed for staining and visualization as described under immunohistochemistry below.
For histology and morphometry, de-paraffinized cross-sections (5 μm) were stained with H&E and evaluated for gross morphology, epidermal and stratum corneum thicknesses. Slides were visualized on a Nikon Eclipse Ti system microscope (Nikon Instruments Inc, Tokyo, Japan), and digital images were captured with an attached CoolSNAP camera (Roper Scientific, Trenton, NJ, USA) linked to a computer. The sections were systematically sampled for digital images at constant intervals with a 20 × objective. The thickness in terms of the area of the entire epidermis (total epidermal thickness indicating the sum of the viable epidermis and the stratum corneum) and the stratum corneum were measured using Image J 1.45 software (NIH, Bethesda, MD, USA), and five measurements were obtained per treatment group per experiment. Differences in the intensity of keratohyalin granules were evaluated by comparing the punctate granular staining intensity of the EE granular layer keratinocytes under the microscope between control and Del-treated tissues. Images were exported as TIFF files, and figures were prepared using Adobe Photoshop and Illustrator software. Data obtained from three to four separate EE cultures were analysed for each group, and a total of three experiments were performed.
For immunostaining, cross-sections were processed as described elsewhere (31). In brief, slides were subjected to antigen retrieval by boiling in 10 mm citrate buffer pH 6.0 at 500 W for 10 min, cooled to rt and blocked with 2% BSA/2.5% normal goat serum (DAKO, Glostrup, Denmark) for 30 min and incubated with primary antibodies (Table S1) overnight at 4°C Biotinylated secondary antibodies (1:500; Vector Laboratories, Burlingame, CA, USA) were detected with Vectastain ABC technique. Diaminobenzidine (DAB) was used as chromogenic substrate, and slides were counterstained with weak solution of haematoxylin, dehydrated, mounted in xylene-based mounting medium (Fisher Diagnostics, Middletown, VA, USA), visualization and analyzed as described above.
Preparation of protein lysates, SDS-PAGE and immunoblot analysis
Normal human epidermal keratinocytes were harvested at 24 and 48 h and whole cell lysates were prepared and western blots were carried out as described elsewhere (32,33). In brief, cells were washed with cold PBS (10 mm, pH 7.4), incubated in ice-cold RIPA lysis buffer, with freshly added Na3VO4 (100 mm), PMSF (1 mm) and protease inhibitor cocktail Set III (Calbiochem, La Jolla, CA, USA) on ice for 30 min. Cells were scraped, collected in a microfuge tube, disaggregated through a 22½-G needle, and lysates were cleared by centrifugation at 13 000 g for 25 min at 4°C, and the supernatants were stored at −80°C The lysates protein content was measured by the BCA protein assay kit as per the manufacturer's protocol, and western blot analysis was performed as described elsewhere (34). In brief, equal quantities of protein were electrophoresed on denaturing and reducing polyacrylamide gels transferred onto nitrocellulose membranes and were immunoblotted after blocking by incubation with the corresponding primary antibodies overnight followed by appropriate HRP-conjugated secondary antibodies. Secondary antibody binding was visualized by chemiluminescent detection system using ECL SuperSignal West Pico kit, and developed by the BioRad UV-trans Camera or on film. Each blot was stripped and reprobed with β-actin to assess equal protein loading, and data are presented as the relative density of protein bands normalized to β-actin.
RNA isolation, complementary DNA synthesis and PCR
Isolation of mRNA and the reverse transcription-polymerase chain reaction (RT-PCR) was performed as described earlier (35). Briefly RNA was isolated from near-confluent keratinocytes treated with/without Del (0–40 μm) for 24–48 h using RNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to manufacturer's protocol. Reverse transcription-PCR was performed using iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA) according to the manufacturer's instructions using M-MLV reverse transcriptase (Life Technologies, Gaithersburg, MD, USA) and an oligo (dt) primer (Promega) as described elsewhere (36). RNase-free water and RNasin, a ribonuclease inhibitor (Promega), were used in all steps involving RNA.
The RT-PCR standardization kit was obtained from Epicenter Biotechnologies and the cDNAs were stored at −70°C until later used. The RT-PCR consisted of a 0.4 μm final concentration of primers and the reaction steps for procaspase-14 was 35 cycles of denaturation (94°C; 10 min), annealing (60°C; 30 s) and elongation (72°C; 75 s) with final extension at 72°C. Human involucrin, β-actin, TG-1 and TG-2 reaction steps consisted of 25 cycles of denaturation (94°C; 10 min), annealing (55°C; 30 s) and elongation (68°C; 90 s) with final extension at 72°C. For quantitative purposes, all samples were initially amplified for 20–40 cycles which produced a linear response for selection of appropriate numbers of cycles for each gene. Amplicons were detected by electrophoresis on a 1.5% agarose gel containing ethidium bromide. The primers and sequences used are listed in Table S2.
Statistical analysis
Results are presented as mean ± SD. Statistical significance was determined by one-way analysis of variance (ANOVA) with Bonferroni post-test for multiple comparisons or unpaired Student's post hoc t-test, using Graph Pad Prism version-4.03 software (San Diego, CA, USA) and P ≤ 0.05 was considered to be statistically significant.
Results
Del treatment of NHEK regulates involucrin promoter activity and induces the expression of differentiation markers in a dose- and time-dependent manner
To understand the role of Del in keratinocyte differentiation, we first examined its effect in submerged keratinocyte cultures. Because differentiation-related structural proteins are key players of epidermal differentiation, we assessed whether Del regulates the activity of hINV promoter. The differentiation marker involucrin is a precursor of keratinocyte cornified cell envelope that is selectively expressed in suprabasal epidermal layers (15,37). As shown in Fig. 1a, Del (0–40 μm) treatment of NHEKs for 24 h resulted in increase in hINV promoter activity. We also found that Del treatment elicited a dose-dependent increase in mRNA and protein expression of markers of NHEK differentiation such as involucrin (Fig. 1b–c), procaspase 14 (Fig. 1d–e) and TGM1 (Fig. 1f–g), but not TGM2 (Fig. S1). Furthermore, treatment with Del also induced the expression of other differentiation markers, including profilaggrin and keratin 1 (Fig. S2).
Del treatment of NHEK modulates morphology, proliferation and viable cell numbers
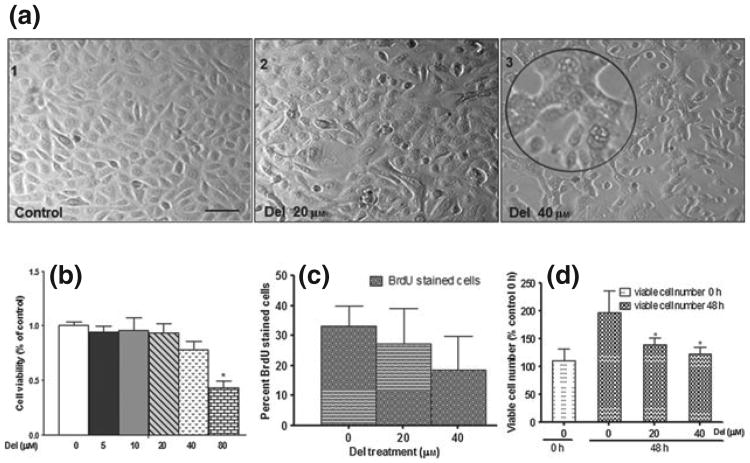
Differentiation is generally associated with an obligatory loss of proliferative capacity (16). We, therefore, examined the effects of Del treatment on growth and morphology of NHEKs, which is characterized by individual cobblestone-like cell morphology that develops into loose, less structured colonies. In contrast, treatment with Del resulted in concentration-dependent (20–40 μm) morphological changes characterized by multivacuolated cells in strongly adherent flattened colonies (Fig. 2a), and did not significantly alter cell viability (20–40 μm), except at higher dose (80 μm) used as positive control for cytotoxicity (Fig. 2b). To further characterize these effects, NHEKs were cultured with or without the addition of Del (20–40 μm), pulse-labelling with BrdU for 6 h and analysed the percentage of cells that traversed the S phase by immunocytochemistry. The results demonstrated that Del treatment of submerged NHEK tend to decreased cell proliferation, although not statistically significant (Fig. 2c). To further validate these observations, similar number of NHEKs were plated and cultured in medium with or without Del, and the number of viable cells was determined 48 h post-treatment. Again, in agreement with previous experiments, the viability of Del-treated keratinocytes was not compromised [Fig. 2c, control 0 h and Del (40 μm), 48 h]. However the numbers of viable cells were decreased in Del-treated groups when compared to untreated control (48 h; Fig. 2d). Taken together, these results demonstrate that the Del treatment led to decreased proliferation of NHEK at these selected doses.
Figure 2.
Del treatment of normal human epidermal keratinocyte (NHEK) modulates morphology, growth, proliferation and viable cell number. (a) Phase contrast photomicrographs of control NHEK (a1) and NHEKs treated with Del 20 μm (a2) and 40 μm (a3). Near-confluent NHEKs were treated for 48 h with or without Del (0–40 μm). Inset (a3). Magnified image showing multivacuolated and stretched adherent cells. (b) Histograms representing effect of Del treatment on cell growth by MTT assay. Normal human epidermal keratinocytes were treated with Del (inset; 0–80 μm) for 48 h, (*P < 0.05). (c) Histograms representing effect of Del treatment on cell proliferation by 5-bromo-2-deoxyuridine (BrdU) labelling. Cells were treated with Del (0–40 μm) for 48 h and then the medium was supplemented with 10 μm BrdU labelling reagent for 6 h. (d) Histograms representing effect of Del treatment on viable cell number using the trypan blue dye exclusion assay. Normal human epidermal keratinocytes were treated with Del (0–40 μm) for 48 h and viable cell number determined and expressed as percentage cell number harvested (48 h) in relation to the cell number present at the time of initiation of treatment (0 h), (*P < 0.05). The Del concentration of 80 μm was used as a positive control for cytotoxicity and apoptosis.
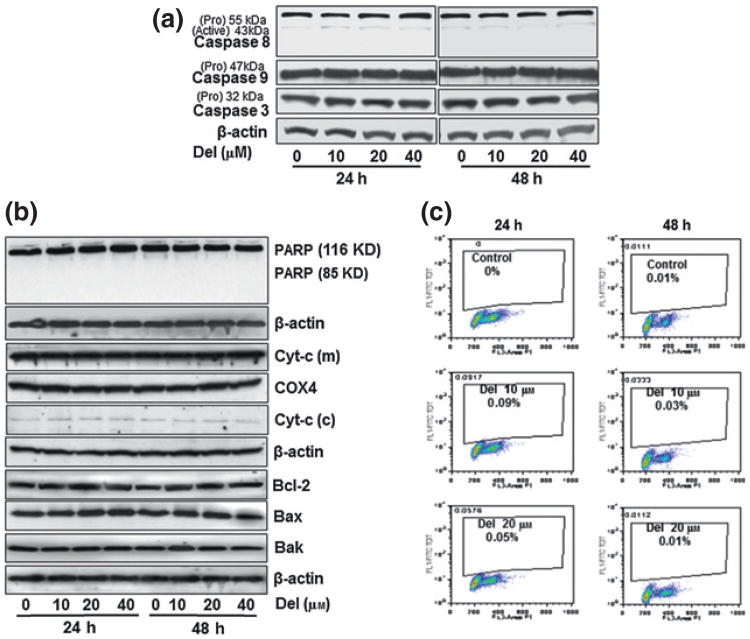
Del treatment of NHEK does not induce apoptosis
Immunoblot, Annexin-V/PI staining and flow cytometric analyses consistently revealed that Del (10–40 μm) treatment of NHEK did not induce apoptosis at the time points investigated as it did not result in the activation of caspases -3, -8 or -9 (Fig. 3a). As an additional measure of the apoptotic status, treatment with Del did not show any alteration in the poly(ADP)-ribose polymerase (PARP; 116 KD) levels, downstream of caspase-3, with no increase observed in the cleaved PARP product (85 KD; Fig. 3b). Del did not exhibit any effect on the protein expression of members of the Bcl-2 family including the proapoptotic Bax and Bak or the antiapoptotic Bcl-2 protein (Fig. 3b). Moreover, no change in cytochrome c protein expression in the cytosolic and mitochondrial fractions was observed as compared to the untreated NHEKs (Fig. 3b). Furthermore, apoptosis induction analysed by immunofluorescence using Annexin-V and FACS analysis confirmed that Del did not induce apoptosis (Fig. S3) and did not affect the sub-G1 population of cells in NHEK (Fig. 3c). The Del dose (80 μm) shown was used as positive control which induce cytotoxic and apoptotic effects on NHEK.
Figure 3.
Del treatment of normal human epidermal keratinocyte (NHEK) does not induce apoptosis nor alter cell cycle profile. Normal human epidermal keratinocytes were seeded, grown to near confluence and treated with or without Del (10–40 μm) for 24 or 48 h. (a) Immunoblot analysis of cell lysates probed with antibodies specific for human caspases -3, -8 & -9 (b) Immunoblot analysis of cell lysates probed with antibodies specific for human poly(ADP)-ribose polymerase, cytosolic and mitochondrial cytochrome c, Bcl2, Bax and Bak. The β-actin or COX4 antibodies were used to reprobe to confirm equal protein loading. (c) Flow cytometric analysis of propidium iodide stained NHEKs treated with or without Del (10–20 μm) for 48 h to show the percentage of sub-G1, cells. These experiments were repeated at least three times and only one representative data is presented.
Del treatment of NHEK enhances cornification in a 3D reconstituted EE model
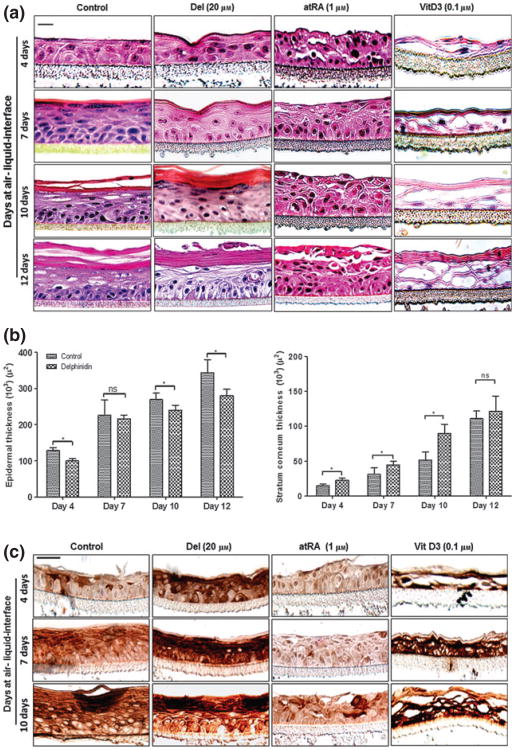
Our observations in submerged monolayer cultures of NHEKs suggest that Del could modulate differentiation of keratinocytes under physiological conditions. In fact, NHEK differentiation in submerged cultures over time is incomplete, but when cultured under 3D organotypic conditions can closely mimic the differentiation pattern seen under physiological conditions, which allows better evaluation of test substances. We therefore determined the effect of Del in a 3D human reconstituted EE model of cornification and compared it with atRA and vitD3, well-known physiological modulators of keratinocyte differentiation.
Control EEs at 4 days showed only minor development of the cornified layers, whereas evidence of more consolidated stratum corneum was observed in the Del-treated EEs (Fig. 4a). The 7–12 days Del-treated reconstructs exhibited more keratohyalin granules and lesser parakeratosis compared to controls as determined by comparative scoring of the proportion of staining intensity of the dark granular keratinocytes on haematoxylin and eosin (H&E) stained sections in control compared to Del-treated group. However, as expected treatment with atRA impaired the cornified cell envelope formation, which resulted in characteristic thickened viable cell layers, consisting of scattered piles of keratinocytes throughout the developing epidermis (Fig. 4a). The vitD3-treated reconstructs showed a more rapidly formed epidermis with a strongly differentiated epithelium already at day 4, but with much less defined epidermal layers compared to the corresponding untreated controls and Del-treated tissues (Fig. 4a). Furthermore, Del treatment of the reconstructed tissues reduced epidermal thickness (Fig. 4b, left panel) and also resulted in increased thickness of the stratum corneum (Fig. 4b, right panel).
Figure 4.
Del treatment enhances cornification and induces caspase-14 expression in the developing 3D epidermal equivalent (EE) model. Normal human epidermal keratinocyte were grown on Millipore PCF cell culture inserts at air–liquid interface to generate a 3D EE treated with or without test compounds as described above, and harvested after 4, 7, 10 and 12 days for histomorphometry and thickness of the EE. (a) Representative H&E photomicrographs of histology of organotypic epidermis untreated controls (left panel) or treated with Del (second panel from left), all-trans retinoic acid (atRA)-treated (third panel from left) or 1, 25(OH)2 vitamin D3 (vitD3)-treated (right panel). (b) Histograms represent the thicknesses of the total epidermis (left panel) and stratum corneum (right panel) of 4, 7, 10 and 12-days-old organotypic epidermis treated with or without Del and analysed as described in Materials and methods. Data represent means ± SD of three independent experiments each performed in triplicate. Statistical analysis was performed using ANOVA (Bonferroni's multiple comparison test; *P < 0.05). (c) Representative micrographs of immunostaining showing caspase-14 expression in developing 3D EE at 4, 7 and 10 days after air-liquid interface. Untreated controls (left panel) or treated with Del (second panel from left) or atRA-treated (third panel from left) and vitD3 (right panel). Bar = 20 μm.
Del treatment of developing 3D reconstituted EE model elicits an enhanced expression of caspase-14 and other differentiation markers
Considering that NHEKs are at the forefront of the body's defense against oxidative insults, and that Del inhibits their proliferation and induces differentiation, we next examined its effects on specific markers of differentiation known to be associated with hyperproliferative disorders such as psoriasis (38) and differentiation. We examined in this 3D EEs by immunohistochemistry caspase-14 expression, which is often down-regulated in psoriatic lesions and keratin 1. An intermittent patchy and intermediate suprabasal staining pattern was observed in the control EEs at 4 days and was increased at 7 and 10 days (Fig. 4c, control panel). At all time points investigated, Del treatment enhanced caspase-14 and Keratin 1 (K1) protein expression in the developing epidermis relative to the untreated controls (Fig. 4c, Del panel and Fig. S4). Moreover, vitD3-treated reconstructs also showed very intense caspase-14 staining clearly evident at day 4, which was significantly increased throughout the time points studied (Fig. 4c, vitD3 panel). In contrast, in our system, and as previously reported in other studies, atRA treatment (29) impaired cornification and exhibited a complete absence of caspase-14 staining up to 7 days, but resulted in a subtle induction at day 10 (Fig. 4c, atRA panel).
Discussion
The most significant finding of this study is the demonstration that Del treatment of NHEKs promotes differentiation both in submerged cultures and 3D EEs model resulting in increased expression of the studied markers of keratinocyte differentiation. Further, evidence is also presented that Del-induced differentiation of human EEs model is associated with an increase in caspase-14 expression, a protease tightly regulated during keratinization and down-regulated in hyperproliferative skin disorders including psoriasis.
Del increased the expression of involucrin promoter activity, a precursor of the keratinocyte cornified cell envelope specifically expressed in the suprabasal layers of the epidermis (10,15,37,39). In addition, the mRNA and protein levels of the major transglutaminase isoform, TGM1, amongst several other markers expressed by NHEKs, responsible for cornified cell envelope assembly, and expressed in a differentiation-dependent manner (40), was selectively increased by Del treatment. However, the expression of TGM2, an isoform not generally associated with differentiation, but induced by retinoic acid (41), was not altered by Del treatment.
Apart from cornification, keratinocytes can also undergo cell death through apoptosis, a cell suicide process with characteristic morphological features that include cell shrinkage, membrane blebbing, apoptotic bodies and phagocytosis (42–44). During apoptosis, activation of procaspases-3, -8 and -9 leads to the downstream cleavage of PARP and followed by destruction of cell organelles, nuclear condensation and DNA fragmentation (44). We showed previously that Del is an anticancer agent whose biological role is attributable to its strong antioxidant and proapoptotic activities (25,45,46). Our earlier study suggested that Del protects HaCaT keratinocytes and murine skin against apoptosis and UV-mediated oxidative stress (19). In the current study, we observed that Del does not promote the cleavage of procaspases-3, 8 or 9 or the release of cytochrome c nor modulates the levels of Bcl2, Bax or Bak in NHEK at doses where it promotes differentiation.
Caspase-14 is involved in both the proteolysis of pro-FLG into multiple units of FLG monomers (12,13) and further proteolysis of these monomers into free amino acids (47). In fact, it's down-regulation is often unequivocally implicated in dermatoses with impaired skin barrier function, for which available conventional treatment options are limited, necessitating the development of novel treatment strategies (48,49). Here, we observed an increase in procaspase-14 expression by Del treatment in submerged NHEK cultures, and no processing of procaspase-14 was detected with or without Del treatment similar to previous studies (29,30,50).
Importantly, we observed in the 3D EE model that Del-induced differentiation. Histo-morphometric analysis demonstrated that Del treatment led to accelerated cornification resulting in decreased epidermal thickness and increased stratum corneum thickness. Immunohistochemical analysis revealed that caspase-14 expression was strongly induced in Del-treated EEs as early as 4 days which remained sustained up to 10 days compared to control EEs (Fig. 4c). In our studies, 3D EEs treated with Del or vitD3 exhibited increased caspase-14 expression, whereas atRA treatment showed a decreased expression. Of note, analogues of vitD3 for example, calcipotriol are mainstay therapy for psoriasis, and we show here that in addition to increasing caspase-14 expression, the architecture of Del-treated EEs closely resembled that of untreated controls when compared to the rapidly cornified but less preserved morphology of vitD3 treated EEs. This suggests that Del holds promise for its effects on hyperproliferative/inflammatory skin conditions (51). One advantage of Del over atRA, which has several side effects associated with teratogenicity, is that it is a non-toxic, naturally occurring plant-derived ingredient that is widely present in diets commonly consumed by humans.
Based on our observations, it is tempting to suggest that Del can be a useful agent for the treatment of aberrant keratinizing skin diseases such as wound healing, psoriasis and ichthyoses. In fact, we (unpublished observation) and several others have found that NHEKs in psoriatic lesions, exhibit reduced caspase-14 expression in the differentiating skin layer (38,52,53), often correlating with the association of caspase-14 expression and terminal differentiation. Based on these data, we envision that topical or systemic application of preparations containing Del can result in enhanced cornification and reduce apoptosis offering beneficial effects to diseased skin. Additional studies are warranted to assess these possibilities and to understand the detailed molecular mechanism of action of Del and fully delineate the signal transduction pathways involved.
Overall, this study reports that Del promotes NHEK terminal differentiation associated with increases in differentiation specific responses not related to apoptosis, but critical to the homeostatic maintenance of normal epidermal barrier function.
Supplementary Material
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Del treatment of normal human epidermal keratinocyte does not alter the gene and protein expression of TGM2.
Figure S2. Del treatment of normal human epidermal keratinocyte induces the protein expression of several differentiation markers.
Figure S3. Del treatment of normal human epidermal keratinocyte does not induce apoptosis and necrosis.
Figure S4. Del treatment enhances cornification and induces keratin-1 expression in the developing 3D epidermal equivalent model.
Table S1. The antibodies used and their sources are listed below.
Table S2. Primer sequences for PCR analysis.
Appendix S1. Determination of cell growth, viability, proliferation, apoptosis and cell cycle.
Acknowledgments
Work in HM's laboratory was supported by US PHS Grant RO1 AR 059742; IAS was supported by T32AR055893 and ACS grant 120038-MRSG-11-019-01-CNE; and FA was supported by R21 AT004966.
Abbreviations
- atRA
all-trans retinoic acid
- BSA
bovine serum albumin
- Del
delphinidin
- DMSO
dimethyl sulphoxide
- vitD3
1, 25(OH)2 vitamin D3
- PARP
poly(ADP)-ribose polymerase
- RT-PCR
reverse transcription-polymerase chain reaction
- Delphinidin
generic name: chemical name: (2R,3R)-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-1(2H)-benzopyran-3,5,7-triol 3-(3,4,5-trihydroxybenzoate)
- EE
epidermal equivalent
- NHEK
normal human epidermal keratinocyte
- FLG
filaggrin
- PBS
phosphate-buffered saline
- PBST
0.1% Tween 20 in PBS
Footnotes
Author contributions: JCC, FA and HM designed the research study. JCC, IAS, DNS, NK, SS and BTB performed the research. JCC, FA, IAS, DNS, GSW, VMA and HM analysed the data. JCC wrote the paper and all authors commented and approved the final draft.
Conflict of interests: The authors have declared no conflicting interests.
References
- 1.Elias PM. Curr Allergy Asthma Rep. 2008;8:299–305. doi: 10.1007/s11882-008-0048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feingold KR, Schmuth M, Elias PM. J Invest Dermatol. 2007;127:1574–1576. doi: 10.1038/sj.jid.5700774. [DOI] [PubMed] [Google Scholar]
- 3.Presland RB, Coulombe PA, Eckert RL, et al. J Invest Dermatol. 2004;123:603–606. doi: 10.1111/j.0022-202X.2004.23226.x. [DOI] [PubMed] [Google Scholar]
- 4.Sandilands A, Sutherland C, Irvine AD, et al. J Cell Sci. 2009;122:1285–1294. doi: 10.1242/jcs.033969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Segre JA. J Clin Invest. 2006;116:1150–1158. doi: 10.1172/JCI28521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Candi E, Schmidt R, Melino G. Nat Rev Mol Cell Biol. 2005;6:328–340. doi: 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- 7.Denecker G, Hoste E, Gilbert B, et al. Nat Cell Biol. 2007;9:666–674. doi: 10.1038/ncb1597. [DOI] [PubMed] [Google Scholar]
- 8.Nemes Z, Steinert PM. Exp Mol Med. 1999;31:5–19. doi: 10.1038/emm.1999.2. [DOI] [PubMed] [Google Scholar]
- 9.Rice RH, Green H. Cell. 1977;11:417–422. doi: 10.1016/0092-8674(77)90059-9. [DOI] [PubMed] [Google Scholar]
- 10.Rice RH, Green H. Cell. 1979;18:681–694. doi: 10.1016/0092-8674(79)90123-5. [DOI] [PubMed] [Google Scholar]
- 11.Robinson NA, LaCelle PT, Eckert RL. J Invest Dermatol. 1996;107:101–107. doi: 10.1111/1523-1747.ep12298323. [DOI] [PubMed] [Google Scholar]
- 12.Denecker G, Ovaere P, Vandenabeele P, et al. J Cell Biol. 2008;180:451–458. doi: 10.1083/jcb.200709098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lippens S, Denecker G, Ovaere P, et al. Cell Death Differ. 2005;12(Suppl 2):1497–1508. doi: 10.1038/sj.cdd.4401722. [DOI] [PubMed] [Google Scholar]
- 14.Balasubramanian S, Eckert RL. J Biol Chem. 2004;279:24007–24014. doi: 10.1074/jbc.M314331200. [DOI] [PubMed] [Google Scholar]
- 15.Balasubramanian S, Efimova T, Eckert RL. J Biol Chem. 2002;277:1828–1836. doi: 10.1074/jbc.M110376200. [DOI] [PubMed] [Google Scholar]
- 16.Balasubramanian S, Sturniolo MT, Dubyak GR, et al. Carcinogenesis. 2005;26:1100–1108. doi: 10.1093/carcin/bgi048. [DOI] [PubMed] [Google Scholar]
- 17.Efimova T, LaCelle P, Welter JF, et al. J Biol Chem. 1998;273:24387–24395. doi: 10.1074/jbc.273.38.24387. [DOI] [PubMed] [Google Scholar]
- 18.Balasubramanian S, Eckert RL. Toxicol Appl Pharmacol. 2007;224:214–219. doi: 10.1016/j.taap.2007.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Afaq F, Syed DN, Malik A, et al. J Invest Dermatol. 2007;127:222–232. doi: 10.1038/sj.jid.5700510. [DOI] [PubMed] [Google Scholar]
- 20.Fotsis T, Pepper MS, Aktas E, et al. Cancer Res. 1997;57:2916–2921. [PubMed] [Google Scholar]
- 21.Mazza G. Crit Rev Food Sci Nutr. 1995;35:341–371. doi: 10.1080/10408399509527704. [DOI] [PubMed] [Google Scholar]
- 22.Nagase H, Sasaki K, Kito H, et al. Planta Med. 1998;64:216–219. doi: 10.1055/s-2006-957412. [DOI] [PubMed] [Google Scholar]
- 23.Martin S, Favot L, Matz R, et al. Biochem Pharmacol. 2003;65:669–675. doi: 10.1016/s0006-2952(02)01568-x. [DOI] [PubMed] [Google Scholar]
- 24.Syed DN, Afaq F, Sarfaraz S, et al. Toxicol Appl Pharmacol. 2008;231:52–60. doi: 10.1016/j.taap.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yun JM, Afaq F, Khan N, et al. Mol Carcinog. 2009;48:260–270. doi: 10.1002/mc.20477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chamcheu JC, Lorie EP, Akgul B, et al. J Dermatol Sci. 2009;53:198–206. doi: 10.1016/j.jdermsci.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Chamcheu JC, Pihl-Lundin I, Mouyobo CE, et al. Br J Dermatol. 2011;164:263–272. doi: 10.1111/j.1365-2133.2010.10092.x. [DOI] [PubMed] [Google Scholar]
- 28.Van Gele M, Geusens B, Speeckaert R, et al. Exp Dermatol. 2011;20:773–775. doi: 10.1111/j.1600-0625.2011.01319.x. [DOI] [PubMed] [Google Scholar]
- 29.Rendl M, Ban J, Mrass P, et al. J Invest Dermatol. 2002;119:1150–1155. doi: 10.1046/j.1523-1747.2002.19532.x. [DOI] [PubMed] [Google Scholar]
- 30.Lippens S, Kockx M, Denecker G, et al. Am J Pathol. 2004;165:833–841. doi: 10.1016/S0002-9440(10)63346-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siddiqui IA, Shukla Y, Adhami VM, et al. Pharm Res. 2008;25:2135–2142. doi: 10.1007/s11095-008-9553-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saleem M, Maddodi N, Abu Zaid M, et al. Clin Cancer Res. 2008;14:2119–2127. doi: 10.1158/1078-0432.CCR-07-4413. [DOI] [PubMed] [Google Scholar]
- 33.Siddiqui IA, Malik A, Adhami VM, et al. Oncogene. 2008;27:2055–2063. doi: 10.1038/sj.onc.1210840. [DOI] [PubMed] [Google Scholar]
- 34.Siddiqui IA, Adhami VM, Afaq F, et al. J Cell Biochem. 2004;91:232–242. doi: 10.1002/jcb.10737. [DOI] [PubMed] [Google Scholar]
- 35.Chamcheu JC, Navsaria H, Pihl-Lundin I, et al. J Invest Dermatol. 2011;131:1684–1691. doi: 10.1038/jid.2011.93. [DOI] [PubMed] [Google Scholar]
- 36.Torma H, Geijer S, Gester T, et al. Toxicol In Vitro. 2006;20:472–479. doi: 10.1016/j.tiv.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 37.Efimova T, Eckert RL. J Biol Chem. 2000;275:1601–1607. doi: 10.1074/jbc.275.3.1601. [DOI] [PubMed] [Google Scholar]
- 38.Hsu S, Qin H, Dickinson D, et al. In Vivo. 2007;21:279–283. [PubMed] [Google Scholar]
- 39.Banks-Schlegel S, Green H. J Cell Biol. 1981;90:732–737. doi: 10.1083/jcb.90.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thacher SM. J Invest Dermatol. 1989;92:578–584. [PubMed] [Google Scholar]
- 41.Phillips MA, Qin Q, Mehrpouyan M, et al. Biochemistry. 1993;32:11057–11063. doi: 10.1021/bi00092a015. [DOI] [PubMed] [Google Scholar]
- 42.Gandarillas A. Exp Gerontol. 2000;35:53–62. doi: 10.1016/s0531-5565(99)00088-1. [DOI] [PubMed] [Google Scholar]
- 43.Gandarillas A, Goldsmith LA, Gschmeissner S, et al. Exp Dermatol. 1999;8:71–79. doi: 10.1111/j.1600-0625.1999.tb00350.x. [DOI] [PubMed] [Google Scholar]
- 44.Zimmermann KC, Bonzon C, Green DR. Pharmacol Ther. 2001;92:57–70. doi: 10.1016/s0163-7258(01)00159-0. [DOI] [PubMed] [Google Scholar]
- 45.Siddiqui IA, Adhami VM, Murtaza I, et al. Cell Cycle. 2008;7:3320–3326. doi: 10.4161/cc.7.21.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hafeez BB, Siddiqui IA, Asim M, et al. Cancer Res. 2008;68:8564–8572. doi: 10.1158/0008-5472.CAN-08-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoste E, Kemperman P, Devos M, et al. J Invest Dermatol. 2011;131:2233–2241. doi: 10.1038/jid.2011.153. [DOI] [PubMed] [Google Scholar]
- 48.Howell MD, Kim BE, Gao P, et al. J Allergy Clin Immunol. 2007;120:150–155. doi: 10.1016/j.jaci.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palmer CN, Irvine AD, Terron-Kwiatkowski A, et al. Nat Genet. 2006;38:441–446. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- 50.Eckhart L, Declercq W, Ban J, et al. J Invest Dermatol. 2000;115:1148–1151. doi: 10.1046/j.1523-1747.2000.00205.x. [DOI] [PubMed] [Google Scholar]
- 51.de Koning HD, Bergboer JG, van den Bogaard EH, et al. Exp Dermatol. 2012;21:961–964. doi: 10.1111/exd.12037. [DOI] [PubMed] [Google Scholar]
- 52.Lippens S, Kockx M, Knaapen M, et al. Cell Death Differ. 2000;7:1218–1224. doi: 10.1038/sj.cdd.4400785. [DOI] [PubMed] [Google Scholar]
- 53.Walsh DS, Borke JL, Singh BB, et al. J Dermatol Sci. 2005;37:61–63. doi: 10.1016/j.jdermsci.2004.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Del treatment of normal human epidermal keratinocyte does not alter the gene and protein expression of TGM2.
Figure S2. Del treatment of normal human epidermal keratinocyte induces the protein expression of several differentiation markers.
Figure S3. Del treatment of normal human epidermal keratinocyte does not induce apoptosis and necrosis.
Figure S4. Del treatment enhances cornification and induces keratin-1 expression in the developing 3D epidermal equivalent model.
Table S1. The antibodies used and their sources are listed below.
Table S2. Primer sequences for PCR analysis.
Appendix S1. Determination of cell growth, viability, proliferation, apoptosis and cell cycle.