Abstract
Depression is a major contributor to the global burden of disease and disability, yet it is poorly understood. Here we review data supporting a novel theoretical model for the biology of depression. In this model, a stressful life event leads to microdamage in the brain. This damage triggers an injury repair response consisting of a neuroinflammatory phase to clear cellular debris, and a spontaneous tissue regeneration phase involving neurotrophins and neurogenesis. During healing, released inflammatory mediators trigger sickness behavior and psychological pain via mechanisms similar to those that produce physical pain during wound healing. The depression remits if the neuronal injury repair process resolves successfully. Importantly, however, the acute psychological pain and neuroinflammation often transition to chronicity and develop into pathological depressive states. This hypothesis for depression explains substantially more data than alternative models, including why emerging data show that analgesic, anti-inflammatory, pro-neurogenic and pro-neurotrophic treatments have antidepressant effects. Thus, an acute depressive episode can be conceptualized as a normally self-limiting but highly error-prone process of recuperation from stress-triggered neuronal microdamage.
Keywords: life event, stress, emotionally traumatic brain injury, eTBI, remodeling, neuroinflammation, cytokines, psychological hyperalgesia, sickness behavior, central sensitization, psychological nociceptors, psychological hyperalgesic priming, depression, antidepressants, neurogenesis, neurotrophins
1. Introduction
Depression is projected to become the second biggest contributor to the global burden of disease and disability by the year 2020 (World Health Organization 2009), yet significant unmet need for treatment exists (Greden 2002). Studies that have used modern techniques to assess depressive episodes in the general population concur that 13 – 16% of adult United States residents meet criteria for having experienced Major Depressive Disorder so far in their lives (Hasin et al. 2005, Kessler et al. 2003), perhaps reaching 20% when extrapolated to the entire lifespan. In these general population samples, the average depressive episode is 3 or 4 months in duration (Eaton et al. 2008, Kessler et al. 2003, Spijker et al. 2002). About half of the first time sufferers will recover and never experience a recurrence (Eaton et al. 2008), although approximately 20% of depressive episodes run a chronic course lasting two years or longer (Spijker et al. 2002). These data reveal that depression in the general population, almost half of which is left untreated (Eaton et al. 2008, Hasin et al. 2005, Kessler et al. 2003), is more common, briefer in duration, and less often recurrent than is apparent from studies of clinical samples.
In this article, we review findings that shape our understanding of the biology of depression, including that depression is often triggered by stressful life events (section 2), that stress triggers neuronal microdamage (section 3) and neuroinflammatory activation (section 4) in the brain, and that inflammatory mediators can induce depressive symptoms (section 5). In addition, we review evidence that anti-inflammatory treatments are emerging as having antidepressant effects (section 6), and that antidepressant treatments increase neurogenesis, neurotrophins and neuronal plasticity (section 7). By utilizing these findings in diverse ways, a variety of theoretical models (reviewed in section 8) have proposed that different malfunctions lead to depression. However, no single view has garnered a widespread consensus, leaving the field without a unified theoretical framework that organizes the disparate findings and guides future research.
Because it is difficult to understand dysfunction of any process without an appreciation of what the healthy functioning of the process is, our approach in section 9 is to use these same findings to elaborate a theoretical model for the proper biological functioning of the response to stressful events. In this theoretical model, a healthy response to stress-induced neuronal microdamage consists of an injury repair process with inflammatory-mediated demolition and stem cell-facilitated regrowth. The inflammatory mediators create an episode of psychological pain and sickness behavior which comprise depressive symptoms. In using this injury repair model to refine existing hypotheses about pathology in depression, we suggest in section 10 that this normally self-limiting repair response may become chronic or exaggerated by similar mechanisms to those that commonly lead to chronic inflammatory and pathological pain conditions.
Implications of this brain injury repair model for depression are discussed in section 11. For example, because our theoretical model invokes physical pain mechanisms for psychological pain, it offers biological scenarios explaining why analgesics appear to have some antidepressant effects, and why depression shares features with a family of disorders involving central sensitization of pain pathways and hyperalgesic priming. Because our theoretical model proposes that depressive symptoms are a result of inflammatory mediators released during repair of stress-induced brain injury, it offers an explanation for why brain injury induced by means other than stress also results in depression at a high rate. Regarding drug discovery, this model underscores that brain injury, neuroinflammation, and pain mechanisms may represent therapeutic targets for depression. Finally, we propose the additional hypotheses that a function accomplished during the acute depressive episode is to dismantle neural circuitry underlying behavior that has been rendered disadvantageous by the life event and to grow neural tissue mediating new behavioral strategies (section 11.2); and that the degree of depressogenicity of the stressor is related to the extent, type and neuroanatomical location of the remodeling (section 11.3). Finally, we suggest that the graded nature of the response can explain the common sense notion that depression is on a continuum with normal sadness.
A note about terminology: The criteria by which a typical reaction to a harrowing event or environment is distinguished from a mental “disorder” is the topic of much controversy, e.g. (Kendler et al. 2008, Maj 2008, Wakefield et al. 2007). Therefore, throughout this review, we will use the general terms “depression” and “depressive episode” to refer to the full range of severity of depressive symptoms, including both those that do and do not reach the DSM-IV-TR (American Psychiatric Association 2000) criteria for “Major Depressive Disorder” and “Major Depressive Episode”.
2. Data suggest that stressful life events can precipitate depressive episodes in humans
An association between stressful life events and depressive episodes has long been noted (Hammen 2005, Paykel 2001) (for reviews). The onset of the first episode of depression is preceded by a severe life event in 70–80% of cases (Brown et al. 1986, Brown et al. 1995, Kendler et al. 1999). To address causality, some studies have focused on events that are judged to be “bad luck” or “fateful” to exclude events that might have been brought on by the person's own potential prodromal dysfunction. The odds that a person with major depression has experienced a disruptive, fateful event have been measured at 2.5 times that of community residents who have no apparent depression (Shrout et al. 1989). In a separate study, events judged to have not resulted from the patients own behavior strongly predicted the occurrence of an onset of major depression at an odds ratio of 2.33 (Kendler et al. 1999). In populations subject to mass conflict and displacement in which the number of potentially traumatic events experienced was positively associated with depression, time since conflict was negatively associated (Steel et al. 2009) (for meta-analysis). These findings support the notion that causality can flow from the stressful event to the depressive episode.
Examples of depression-associated stressors include physiological and psychological events, such as transitioning to menopause (Cohen et al. 2006, Freeman et al. 2004, Freeman et al. 2006), experiencing major health problems such as myocardial infarction (Ziegelstein 2001) (for review), having a baby (Paulson and Bazemore 2010)(Robertson et al. 2004) (for review), and caregiving for a loved one with degenerative disease (Mahoney et al. 2005).
Simple, one-way causality is not the whole story, however. Stressful life events may also be a consequence of depression. Supporting this possibility, people with a history of depression are significantly more likely, even in periods of remission, to experience high levels of subsequent episodic life events to which they themselves contributed (Hammen 2005) (for review). Causality may sometimes run bidirectionally, as has been suggested for the myocardial infarction - depression link (Lippi et al. 2009) (for review). While life events can be both a cause and consequence of depression, causality may also come from a third factor. Supporting this possibility, people who are high on the personality trait of neuroticism are at greater overall risk of major depression and are more sensitive to depressogenic effects of adversity (Kendler et al. 2004). Furthermore, causality may shift over the course of the illness. This contention is supported by the finding that first episodes of depression are more strongly associated with major life stress than subsequent episodes, which has led to a kindling hypothesis (Kendler et al. 2000) (Monroe and Harkness 2005) (for review). Finally, not all stressful life events elicit depression in all people each time such an event occurs (Bonanno 2004).
Therefore, stressful life events and depression likely share a complex relationship. Within this complexity, however, the most recent review of the field concludes that overall, the evidence indicates that a robust proportion of the association is due to causality flowing in the direction of the event to depression (Hammen 2005) (for review). Establishing this type of causality is important for developing a theoretical model of depression because it suggests that stress can initiate a cascade of biological events that lead to depression.
3. Stress can trigger remodeling and microdamage in the brain
“..one is….an unwilling witness of an execution, the disintegration of one's own personality…this phase comes to a dead-end, eventually, and is succeeded by vacuous quiet. In this you can try to estimate what has been sheared away and what is left” (Fitzgerald 1996) describing a depressive episode he had experienced.
A finding that has emerged with particular clarity over decades of research is that stress reshapes the physical structure of the brain (Arnsten 2009, Lupien et al. 2009, McEwen 2007, Rodrigues et al. 2009) (for reviews). Stress triggers increases in markers of apoptosis (Bachis et al. 2008, Jalalvand et al. 2008, Lucassen et al. 2001), decreases in neurogenesis (Alonso et al. 2004, Bain et al. 2004, Bland et al. 2006, Chen H. et al. 2006, Cherng et al. 2010, Czeh et al. 2001, Czeh et al. 2002, Czeh et al. 2007, Dagyte et al. 2009, Ferragud et al. 2010, Goshen et al. 2008, Gould et al. 1997, Heine et al. 2004, Hill et al. 2006, Koo and Duman 2008, Lagace et al. 2010, Lee K.J. et al. 2006, Luo et al. 2005, Malberg and Duman 2003, Oomen et al. 2007, Pham et al. 2003, Rosenbrock et al. 2005, Thomas et al. 2007, Torner et al. 2009, Westenbroek et al. 2004, Xu H. et al. 2006, Zhou et al. 2007) but see (Parihar et al. 2009), and region-specific changes in brain derived neurotrophic factor (BDNF), both increases (Aguilar-Valles et al. 2005, Berton et al. 2006, Bland et al. 2005, Bland et al. 2007, Charrier et al. 2006, Dagnino-Subiabre et al. 2006, Givalois et al. 2001, Givalois et al. 2004, Hammack et al. 2009, Lee Y. et al. 2006, Li et al. 2007, Marmigere et al. 2003, Molteni et al. 2009, Pardon et al. 2005, Rage et al. 2002, Schulte-Herbruggen et al. 2009) and decreases (Adlard and Cotman 2004, Aleisa et al. 2006, Bland et al. 2007, Cavus and Duman 2003, Charrier et al. 2006, Chen JX et al. 2008, Duric and McCarson 2005, Duric and McCarson 2006, Dzitoyeva et al. 2008, Fuchikami et al. 2009, Gronli et al. 2006, Kozlovsky et al. 2007, Li et al. 2009, Luo et al. 2004, Murakami et al. 2005, Park et al. 2009, Scaccianoce et al. 2003, Smith et al. 1995, Takeda et al. 2006, Vaidya et al. 1999, van Donkelaar et al. 2009, Vollmayr et al. 2001, Xu et al. 2002, Xu et al. 2004, Xu H. et al. 2006, Xu Y. et al 2006, Yun et al. 2002), with a minority of studies detecting no change (Allaman et al. 2008, Lucca et al. 2008).
In studies that have directly examined neuronal structure, stress was found to induce neurite growth in certain brain regions e.g. (Dias-Ferreira et al. 2009, Liston et al. 2006, Vyas et al. 2002). However, many more studies have documented that stress leads to a reduction of dendritic, spine and synaptic material in the hippocampus and prefrontal cortex. Some studies have referred to this morphological change with stress as “atrophy” e.g. (Conrad et al. 1999, Galea et al. 1997, Lambert et al. 1998, Liu and Aghajanian 2008, Magarinos and McEwen 1995a, Magarinos and McEwen 1995b, Magarinos et al. 1996, Ramkumar et al. 2008, Watanabe et al. 1992a, Watanabe et al. 1992b). Some have called it “retraction” e.g. (Conrad 2006, Izquierdo et al. 2006, McLaughlin et al. 2005, McLaughlin et al. 2009, Radley et al. 2005). Some call it “loss” e.g. (Chen Y. et al. 2008, Radley et al. 2006, Sandi et al. 2003). Others have used the term “damage” e.g. (McEwen and Magarinos 2001, McEwen 2002, Sapolsky 1996, Sousa et al. 2000, Sunanda et al. 1997). For the purposes of this review, we will refer to the stress-induced reduction of neuronal material as “microdamage”.
Most studies addressing structural change with stress have used chronic stress protocols, but structural changes have also been observed after brief stressors. For example, in the hippocampus, a reduction in dendritic spines can be seen within hours of either onset of restraint stress (Chen Y. et al. 2008) or intermittent tailshock (Shors, et al. 2001), and brief social defeat stress induces a reduction in apical dendritic length (Kole, et al. 2004). Loss of dendritic length has also been seen in the infralimbic cortex after a single ten minute episode of swim stress (Izquierdo et al. 2006).
These structural changes can be transient. After cessation of stress, some replacement of missing tissue can be observed within 30 days (Conrad et al. 1999, Goldwater et al. 2009, Radley et al. 2005, Sousa et al. 2000). The stress-induced loss of neuronal material can be reversed more quickly if a new learning (Sandi et al. 2003) or rewarding (Ramkumar et al. 2008) experience is provided, suggesting that learning and reward counter the effects of stress on brain structure.
Structural changes are also seen in the brains of depressed patients (Ebmeier et al. 2006) (for review). The best studied change is reduced hippocampal volume, which has been confirmed in three meta-analyses (Campbell et al. 2004, McKinnon et al. 2009, Videbech and Ravnkilde 2004). The reduced hippocampal volume in depression may not be apparent if the duration of illness is less than two years or fewer than two episodes (McKinnon et al. 2009) for meta-analysis). This volume loss is significantly positively correlated with the total number of previous episodes (Videbech and Ravnkilde 2004) for meta-analysis). Thus, the data suggest that the reduced volume generally occurs after disease onset (McKinnon et al. 2009), and may be a consequence of repeated episodes of depression (Videbech and Ravnkilde 2004). In addition, there is also evidence that among people who have familial risk of depression but have not yet had a first episode, some hippocampal volume decrease preexists (Baare et al. 2010, Chen M.C. et al. 2010, Rao et al. 2009). In a recent review analyzing various possible explanations for the hippocampal volume decrease in depression, alterations in dendritic, axonal, and synaptic components, as well as putative glial changes were considered to be more consistent with the data (including postmortem analyses) than massive neuronal loss or a suppression of neurogenesis. However, shifts in fluid balance or changes in the extracellular space could not be excluded (Czeh and Lucassen 2007) (for review). Some evidence suggests that effective antidepressant treatment reverses hippocampal volume changes (Ebmeier et al. 2006) (for review).
Thus, a picture is emerging that stress can induce structural remodeling including reversible microdamage in the rodent brain, a phenomenon that may be echoed in human depression. Even brief stressors have been found to elicit such structural change in the rodent.
4. Stress may stimulate the neuroinflammatory system
Evidence of inflammatory activation can be observed in both blood and brain of stressed rodents and humans. For example, acute stress increases markers of inflammation in the blood circulation of rodents e.g. (Grippo et al. 2005, Hale et al. 2003, Johnson et al. 2005). Stress induces cytokine responses in various rodent brain regions e.g. (Barnum et al. 2008, Blandino et al. 2006, Blandino et al. 2009, Goshen et al. 2008, Grippo et al. 2005, Johnson et al. 2005, Kwon et al. 2008, Murray and Lynch 1998, Nguyen et al. 1998) (Garcia-Bueno et al. 2008, Munhoz et al. 2008) (for reviews). Although there have been inconsistencies across studies, these may be attributed to varying stressor characteristics (Deak et al. 2005) and to regional selectivity in the response to a given stressor (Blandino et al. 2009). In addition to cytokine responses, both acute and chronic stress induce inflammatory signs in microglia (the resident inflammatory cells of the brain) such as increases in microglial proliferation (Nair and Bonneau 2006), morphological activation (Sugama et al. 2007, Sugama et al. 2009), activation marker expression (Frank et al. 2007), and decreases in a marker of microglial quiescence (Blandino et al. 2009) in various brain regions. In addition, stress increases the number of microglia in certain stress-sensitive brain regions, and triggers a marked transition of microglia from a ramified-resting state to a non-resting state (Tynan et al. 2010). There is even one report of recruitment of bone-marrow derived cells into the hippocampus during stress (Brevet et al. 2010), suggesting that stress may trigger full blown neuroinflammation rather than merely activating brain cytokine signaling. Treatment with the putative microglial inhibitor, minocycline, prevented the stress-induced rise in interleukin (IL) -1 expression (Blandino et al. 2006, Blandino et al. 2009), suggesting that the stress-induced activation of microglia may be responsible for some of the cytokine responses. In humans, acute stress induces a robust increase in inflammatory cytokines, IL-6 and IL-1 in the blood circulation (Steptoe et al. 2007) (for review and meta-analysis). In addition, one study found elevated levels of the inflammatory mediator, substance P, in cerebrospinal fluid of stressed human subjects (Geracioti et al. 2006).
In depressed patients, many, but not all studies have found signs of inflammatory activation in the blood circulation. Three recent meta-analyses (Dowlati et al. 2009, Howren et al. 2009, Zorrilla et al. 2001) concur that overall, the data support the conclusion that the hallmarks of inflammatory activation are present in the blood circulation of depressed subjects. Evidence for altered levels of inflammatory mediators in cerebrospinal fluid of depressed patients has been inconsistent e.g. (Carpenter et al. 2004, Deuschle et al. 2005, Geracioti et al. 2006, Levine et al. 1999, Lindqvist et al. 2009).
Note that the presence of inflammatory markers in the blood circulation does not necessarily indicate a peripheral site for the inflammation, as activation of the resident inflammatory system within the brain also results in inflammatory markers in the blood. For example, elevations of circulating cytokines occur in other disorders with solely central nervous system (CNS) inflammation, such as Alzheimer's disease (Alvarez et al. 2007, Bonotis et al. 2008, De Luigi et al. 2002, Licastro et al. 2000, Lombardi et al. 1999, Sala et al. 2003, Zuliani et al. 2007) and stroke (Allard et al. 2004, Castellanos et al. 2004, Di Napoli et al. 2001, Intiso et al. 2004, Lynch et al. 2004, Pedersen et al. 2004, Reynolds et al. 2003, Rost et al. 2001, Silvestri et al. 2004, Smith et al. 2004).
Taken together, the studies reviewed in this section suggest that stress elicits evidence of inflammatory activation in the rodent brain with inflammatory signs in the blood circulation of stressed and depressed people. The mechanism by which stress might induce neuroinflammatory responses is not yet clear. It has been suggested that inflammatory activity in the brain is induced, paradoxically, by the usually anti-inflammatory glucocorticoids that are released during stress (Sorrells and Sapolsky 2007) (for review). Other data suggests that catecholamines, such as norepinephrine, are required for stress to induce biomarkers of inflammatory activity in the brain (Blandino et al. 2006, Blandino et al. 2009, Johnson et al. 2005, Miller 2007, Miller et al. 2009). We suggest the additional possibility that the stress-induced microdamage in the brain described in section 3 above may be a stimulus that contributes to activation of the neuroinflammatory system.
5. Inflammatory mediators can induce depressive symptoms
A body of evidence shows that inflammatory mediators, such as cytokines, are potent modulators of behavior and affect. For example, people who are treated with the inflammatory mediators interferon-α or IL-2 in the course of therapy for various medical conditions unrelated to mood, develop Major Depression during treatment at a frequency of 23 – 45% (Hauser et al. 2002, Horikawa et al. 2003, Musselman et al. 2001, Robaeys et al. 2007) (Lotrich 2009) (for review). Of those with preexisting depression before cytokine treatment began, most exhibited a worsening of depressive symptomatology (Beratis et al. 2005). Depressive symptoms in cytokine-treated patients respond to antidepressant medication (Musselman et al. 2001), suggesting biological similarity of cytokine-induced depression to depression in general. Even in healthy human volunteers, experimental exposure to an inflammatory stimulus, such as vaccination, elicits depressed mood (Eisenberger et al. 2009, Eisenberger et al. 2010, Harrison et al. 2009, Strike et al. 2004, Wright et al. 2005). In experimental animals, injection of cytokines or lipopolysacharide (LPS, a bacterial endotoxic cell wall component which induces cytokine release) generally leads to depressive behavior in the forced swim and tail suspension test (Dunn and Swiergiel 2005, Frenois et al. 2007, Godbout et al. 2008), but see (Deak et al. 2005).
The capacity of inflammatory mediators to induce depression is further supported by the study of individual depressive symptoms. For example, inflammatory mediators induce anhedonia in rodents as measured by decreased sucrose consumption or preference (De La Garza 2005) (for review). However, sucrose related measures can be confounded with anorexia, a symptom that is also induced by inflammatory mediators. Therefore, intracranial self-stimulation reward has been used as an alternative measure of reward function. Using this procedure, several studies have verified that injection of cytokines or LPS generally results in decreased reward reactivity (Anisman et al. 1996, Anisman et al. 1998, Barr et al. 2003, Borowski et al. 1998, Miguelez et al. 2004), but see (Kentner et al. 2007).
Sleep and appetite disturbances and fatigue, which characterize depression (American Psychiatric Association 2000), are part of a cytokine-triggered syndrome termed “sickness behavior” that also occurs when an organism has an infection (Dantzer et al. 2008, Konsman et al. 2002) (for reviews). It is now thought that the brain recognizes cytokines as molecular broadcasts of injury or infection, reorganizes the individual's behavior in ways that promote recuperation, and that sickness behavior reflects this reprioritization (Dantzer et al. 2008, Konsman et al. 2002). Specifically, cytokines have been found to mediate the changes in sleep produced by infection (Imeri and Opp 2009) (for review). Evidence also supports a role for cytokines in mediating the appetite changes in depression (Andreasson et al. 2007) (for review).
Difficulty concentrating or thinking clearly is a symptom of depression (American Psychiatric Association 2000) that may also be a consequence of inflammatory activation. Acute cognitive impairments have been noted in many situations in which the inflammatory system is activated (Dantzer et al. 2008) (for review), such as after cytokine injection (Rachal Pugh et al. 2001) (for review), peripheral infection e.g. (Sparkman et al. 2006), tissue injury, such as major surgery (Wan et al. 2007), and chronic inflammatory conditions (Dimopoulos et al. 2006). More specifically, inflammatory molecules affect synaptic plasticity (Boulanger 2009) (for review).
Somatic complaints, including diarrhea (Sugahara et al. 2004), nausea (Haug et al. 2002), aches (Lecrubier 2006), and fever (Sugahara et al. 2004), are often associated with depression. These symptoms are also commonly experienced in flu-like illness, and are all thought to be induced by inflammatory mediators (Eccles 2005, Elmquist et al. 1997, Musch et al. 2002).
What is the route by which inflammatory mediators influence behavior? In the case of sickness, the symptoms are triggered by cytokines originating in the periphery. Several pathways have been discovered that transmit the inflammatory signal from the periphery to the brain but engagement of these immune-to-brain communication pathways ultimately leads to the production of proinflammatory cytokines by microglial cells of the brain (Dantzer et al. 2008) (for review). In the case of stress-induced depression, a more parsimonious mechanism for cytokine-induced behavioral change is possible. The inflammatory mediators could originate from microglia within the brain.
To recap this section, inflammatory mediators administered to experimental animals induce depressive symptoms, including anhedonia, sleep, appetite and activity level disturbances, cognitive deficits, and other flu-like complaints. Furthermore, in humans, administration of inflammatory mediators can trigger the entire Major Depressive syndrome. These data have led recent reviews to concur that inflammatory mediators can play a role in the generation of depressive symptoms (Dantzer et al. 2008, Miller et al. 2009, Raison et al. 2006).
6. Anti-inflammatory manipulations may have antidepressant effects
Evidence suggests that various anti-inflammatory manipulations have antidepressant effects in experimental animals and in humans. For example, genetic knockout of IL-6 in mice reduces depressive-like behavior in the forced swim, tail suspension, learned helplessness, and sucrose preference tests (Chourbaji et al. 2006). Knockout of IL-1 receptor blocked stress-induced depressive-like behavior in the sucrose preference and social exploration tests (Goshen et al. 2008). IL-1 receptor antagonist delivered to the rodent brain blocks stress-induced depressive behaviors such as escape deficits (Maier and Watkins 1995), anhedonia (Goshen et al. 2008, Koo and Duman 2008) and reduction of social behavior (Arakawa et al. 2009, Goshen et al. 2008).
There is some evidence for an effect of nonsteroidal anti-inflammatory medications (NSAIDs) on mood (Brunello et al. 2006, Ketterer et al. 1996, Onder et al. 2004). Positive effects of NSAIDs on mood have been noted in humans during therapy for psoriasis (Krishnan R. et al. 2007, Tyring et al. 2006). A small study using the anti-inflammatory agent acetylsalicylic acid found a shortened onset of action of antidepressants as well as an augmentation of their therapeutic effects in humans (Mendlewicz et al. 2006) and in an animal model of depression (Brunello et al. 2006). Likewise, adjunctive treatment with the anti-inflammatory cyclooxygenase-2 inhibitor celecoxib showed superiority over antidepressant alone in the treatment of major depression (Akhondzadeh et al. 2009), and may produce a rapid-onset antidepressant effect in bipolar patients (Nery et al. 2008). Muller and colleagues found that celecoxib has therapeutic effects in major depression in a double-blind, randomized, placebo-controlled add-on pilot study to reboxetine, a selective norepinephrine reuptake inhibitor (Muller et al. 2006). An antidepressant effect of celecoxib has also been reported in an animal model of depression (Guo et al. 2009). Several anti-inflammatory manipulations, including injection of the NSAID, indomethacin, relieved depressive-like symptoms in the rodent maternal separation model (Hennessy, Deak et al. 2009) (for review).
Minocycline, which has powerful anti-neuroinflammatory properties (Tikka et al. 2001, Yrjanheikki et al. 1998, Yrjanheikki et al. 1999), is reported to have an antidepressant effect in a human case (Levine et al. 1996) and in animal models of antidepressant activity (Molina-Hernandez et al. 2008a, Molina-Hernandez et al. 2008b, Pae et al. 2008), but see (Deak et al. 2005). Further, positive effects of anti-cytokine antibodies, infliximab, on mood in humans have been noted during treatment of Crohn's disease (Lichtenstein et al. 2002).
Systemically administered steroidal anti-inflammatory drugs, such as prednisone, have also been noted to affect mood. For example, in one series of human cases, prednisone augmentation of antidepressant therapy showed promise in treatment-resistant depression (Bouwer et al. 2000). Patients taking prednisone for various health concerns are counseled that they may have to endure “inappropriate happiness” as a side effect (U.S. National Library of Medicine and the National Institutes of Health 2009). While it appears that short-term treatment with high-dose prednisone often leads to mania and hypomania, long-term treatment leads more often to depression (Bolanos et al. 2004, Brown et al. 2002) (Brown and Suppes 1998, Brown and Chandler 2001) (for reviews). In accord with potential opposing effects of acute versus chronic glucocorticoids on mood, together with evidence of chronic hypercortisolemia in some depressed people (Gillespie and Nemeroff 2005), both administration of steroidal drugs (Bouwer et al. 2000) as well as blockade of endogenous cortisol secretion (Gallagher et al. 2008) are being pursued as potential antidepressant therapies.
In addition, to these examples of anti-inflammatory treatments that may have antidepressant effects, there is evidence that the antidepressant treatment, Vagal Nerve Stimulation (VNS), may have anti-neuroinflammatory activity. Despite some controversy, VNS has received United States Food and Drug Administration (FDA) approval for the treatment of refractory depression and a recent review continues to supports its usefulness (Rush and Siefert 2009) (for review). The mechanism of action of VNS is open to speculation. Several hypotheses have been ventured, such as that its effectiveness is due to the anticonvulsant effects of VNS, or due to neural connections between the vagus and brain regions that regulate serotonin and norepinephrine (Groves and Brown 2005, Nemeroff et al. 2006) (for reviews). An alternative possibility is that VNS effectiveness in depression is attributable to its effects on the inflammatory system (Corcoran et al. 2005, Das 2007). VNS is effective against a wide variety of conditions with inflammatory features, such as endotoxemia (Borovikova et al. 2000), experimental sepsis, ischemia/reperfusion injury, hemorrhagic shock, arthritis, and other inflammatory syndromes (Tracey 2007) (for review). In addition to these effects in the periphery, VNS inhibits neuronal damage after cerebral ischemia (Masada et al. 1996) and reduces infarct size (Ay et al. 2009). Following traumatic brain injury, VNS protects gamma aminobutyric acid (GABA) neurons (Neese et al. 2007), enhances motor and cognitive recovery (Smith et al. 2005, Smith et al. 2006) and attenuates cortical edema (Clough et al. 2007). These data suggest that VNS may have anti-inflammatory and neuroprotective effects in the brain.
Thus, evidence indicates that anti-inflammatory manipulations have antidepressant actions in humans and animals. Therefore, in addition to inflammatory mediators being sufficient to induce depressive symptoms as reviewed in section 5 above, the evidence reviewed in this section suggests that inflammatory mediators are sometimes necessary for depressive symptoms.
7. A therapeutic mechanism of action for antidepressant treatments may involve neuronal plasticity, neurogenesis and neurotrophins
The degree of efficacy of antidepressant medications in the treatment of depression has been a matter of debate. Two recent meta-analyses (Fournier et al. 2010, Kirsch et al. 2008) that circumvent problems of publication bias (Turner et al. 2008) concur that while the magnitude of benefit of antidepressant medication compared with placebo may be minimal or nonexistent, on average, in patients with mild or moderate symptoms, for patients on the upper end of very severe depression, the benefit of medications over placebo is statistically and clinically significant. The possibility remains that an effect of antidepressants on mild to moderate depression was obscured by a substantial and growing placebo effect (Walsh et al. 2002), which may include a high spontaneous resolution rate (Andrews 2001, Hrobjartsson and Gotzsche 2001). Nonetheless, the meta-analyses provide some rationale for continuing research into the mechanism of action of current antidepressant medications.
Antidepressant treatments influence plasticity at the electrophysiological and structural levels. Chronic administration of the selective serotonin reuptake inhibitor and antidepressant, fluoxetine, restores ocular dominance plasticity in the adult visual cortex as assessed electrophysiologically and behaviorally (Maya Vetencourt et al. 2008). Furthermore, fluoxetine protected hippocampus synaptic plasticity during conditioned fear stress (Spennato et al. 2008). In addition, fluoxetine and the tricyclic antidepressant, imipramine, induce structural changes in the hippocampus (Bessa et al. 2009, Chen F. et al. 2008, Hajszan et al. 2005), the somatosensory cortex (Guirado et al. 2009) and the prefrontal cortex (PFC) (Bessa et al. 2009).
Increases in neurogenesis result from treatment with all major classes of antidepressant drugs e.g.(Pechnick et al. 2008, Wang et al. 2008, Yanpallewar et al. 2010) (Zhao et al. 2008) (for review), as well as with other antidepressant interventions, such as exercise e.g. (Stranahan et al. 2006, van Praag et al. 2005) and electroconvulsive therapy e.g. (Perera et al. 2007, Segi-Nishida et al. 2008). Antidepressant treatment-induced neurogenesis has been reported in humans (Boldrini et al. 2009), non-human primates (Perera et al. 2007), tree shrews (Czeh et al. 2001), and rodents (Zhao et al. 2008) (for review), albeit not in all strains. One survey found that fluoxetine increases hippocampal cell proliferation only in those mouse strains that also show a positive behavioral response to treatment (Miller et al. 2008). In one strain of rat however, the tricyclic antidepressant nortriptyline induced an antidepressant behavioral change in the forced swim test while no increase in neurogenesis was detected (Petersen et al. 2009). In that strain (the genetic depression model, Flinders Sensitive Line) neurogenesis was already elevated compared to the Flinders resistant line. Thus in general, but not without exception, antidepressant-induced behavioral change is accompanied by increased neurogenesis.
Importantly, evidence indicates that in some instances, the behavioral effects of antidepressant drugs depend on neurogenesis (Zhao et al. 2008) (for review). Antidepressants fail to elicit behavioral effects when neurogenesis is blocked by localized x-irradiation (Airan et al. 2007, David et al. 2009, Santarelli et al. 2003, Surget et al. 2008, Wang et al. 2008), by genetic manipulation of receptor tyrosine kinase trk B on neural progenitor cells (Li et al. 2008), or by pharmacological inhibition of vascular endothelial growth factor (VEGF) receptor Flk-1 (Warner-Schmidt and Duman 2007). The dependence of antidepressant-induced behavioral change on neurogenesis has been found in various strains of mouse (David et al. 2009, Li et al. 2008, Santarelli et al. 2003, Surget et al. 2008, Wang et al. 2008) and rat (Airan et al. 2007, Warner-Schmidt and Duman 2007). Neurogenesis-dependence has been demonstrated for fluoxetine (Airan et al. 2007, David et al. 2009, Li et al. 2008, Santarelli et al. 2003, Surget et al. 2008, Wang et al. 2008), imipramine (Li et al. 2008, Santarelli et al. 2003, Surget et al. 2008) and the tricyclic antidepressant, desipramine (Warner-Schmidt and Duman 2007). This neurogenesis-dependence has been seen for antidepressant effects on a variety of behavioral endpoints, such as grooming (Surget et al. 2008), anhedonia (Warner-Schmidt and Duman 2007), immobility in the forced swim test (Airan et al. 2007, Warner-Schmidt and Duman 2007) and tail suspension test (Li et al. 2008), novelty suppressed feeding (David et al. 2009, Li et al. 2008, Santarelli et al. 2003, Surget et al. 2008, Wang et al. 2008, Warner-Schmidt and Duman 2007), and learned helplessness (Warner-Schmidt and Duman 2007). It should be noted, however, that a minority of studies did not find any neurogenesis-dependence to antidepressant effects on behavior (Bessa et al. 2009, Holick et al. 2008), and the dependence appears to vary in behavioral endpoint-specific (David et al. 2009), and antidepressant-specific (Surget et al. 2008) manner.
A similar set of findings has been obtained for BDNF. Chronic exposure to a variety of antidepressant treatments increases BDNF expression in various brain regions e.g. (Conti et al. 2002, Maya Vetencourt et al. 2008, Nibuya et al. 1995, Tsankova et al. 2006, Yanpallewar et al. 2010) and even in the blood circulation of antidepressant-treated human subjects (Brunoni et al. 2008, Sen et al. 2008) (for meta-analyses). Infusions of BDNF into the midbrain (Siuciak et al. 1997), hippocampus (Shirayama et al. 2002, Sirianni et al. 2010), and ventricle (Hoshaw et al. 2005) elicit antidepressant effects in the forced swim test and learned helpless procedure, although opposite effects have been reported for infusion of BDNF into the ventral tegmental area (Eisch et al. 2003).
Studies have shown that the effects of antidepressant drugs on behavior depend on BDNF or its receptor (Chen Z.Y. et al. 2006, Ibarguen-Vargas et al. 2009, Rantamaki et al. 2007, Saarelainen et al. 2003) and have localized this dependence to the forebrain (Monteggia et al. 2004, Monteggia et al. 2007), to the dentate gyrus within the forebrain (Adachi et al. 2008), and to neural progenitor cells within the dentate gyrus (Li et al. 2008). This set of findings intersects with the data showing neurogenesis-dependence of antidepressant drug effects on behavior, as well as with data showing that BDNF generally increases neurogenesis (Henry et al. 2007, Lee et al. 2002, Mohapel et al. 2005, Pencea et al. 2001, Rasika et al. 1999, Rossi et al. 2006, Schabitz et al. 2007, Scharfman et al. 2005) but see (Galvao et al. 2008, Larsson et al. 2002).
In addition to these effects on plasticity, neurogenesis and BDNF, antidepressants exert anti-inflammatory (Abdel-Salam et al. 2003, Abdel-Salam et al. 2004, Brustolim et al. 2006, Diamond et al. 2006, Kubera et al. 2001, Maes et al. 1999, Roumestan et al. 2007) and anti-neuroinflammatory effects (Hashioka et al. 2007, Hwang et al. 2008, Lim et al. 2009, O'Sullivan et al. 2009, Tai et al. 2006, Vollmar et al. 2008), and provide neuroprotection (Jin et al. 2009, Lim et al. 2009, Peng et al. 2008) (Lauterbach et al. 2010, Mostert et al. 2008) (for reviews).
Overall, recent literature reviews concur that increases in neurotrophins and neurogenesis are required for at least some behavioral effects of some antidepressants in some strains (Eisch et al. 2008, Krishnan and Nestler 2008, Martinowich et al. 2007, Sahay and Hen 2007, Zhao et al. 2008) (for reviews). In addition to the trophic effects, antidepressants appear to have anti-neuroinflammatory and neuroprotective effects.
8. Several theoretical models for the pathophysiology of depression have been built with these findings
The above sections review an evidence base of moderate strength for each of the following six conclusions. In humans, depression is often triggered by a stressful life event. Stress induces microdamage and remodeling in the brain. Stress induces signs of neuroinflammatory activation. Inflammatory mediators can trigger many depressive symptoms. Anti-inflammatory treatments may have antidepressant effects. Finally, antidepressants affect neuronal plasticity and, under some circumstances, the effects of antidepressants on behavior are dependent on neurogenesis and neurotrophins. Several authors have used the above findings to develop theoretical models for the pathophysiology of depression.
In the “neurogenesis hypothesis of depression”, (i) the decrease in neurogenesis seen with stress (ii) coupled with the loss of hippocampal volume seen in depressed patients and (iii) the involvement of neurogenesis in the effects of antidepressant drugs on behavior supported the following hypothesis: stress-induced reductions in neurogenesis might be an important causal factor in precipitating depressive episodes (Jacobs et al. 2000, Jacobs 2002). However, in subsequent studies, there was no indication that blockade of neurogenesis by brain irradiation leads to a depressive phenotype, at least in the forced swim test (Airan et al. 2007, Holick et al. 2008). Likewise, eliminating neurogenesis did not increase sensitivity to the depressive effect of unpredictable chronic mild stress as measured on several behavioral tests (Surget et al. 2008). In addition, there are a number of indirect arguments against the possibility that impaired neurogenesis could cause depressive phenotypes (Decarolis and Eisch 2010, Sahay and Hen 2007, Sapolsky 2004) (for reviews). Taken together, recent reviews agree that although the findings are firm that stress leads to decreased neurogenesis, and that behavioral effects of antidepressants often require neurogenesis, decreased neurogenesis is not likely to be a causal factor in precipitating depression (Krishnan and Nestler 2008, Sahay and Hen 2007).
The BDNF saga is similar to that of neurogenesis. The original version of the “neurotrophin hypothesis of depression” proposed that decreased expression of BDNF contributes to depression (Duman and Monteggia 2006). However, in general, little effect of genetically reduced BDNF signaling was seen on tests reflecting depressive-like behavior (Adachi et al. 2008, Chourbaji et al. 2004, Li et al. 2008, MacQueen et al. 2001, Monteggia et al. 2004, Saarelainen et al. 2003, Zorner et al. 2003) but see (Monteggia et al. 2007). Data on whether reductions in BDNF increase sensitivity to stress-induced depressive behavior are mixed e.g. (Advani et al. 2009, Ibarguen-Vargas et al. 2009). Overall, recent reviews concur that data do not support the hypothesis that reduced BDNF signaling can cause depressive-like behavior, despite firm findings that BDNF is regulated by stress and that BDNF is required for antidepressant effects (Krishnan and Nestler 2008, Martinowich et al. 2007).
The “cellular plasticity hypothesis of depression” (Kempermann and Kronenberg 2003) incorporates features of both the neurotrophin and neurogenesis hypotheses to overcome the limitations of either hypothesis alone, but is has not explicitly incorporated the data implicating the inflammatory system in depression.
Likewise other hypotheses contain valuable additional insights but are still incomplete. For example, the “hygiene hypothesis”, and more accurately the “old friends hypothesis”, argues that an immunoregulatory failure precipitated by the unprecedentedly hygienic environment of developed nations is responsible for the rising incidence of chronic inflammatory disorders (Guarner et al. 2006, Rook 2007, Rook 2009). Invoking data on the involvement of the inflammatory system in depression, Rook (Rook and Lowry 2008, Rook 2009) has extended this hypothesis to explain the rising incidence of depression. This hypothesis persuasively argues that this immunoregulatory failure could increase vulnerability to stress-related depression, but the hypothesis has not yet been further elaborated to incorporate the neurotrophin, neurogenesis and plasticity data.
In the “macrophage hypothesis of depression”, Leonard (Leonard 2001) argues that stress-induced hypersecretion of glucocorticoids results in a malfunctioning of macrophages and dysregulated release of cytokines which in turn increases glucocorticoids further and leads to dysregulation of noradrenergic and serotonergic neurotransmission and sickness behavior. According to this hypothesis, antidepressants have anti-inflammatory effects on macrophages which lead to a normalization of glucocorticoid release and downstream effects on central neurotransmission. This is an older hypothesis that does not, as is, incorporate the recent data on neurogenesis, neurotrophin or stress-induced structural changes.
In an elaboration of the “Mayberg model” by Stone and colleagues (Stone et al. 2008), stressful experiences lead to prolonged hypoactivity in the brain's reward circuitry, which in turn reduces neurotrophic and neurogenic support in these regions, leading to disuse atrophy. Simultaneously, the stressful experience leads to overstimulation of the stress circuitry. Stress-sensitizing and reward-inhibiting actions of cytokines contribute to depression. Antidepressants lead to a normalization of these effects and gradually overcome the atrophy.
In the inflammation and neurodegenerative hypothesis of depression (Maes et al. 2009) an inflammatory processes triggered by stress enhances neurodegeneration and reduces neurogenesis.
The diversity of these theoretical models reviewed in this section highlights the fact that the phenomena of stress, microdamage in the brain, cytokines, reduced neurogenesis, neuroinflammation, and neurotrophic factors, are reciprocally interconnected in many different ways. Many of these theoretical models are not mutually exclusive and very little data exist to distinguish one from another. In fact, these models could conceivably all represent individual facets of a complex of interconnected pathologies that stress can trigger in the brain.
9. These findings can also be assembled into a theoretical scenario for a healthy response to stressful life events
In all of the theoretical models described in section 8, it is assumed that depression is a result of some kind of malfunction. It may be easier to predict how a response can malfunction, however, after first comprehending the cascade of events that comprise its proper function. Therefore, we now use the above findings to address what the healthy response to stressful life events might be and use that as a framework to clarify and organize ideas of how dysfunction occurs.
9.1 A healthy response to tissue damage includes wound repair during an episode of recuperative behavior
“The complex of melancholia behaves like an open wound…” (Freud 1917/1957)
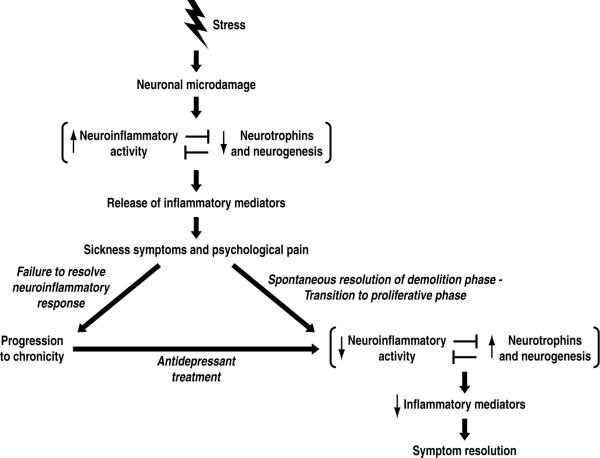
To build a theoretical model for a healthy response to a stressful event we will start with the stress-induced microdamage in the brain and ask what a typical response to this damage might be (Fig 1). Generally, when tissue in the body is damaged, a three-stage wound repair process is triggered. An initial phase of wound healing is the demolition phase, in which devitalized tissue is removed and proinflammatory cytokines are released. With the wound cleared of debris, the release of growth factors from various sources triggers a second, regenerative phase of wound repair in which new tissue is formed (Gurtner et al. 2008) (for review). This process involves stem cells which are resident in virtually all tissue types. These cells provide daughter cells that differentiate and directly participate in the structural repair of the wound, and they also supply secreted factors that downregulate the inflammatory response (Stappenbeck and Miyoshi 2009, Uccelli et al. 2007) (for reviews). Finally, there is a third refinement phase that can take months to years (Gurtner et al. 2008) (for review).
Figure 1.
Theoretical model for depression. In this model, a major adverse stressful life event (reviewed in section 2) leads to neuronal microdamage such as reduction of dendritic length, spines and branching in the hippocampus and prefrontal cortex (section 3). Such microdamage elicits a neuroinflammatory response (section 4), which inhibits neurogenesis and neurotrophin activity (section 3). The activated neuroinflammatory system releases inflammatory mediators which elicit sickness symptoms (section 5). In addition, these neuroinflammatory mediators hypersensitize psychological pain circuits by a similar mechanism to that by which they are known to hypersensitize physical pain circuits in the context of bodily injury (section 9.2). If the neuroinflammatory response fails to resolve, the depressive episode becomes chronic (section 10). On the other hand, a healthy inflammatory response will spontaneously resolve and transition to the proliferative phase of injury repair (section 9.1). In this transition, the decreased neuroinflammatory activity releases inhibition of neurotrophin activity and neurogenesis, and these trophic processes increase. As the injury repair nears completion, the release of proinflammatory mediators decrease, allowing depressive symptoms to remit. Because antidepressant treatments have anti-neuroinflammatory effects and lead to an increase in neurogenesis and neurotrophin expression (section 7), these treatments promote resolution of the injury repair response.
A similar process takes place after brain injury (Wieloch and Nikolich 2006) (for review). After stroke, for example, a neuroinflammatory response is triggered involving activation of microglia, the resident inflammatory cells in the brain (Kreutzberg 1996) (for review). After activation, microglia migrate to the site of injury, release proinflammatory cytokines and phagocytose cellular debris (Kreutzberg 1996) (for review). In the region of reversible stroke damage called the penumbra, those neurons that do not die lose dendritic spines (Murphy and Corbett 2009) (for review), as has also been seen after stress (Chen Y. et al. 2008, Goldwater et al. 2009, Hajszan et al. 2009, Radley et al. 2006). At some point, microglia begin to release growth factors, such as BDNF (Madinier et al. 2009, Rickhag et al. 2007, Sato et al. 2009) which can increase neurite outgrowth and sprouting in the injured brain (Batchelor et al. 1999, Batchelor et al. 2000, Batchelor et al. 2008, Chen et al. 2005, Mamounas et al. 2000). In general, functional recovery from stroke is enhanced by (Muller et al. 2008, Schabitz et al. 2004, Schabitz et al. 2007) and is in fact dependent on (Chen et al. 2005, Ploughman et al. 2009) this BDNF signaling, but see (Nygren et al. 2006). By a few weeks after the stroke, dendritic spine turnover and synaptogenesis are apparent and new functional connections proliferate (Murphy and Corbett 2009) (for review). These changes can occur in the perinfarct, as well as connected areas (Carmichael 2006) (for review).
Although neuroinflammation transiently inhibits neurogenesis (Ekdahl et al. 2003, Monje et al. 2003, Zhao et al. 2008) (for review), injury eventually increases neurogenesis, e.g. (Arvidsson et al. 2002, Ohira et al. 2009, Parent et al. 2002, Yu et al. 2008) presumably in the later regenerative phase of brain injury repair. Although neurogenesis only occurs in a small number of brain sites, in the case of stroke the neural progenitors are able to migrate great distances to the site of neuroinflammation, a process that is regulated by chemokines (Belmadani et al. 2006). These newborn neurons have been demonstrated to take up residence at the site of injury and integrate synaptically (Arvidsson et al. 2002, Yamashita et al. 2006, Zhang et al. 2007) (Massouh and Saghatelyan 2010) (for review). In addition to this cell replacement role in repair of the injury, neural stem cells also protect the CNS from inflammatory damage in other ways (Martino and Pluchino 2006) (for review), including immunomodulation (Pluchino et al. 2005, Pluchino et al. 2009). These effects presumably help resolve the neuroinflammatory phase and promote the growth phase in the course of brain injury repair. Some months after the stroke, refinement of synaptic connections occurs (Murphy and Corbett 2009). The end result is that, albeit to a limited extent, neurons have been rewired, function has been recovered and the brain has self-repaired (Murphy and Corbett 2009).
In general, wound repair responses are graded on a continuum from para-inflammation to full-blown inflammation. The body reacts to cellular stress, malfunction, or microdamage with para-inflammation to help the tissue restore functionality (Medzhitov 2008) (for review). Mild cases can be handled by tissue-resident macrophages, while more severe damage requires recruitment of leukocytes and plasma proteins from the circulation as occurs in full blown inflammation. The degree of activation will determine whether the inflammatory response is detectable using common biomarkers (Medzhitov 2008).
Another component of the healthy response to injury is behavioral change that supports recuperation. For example an injured primate might reduce foraging, rest in a safe place and tend its wounds (Dittus and Ratnayeke 1989). As discussed in section 5, a similar behavioral syndrome is triggered by cytokines during infection and is typically called “sickness behavior” (Dantzer and Kelley 2007) (for review) but could equally well be termed “convalescent behavior”.
Taken together, the healthy response to brain injury likely involves many of the phenomena that have emerged as important in depression, stress, and antidepressant response. These phenomena include neuroinflammatory activation, cytokines, sickness behavior, BDNF, neurogenesis, and plasticity. Therefore, a theoretical model for the healthy response to stress-induced mild brain injury is as follows: An initial neuroinflammatory phase of brain injury repair inhibits growth while clearing the lesion of cellular debris. During this initial phase, released proinflammatory cytokines induce a motivational reprioritization, sickness behavior, that promotes convalescence. As the repair process transitions to the regenerative phase, trophic influences and neurogenesis gain dominance and inhibit proinflammatory processes. Sickness symptoms ultimately resolve and a final refinement phase of the repair response is carried out (Fig. 1).
9.2 Response to tissue damage includes inflammatory pain and central sensitization of pain pathways: candidate mechanisms for psychological pain during acute depressive episodes
“the gray drizzle of horror induced by depression takes on the quality of physical pain” (Styron 1990)
Postulating an injury repair process for stress-induced microdamage suggests possible molecular mechanisms for the psychological pain in depression. Inflammatory pain is produced during the wound repair process by a large number of mediators in the “inflammatory soup” which act through their respective receptors and signaling cascades to phosphorylate TRP (transient receptor potential) and voltage-gated sodium channels (Hucho and Levine 2007). This modification alters the thresholds and kinetics of the channels, thereby increasing the sensitivity of the nociceptive neurons. For example, one of these pathways to hyperalgesia (hypersensitivity to pain) is demonstrated by the familiar analgesic effectiveness of NSAIDs. NSAIDs target cyclooxygenase, the enzyme that synthesizes prostaglandins. Prostaglandin E2 (PGE2) is released from activated macrophages after an injury. PGE2 then interacts with its G-protein-coupled receptor on the surface of pain fibers. The resulting stimulation of cAMP (cyclic adenosine monophosphate) production leads to activation of protein kinase A and phosphorylation of sodium channel Nav1.8, thereby reducing the activation threshold and increasing responsiveness of the nociceptors (Hucho and Levine 2007). In addition to cyclooxygenase inhibition, selective pharmacological blockade of Nav1.8 sodium channels produces antinociception in animal models of neuropathic and inflammatory pain (Jarvis et al. 2007). This type of inflammatory pain lasts the duration of the wound repair process and resolves upon successful healing.
The above mechanisms describe inflammatory pain as it occurs on peripheral neurons at the site of injury. After such a peripheral injury, a similar process can also occur on neurons within the CNS, further augmenting the hypersensitivity to pain. Peripheral injury stimulates glia in the CNS to release inflammatory mediators, such as tumor necrosis factor (TNF) -α, IL-6, IL-1β, prostaglandin, bradykinin, and monocyte chemoattractant protein-1 which increase the sensitivity of central pain pathways (McMahon and Malcangio 2009) (for review). Thus, a neuroinflammatory mechanism contributes to the sensitization of these central pathways (Gao et al. 2009, Hains and Waxman 2006, Harvey et al. 2004, Kawasaki et al. 2008, Kohno et al. 2008, Samad et al. 2001, Watkins et al. 2001). This phenomenon of enhanced sensitivity to pain that is mediated by changes in the central, rather than peripheral, nervous system is called “central sensitization” of pain pathways. Note that “central sensitization” of pain pathways should not be confused with sensitization to the effects of drugs of abuse or of psychiatric medication.
Might these mechanisms of central sensitization to inflammatory pain also apply to the psychological pain of depression? A molecular overlap is supported by the finding that IL-1 signaling is increased in the brain with stress (Barnum et al. 2008, Blandino et al. 2006, Blandino et al. 2009, Deak et al. 2005, Goshen et al. 2008, Grippo et al. 2005, Johnson et al. 2005, Kwon et al. 2008, Murray and Lynch 1998, Nguyen et al. 1998), is necessary in the brain for stress-induced depressive symptoms (Arakawa et al. 2009, Goshen et al. 2008, Koo and Duman 2008, Maier and Watkins 1995), and is known under other circumstances to rapidly and directly induces pain hypersensitivity in the periphery (Binshtok et al. 2008), and to participate in central sensitization to pain (Samad et al. 2001) (Kawasaki et al. 2008) (for review).
In further support of the possibility of shared mechanisms between physical and psychological pain, it has been proposed on theoretical grounds that psychological pain serves a related function to physical pain in motivating avoidance of certain types of evolutionary fitness-detracting threat (Thornhill and Thornhill 1989). Empirical evidence bolsters the theoretical similarity between physical and psychological pain. For example, functional magnetic resonance imaging (fMRI) studies suggests neuroanatomical overlap in the processing of physical pain and psychological pain, such as from social rejection (Eisenberger et al. 2003), envy (Takahashi et al. 2009) dread (Berns et al. 2006), empathy for someone else's pain (Singer et al. 2004), and the placebo-responsive component of physical pain (Wager et al. 2004). Behavioral, cognitive, linguistic and psychological evidence also suggests that physical and social pain operate via common mechanisms (Eisenberger and Lieberman 2004, Macdonald and Leary 2005) (for reviews) (Panksepp 2003) (for comment).
In concluding this section, we propose that, in the context of stress-induced microdamage in the brain, similar molecular and cellular mechanisms that have been elucidated for central sensitization to inflammatory pain may contribute to psychological pain in depression. Thus, in accord with bodily pain, the terms “psychache” (Lester 2000) or “psychological hyperalgesia”, and “psychological allodynia” (allodynia is pain triggered by stimuli that are not normally painful), and even aching emotional numbness may apply to different characteristics of psychological pain in depression. In addition to central sensitization of psychological pain pathways in depression, we propose that central sensitization also occurs in physical pain pathways, giving rise to the physical pain complaints that are common in depression (Lecrubier 2006).
10. Both pain and inflammation are vulnerable to chronicity: candidate mechanisms for dysfunctional depression
Productive and unproductive depression (reflects) the success or failure of a vital process (Gut 1989)
Chronic inflammation is a common mechanism of disease, with the clinical presentation of the disease varying widely depending on which tissue is involved. For example, chronic inflammatory activity can affect lungs, heart, scalp, skin, gums, joints, arteries, bones, muscle, tendon, intestines, as well as CNS sites like spinal cord, caudate putamen, nigrostriatal dopaminergic pathway and cortex. Chronic inflammatory activity at these sites has been suggested to contribute, respectively, to asthma, myocarditis, alopecia, psoriasis, gingivitis, arthritis, atherosclerosis, osteoporosis, myositis, tendonitis, inflammatory bowel disease, as well as Amyotrophic Lateral Sclerosis, Huntington's, Parkinson's and Alzheimer's disease (Frank-Cannon et al. 2009). Almost any tissue is at risk for becoming chronically inflamed, with inflammation at each site giving rise to a unique symptomatology.
The pathological potential for inflammation is unprecedented as a physiological process (Medzhitov 2008) (for review). Even in a well controlled inflammatory response, collateral damage to surrounding healthy tissue is an unavoidable consequence (Medzhitov 2008) (for review). Thus inflammation is both a cause and consequence of tissue damage. When the work of the acute inflammatory response is accomplished, a successful inflammatory reaction will swiftly resolve, thus limiting collateral damage. Resolution is not merely a passive termination of inflammation, but rather an active, tightly coordinated biochemical process involving pro-resolution mediators, some of which are biosynthesized from omega-3 fatty acids (Serhan et al. 2008) (for review).
Chronic inflammation arises under certain circumstances. Chronic persistence of an infectious, injurious, insoluble or antigenic agent can drive chronic inflammation. It has also been suggested that genetic or lifestyle-mediated interference with resolution (Lawrence and Gilroy 2007) or tolerance (Rook 2009) might contribute to chronic inflammatory disorders.
Just as inflammatory responses are prone to becoming chronic, so too are pain responses. Chronic pain develops in a substantial fraction of people who experience common surgeries (10–50%) (Kehlet et al. 2006) (for review), serious bodily injury (44%) (Jenewein et al. 2009), and even mild traumatic brain injury (75%) (Nampiaparampil 2008) (for review). In addition, sometimes chronic pain develops without any obvious precipitant (Burton 2003).
Several mechanisms have been elucidated for the transition from acute to chronic or exaggerated pain. If an injury involves damage to the nervous system, the response leading to inflammatory central sensitization is much exaggerated and creates pain that can persist well beyond the completion of the wound healing process (Latremoliere and Woolf 2009) (for review). In addition to this neuropathic central sensitization, an interrelated phenomenon in chronic pain is hyperalgesic priming. In hyperalgesic priming, an acute inflammatory insult can prime nociceptors (pain neurons) to develop an exaggerated hypersensitivity to pain upon future inflammatory insult (Reichling and Levine 2009) (for review).
We suggest that these mechanisms for chronic inflammation and exaggerated pain may also occur during the depressive response, rendering the response pathological (Fig 1.). First regarding chronic inflammation, in our theoretical model, the stressful life event is injurious to the brain. If a chronic stressor is unavoidable, unpredictable, or uncontrollable, we argue it would be persistently injurious to the brain, driving chronic neuroinflammation, and chronic depression. An alternative route to chronic depression might be via immunoregulatory failure precipitated by the high contemporary level of hygiene as proposed by Rook and Lowry (Rook and Lowry 2008). In our theoretical model, however, we suggest that the CNS is the site of the chronic inflammation giving rise to chronic depression, while the Rook and Lowry hypothesis assumes a diffuse peripheral origin for the chronic inflammation that promotes depression.
Regarding chronic pain mechanisms, since injury to nervous tissue leads to exaggerated central sensitization of physical pain circuits, we argue that the stress-induced injury to nervous tissue has a high risk of leading to neuropathic central sensitization of psychological pain circuits and thus to chronic depression. In addition, although the mechanism of hyperalgesic priming was originally described in peripheral nociceptors, it could, theoretically, apply to central “psychological nociceptors” as well.
In sum, if the proper functioning of the response to stress-induced microdamage involves pain mechanisms and inflammatory activity, then we propose that dysfunction of this response likely occurs via the common complications of pain and inflammation, such as conversion to exaggerated and chronic states.
11. Implications, predictions and future directions
Because our model proposes molecular similarity between the mechanisms of physical and psychological pain (section 9.2 and 10 above), it offers a molecular basis to explain suggestive data that analgesics may be effective in depression.
For example, opiates were used routinely to treat depression until the 1950s when tricyclic antidepressants were introduced (Ban 2001) (for review). Several small recent studies continue to support the role of opiates as effective, durable, and rapid therapeutic agents in the treatment of depression (Tenore 2008) (for review). Additional evidence that analgesics may have antidepressant actions comes from subanesthetic doses of ketamine which act as an analgesic (Annetta et al. 2005). Robust and rapid antidepressant effects result from a single intravenous dose of ketamine in treatment-resistant major depression (Skolnick et al. 2009) for review.
Depression is often grouped with a large family of other disorders that includes chronic fatigue syndrome, fibromyalgia, and irritable bowel syndrome. This grouping is based on a number of different criteria including frequent comorbidity with each other (Whitehead et al. 2002), shared response to antidepressant treatments (Hudson and Pope 1990), common risk factors such as childhood maltreatment (Tietjen et al. 2009), coaggregation in families (Hudson et al. 2003), evidence of stress intolerance (Van Houdenhove and Luyten 2009), and absence of ongoing peripheral organ pathology (Burton 2003). It is thought that these shared features reflect a common, cryptic biopsychosocial etiology, the nature of which has not yet been firmly established. It has also been suggested that this family of disorders may be united by the phenomenon of hyperalgesic priming (Reichling and Levine 2009) or central sensitization (Yunus 2007). Because in our theoretical model, hyperalgesic priming and central sensitization to physical and psychological contribute to depression, it offers a biological scenario explaining why depression seems to be part of this family of syndromes.
Because our theoretical model proposes that stress-induced microdamage in the brain triggers a cascade of neurobiological events leading to the depressive episode, this might explain data indicating that brain injury induced by means other than stress also precipitates depression at a high rate. For example, a few preliminary studies in experimental animals have noted that depressive-like behaviors follow traumatic or ischemic brain injury, although the studies are not well controlled for confounds (Kato et al. 2000, Milman et al. 2005, Pandey et al. 2009, Shapira et al. 2007) but see (Jones et al. 2008). In humans, depression is a common sequelae of traumatic brain injury (Bombardier et al. 2010), even when mild (Ryan and Warden 2003). In fact, the symptom overlap between depression and the common “postconcussion syndrome” is substantial (Bryant 2008, Hoge et al. 2008). Furthermore, there is evidence that late-life depression can be precipitated by accumulated small silent cerebral infarctions that appear as white matter hyperintensities on magnetic resonance imaging (MRI) scans, a phenomenon termed “vascular depression” (Alexopoulos et al. 1997, Sheline et al. 2010, Thomas et al. 2002) (Santos et al. 2009) (for review). Moreover, a third of survivors of overt stroke develop post-stroke depression (Lenzi et al. 2008) (for review).
Our theoretical model encourages testing of emerging drugs that target brain injury, neuroinflammation and pain for antidepressant effects. For example, an acute depressive episode brought on by an acute stressful life event may respond to treatments that target traumatic or ischemic brain injury e.g. (Xiong et al. 2009), acute inflammation, inflammatory pain, or acute central sensitization to pain. Our theoretical model argues that chronic depression resulting from exposure to chronic unavoidable, unpredictable or uncontrollable stressors, such as in the chronic mild stress animal paradigm (Willner 2005), may respond to agents that are effective in chronic neuroinflammation or neurodegenerative disease. Occurrences of depression in which a depressive reaction to a stressor is worsened by a prior psychologically traumatic event, such as after repeated maternal separation of guinea pig pups (Hennessy, Shiml-Webb, et al. 2009), may respond to emerging neuropathic pain treatments (Dray 2008) and inhibitors of Protein Kinase C epsilon, which block hyperalgesic priming (Reichling and Levine 2009). Finally, since our model argues that inflammatory activity within the brain often contributes to depressive symptoms, the extent to which anti-inflammatory and pro-resolution treatments pass the blood brain barrier may influence their antidepressant efficacy. Thus, while this model proposes a common core cascade in depression involving brain injury, neuroinflammation, and psychological pain, within this core, different therapeutic opportunities exist in different types of depression and are highlighted by different animal paradigms.
11.1 What are the mechanisms by which stress produces neuronal microdamage?
In our theoretical model, it is assumed that acute stress induces neuronal microdamage by some unknown mechanism, and that this microdamage in turn induces neuroinflammatory activity. This assumption requires experimental confirmation because any observed differences between the healthy and affected individuals may always represent either the cause or the consequence of the disease. For example, neuroinflammation may be the brain's attempt to repair the stress-induced microdamage as we have hypothesized, but it is also possible that the observed neuroinflammation may instead be the cause the neuronal microdamage in the first place.
In resolving this classic conundrum, it may be important to consider data from acute and chronic paradigms separately. It is possible that some unknown mechanism initially induces the microdamage in acute depression, but in cases that progress to chronic depression, the initial neuronal microdamage is maintained by a self-perpetuating cycle of inflammation-induced tissue destruction that results when the acute neuroinflammatory response fails to resolve for any reason.
Studies using chronic paradigms suggest the involvement of chronic glucocorticoids. Chronic glucocorticoid exposure produces loss of apical dendritic length and branching in the hippocampus that mimics chronic stress-induced microdamage e.g. (Conrad et al. 2007). Chronic stress-induced microdamage was prevented by cyanoketone, a steroid synthesis blocker (Magarinos and McEwen 1995a). Further dissection of the pathway suggests a dependence on serotonin and glutamate, as the microdamage that follows chronic glucocorticoid or chronic stress exposure is reduced by tianeptine, an enhancer of serotonin reuptake (Conrad et al. 1999, Magarinos et al. 1999, McEwen et al. 1997, Watanabe et al. 1992c), and by phenytoin, an inhibitor of excitatory amino acid release and action (Magarinos and McEwen 1995a, McEwen et al. 1997, Watanabe et al. 1992a). There are also scattered reports of chronic stress-induced microdamage being reduced by a variety of other manipulations, such as protein kinase C inhibition (Hains et al. 2009), by enhancement of GABAergic tone (Magarinos et al. 1999), by antioxidants (Lee Y.J. et al. 2006), by estradiol (McLaughlin et al. 2010), and by disruption of the tissue plasminogen activator gene (Bennur et al. 2007). These studies provide many tools by which a causal chain of events between chronic stress and microdamage can start to be assembled.
On the other hand, with acute stress, dendritic microdamage can be produced via a mechanism upstream from gluccocorticoids. A corticotropin releasing hormone (CRH) receptor 1 blocker inhibits acute stress-induced spine loss (Chen Y. et al. 2008, Chen Y. et al. 2010). CRH, which is released with stress not only from the hypothalamus, but also within the hippocampus, induces rapid spine loss and dendritic regression in hippocampal cultures (Chen Y. et al. 2008, Chen Y. et al. 2010). Because this effect can be seen in culture, CRH's acute effects cannot be mediated via adrenal glucocorticoids. Further exploring these pathways from acute and chronic stress to neuronal microdamage is an important future direction.
11.2 Is a function of the acute depressive episode to dismantle neural circuitry that has been rendered disadvantageous, such as by a life event, and to grow neural tissue mediating new behavioral strategies?
I speak of “productive depression” when at the end of a period of being depressed there is evidence…that…some behavior has been reorganized, some plan revised, so that following the depressed episode we function more effectively …” (Gut 1989)
“… if her patients did not achieve some sort of restructuring, they became chronically ill. Something had interfered with the resolution of their depressed response, she felt, so that it lost its self-limiting quality and became autonomous and self-perpetuating. Again we can use the analogy of the immune response to explain this. If the immune response … does not resolve normally, it may become a problem in its own right – an immune disorder.” (Zuess 2003)
The data we have reviewed so far would suggest that the brain is extremely vulnerable to stress-induced microdamage. Yet to be so easily injured would seem maladaptive. In the case of an acute depressive episode, might stress-induced microdamage in the brain actually reflect neurite autodestruction that serves some sort of adaptive function? Evidence suggests that the stress-induced structural remodeling that has been seen at specific brain sites, such as the hippocampus, prefrontal cortex, and amygdala (reviewed in section 3 above) may be the structural correlates of long-term behavioral adaptations to the stressor. For example, stress-induced structural change in the hippocampus is accompanied by change in hippocampal-dependent behavioral tasks, in general enhancing hippocampal-dependent fear-related memory but impairing hippocampal-dependent memories acquired outside of a fear-conditioning context (Kim and Diamond 2002) (for review). In another example, stress was found to induce selective impairment of attention set-shifting, with no change in reversal learning, with corresponding structural changes in the two brain regions thought to mediate these behavioral tasks, the medial prefrontal cortex (mPFC) and orbitofrontal cortex, respectively (Liston et al. 2006). The mPFC is also implicated in extinction of conditioned fear. Stress attenuates this extinction in a manner that corresponds with mPFC dendritic retraction (Izquierdo et al. 2006). In another study (Vyas et al. 2002), two different stress protocols that elicited contrasting behavioral effects in the elevated plus maze were accompanied by contrasting structural changes, such that enhanced anxiety-like behavior was accompanied by sprouting in the amygdala (a key structure in the fear circuit (Rodrigues et al. 2009) (for review). Increases in anxiety, changes in attention, a learning bias for fear-related memories, and a resistance to their extinction that are observed after threatening laboratory stressors could function, in a natural setting, to promote vigilance and aversive memories that enable the animal to avoid future harm from similar stressors. Thus, a possible function for stress induced remodeling in the brain is to create long-term behavioral adaptations to the stressor.
Is there any evidence that such long-term behavioral adaptations are created during an episode of depressive symptoms? Some support comes from work on a social defeat paradigm in mice. In response to social defeat stress, half of the exposed animals exhibited a transient depressive-like episode and a persistent change in social avoidance behavior. The other half manifested neither of these changes and could therefore be considered resilient to defeat stress (Krishnan V. et al. 2007). Although the authors of those studies interpret these findings as indicating that the persistent social avoidance behavior reflects maladaptive functioning (Krishnan V. et al. 2007, Lagace et al. 2010), another interpretation is possible. Both the resilient and the depressive/avoidance behavioral trajectories may be evolutionarily adaptive under different circumstances. The persistent social avoidance could be a long-term behavioral adaptation to defeat stress that serves to protect the animal from future aggression. In contrast, the resilient trajectory might be evolutionarily adaptive if the costs of the depressive/avoidance behavioral change outweigh its benefits. These studies were done in an inbred strain suggesting that it was not genetic differences that dictated which of the trajectories were followed. Because the persistent social avoidance behavior developed only in the subset of animals that displayed the transient depressive-like episode (Krishnan V. et al. 2007), our alternative interpretation is consistent with the possibility that the neural underpinnings of long-term behavioral adaptations to the stressor are created during an episode of depressive symptoms.
Is a neural injury repair process required for the development of long-term behavioral adaptation to the stressor? In this social defeat paradigm, both the resilient and the depressive/avoidant groups showed a transient decrease in neurogenesis which resolved by 24 hours after cessation of defeat stress (Lagace et al. 2010). While the resilient subset showed no further changes in neurogenesis, the depressive/avoidant subset began to demonstrate subsequent compensatory increases in neurogenesis (Lagace et al. 2010). Neurogenesis proved to be important for the development of the long-term behavioral change because if neurogenesis were ablated via irradiation, then the development of the persistent social avoidance behavior was attenuated (Lagace et al. 2010). Similar results were seen for BDNF (Berton et al. 2006, Krishnan V. et al. 2007). The spontaneous increase in neurogenesis and BDNF that were detected only in the subset that were experiencing the depressive-like episode supports our wound healing model of the depressive episode. Furthermore, the requirement of neurogenesis and BDNF for the full expression of the persistent social avoidance introduces the possibility that the growth phase of the stress-induced injury repair process may be involved in creating the neuronal underpinnings of long-term behavioral adaptations.
What effect do antidepressant drugs have on the development of long-term behavioral adaptations to the stressor? In this social defeat paradigm, the depressive-like episode resolves spontaneously, without requiring antidepressant medication. However, if the animals were chronically treated with the antidepressants imipramine or fluoxetine, then the persistence of the social avoidance behavior was blocked or attenuated (Berton et al. 2006). These findings were interpreted by the authors of those studies as further indication that the persistent social avoidance is a maladaptive component of depressive symptomatology (Lagace et al. 2010). Another possible interpretation, however, is that by hastening the resolution of an acute depressive episode, antidepressants can disrupt a function of the episode, which is a slow delicate process of developing the neural underpinnings of long-term behavioral adaptations to the stressor.
Another paradigm in which such a “behavioral metamorphosis” hypothesis for depression could be tested is the maternal separation model in guinea pigs. Soon after pups are isolated from their mothers, the pups begin to display depressive-like behavior that is inhibited by anti-inflammatory treatments (Hennessy, Deak, et al. 2009). Do the pups develop long-term behavioral adaptations to separation, such as a reduction in attachment behavior, during the course of this depressive-like episode? There is some evidence that pups respond differently to the presence of the mother when tested after two weeks of a similar separation protocol, relative to those pups who had not been separated from their mothers (Hennessy and Morris 2005). Does inhibition of neuroinflammation, neurogenesis or BDNF during the depressive-like episode inhibit any subsequent reduction of attachment behavior? Can microglial activation, focal cytokine release and neural structural changes in the brain be detected in parallel with the behavioral change?
Thus, in addition to our theoretical model whereby a stressful event elicits a brain injury repair process involving neuroinflammatory-assisted demolition and subsequent neurogenesis-and BDNF-facilitated growth, here we propose a testable “behavioral metamorphosis” hypothesis for depression: This injury repair response that occurs during an episode of depressive symptoms ultimately functions to remove circuitry that has been rendered disadvantageous and to create the neuronal underpinnings of long-term adaptive behavioral change. Such a behavioral metamorphosis function for depression may also apply to depressions that are triggered by situations other than stressful life events, such as by commitment to unreachable goals (Nesse 2000).
11.3 Is the depressogenicity of a stressor related to the extent, type and neuroanatomical location of the remodeling that it elicits?
“Now in what consists the work which mourning performs?…Each single one of the memories and hopes which bound the (reward motivation) to the (lost) object is brought up … and the detachment of the (reward motivation) from it accomplished. … loss in melancholia would also result in an inner labour of the same kind… If the object had not this great significance, strengthened by a thousand links, to the ego, the loss of it would be no meet cause for either mourning or melancholia.” (Freud 1917/1957)
What features of the stressor influence severity of the depressive response? Some data are consistent with the possibility that the extent of behavioral remodeling that is triggered by the stressor may influence the depressogenicity of that stressor. In humans, for example, the dependency of the event on the patient's own behavior influences the event's depressogenicity. If the patient was judged to have contributed to the adverse event, its association with the depressive episode is significantly greater than if the event was judged to have been just “bad luck” (Kendler et al. 1999). Furthermore, events that remove from a person the possibility of enacting a behavioral role, such as being devalued in the role of spouse, parent, or breadwinner, are strongly linked to depressive episodes (Kendler et al. 2003).
What features of a stressor promote a depressive trajectory as opposed to alternative responses to stressful events, such as post traumatic stress disorder (PTSD), or Somatization Disorder? There is some evidence that events involving physical/sexual abuse are more likely to precede Somatization Disorders than to precede depression e.g. (Modestin et al. 2005, Spitzer et al. 2008). Events that represent danger are more likely to precede bouts of anxiety disorders than depression, while loss of a relationship and other types of losses are especially depressogenic (Hammen 2005, Kendler et al. 2003) (for review). The finding that loss is more depressogenic than other types of stressors is consistent with many decades of clinical observation e.g. (Freud 1917/1957). Thus, different classes of stressor seem to elicit different predominant symptoms.
Interpreting these data in the context of our theoretical work, we propose three hypotheses. First, we propose that different classes of stressors trigger remodeling of different circuits. For example, an event that represents a loss of a source of reward might trigger demolition along the reward circuit, which includes among other sites the hippocampus and prefrontal/infralimbic cortex (Haber and Knutson 2010) (for review). An event that represents a new source of danger might trigger remodeling along the fear circuit, which includes the amygdala (Wilensky et al. 2006).
Second, as our theoretical model proposes focal inflammatory activity at the sites of remodeling with localized release of inflammatory mediators there, we propose that which circuit the inflammatory mediators are localized to influences which symptoms predominate. Just as local inflammatory mediators can alter the threshold for firing of neurons in pain circuits, we argue that these molecules can alter the threshold for firing of neurons of other circuits. For example, raising the threshold for firing of neurons in the reward circuit may lead to anhedonia. Lowering the threshold for firing of neurons in the fear circuit may lead to anxiety. Inflammatory mediators at other neuroanatomical sites might give rise to predominantly somatic symptoms.
Third, just as the duration and severity of pain responses vary with the extent of bodily injury being repaired, we propose that the duration and severity of depressive symptoms would relate to the extent of the “injury” resulting from the demolition of neural tissue. Thus, a minor adverse event, such as a disappointment, might trigger only modest neural demolition, such as dismantling neural tissue underlying an erroneous reward prediction, induce only subtle activation of the inflammatory system such as para-inflammation, which might induce only brief and mild depressive symptoms like sadness. On the other hand, the loss of a major source of reward might trigger extensive demolition that induces the inflammatory system to grade up the continuum toward a full-blown wound repair response. Under these circumstances, the episode of depressive symptoms would be more severe and longer lasting, perhaps sometimes reaching the DSM criteria for a Major Depressive Episode.
These three hypotheses propose mechanisms by which the depressogenicity of a stressor may be influenced by the extent and neuroanatomical location of the injury that the stressor elicits. This provides a basis for the common sense notion that depression is at the extreme end of a continuum that includes ordinary states such as disappointment, sadness and distress.
12. Limitations
Although our theoretical model integrates six bodies of data (sections 2–7), it does not propose how to integrate data showing that the hypothalamo-pituitary-adrenal (HPA) axis, female gender, genetics, and the personality trait of neuroticism play roles in risk for depression. Although our theoretical model proposes explanations for acute stress-induced depressive episodes, chronic depression, late-life vascular depression, post-stroke depression, post-concussion depression, the increased risk of depression after traumatic childhood experiences, and for the full range of symptoms severity, our model does not address Seasonal Affective Disorder, Bipolar Disorder, or Premenstrual Dysphoria. Further, this model does not propose an explanation for why the hippocampus and prefrontal cortex are particularly susceptible to stress-induced microdamage. In addition, the model does not propose molecular mechanisms by which serotonin or norepinephrine reuptake inhibitors lead to enhancement of neurogenesis, BDNF, and plasticity and have anti-inflammatory and neuroprotective actions. Nor does it explain the antidepressant efficacy of Cognitive Behavioral Therapy, Electroconvulsive Therapy, or Transcranial Magnetic Stimulation. The model presented here does not propose what specific symptom criteria could be used to distinguish a well-functioning acute depressive response from a malfunctioning response. Nor does it indicate what cutoff would distinguish acute from chronic depression.
We do not discuss the large and growing field showing differential fMRI responses in various brain regions of depressed versus nondepressed individuals. One possibility is that these fMRI differences may be the neurobiological correlates of the differences in affective processing that are produced by cytokines and contribute to the motivational reprioritization of sickness behavior (Harrison et al. 2009).
Although the hypothesis elaborated in the present article suggests a molecular mechanism for psychological pain and physical pain in depression, it does not propose a molecular mechanism for other symptoms or common comorbidities of depression, such as anxiety (Shorter and Tyrer 2003), irritability (Snaith and Taylor 1985), guilt (American Psychiatric Association 2000), and worthlessness (American Psychiatric Association 2000), nor does it provide a mechanism by which inflammatory mediators in the brain induce the sickness symptoms that are common in depression, such as sleep and appetite disturbances (American Psychiatric Association 2000), fatigue (American Psychiatric Association 2000), nausea (Haug et al. 2002), diarrhea (Sugahara et al. 2004), or fever (Sugahara et al. 2004). In a separate manuscript in preparation (Wager-Smith 2010) we present a theoretical model whereby these symptoms respresent a family of behavioral defenses that are controlled by neuronal circuits for which the trigger threshold can be altered by inflammatory mediators.
Two additional symptoms of depression have not been discussed in this review nor in the manuscript just mentioned: recurrent thoughts of death/suicide and psychomotor retardation/agitation (American Psychiatric Association 2000).
Thus, although we have integrated many bodies of data into our theoretical model for depression, many relevant findings and symptoms of depression remain to be incorporated. Many hypotheses and predictions that emerge from this model will need to be tested before it can be determined whether this model is a solid foundation upon which a more complete understanding of depression can be built.
13. Conclusion
In this article, we have reviewed data supporting a theoretical model for the biological etiology of depression. This model uses a novel scenario for the healthy functioning of the response to stress in order to predict sources of pathology in depression. In this model, what could be considered emotionally traumatic brain injury, eTBI, triggers a neuroinflammatory-facilitated injury repair response. The sickness symptoms of depression are induced by the released inflammatory mediators which also produce central sensitization of psychological pain circuits. As after stroke, the injury triggers neurogenesis at remote brain sites and produces newborn neurons that migrate to the sites of injury across the brain. The depressive symptoms remit if the healing process is successful, if the neuroinflammatory response resolves rather than becoming chronic, and if the transition to an exaggerated psychological pain state is avoided. This depressive response is graded so that weak stressors would induce only mild and short-lived depressive symptoms like ordinary sadness.
However, pathological outcomes develop frequently, as they do after serious bodily injury, and can lead to chronic psychological pain and lasting disability. If chronic neuroinflammation occurs, inflammatory-mediated destruction of tissue at some brain sites can lead to loss of material that is detectable macroscopically. Furthermore, hyperalgesic priming can lead to exaggerated reactions to trivial stressors, allowing future depressions to develop without any apparent precipitating event.
According to one hypothesis that emerges from this theoretical model, this stressful life event-triggered injury repair process may serve a greater function, that is to dismantle neural circuitry that has been rendered disadvantageous by the life event, and to generate neural underpinnings of an updated behavioral program. Within this view, if a behavioral or physiological solution to the stressful predicament is not possible, this might provide an additional route to dysfunction of the depressive response.
These various types of exaggerated and malfunctioning depressive responses will likely be overrepresented in the subset of individuals that seek treatment. If individuals with such pathological depression receive antidepressant medications, these treatments have a number of effects that may tip a growth/pruning balance in the brain by inhibiting destruction and promoting growth of neural tissue (Fig. 1). Such a constellation of effects would be expected to accelerate, but not to instantaneously terminate, the healing process. This may explain the several weeks-long delay in apparent antidepressant efficacy. On the other hand, if an acute, well-functioning depressive episode is treated with antidepressants, such accelerated healing might disrupt the delicate process of creating the long-term neural and behavioral changes that were necessitated by the stressor.
Our theoretical model for depression suggests answers to many questions such as what is the source of inflammatory mediators that have been detected in the blood circulation of depressed patients (brain injury), how does vagal nerve stimulation exert antidepressant efficacy (through a anti-neuroinflammatory effects), how does traumatic and ischemic brain injury lead to depression (the brain injury repair process induces depression), why does depression share features with a family of other chronic disorders (they share a central sensitization or hyperalgesic priming pathophysiology), how do analgesic and anti-inflammatory drugs exert antidepressant effects (via shared mechanisms for psychological and physical pain). Our theoretical model encourages drug discovery efforts for depression to include testing emerging analgesic, anti-neuroinflammatory and neuroprotective agents in animal depression paradigms that are chosen to match each of their unique hypothesized etiologies with the process that the drug targets.
The concept, that a healthy function of a depressive episode is to accomplish some sort of enduring psychological transformation, has a long history in an extremely broad variety of disciplines, both scientific and nonscientific. For example, psychoanalytic observations have led to the proposal that depression consists of an inner labor that allows a person to detach from some entity which he/she had become attached to but had subsequently lost (Freud 1917/1957) and that resolution of depression involves some type of inner restructuring such as behavioral reorganization, revision of plans, or abandoned strivings (Gut 1989). Animal and human psychological studies led to the proposal that depression is a stage in the incentive-disengagement cycle during which the individual terminates a commitment to pursue a particular incentive (Klinger 1975). An evolutionary argument posits that the low mood in depression can foster disengagement from unreachable goals (Nesse 2000). An integration of each of these notions of depression with conceptions drawn from other sources including Native American spiritual traditions and the philosophy of homeopathic medicine led to the proposal that depression is a healing response that functions as a mediator of personal transformation (Zuess 2003). Observations in clinical sociology indicate that depression enables a change in social roles (Fein 1990). A neuropsychoanalytic argument holds that sadness functions to decouple outdated stimulus-reward associations, thereby correcting reward prediction errors (Freed and Mann 2007, Freed 2009). Each of these disparate perspectives also emphasize that depressive responses sometimes fail in their adaptive function, go awry and create clinical depressive illness. Our theoretical model for depression suggests molecular and cellular processes that may underlie this widely proposed psychological transformation function and its vulnerability to dysfunction.
In our scenario, the depressive episode can be considered analogous to a period of convalescence from a serious injury, such as a broken pelvis. If one were unfortunate enough to have to endure an unmedicated recuperation from this analogous injury, a two or more week long period of clinically significant distress and impaired social and occupational functioning would certainly be expected. It would not be hard to imagine that most of the day, nearly every day, the victim of one of these incidents would experience markedly diminished interest in almost all activities, diminished ability to concentrate, appetite disturbances resulting in significant weight loss or gain, insomnia or hypersomnia, fatigue, as well as pain, anxiety, irritability, and an unwillingness to get out of bed. Despite meeting the DSM criteria for Major Depressive Disorder, a diagnosis of a “disorder” would not be given here because an exclusion criterion is met (American Psychiatric Association 2000): The symptoms are due to a medical condition - a major injury. Future study will discern whether the DSM inclusion criteria for Major Depressive Disorder can be reached by a well-functioning depressive response to severe stress-induced neuronal injury in the absence of any mental illness, in addition to being reached by individuals with a malfunctioning depressive response.
Acknowledgements
The authors would like to thank Mr. Michael Arends for editorial assistance, Mrs. Jessica Benedict and Ms. Amy Tan for technical assistance with unpublished experimental work that led to this hypothesis, Dr. John Walker for performing DNA microarray analysis on those unpublished experiments, Dr. Steve Kay and Dr. George Koob for mentoring and supporting related projects that helped shape these ideas and for comments on early drafts of the manuscript. We would also like to thank an extraordinarily knowledgeable anonymous reviewer of the first submitted version of this review for providing a rapid, thorough, detailed and insightful critique that led to a complete reworking of the review into its present, much improved form. We also sincerely thank Drs. Geoffrey Miller, Gary Wayman, Naomi Wray, Mark Mayford, Lewis Wolpert, Graham Rook, Michael Hennessy, Jaak Panksepp, Dimitri Grigoriadis, Jonathan Zuess and Robert McArthur for feedback on previous drafts of this review. We are also grateful to Drs. Charles Raison, Chris Lowry, and Graham Rook for sharing their relevant work before publication. Many thanks to Drs. Michael Hennessy, Terrence Deak, Stanley T. Carmichael, Matthew O. Fraser, Eric Klinger, Jonathan Zuess, Constance Hammen, and Randy Nesse for helpful discussions. This work was supported by National Institutes of Health National Research Service Award F32 DA017403-01A1 to KWS and research grant R01MH62527-02 to AM from the National Institute of Mental Health.
References
- Abdel-Salam OM, Nofal SM, El-Shenawy SM. Evaluation of the anti-inflammatory and anti-nociceptive effects of different antidepressants in the rat. Pharmacol Res. 2003;48:157–65. doi: 10.1016/s1043-6618(03)00106-3. [DOI] [PubMed] [Google Scholar]
- Abdel-Salam OM, Baiuomy AR, Arbid MS. Studies on the anti-inflammatory effect of fluoxetine in the rat. Pharmacol Res. 2004;49:119–31. doi: 10.1016/j.phrs.2003.07.016. [DOI] [PubMed] [Google Scholar]
- Adachi M, Barrot M, Autry AE, Theobald D, Monteggia LM. Selective loss of brain-derived neurotrophic factor in the dentate gyrus attenuates antidepressant efficacy. Biol Psychiatry. 2008;63:642–9. doi: 10.1016/j.biopsych.2007.09.019. Epub 2007 Nov 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adlard PA, Cotman CW. Voluntary exercise protects against stress-induced decreases in brain-derived neurotrophic factor protein expression. Neuroscience. 2004;124:985–92. doi: 10.1016/j.neuroscience.2003.12.039. [DOI] [PubMed] [Google Scholar]
- Advani T, Koek W, Hensler JG. Gender differences in the enhanced vulnerability of BDNF+/− mice to mild stress. Int J Neuropsychopharmacol. 2009;12:583–8. doi: 10.1017/S1461145709000248. Epub 2009 Apr 3. [DOI] [PubMed] [Google Scholar]
- Aguilar-Valles A, Sanchez E, de Gortari P, Balderas I, Ramirez-Amaya V, Bermudez-Rattoni F, Joseph-Bravo P. Analysis of the stress response in rats trained in the water-maze: differential expression of corticotropin-releasing hormone, CRH-R1, glucocorticoid receptors and brain-derived neurotrophic factor in limbic regions. Neuroendocrinology. 2005;82:306–19. doi: 10.1159/000093129. Epub 2006 May 4. [DOI] [PubMed] [Google Scholar]
- Airan RD, Meltzer LA, Roy M, Gong Y, Chen H, Deisseroth K. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science. 2007;317:819–23. doi: 10.1126/science.1144400. Epub 2007 Jul 5. [DOI] [PubMed] [Google Scholar]
- Akhondzadeh S, Jafari S, Raisi F, Nasehi AA, Ghoreishi A, Salehi B, Mohebbi-Rasa S, Raznahan M, Kamalipour A. Clinical trial of adjunctive celecoxib treatment in patients with major depression: a double blind and placebo controlled trial. Depress Anxiety. 2009;26:607–611. doi: 10.1002/da.20589. [DOI] [PubMed] [Google Scholar]
- Aleisa AM, Alzoubi KH, Gerges NZ, Alkadhi KA. Chronic psychosocial stress-induced impairment of hippocampal LTP: possible role of BDNF. Neurobiol Dis. 2006;22:453–62. doi: 10.1016/j.nbd.2005.12.005. Epub 2006 Mar 10. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. `Vascular depression' hypothesis. Arch Gen Psychiatry. 1997;54:915–22. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- Allaman I, Papp M, Kraftsik R, Fiumelli H, Magistretti PJ, Martin JL. Expression of brain-derived neurotrophic factor is not modulated by chronic mild stress in the rat hippocampus and amygdala. Pharmacol Rep. 2008;60:1001–7. [PubMed] [Google Scholar]
- Allard L, Lescuyer P, Burgess J, Leung KY, Ward M, Walter N, Burkhard PR, Corthals G, Hochstrasser DF, Sanchez JC. ApoC-I and ApoC-III as potential plasmatic markers to distinguish between ischemic and hemorrhagic stroke. Proteomics. 2004;4:2242–51. doi: 10.1002/pmic.200300809. [DOI] [PubMed] [Google Scholar]
- Alonso R, Griebel G, Pavone G, Stemmelin J, Le Fur G, Soubrie P. Blockade of CRF(1) or V(1b) receptors reverses stress-induced suppression of neurogenesis in a mouse model of depression. Mol Psychiatry. 2004;9:278–86. 224. doi: 10.1038/sj.mp.4001464. [DOI] [PubMed] [Google Scholar]
- Alvarez A, Cacabelos R, Sanpedro C, Garcia-Fantini M, Aleixandre M. Serum TNF-alpha levels are increased and correlate negatively with free IGF-I in Alzheimer disease. Neurobiol Aging. 2007;28:533–6. doi: 10.1016/j.neurobiolaging.2006.02.012. Epub 2006 Mar 29. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th edition. American Psychiatric Association; Washington, DC: 2000. text revision. [Google Scholar]
- Andreasson A, Arborelius L, Erlanson-Albertsson C, Lekander M. A putative role for cytokines in the impaired appetite in depression. Brain Behav Immun. 2007;21:147–52. doi: 10.1016/j.bbi.2006.08.002. Epub 2006 Sep 22. [DOI] [PubMed] [Google Scholar]
- Andrews G. Placebo response in depression: bane of research, boon to therapy. Br J Psychiatry. 2001;178:192–4. doi: 10.1192/bjp.178.3.192. [DOI] [PubMed] [Google Scholar]
- Anisman H, Kokkinidis L, Merali Z. Interleukin-2 decreases accumbal dopamine efflux and responding for rewarding lateral hypothalamic stimulation. Brain Res. 1996;731:1–11. doi: 10.1016/0006-8993(96)00460-x. [DOI] [PubMed] [Google Scholar]
- Anisman H, Kokkinidis L, Borowski T, Merali Z. Differential effects of interleukin (IL)-1beta, IL-2 and IL-6 on responding for rewarding lateral hypothalamic stimulation. Brain Res. 1998;779:177–87. doi: 10.1016/s0006-8993(97)01114-1. [DOI] [PubMed] [Google Scholar]
- Annetta MG, Iemma D, Garisto C, Tafani C, Proietti R. Ketamine: new indications for an old drug. Curr Drug Targets. 2005;6:789–94. doi: 10.2174/138945005774574533. [DOI] [PubMed] [Google Scholar]
- Arakawa H, Blandino P, Jr., Deak T. Central infusion of interleukin-1 receptor antagonist blocks the reduction in social behavior produced by prior stressor exposure. Physiol Behav. 2009;98:139–46. doi: 10.1016/j.physbeh.2009.04.024. Epub 2009 May 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–22. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–70. doi: 10.1038/nm747. Epub 2002 Aug 5. [DOI] [PubMed] [Google Scholar]
- Ay I, Lu J, Ay H, Gregory Sorensen A. Vagus nerve stimulation reduces infarct size in rat focal cerebral ischemia. Neurosci Lett. 2009;459:147–51. doi: 10.1016/j.neulet.2009.05.018. Epub 2009 May 13. [DOI] [PubMed] [Google Scholar]
- Baare WF, Vinberg M, Knudsen GM, Paulson OB, Langkilde AR, Jernigan TL, Kessing LV. Hippocampal volume changes in healthy subjects at risk of unipolar depression. J Psychiatr Res. 2010 doi: 10.1016/j.jpsychires.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Bachis A, Cruz MI, Nosheny RL, Mocchetti I. Chronic unpredictable stress promotes neuronal apoptosis in the cerebral cortex. Neurosci Lett. 2008;442:104–8. doi: 10.1016/j.neulet.2008.06.081. Epub 2008 Jul 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain MJ, Dwyer SM, Rusak B. Restraint stress affects hippocampal cell proliferation differently in rats and mice. Neurosci Lett. 2004;368:7–10. doi: 10.1016/j.neulet.2004.04.096. [DOI] [PubMed] [Google Scholar]
- Ban TA. Pharmacotherapy of depression: a historical analysis. J Neural Transm. 2001;108:707–16. doi: 10.1007/s007020170047. [DOI] [PubMed] [Google Scholar]
- Barnum CJ, Blandino P, Jr., Deak T. Social status modulates basal IL-1 concentrations in the hypothalamus of pair-housed rats and influences certain features of stress reactivity. Brain Behav Immun. 2008;22:517–27. doi: 10.1016/j.bbi.2007.10.004. Epub 2007 Nov 26. [DOI] [PubMed] [Google Scholar]
- Barr AM, Song C, Sawada K, Young CE, Honer WG, Phillips AG. Tolerance to the anhedonic effects of lipopolysaccharide is associated with changes in syntaxin immunoreactivity in the nucleus accumbens. Int J Neuropsychopharmacol. 2003;6:23–34. doi: 10.1017/S146114570200319X. [DOI] [PubMed] [Google Scholar]
- Batchelor PE, Liberatore GT, Wong JY, Porritt MJ, Frerichs F, Donnan GA, Howells DW. Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. J Neurosci. 1999;19:1708–16. doi: 10.1523/JNEUROSCI.19-05-01708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor PE, Liberatore GT, Porritt MJ, Donnan GA, Howells DW. Inhibition of brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor expression reduces dopaminergic sprouting in the injured striatum. Eur J Neurosci. 2000;12:3462–8. doi: 10.1046/j.1460-9568.2000.00239.x. [DOI] [PubMed] [Google Scholar]
- Batchelor PE, Wills TE, Hewa AP, Porritt MJ, Howells DW. Stimulation of axonal sprouting by trophic factors immobilized within the wound core. Brain Res. 2008;1209:49–56. doi: 10.1016/j.brainres.2008.02.098. Epub 2008 Mar 10. [DOI] [PubMed] [Google Scholar]
- Belmadani A, Tran PB, Ren D, Miller RJ. Chemokines regulate the migration of neural progenitors to sites of neuroinflammation. J Neurosci. 2006;26:3182–91. doi: 10.1523/JNEUROSCI.0156-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennur S, Shankaranarayana Rao BS, Pawlak R, Strickland S, McEwen BS, Chattarji S. Stress-induced spine loss in the medial amygdala is mediated by tissue-plasminogen activator. Neuroscience. 2007;144:8–16. doi: 10.1016/j.neuroscience.2006.08.075. Epub 2006 Oct 16. [DOI] [PubMed] [Google Scholar]
- Beratis S, Katrivanou A, Georgiou S, Monastirli A, Pasmatzi E, Gourzis P, Tsambaos D. Major depression and risk of depressive symptomatology associated with short-term and low-dose interferon-alpha treatment. J Psychosom Res. 2005;58:15–8. doi: 10.1016/j.jpsychores.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Berns GS, Chappelow J, Cekic M, Zink CF, Pagnoni G, Martin-Skurski ME. Neurobiological substrates of dread. Science. 2006;312:754–8. doi: 10.1126/science.1123721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–8. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Bessa JM, Ferreira D, Melo I, Marques F, Cerqueira JJ, Palha JA, Almeida OF, Sousa N. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry. 2009;14:764–73. 739. doi: 10.1038/mp.2008.119. Epub 2008 Nov 4. [DOI] [PubMed] [Google Scholar]
- Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, Brenner GJ, Ji RR, Bean BP, Woolf CJ, Samad TA. Nociceptors are interleukin-1beta sensors. J Neurosci. 2008;28:14062–73. doi: 10.1523/JNEUROSCI.3795-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland ST, Schmid MJ, Der-Avakian A, Watkins LR, Spencer RL, Maier SF. Expression of c-fos and BDNF mRNA in subregions of the prefrontal cortex of male and female rats after acute uncontrollable stress. Brain Res. 2005;1051:90–9. doi: 10.1016/j.brainres.2005.05.065. [DOI] [PubMed] [Google Scholar]
- Bland ST, Schmid MJ, Greenwood BN, Watkins LR, Maier SF. Behavioral control of the stressor modulates stress-induced changes in neurogenesis and fibroblast growth factor-2. Neuroreport. 2006;17:593–7. doi: 10.1097/00001756-200604240-00008. [DOI] [PubMed] [Google Scholar]
- Bland ST, Tamlyn JP, Barrientos RM, Greenwood BN, Watkins LR, Campeau S, Day HE, Maier SF. Expression of fibroblast growth factor-2 and brain-derived neurotrophic factor mRNA in the medial prefrontal cortex and hippocampus after uncontrollable or controllable stress. Neuroscience. 2007;144:1219–28. doi: 10.1016/j.neuroscience.2006.11.026. Epub 2006 Dec 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandino P, Jr., Barnum CJ, Deak T. The involvement of norepinephrine and microglia in hypothalamic and splenic IL-1beta responses to stress. J Neuroimmunol. 2006;173:87–95. doi: 10.1016/j.jneuroim.2005.11.021. Epub 2006 Jan 4. [DOI] [PubMed] [Google Scholar]
- Blandino P, Jr., Barnum CJ, Solomon LG, Larish Y, Lankow BS, Deak T. Gene expression changes in the hypothalamus provide evidence for regionally-selective changes in IL-1 and microglial markers after acute stress. Brain Behav Immun. 2009;21:21. doi: 10.1016/j.bbi.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Blizzard CA, Chuckowree JA, King AE, Hosie KA, McCormack GH, Chapman JA, Vickers JC, Dickson TC. Focal Damage to the Adult Rat Neocortex Induces Wound Healing Accompanied by Axonal Sprouting and Dendritic Structural Plasticity. Cereb Cortex. 2010;28 doi: 10.1093/cercor/bhq091. [DOI] [PubMed] [Google Scholar]
- Bolanos SH, Khan DA, Hanczyc M, Bauer MS, Dhanani N, Brown ES. Assessment of mood states in patients receiving long-term corticosteroid therapy and in controls with patient-rated and clinician-rated scales. Ann Allergy Asthma Immunol. 2004;92:500–5. doi: 10.1016/S1081-1206(10)61756-5. [DOI] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Hen R, Rosoklija GB, Dwork AJ, John Mann J, Arango V. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology. 2009;34:2376–89. doi: 10.1038/npp.2009.75. Epub 2009 Jul 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombardier CH, Fann JR, Temkin NR, Esselman PC, Barber J, Dikmen SS. Rates of major depressive disorder and clinical outcomes following traumatic brain injury. Jama. 2010;303:1938–45. doi: 10.1001/jama.2010.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanno GA. Loss, trauma, and human resilience: have we underestimated the human capacity to thrive after extremely aversive events? Am Psychol. 2004;59:20–8. doi: 10.1037/0003-066X.59.1.20. [DOI] [PubMed] [Google Scholar]
- Bonotis K, Krikki E, Holeva V, Aggouridaki C, Costa V, Baloyannis S. Systemic immune aberrations in Alzheimer's disease patients. J Neuroimmunol. 2008;193:183–7. doi: 10.1016/j.jneuroim.2007.10.020. Epub 2007 Nov 26. [DOI] [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–62. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- Borowski T, Kokkinidis L, Merali Z, Anisman H. Lipopolysaccharide, central in vivo biogenic amine variations, and anhedonia. Neuroreport. 1998;9:3797–802. doi: 10.1097/00001756-199812010-00006. [DOI] [PubMed] [Google Scholar]
- Boulanger LM. Immune proteins in brain development and synaptic plasticity. Neuron. 2009;64:93–109. doi: 10.1016/j.neuron.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Bouwer C, Claassen J, Dinan TG, Nemeroff CB. Prednisone augmentation in treatment-resistant depression with fatigue and hypocortisolaemia: a case series. Depress Anxiety. 2000;12:44–50. doi: 10.1002/1520-6394(2000)12:1<44::AID-DA6>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Brevet M, Kojima H, Asakawa A, Atsuchi K, Ushikai M, Ataka K, Inui A, Kimura H, Sevestre H, Fujimiya M. Chronic foot-shock stress potentiates the influx of bone marrow-derived microglia into hippocampus. J Neurosci Res. 2010 doi: 10.1002/jnr.22362. [DOI] [PubMed] [Google Scholar]
- Brown ES, Suppes T. Mood symptoms during corticosteroid therapy: a review. Harv Rev Psychiatry. 1998;5:239–46. doi: 10.3109/10673229809000307. [DOI] [PubMed] [Google Scholar]
- Brown ES, Chandler PA. Mood and cognitive changes during systemic corticosteroid therapy. Prim Care Companion J Clin Psychiatry. 2001;3:17–21. doi: 10.4088/pcc.v03n0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ES, Suppes T, Khan DA, Carmody TJ., 3rd Mood changes during prednisone bursts in outpatients with asthma. J Clin Psychopharmacol. 2002;22:55–61. doi: 10.1097/00004714-200202000-00009. [DOI] [PubMed] [Google Scholar]
- Brown GW, Bifulco A, Harris T, Bridge L. Life stress, chronic subclinical symptoms and vulnerability to clinical depression. J Affect Disord. 1986;11:1–19. doi: 10.1016/0165-0327(86)90054-6. [DOI] [PubMed] [Google Scholar]
- Brown GW, Harris TO, Hepworth C. Loss, humiliation and entrapment among women developing depression: a patient and non-patient comparison. Psychol Med. 1995;25:7–21. doi: 10.1017/s003329170002804x. [DOI] [PubMed] [Google Scholar]
- Brunello N, Alboni S, Capone G, Benatti C, Blom JM, Tascedda F, Kriwin P, Mendlewicz J. Acetylsalicylic acid accelerates the antidepressant effect of fluoxetine in the chronic escape deficit model of depression. Int Clin Psychopharmacol. 2006;21:219–25. doi: 10.1097/00004850-200607000-00004. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Lopes M, Fregni F. A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. Int J Neuropsychopharmacol. 2008;11:1169–80. doi: 10.1017/S1461145708009309. Epub 2008 Aug 28. [DOI] [PubMed] [Google Scholar]
- Brustolim D, Ribeiro-dos-Santos R, Kast RE, Altschuler EL, Soares MB. A new chapter opens in anti-inflammatory treatments: the antidepressant bupropion lowers production of tumor necrosis factor-alpha and interferon-gamma in mice. Int Immunopharmacol. 2006;6:903–7. doi: 10.1016/j.intimp.2005.12.007. Epub 2006 Jan 25. [DOI] [PubMed] [Google Scholar]
- Bryant RA. Disentangling mild traumatic brain injury and stress reactions. N Engl J Med. 2008;358:525–7. doi: 10.1056/NEJMe078235. Epub 2008 Jan 30. [DOI] [PubMed] [Google Scholar]
- Burton C. Beyond somatisation: a review of the understanding and treatment of medically unexplained physical symptoms (MUPS) Br J Gen Pract. 2003;53:231–9. [PMC free article] [PubMed] [Google Scholar]
- Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- Carmichael ST. Cellular and molecular mechanisms of neural repair after stroke: making waves. Ann Neurol. 2006;59:735–42. doi: 10.1002/ana.20845. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Heninger GR, Malison RT, Tyrka AR, Price LH. Cerebrospinal fluid interleukin (IL)-6 in unipolar major depression. J Affect Disord. 2004;79:285–9. doi: 10.1016/S0165-0327(02)00460-3. [DOI] [PubMed] [Google Scholar]
- Castellanos M, Leira R, Serena J, Blanco M, Pedraza S, Castillo J, Davalos A. Plasma cellular-fibronectin concentration predicts hemorrhagic transformation after thrombolytic therapy in acute ischemic stroke. Stroke. 2004;35:1671–6. doi: 10.1161/01.STR.0000131656.47979.39. Epub 2004 May 27. [DOI] [PubMed] [Google Scholar]
- Cavus I, Duman RS. Influence of estradiol, stress, and 5-HT2A agonist treatment on brain-derived neurotrophic factor expression in female rats. Biol Psychiatry. 2003;54:59–69. doi: 10.1016/s0006-3223(03)00236-1. [DOI] [PubMed] [Google Scholar]
- Charrier C, Chigr F, Tardivel C, Mahaut S, Jean A, Najimi M, Moyse E. BDNF regulation in the rat dorsal vagal complex during stress-induced anorexia. Brain Res. 2006;1107:52–7. doi: 10.1016/j.brainres.2006.05.099. Epub 2006 Jul 18. [DOI] [PubMed] [Google Scholar]
- Chen F, Madsen TM, Wegener G, Nyengaard JR. Changes in rat hippocampal CA1 synapses following imipramine treatment. Hippocampus. 2008;18:631–9. doi: 10.1002/hipo.20423. [DOI] [PubMed] [Google Scholar]
- Chen H, Pandey GN, Dwivedi Y. Hippocampal cell proliferation regulation by repeated stress and antidepressants. Neuroreport. 2006;17:863–7. doi: 10.1097/01.wnr.0000221827.03222.70. [DOI] [PubMed] [Google Scholar]
- Chen J, Zacharek A, Zhang C, Jiang H, Li Y, Roberts C, Lu M, Kapke A, Chopp M. Endothelial nitric oxide synthase regulates brain-derived neurotrophic factor expression and neurogenesis after stroke in mice. J Neurosci. 2005;25:2366–75. doi: 10.1523/JNEUROSCI.5071-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JX, Li W, Zhao X, Yang JX. Effects of the Chinese traditional prescription Xiaoyaosan decoction on chronic immobilization stress-induced changes in behavior and brain BDNF, TrkB, and NT-3 in rats. Cell Mol Neurobiol. 2008;28:745–55. doi: 10.1007/s10571-007-9169-6. Epub 2007 Jul 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MC, Hamilton JP, Gotlib IH. Decreased hippocampal volume in healthy girls at risk of depression. Arch. 2010;67:270–6. doi: 10.1001/archgenpsychiatry.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Dube CM, Rice CJ, Baram TZ. Rapid loss of dendritic spines after stress involves derangement of spine dynamics by corticotropin-releasing hormone. J Neurosci. 2008;28:2903–11. doi: 10.1523/JNEUROSCI.0225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Rex CS, Rice CJ, Dube CM, Gall CM, Lynch G, Baram TZ. Correlated memory defects and hippocampal dendritic spine loss after acute stress involve corticotropin-releasing hormone signaling. Proc Natl Acad Sci U S A. 2010;107:13123–8. doi: 10.1073/pnas.1003825107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, Hempstead BL, Lee FS. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–3. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherng CG, Lin PS, Chuang JY, Chang WT, Lee YS, Kao GS, Lai YT, Yu L. Presence of conspecifics and their odor-impregnated objects reverse stress-decreased neurogenesis in mouse dentate gyrus. J Neurochem. 2010;112:1138–46. doi: 10.1111/j.1471-4159.2009.06505.x. [DOI] [PubMed] [Google Scholar]
- Chourbaji S, Hellweg R, Brandis D, Zorner B, Zacher C, Lang UE, Henn FA, Hortnagl H, Gass P. Mice with reduced brain-derived neurotrophic factor expression show decreased choline acetyltransferase activity, but regular brain monoamine levels and unaltered emotional behavior. Brain Res Mol Brain Res. 2004;121:28–36. doi: 10.1016/j.molbrainres.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Chourbaji S, Urani A, Inta I, Sanchis-Segura C, Brandwein C, Zink M, Schwaninger M, Gass P. IL-6 knockout mice exhibit resistance to stress-induced development of depression-like behaviors. Neurobiol Dis. 2006;23:587–94. doi: 10.1016/j.nbd.2006.05.001. Epub 2006 Jul 12. [DOI] [PubMed] [Google Scholar]
- Clough RW, Neese SL, Sherill LK, Tan AA, Duke A, Roosevelt RW, Browning RA, Smith DC. Cortical edema in moderate fluid percussion brain injury is attenuated by vagus nerve stimulation. Neuroscience. 2007;147:286–93. doi: 10.1016/j.neuroscience.2007.04.043. Epub 2007 Jun 1. [DOI] [PubMed] [Google Scholar]
- Cohen LS, Soares CN, Vitonis AF, Otto MW, Harlow BL. Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Arch Gen Psychiatry. 2006;63:385–90. doi: 10.1001/archpsyc.63.4.385. [DOI] [PubMed] [Google Scholar]
- Conrad CD, LeDoux JE, Magarinos AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113:902–13. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Conrad CD. What is the functional significance of chronic stress-induced CA3 dendritic retraction within the hippocampus? Behav Cogn Neurosci Rev. 2006;5:41–60. doi: 10.1177/1534582306289043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, McLaughlin KJ, Harman JS, Foltz C, Wieczorek L, Lightner E, Wright RL. Chronic glucocorticoids increase hippocampal vulnerability to neurotoxicity under conditions that produce CA3 dendritic retraction but fail to impair spatial recognition memory. J Neurosci. 2007;27:8278–85. doi: 10.1523/JNEUROSCI.2121-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti AC, Cryan JF, Dalvi A, Lucki I, Blendy JA. cAMP response element-binding protein is essential for the upregulation of brain-derived neurotrophic factor transcription, but not the behavioral or endocrine responses to antidepressant drugs. J Neurosci. 2002;22:3262–8. doi: 10.1523/JNEUROSCI.22-08-03262.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran C, Connor TJ, O'Keane V, Garland MR. The effects of vagus nerve stimulation on pro- and anti-inflammatory cytokines in humans: a preliminary report. Neuroimmunomodulation. 2005;12:307–9. doi: 10.1159/000087109. [DOI] [PubMed] [Google Scholar]
- Czeh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci U S A. 2001;98:12796–801. doi: 10.1073/pnas.211427898. Epub 2001 Oct 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeh B, Welt T, Fischer AK, Erhardt A, Schmitt W, Muller MB, Toschi N, Fuchs E, Keck ME. Chronic psychosocial stress and concomitant repetitive transcranial magnetic stimulation: effects on stress hormone levels and adult hippocampal neurogenesis. Biol Psychiatry. 2002;52:1057–65. doi: 10.1016/s0006-3223(02)01457-9. [DOI] [PubMed] [Google Scholar]
- Czeh B, Lucassen PJ. What causes the hippocampal volume decrease in depression? Are neurogenesis, glial changes and apoptosis implicated? Eur Arch Psychiatry Clin Neurosci. 2007;257:250–60. doi: 10.1007/s00406-007-0728-0. [DOI] [PubMed] [Google Scholar]
- Czeh B, Muller-Keuker JI, Rygula R, Abumaria N, Hiemke C, Domenici E, Fuchs E. Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology. 2007;32:1490–503. doi: 10.1038/sj.npp.1301275. Epub 2006 Dec 13. [DOI] [PubMed] [Google Scholar]
- Dagnino-Subiabre A, Zepeda-Carreno R, Diaz-Veliz G, Mora S, Aboitiz F. Chronic stress induces upregulation of brain-derived neurotrophic factor (BDNF) mRNA and integrin alpha5 expression in the rat pineal gland. Brain Res. 2006;1086:27–34. doi: 10.1016/j.brainres.2006.02.118. Epub 2006 Apr 13. [DOI] [PubMed] [Google Scholar]
- Dagyte G, Van der Zee EA, Postema F, Luiten PG, Den Boer JA, Trentani A, Meerlo P. Chronic but not acute foot-shock stress leads to temporary suppression of cell proliferation in rat hippocampus. Neuroscience. 2009;162:904–13. doi: 10.1016/j.neuroscience.2009.05.053. Epub 2009 May 29. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21:153–60. doi: 10.1016/j.bbi.2006.09.006. Epub 2006 Nov 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das UN. Vagus nerve stimulation, depression, and inflammation. Neuropsychopharmacology. 2007;32:2053–4. doi: 10.1038/sj.npp.1301286. [DOI] [PubMed] [Google Scholar]
- David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, Drew M, Craig DA, Guiard BP, Guilloux JP, Artymyshyn RP, Gardier AM, Gerald C, Antonijevic IA, Leonardo ED, Hen R. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–93. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Garza R., 2nd Endotoxin- or pro-inflammatory cytokine-induced sickness behavior as an animal model of depression: focus on anhedonia. Neurosci Biobehav Rev. 2005;29:761–70. doi: 10.1016/j.neubiorev.2005.03.016. [DOI] [PubMed] [Google Scholar]
- De Luigi A, Pizzimenti S, Quadri P, Lucca U, Tettamanti M, Fragiacomo C, De Simoni MG. Peripheral inflammatory response in Alzheimer's disease and multiinfarct dementia. Neurobiol Dis. 2002;11:308–14. doi: 10.1006/nbdi.2002.0556. [DOI] [PubMed] [Google Scholar]
- Deak T, Bellamy C, D'Agostino LG, Rosanoff M, McElderry NK, Bordner KA. Behavioral responses during the forced swim test are not affected by anti-inflammatory agents or acute illness induced by lipopolysaccharide. Behav Brain Res. 2005;160:125–34. doi: 10.1016/j.bbr.2004.11.024. Epub 2004 Dec 29. [DOI] [PubMed] [Google Scholar]
- Deak T, Bordner KA, McElderry NK, Barnum CJ, Blandino P, Jr., Deak MM, Tammariello SP. Stress-induced increases in hypothalamic IL-1: a systematic analysis of multiple stressor paradigms. Brain Res Bull. 2005;64:541–56. doi: 10.1016/j.brainresbull.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Decarolis NA, Eisch AJ. Hippocampal neurogenesis as a target for the treatment of mental illness: A critical evaluation. Neuropharmacology. 2010;58:884–93. doi: 10.1016/j.neuropharm.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuschle M, Sander P, Herpfer I, Fiebich BL, Heuser I, Lieb K. Substance P in serum and cerebrospinal fluid of depressed patients: no effect of antidepressant treatment. Psychiatry Res. 2005;136:1–6. doi: 10.1016/j.psychres.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Di Napoli M, Papa F, Bocola V. Prognostic influence of increased C-reactive protein and fibrinogen levels in ischemic stroke. Stroke. 2001;32:133–8. doi: 10.1161/01.str.32.1.133. [DOI] [PubMed] [Google Scholar]
- Diamond M, Kelly JP, Connor TJ. Antidepressants suppress production of the Th1 cytokine interferon-gamma, independent of monoamine transporter blockade. Eur Neuropsychopharmacol. 2006;16:481–90. doi: 10.1016/j.euroneuro.2005.11.011. Epub 2006 Jan 4. [DOI] [PubMed] [Google Scholar]
- Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, Costa RM, Sousa N. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325:621–5. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- Dimopoulos N, Piperi C, Salonicioti A, Mitropoulos P, Kallai E, Liappas I, Lea RW, Kalofoutis A. Indices of low-grade chronic inflammation correlate with early cognitive deterioration in an elderly Greek population. Neurosci Lett. 2006;398:118–23. doi: 10.1016/j.neulet.2005.12.064. Epub 2006 Jan 19. [DOI] [PubMed] [Google Scholar]
- Dittus W, Ratnayeke S. Individual and social behavioral responses to injury in wild toque macaques (Macaca Sinica) International Journal of Primatology. 1989;10:215–234. [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2009;67:446–57. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Dray A. Neuropathic pain: emerging treatments. Br J Anaesth. 2008;101:48–58. doi: 10.1093/bja/aen107. Epub 2008 May 28. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–27. doi: 10.1016/j.biopsych.2006.02.013. Epub 2006 Apr 21. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH. Effects of interleukin-1 and endotoxin in the forced swim and tail suspension tests in mice. Pharmacol Biochem Behav. 2005;81:688–93. doi: 10.1016/j.pbb.2005.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duric V, McCarson KE. Hippocampal neurokinin-1 receptor and brain-derived neurotrophic factor gene expression is decreased in rat models of pain and stress. Neuroscience. 2005;133:999–1006. doi: 10.1016/j.neuroscience.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Duric V, McCarson KE. Effects of analgesic or antidepressant drugs on pain- or stress-evoked hippocampal and spinal neurokinin-1 receptor and brain-derived neurotrophic factor gene expression in the rat. J Pharmacol Exp Ther. 2006;319:1235–43. doi: 10.1124/jpet.106.109470. Epub 2006 Sep 6. [DOI] [PubMed] [Google Scholar]
- Dzitoyeva S, Imbesi M, Uz T, Dimitrijevic N, Manev H, Manev R. Caffeic acid attenuates the decrease of cortical BDNF transcript IV mRNA induced by swim stress in wild-type but not in 5-lipoxygenase-deficient mice. J Neural Transm. 2008;115:823–7. doi: 10.1007/s00702-008-0034-7. Epub 2008 Feb 28. [DOI] [PubMed] [Google Scholar]
- Eaton WW, Shao H, Nestadt G, Lee HB, Bienvenu OJ, Zandi P. Population-based study of first onset and chronicity in major depressive disorder. Arch Gen Psychiatry. 2008;65:513–20. doi: 10.1001/archpsyc.65.5.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebmeier KP, Donaghey C, Steele JD. Recent developments and current controversies in depression. Lancet. 2006;367:153–67. doi: 10.1016/S0140-6736(06)67964-6. [DOI] [PubMed] [Google Scholar]
- Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5:718–25. doi: 10.1016/S1473-3099(05)70270-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ, Bolanos CA, de Wit J, Simonak RD, Pudiak CM, Barrot M, Verhaagen J, Nestler EJ. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: a role in depression. Biol Psychiatry. 2003;54:994–1005. doi: 10.1016/j.biopsych.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Cameron HA, Encinas JM, Meltzer LA, Ming GL, Overstreet-Wadiche LS. Adult neurogenesis, mental health, and mental illness: hope or hype? J Neurosci. 2008;28:11785–91. doi: 10.1523/JNEUROSCI.3798-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–2. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD. Why rejection hurts: a common neural alarm system for physical and social pain. Trends Cogn Sci. 2004;8:294–300. doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. An fMRI study of cytokine-induced depressed mood and social pain: the role of sex differences. Neuroimage. 2009;47:881–90. doi: 10.1016/j.neuroimage.2009.04.040. Epub 2009 Apr 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Mashal NM, Irwin MR. Inflammation and social experience: An inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain Behav Immun. 2010;24:558–63. doi: 10.1016/j.bbi.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100:13632–7. doi: 10.1073/pnas.2234031100. Epub 2003 Oct 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmquist JK, Scammell TE, Saper CB. Mechanisms of CNS response to systemic immune challenge: the febrile response. Trends Neurosci. 1997;20:565–70. doi: 10.1016/s0166-2236(97)01138-7. [DOI] [PubMed] [Google Scholar]
- Fein M. Role Change: A Resocialization Perspective. Praeger Publishers; New York: 1990. [Google Scholar]
- Ferragud A, Haro A, Sylvain A, Velazquez-Sanchez C, Hernandez-Rabaza V, Canales JJ. Enhanced habit-based learning and decreased neurogenesis in the adult hippocampus in a murine model of chronic social stress. Behav Brain Res. 2010;210:134–9. doi: 10.1016/j.bbr.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Fitzgerald FS. The Crack-up. Quality Paperback Book Club; New York: 1996. [Google Scholar]
- Fournier JC, DeRubeis RJ, Hollon SD, Dimidjian S, Amsterdam JD, Shelton RC, Fawcett J. Antidepressant drug effects and depression severity: a patient-level meta-analysis. Jama. 2010;303:47–53. doi: 10.1001/jama.2009.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun. 2007;21:47–59. doi: 10.1016/j.bbi.2006.03.005. Epub 2006 May 2. [DOI] [PubMed] [Google Scholar]
- Frank-Cannon TC, Alto LT, McAlpine FE, Tansey MG. Does neuroinflammation fan the flame in neurodegenerative diseases? Mol Neurodegener. 2009;4:47. doi: 10.1186/1750-1326-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed PJ, Mann JJ. Sadness and loss: toward a neurobiopsychosocial model. Am J Psychiatry. 2007;164:28–34. doi: 10.1176/ajp.2007.164.1.28. [DOI] [PubMed] [Google Scholar]
- Freed PJ. Is Sadness an Evolutionarily Conserved Brain Mechanism to Dampen Reward Seeking? Deression May Be a “Sadnes Disorder”. Neuropsychoanalysis. 2009;11:61–66. [Google Scholar]
- Freeman EW, Sammel MD, Liu L, Gracia CR, Nelson DB, Hollander L. Hormones and menopausal status as predictors of depression in women in transition to menopause. Arch Gen Psychiatry. 2004;61:62–70. doi: 10.1001/archpsyc.61.1.62. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry. 2006;63:375–82. doi: 10.1001/archpsyc.63.4.375. [DOI] [PubMed] [Google Scholar]
- Frenois F, Moreau M, O'Connor J, Lawson M, Micon C, Lestage J, Kelley KW, Dantzer R, Castanon N. Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology. 2007;32:516–31. doi: 10.1016/j.psyneuen.2007.03.005. Epub 2007 May 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freud S. Mourning and Melancholia. Hogarth Press; London, England: 1917/1957. [Google Scholar]
- Fuchikami M, Morinobu S, Kurata A, Yamamoto S, Yamawaki S. Single immobilization stress differentially alters the expression profile of transcripts of the brain-derived neurotrophic factor (BDNF) gene and histone acetylation at its promoters in the rat hippocampus. Int J Neuropsychopharmacol. 2009;12:73–82. doi: 10.1017/S1461145708008997. Epub 2008 Jun 11. [DOI] [PubMed] [Google Scholar]
- Galea LA, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81:689–97. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- Gallagher P, Malik N, Newham J, Young AH, Ferrier IN, Mackin P. Antiglucocorticoid treatments for mood disorders. Cochrane Database Syst Rev. 2008:CD005168. doi: 10.1002/14651858.CD005168.pub2. [DOI] [PubMed] [Google Scholar]
- Galvao RP, Garcia-Verdugo JM, Alvarez-Buylla A. Brain-derived neurotrophic factor signaling does not stimulate subventricular zone neurogenesis in adult mice and rats. J Neurosci. 2008;28:13368–83. doi: 10.1523/JNEUROSCI.2918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu ZZ, Park JY, Lind AL, Ma Q, Ji RR. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci. 2009;29:4096–108. doi: 10.1523/JNEUROSCI.3623-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bueno B, Caso JR, Leza JC. Stress as a neuroinflammatory condition in brain: damaging and protective mechanisms. Neurosci Biobehav Rev. 2008;32:1136–51. doi: 10.1016/j.neubiorev.2008.04.001. Epub 2008 Apr 11. [DOI] [PubMed] [Google Scholar]
- Geracioti TD, Jr., Carpenter LL, Owens MJ, Baker DG, Ekhator NN, Horn PS, Strawn JR, Sanacora G, Kinkead B, Price LH, Nemeroff CB. Elevated cerebrospinal fluid substance p concentrations in posttraumatic stress disorder and major depression. Am J Psychiatry. 2006;163:637–43. doi: 10.1176/ajp.2006.163.4.637. [DOI] [PubMed] [Google Scholar]
- Gillespie CF, Nemeroff CB. Hypercortisolemia and depression. Psychosom Med. 2005;67:S26–8. doi: 10.1097/01.psy.0000163456.22154.d2. [DOI] [PubMed] [Google Scholar]
- Givalois L, Marmigere F, Rage F, Ixart G, Arancibia S, Tapia-Arancibia L. Immobilization stress rapidly and differentially modulates BDNF and TrkB mRNA expression in the pituitary gland of adult male rats. Neuroendocrinology. 2001;74:148–59. doi: 10.1159/000054681. [DOI] [PubMed] [Google Scholar]
- Givalois L, Arancibia S, Alonso G, Tapia-Arancibia L. Expression of brain-derived neurotrophic factor and its receptors in the median eminence cells with sensitivity to stress. Endocrinology. 2004;145:4737–47. doi: 10.1210/en.2004-0616. Epub 2004 Jul 1. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Moreau M, Lestage J, Chen J, Sparkman NL, J OC, Castanon N, Kelley KW, Dantzer R, Johnson RW. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology. 2008;33:2341–51. doi: 10.1038/sj.npp.1301649. Epub 2007 Dec 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldwater DS, Pavlides C, Hunter RG, Bloss EB, Hof PR, McEwen BS, Morrison JH. Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neuroscience. 2009;164:798–808. doi: 10.1016/j.neuroscience.2009.08.053. Epub 2009 Aug 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, Yirmiya R. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry. 2008;13:717–28. doi: 10.1038/sj.mp.4002055. Epub 2007 Aug 14. [DOI] [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–8. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greden JF. Unmet need: what justifies the search for a new antidepressant? J Clin Psychiatry. 2002;63:3–7. [PubMed] [Google Scholar]
- Grippo AJ, Francis J, Beltz TG, Felder RB, Johnson AK. Neuroendocrine and cytokine profile of chronic mild stress-induced anhedonia. Physiol Behav. 2005;84:697–706. doi: 10.1016/j.physbeh.2005.02.011. Epub 2005 Mar 23. [DOI] [PubMed] [Google Scholar]
- Gronli J, Bramham C, Murison R, Kanhema T, Fiske E, Bjorvatn B, Ursin R, Portas CM. Chronic mild stress inhibits BDNF protein expression and CREB activation in the dentate gyrus but not in the hippocampus proper. Pharmacol Biochem Behav. 2006;85:842–9. doi: 10.1016/j.pbb.2006.11.021. Epub 2007 Jan 3. [DOI] [PubMed] [Google Scholar]
- Groves DA, Brown VJ. Vagal nerve stimulation: a review of its applications and potential mechanisms that mediate its clinical effects. Neurosci Biobehav Rev. 2005;29:493–500. doi: 10.1016/j.neubiorev.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Guarner F, Bourdet-Sicard R, Brandtzaeg P, Gill HS, McGuirk P, van Eden W, Versalovic J, Weinstock JV, Rook GA. Mechanisms of disease: the hygiene hypothesis revisited. Nat Clin Pract Gastroenterol Hepatol. 2006;3:275–84. doi: 10.1038/ncpgasthep0471. [DOI] [PubMed] [Google Scholar]
- Guirado R, Varea E, Castillo-Gomez E, Gomez-Climent MA, Rovira-Esteban L, Blasco-Ibanez JM, Crespo C, Martinez-Guijarro FJ, Nacher J. Effects of chronic fluoxetine treatment on the rat somatosensory cortex: activation and induction of neuronal structural plasticity. Neurosci Lett. 2009;457:12–5. doi: 10.1016/j.neulet.2009.03.104. Epub 2009 Apr 5. [DOI] [PubMed] [Google Scholar]
- Guo JY, Li CY, Ruan YP, Sun M, Qi XL, Zhao BS, Luo F. Chronic treatment with celecoxib reverses chronic unpredictable stress-induced depressive-like behavior via reducing cyclooxygenase-2 expression in rat brain. Eur J Pharmacol. 2009;612:54–60. doi: 10.1016/j.ejphar.2009.03.076. Epub 2009 Apr 6. [DOI] [PubMed] [Google Scholar]
- Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–21. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- Gut E. Productive and Unproductive Depression: Success or Failure of a Vital Process. Basic Books; New York: 1989. [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains AB, Vu MA, Maciejewski PK, van Dyck CH, Gottron M, Arnsten AF. Inhibition of protein kinase C signaling protects prefrontal cortex dendritic spines and cognition from the effects of chronic stress. Proc Natl Acad Sci U S A. 2009;106:17957–62. doi: 10.1073/pnas.0908563106. Epub 2009 Sep 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains BC, Waxman SG. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J Neurosci. 2006;26:4308–17. doi: 10.1523/JNEUROSCI.0003-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajszan T, MacLusky NJ, Leranth C. Short-term treatment with the antidepressant fluoxetine triggers pyramidal dendritic spine synapse formation in rat hippocampus. Eur J Neurosci. 2005;21:1299–303. doi: 10.1111/j.1460-9568.2005.03968.x. [DOI] [PubMed] [Google Scholar]
- Hajszan T, Dow A, Warner-Schmidt JL, Szigeti-Buck K, Sallam NL, Parducz A, Leranth C, Duman RS. Remodeling of hippocampal spine synapses in the rat learned helplessness model of depression. Biol Psychiatry. 2009;65:392–400. doi: 10.1016/j.biopsych.2008.09.031. Epub 2008 Nov 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale KD, Weigent DA, Gauthier DK, Hiramoto RN, Ghanta VK. Cytokine and hormone profiles in mice subjected to handling combined with rectal temperature measurement stress and handling only stress. Life Sci. 2003;72:1495–508. doi: 10.1016/s0024-3205(02)02415-3. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Cheung J, Rhodes KM, Schutz KC, Falls WA, Braas KM, May V. Chronic stress increases pituitary adenylate cyclase-activating peptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST): roles for PACAP in anxiety-like behavior. Psychoneuroendocrinology. 2009;34:833–43. doi: 10.1016/j.psyneuen.2008.12.013. Epub 2009 Jan 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C. Stress and depression. Annu Rev Clin Psychol. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry. 2009;66:407–14. doi: 10.1016/j.biopsych.2009.03.015. Epub 2009 May 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RJ, Depner UB, Wassle H, Ahmadi S, Heindl C, Reinold H, Smart TG, Harvey K, Schutz B, Abo-Salem OM, Zimmer A, Poisbeau P, Welzl H, Wolfer DP, Betz H, Zeilhofer HU, Muller U. GlyR α3: an essential target for spinal PGE2-mediated inflammatory pain sensitization. Science. 2004;304:884–7. doi: 10.1126/science.1094925. [DOI] [PubMed] [Google Scholar]
- Hashioka S, Klegeris A, Monji A, Kato T, Sawada M, McGeer PL, Kanba S. Antidepressants inhibit interferon-γ-induced microglial production of IL-6 and nitric oxide. Exp Neurol. 2007;206:33–42. doi: 10.1016/j.expneurol.2007.03.022. Epub 2007 Mar 30. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Arch Gen Psychiatry. 2005;62:1097–106. doi: 10.1001/archpsyc.62.10.1097. [DOI] [PubMed] [Google Scholar]
- Haug TT, Mykletun A, Dahl AA. The prevalence of nausea in the community: psychological, social and somatic factors. Gen Hosp Psychiatry. 2002;24:81–6. doi: 10.1016/s0163-8343(01)00184-0. [DOI] [PubMed] [Google Scholar]
- Hauser P, Khosla J, Aurora H, Laurin J, Kling MA, Hill J, Gulati M, Thornton AJ, Schultz RL, Valentine AD, Meyers CA, Howell CD. A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Mol Psychiatry. 2002;7:942–7. doi: 10.1038/sj.mp.4001119. [DOI] [PubMed] [Google Scholar]
- Heine VM, Maslam S, Zareno J, Joels M, Lucassen PJ. Suppressed proliferation and apoptotic changes in the rat dentate gyrus after acute and chronic stress are reversible. Eur J Neurosci. 2004;19:131–44. doi: 10.1046/j.1460-9568.2003.03100.x. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Morris A. Passive responses of young guinea pigs during exposure to a novel environment: influences of social partners and age. Dev Psychobiol. 2005;46:86–96. doi: 10.1002/dev.20045. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Deak T, Schiml Webb P. A. Early attachment-figure separation and increased risk for later depression: potential mediation by proinflammatory processes. Neurosci Biobehav Rev. 2009;34:782–90. doi: 10.1016/j.neubiorev.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy MB, SchimlWebb PA, Deak T. Separation, Sickness, and Depression: A New Perspective on an Old Animal Model. Curr Dir Psychol Sci. 2009;18:227–231. doi: 10.1111/j.1467-8721.2009.01641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry RA, Hughes SM, Connor B. AAV-mediated delivery of BDNF augments neurogenesis in the normal and quinolinic acid-lesioned adult rat brain. Eur J Neurosci. 2007;25:3513–25. doi: 10.1111/j.1460-9568.2007.05625.x. [DOI] [PubMed] [Google Scholar]
- Hill MN, Kambo JS, Sun JC, Gorzalka BB, Galea LA. Endocannabinoids modulate stress-induced suppression of hippocampal cell proliferation and activation of defensive behaviours. Eur J Neurosci. 2006;24:1845–9. doi: 10.1111/j.1460-9568.2006.05061.x. [DOI] [PubMed] [Google Scholar]
- Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N Engl J Med. 2008;358:453–63. doi: 10.1056/NEJMoa072972. Epub 2008 Jan 30. [DOI] [PubMed] [Google Scholar]
- Holick KA, Lee DC, Hen R, Dulawa SC. Behavioral effects of chronic fluoxetine in BALB/cJ mice do not require adult hippocampal neurogenesis or the serotonin 1A receptor. Neuropsychopharmacology. 2008;33:406–17. doi: 10.1038/sj.npp.1301399. Epub 2007 Apr 11. [DOI] [PubMed] [Google Scholar]
- Horikawa N, Yamazaki T, Izumi N, Uchihara M. Incidence and clinical course of major depression in patients with chronic hepatitis type C undergoing interferon-alpha therapy: a prospective study. Gen Hosp Psychiatry. 2003;25:34–8. doi: 10.1016/s0163-8343(02)00239-6. [DOI] [PubMed] [Google Scholar]
- Hoshaw BA, Malberg JE, Lucki I. Central administration of IGF-I and BDNF leads to long-lasting antidepressant-like effects. Brain Res. 2005;1037:204–8. doi: 10.1016/j.brainres.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–86. doi: 10.1097/PSY.0b013e3181907c1b. Epub 2009 Feb 2. [DOI] [PubMed] [Google Scholar]
- Hrobjartsson A, Gotzsche PC. Is the placebo powerless? An analysis of clinical trials comparing placebo with no treatment. N Engl J Med. 2001;344:1594–602. doi: 10.1056/NEJM200105243442106. [DOI] [PubMed] [Google Scholar]
- Hucho T, Levine JD. Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron. 2007;55:365–76. doi: 10.1016/j.neuron.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Hudson JI, Pope HG., Jr. Affective spectrum disorder: does antidepressant response identify a family of disorders with a common pathophysiology? Am J Psychiatry. 1990;147:552–64. doi: 10.1176/ajp.147.5.552. [DOI] [PubMed] [Google Scholar]
- Hudson JI, Mangweth B, Pope HG, Jr., De Col C, Hausmann A, Gutweniger S, Laird NM, Biebl W, Tsuang MT. Family study of affective spectrum disorder. Arch Gen Psychiatry. 2003;60:170–7. doi: 10.1001/archpsyc.60.2.170. [DOI] [PubMed] [Google Scholar]
- Hwang J, Zheng LT, Ock J, Lee MG, Kim SH, Lee HW, Lee WH, Park HC, Suk K. Inhibition of glial inflammatory activation and neurotoxicity by tricyclic antidepressants. Neuropharmacology. 2008;55:826–34. doi: 10.1016/j.neuropharm.2008.06.045. Epub 2008 Jun 29. [DOI] [PubMed] [Google Scholar]
- Ibarguen-Vargas Y, Surget A, Vourc'h P, Leman S, Andres CR, Gardier AM, Belzung C. Deficit in BDNF does not increase vulnerability to stress but dampens antidepressant-like effects in the unpredictable chronic mild stress. Behav Brain Res. 2009;202:245–51. doi: 10.1016/j.bbr.2009.03.040. Epub 2009 Apr 5. [DOI] [PubMed] [Google Scholar]
- Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat Rev Neurosci. 2009;10:199–210. doi: 10.1038/nrn2576. Epub 2009 Feb 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intiso D, Zarrelli MM, Lagioia G, Di Rienzo F, Checchia De Ambrosio C, Simone P, Cioffi Dagger RP. Tumor necrosis factor alpha serum levels and inflammatory response in acute ischemic stroke patients. Neurol Sci. 2004;24:390–6. doi: 10.1007/s10072-003-0194-z. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci. 2006;26:5733–8. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, Praag H, Gage FH. Adult brain neurogenesis and psychiatry: a novel theory of depression. Mol Psychiatry. 2000;5:262–9. doi: 10.1038/sj.mp.4000712. [DOI] [PubMed] [Google Scholar]
- Jacobs BL. Adult brain neurogenesis and depression. Brain Behav Immun. 2002;16:602–9. doi: 10.1016/s0889-1591(02)00015-6. [DOI] [PubMed] [Google Scholar]
- Jalalvand E, Javan M, Haeri-Rohani A, Ahmadiani A. Stress- and non-stress-mediated mechanisms are involved in pain-induced apoptosis in hippocampus and dorsal lumbar spinal cord in rats. Neuroscience. 2008;157:446–52. doi: 10.1016/j.neuroscience.2008.08.052. Epub 2008 Sep 4. [DOI] [PubMed] [Google Scholar]
- Jarvis MF, Honore P, Shieh CC, Chapman M, Joshi S, Zhang XF, Kort M, Carroll W, Marron B, Atkinson R, Thomas J, Liu D, Krambis M, Liu Y, McGaraughty S, Chu K, Roeloffs R, Zhong C, Mikusa JP, Hernandez G, Gauvin D, Wade C, Zhu C, Pai M, Scanio M, Shi L, Drizin I, Gregg R, Matulenko M, Hakeem A, Gross M, Johnson M, Marsh K, Wagoner PK, Sullivan JP, Faltynek CR, Krafte DS. A-803467, a potent and selective Nav1.8 sodium channel blocker, attenuates neuropathic and inflammatory pain in the rat. Proc Natl Acad Sci U S A. 2007;104:8520–5. doi: 10.1073/pnas.0611364104. Epub 2007 May 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenewein J, Moergeli H, Wittmann L, Buchi S, Kraemer B, Schnyder U. Development of chronic pain following severe accidental injury. Results of a 3-year follow-up study. J Psychosom Res. 2009;66:119–26. doi: 10.1016/j.jpsychores.2008.07.011. Epub 2008 Dec 16. [DOI] [PubMed] [Google Scholar]
- Jin Y, Lim CM, Kim SW, Park JY, Seo JS, Han PL, Yoon SH, Lee JK. Fluoxetine attenuates kainic acid-induced neuronal cell death in the mouse hippocampus. Brain Res. 2009;1281:108–16. doi: 10.1016/j.brainres.2009.04.053. Epub 2009 May 7. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Greenwood BN, Fleshner M. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience. 2005;135:1295–307. doi: 10.1016/j.neuroscience.2005.06.090. Epub 2005 Sep 13. [DOI] [PubMed] [Google Scholar]
- Jones NC, Cardamone L, Williams JP, Salzberg MR, Myers D, O'Brien TJ. Experimental traumatic brain injury induces a pervasive hyperanxious phenotype in rats. J Neurotrauma. 2008;25:1367–74. doi: 10.1089/neu.2008.0641. [DOI] [PubMed] [Google Scholar]
- Kato M, Iwata H, Okamoto M, Narita H. Focal cerebral ischemia-induced escape deficit in rats is ameliorated by a reversible inhibitor of monoamine oxidase-a: implications for a novel animal model of post-stroke depression. Biol Pharm Bull. 2000;23:406–10. doi: 10.1248/bpb.23.406. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1β, interleukin-6, and tumor necrosis factor-α in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008;28:5189–94. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–25. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kronenberg G. Depressed new neurons--adult hippocampal neurogenesis and a cellular plasticity hypothesis of major depression. Biol Psychiatry. 2003;54:499–503. doi: 10.1016/s0006-3223(03)00319-6. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–41. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Thornton LM, Gardner CO. Stressful life events and previous episodes in the etiology of major depression in women: an evaluation of the “kindling” hypothesis. Am J Psychiatry. 2000;157:1243–51. doi: 10.1176/appi.ajp.157.8.1243. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Hettema JM, Butera F, Gardner CO, Prescott CA. Life event dimensions of loss, humiliation, entrapment, and danger in the prediction of onsets of major depression and generalized anxiety. Arch Gen Psychiatry. 2003;60:789–96. doi: 10.1001/archpsyc.60.8.789. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kuhn J, Prescott CA. The interrelationship of neuroticism, sex, and stressful life events in the prediction of episodes of major depression. Am J Psychiatry. 2004;161:631–6. doi: 10.1176/appi.ajp.161.4.631. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Myers J, Zisook S. Does bereavement-related major depression differ from major depression associated with other stressful life events? Am J Psychiatry. 2008;165:1449–55. doi: 10.1176/appi.ajp.2008.07111757. Epub 2008 Aug 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentner AC, James JS, Miguelez M, Bielajew C. Investigating the hedonic effects of interferon-alpha on female rats using brain-stimulation reward. Behav Brain Res. 2007;177:90–9. doi: 10.1016/j.bbr.2006.10.033. Epub 2006 Nov 28. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) Jama. 2003;289:3095–105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Ketterer MW, Brymer J, Rhoads K, Kraft P, Lovallo WR. Is aspirin, as used for antithrombosis, an emotion-modulating agent? J Psychosom Res. 1996;40:53–8. doi: 10.1016/0022-3999(95)00524-2. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–62. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med. 2008;5:e45. doi: 10.1371/journal.pmed.0050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger E. Consequences of Commitment to and Disengagement from Incentives. Psychological Review. 1975;82:1–25. [Google Scholar]
- Kohno T, Wang H, Amaya F, Brenner GJ, Cheng JK, Ji RR, Woolf CJ. Bradykinin enhances AMPA and NMDA receptor activity in spinal cord dorsal horn neurons by activating multiple kinases to produce pain hypersensitivity. J Neurosci. 2008;28:4533–40. doi: 10.1523/JNEUROSCI.5349-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kole MH, Costoli T, Koolhaas JM, Fuchs E. Bidirectional shift in the cornu ammonis 3 pyramidal dendritic organization following brief stress. Neurosci. 125:337–47. doi: 10.1016/j.neuroscience.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 2002;25:154–9. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- Koo JW, Duman RS. IL-1β is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A. 2008;105:751–6. doi: 10.1073/pnas.0708092105. Epub 2008 Jan 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlovsky N, Matar MA, Kaplan Z, Kotler M, Zohar J, Cohen H. Long-term down-regulation of BDNF mRNA in rat hippocampal CA1 subregion correlates with PTSD-like behavioural stress response. Int J Neuropsychopharmacol. 2007;10:741–58. doi: 10.1017/S1461145707007560. Epub 2007 Feb 12. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–8. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Krishnan R, Cella D, Leonardi C, Papp K, Gottlieb AB, Dunn M, Chiou CF, Patel V, Jahreis A. Effects of etanercept therapy on fatigue and symptoms of depression in subjects treated for moderate to severe plaque psoriasis for up to 96 weeks. Br J Dermatol. 2007;157:1275–7. doi: 10.1111/j.1365-2133.2007.08205.x. Epub 2007 Oct 4. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubera M, Lin AH, Kenis G, Bosmans E, van Bockstaele D, Maes M. Anti-Inflammatory effects of antidepressants through suppression of the interferon-gamma/interleukin-10 production ratio. J Clin Psychopharmacol. 2001;21:199–206. doi: 10.1097/00004714-200104000-00012. [DOI] [PubMed] [Google Scholar]
- Kwon MS, Seo YJ, Lee JK, Lee HK, Jung JS, Jang JE, Park SH, Suh HW. The repeated immobilization stress increases IL-1beta immunoreactivities in only neuron, but not astrocyte or microglia in hippocampal CA1 region, striatum and paraventricular nucleus. Neurosci Lett. 2008;430:258–63. doi: 10.1016/j.neulet.2007.11.006. Epub 2007 Nov 6. [DOI] [PubMed] [Google Scholar]
- Lagace DC, Donovan MH, Decarolis NA, Farnbauch LA, Malhotra S, Berton O, Nestler EJ, Krishnan V, Eisch AJ. Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. Proc Natl Acad Sci U S A. 2010;107:4436–41. doi: 10.1073/pnas.0910072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert KG, Buckelew SK, Staffiso-Sandoz G, Gaffga S, Carpenter W, Fisher J, Kinsley CH. Activity-stress induces atrophy of apical dendrites of hippocampal pyramidal neurons in male rats. Physiol Behav. 1998;65:43–9. doi: 10.1016/s0031-9384(98)00114-0. [DOI] [PubMed] [Google Scholar]
- Larsson E, Mandel RJ, Klein RL, Muzyczka N, Lindvall O, Kokaia Z. Suppression of insult-induced neurogenesis in adult rat brain by brain-derived neurotrophic factor. Exp Neurol. 2002;177:1–8. doi: 10.1006/exnr.2002.7992. [DOI] [PubMed] [Google Scholar]
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterbach EC, Victoroff J, Coburn KL, Shillcutt SD, Doonan SM, Mendez MF. Psychopharmacological neuroprotection in neurodegenerative disease: assessing the preclinical data. J Neuropsychiatry Clin Neurosci. 2010;22:8–18. doi: 10.1176/jnp.2010.22.1.8. [DOI] [PubMed] [Google Scholar]
- Lawrence T, Gilroy DW. Chronic inflammation: a failure of resolution? Int J Exp Pathol. 2007;88:85–94. doi: 10.1111/j.1365-2613.2006.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecrubier Y. Physical components of depression and psychomotor retardation. J Clin Psychiatry. 2006;67:23–6. [PubMed] [Google Scholar]
- Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82:1367–75. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Kim SJ, Kim SW, Choi SH, Shin YC, Park SH, Moon BH, Cho E, Lee MS, Chun BG, Shin KH. Chronic mild stress decreases survival, but not proliferation, of new-born cells in adult rat hippocampus. Exp Mol Med. 2006;38:44–54. doi: 10.1038/emm.2006.6. [DOI] [PubMed] [Google Scholar]
- Lee Y, Duman RS, Marek GJ. The mGlu2/3 receptor agonist LY354740 suppresses immobilization stress-induced increase in rat prefrontal cortical BDNF mRNA expression. Neurosci Lett. 2006;398:328–32. doi: 10.1016/j.neulet.2006.01.021. Epub 2006 Feb 15. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Choi B, Lee EH, Choi KS, Sohn S. Immobilization stress induces cell death through production of reactive oxygen species in the mouse cerebral cortex. Neurosci Lett. 2006;392:27–31. doi: 10.1016/j.neulet.2005.08.065. Epub 2005 Oct 3. [DOI] [PubMed] [Google Scholar]
- Lenzi GL, Altieri M, Maestrini I. Post-stroke depression. Rev Neurol (Paris) 2008;164:837–40. doi: 10.1016/j.neurol.2008.07.010. Epub 2008 Sep 3. [DOI] [PubMed] [Google Scholar]
- Leonard BE. The immune system, depression and the action of antidepressants. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:767–80. doi: 10.1016/s0278-5846(01)00155-5. [DOI] [PubMed] [Google Scholar]
- Lester D. Psychache, depression, and personality. Psychol Rep. 2000;87:940. doi: 10.2466/pr0.2000.87.3.940. [DOI] [PubMed] [Google Scholar]
- Levine J, Cholestoy A, Zimmerman J. Possible antidepressant effect of minocycline. Am J Psychiatry. 1996;153:582. doi: 10.1176/ajp.153.4.582b. [DOI] [PubMed] [Google Scholar]
- Levine J, Barak Y, Chengappa KN, Rapoport A, Rebey M, Barak V. Cerebrospinal cytokine levels in patients with acute depression. Neuropsychobiology. 1999;40:171–6. doi: 10.1159/000026615. [DOI] [PubMed] [Google Scholar]
- Li XH, Liu NB, Zhang MH, Zhou YL, Liao JW, Liu XQ, Chen HW. Effects of chronic multiple stress on learning and memory and the expression of Fyn, BDNF, TrkB in the hippocampus of rats. Chin Med J (Engl) 2007;120:669–74. [PubMed] [Google Scholar]
- Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG, Bassel-Duby R, Parada LF. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59:399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Ji YJ, Jiang H, Liu DX, Zhang Q, Pan F. Effects of unpredictable chronic stress on behavior and brain-derived neurotrophic factor expression in CA3 subfield and dentate gyrus of the hippocampus in different aged rats. Chin Med J (Engl) 2009;122:1564–9. [PubMed] [Google Scholar]
- Licastro F, Pedrini S, Caputo L, Annoni G, Davis LJ, Ferri C, Casadei V, Grimaldi LM. Increased plasma levels of interleukin-1, interleukin-6 and α-1-antichymotrypsin in patients with Alzheimer's disease: peripheral inflammation or signals from the brain? J Neuroimmunol. 2000;103:97–102. doi: 10.1016/s0165-5728(99)00226-x. [DOI] [PubMed] [Google Scholar]
- Lichtenstein GR, Bala M, Han C, DeWoody K, Schaible T. Infliximab improves quality of life in patients with Crohn's disease. Inflamm Bowel Dis. 2002;8:237–43. doi: 10.1097/00054725-200207000-00001. [DOI] [PubMed] [Google Scholar]
- Lim CM, Kim SW, Park JY, Kim C, Yoon SH, Lee JK. Fluoxetine affords robust neuroprotection in the postischemic brain via its anti-inflammatory effect. J Neurosci Res. 2009;87:1037–45. doi: 10.1002/jnr.21899. [DOI] [PubMed] [Google Scholar]
- Lindqvist D, Janelidze S, Hagell P, Erhardt S, Samuelsson M, Minthon L, Hansson O, Bjorkqvist M, Traskman-Bendz L, Brundin L. Interleukin-6 Is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.01.030. in press. [DOI] [PubMed] [Google Scholar]
- Lippi G, Montagnana M, Favaloro EJ, Franchini M. Mental depression and cardiovascular disease: a multifaceted, bidirectional association. Semin Thromb Hemost. 2009;35:325–36. doi: 10.1055/s-0029-1222611. Epub 2009 May 18. [DOI] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–4. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, Aghajanian GK. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci U S A. 2008;105:359–64. doi: 10.1073/pnas.0706679105. Epub 2008 Jan 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi VR, Garcia M, Rey L, Cacabelos R. Characterization of cytokine production, screening of lymphocyte subset patterns and in vitro apoptosis in healthy and Alzheimer's Disease (AD) individuals. J Neuroimmunol. 1999;97:163–71. doi: 10.1016/s0165-5728(99)00046-6. [DOI] [PubMed] [Google Scholar]
- Lotrich FE. Major depression during interferon-alpha treatment: vulnerability and prevention. Dialogues Clin Neurosci. 2009;11:417–25. doi: 10.31887/DCNS.2009.11.4/felotrich. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucassen PJ, Vollmann-Honsdorf GK, Gleisberg M, Czeh B, De Kloet E. R. and Fuchs E. Chronic psychosocial stress differentially affects apoptosis in hippocampal subregions and cortex of the adult tree shrew. Eur J Neurosci. 2001;14:161–6. doi: 10.1046/j.0953-816x.2001.01629.x. [DOI] [PubMed] [Google Scholar]
- Lucca G, Comim CM, Valvassori SS, Pereira JG, Stertz L, Gavioli EC, Kapczinski F, Quevedo J. Chronic mild stress paradigm reduces sweet food intake in rats without affecting brain derived neurotrophic factor protein levels. Curr Neurovasc Res. 2008;5:207–13. doi: 10.2174/156720208786413406. [DOI] [PubMed] [Google Scholar]
- Luo C, Xu H, Li XM. Post-stress changes in BDNF and Bcl-2 immunoreactivities in hippocampal neurons: effect of chronic administration of olanzapine. Brain Res. 2004;1025:194–202. doi: 10.1016/j.brainres.2004.06.089. [DOI] [PubMed] [Google Scholar]
- Luo C, Xu H, Li XM. Quetiapine reverses the suppression of hippocampal neurogenesis caused by repeated restraint stress. Brain Res. 2005;1063:32–9. doi: 10.1016/j.brainres.2005.09.043. Epub 2005 Nov 4. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–45. doi: 10.1038/nrn2639. Epub 2009 Apr 29. [DOI] [PubMed] [Google Scholar]
- Lynch JR, Blessing R, White WD, Grocott HP, Newman MF, Laskowitz DT. Novel diagnostic test for acute stroke. Stroke. 2004;35:57–63. doi: 10.1161/01.STR.0000105927.62344.4C. Epub 2003 Dec 11. [DOI] [PubMed] [Google Scholar]
- Macdonald G, Leary MR. Why does social exclusion hurt? The relationship between social and physical pain. Psychol Bull. 2005;131:202–23. doi: 10.1037/0033-2909.131.2.202. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Ramakrishnan K, Croll SD, Siuciak JA, Yu G, Young LT, Fahnestock M. Performance of heterozygous brain-derived neurotrophic factor knockout mice on behavioral analogues of anxiety, nociception, and depression. Behav Neurosci. 2001;115:1145–53. doi: 10.1037//0735-7044.115.5.1145. [DOI] [PubMed] [Google Scholar]
- Madinier A, Bertrand N, Mossiat C, Prigent-Tessier A, Beley A, Marie C, Garnier P. Microglial involvement in neuroplastic changes following focal brain ischemia in rats. PLoS One. 2009;4:e8101. doi: 10.1371/journal.pone.0008101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Song C, Lin AH, Bonaccorso S, Kenis G, De Jongh R, Bosmans E, Scharpe S. Negative immunoregulatory effects of antidepressants: inhibition of interferon-gamma and stimulation of interleukin-10 secretion. Neuropsychopharmacology. 1999;20:370–9. doi: 10.1016/S0893-133X(98)00088-8. [DOI] [PubMed] [Google Scholar]
- Maes M, Yirmyia R, Noraberg J, Brene S, Hibbeln J, Perini G, Kubera M, Bob P, Lerer B, Maj M. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab Brain Dis. 2009;24:27–53. doi: 10.1007/s11011-008-9118-1. Epub 2008 Dec 16. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995a;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: comparison of stressors. Neuroscience. 1995b;69:83–8. doi: 10.1016/0306-4522(95)00256-i. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS, Flugge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci. 1996;16:3534–40. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magarinos AM, Deslandes A, McEwen BS. Effects of antidepressants and benzodiazepine treatments on the dendritic structure of CA3 pyramidal neurons after chronic stress. Eur J Pharmacol. 1999;371:113–22. doi: 10.1016/s0014-2999(99)00163-6. [DOI] [PubMed] [Google Scholar]
- Mahoney R, Regan C, Katona C, Livingston G. Anxiety and depression in family caregivers of people with Alzheimer disease: the LASER-AD study. Am J Geriatr Psychiatry. 2005;13:795–801. doi: 10.1176/appi.ajgp.13.9.795. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Intracerebroventricular interleukin-1 receptor antagonist blocks the enhancement of fear conditioning and interference with escape produced by inescapable shock. Brain Res. 1995;695:279–82. doi: 10.1016/0006-8993(95)00930-o. [DOI] [PubMed] [Google Scholar]
- Maj M. Depression, bereavement, and “understandable” intense sadness: should the DSM-IV approach be revised? Am J Psychiatry. 2008;165:1373–5. doi: 10.1176/appi.ajp.2008.08071047. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28:1562–71. doi: 10.1038/sj.npp.1300234. Epub 2003 Jul 2. [DOI] [PubMed] [Google Scholar]
- Mamounas LA, Altar CA, Blue ME, Kaplan DR, Tessarollo L, Lyons WE. BDNF promotes the regenerative sprouting, but not survival, of injured serotonergic axons in the adult rat brain. J Neurosci. 2000;20:771–82. doi: 10.1523/JNEUROSCI.20-02-00771.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmigere F, Givalois L, Rage F, Arancibia S, Tapia-Arancibia L. Rapid induction of BDNF expression in the hippocampus during immobilization stress challenge in adult rats. Hippocampus. 2003;13:646–55. doi: 10.1002/hipo.10109. [DOI] [PubMed] [Google Scholar]
- Martino G, Pluchino S. The therapeutic potential of neural stem cells. Nat Rev Neurosci. 2006;7:395–406. doi: 10.1038/nrn1908. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Manji H. and Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10:1089–93. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- Masada T, Itano T, Fujisawa M, Miyamoto O, Tokuda M, Matsui H, Nagao S, Hatase O. Protective effect of vagus nerve stimulation on forebrain ischaemia in gerbil hippocampus. Neuroreport. 1996;7:446–8. doi: 10.1097/00001756-199601310-00017. [DOI] [PubMed] [Google Scholar]
- Massouh M, Saghatelyan A. De-routing neuronal precursors in the adult brain to sites of injury: Role of the vasculature. Neuropharmacology. 2010;58:877–83. doi: 10.1016/j.neuropharm.2009.12.021. [DOI] [PubMed] [Google Scholar]
- Maya Vetencourt JF, Sale A, Viegi A, Baroncelli L, De Pasquale R, O'Leary OF, Castren E, Maffei L. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320:385–8. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Conrad CD, Kuroda Y, Frankfurt M, Magarinos AM, McKittrick C. Prevention of stress-induced morphological and cognitive consequences. Eur Neuropsychopharmacol. 1997;7:S323–8. doi: 10.1016/s0924-977x(97)00064-3. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Magarinos AM. Stress and hippocampal plasticity: implications for the pathophysiology of affective disorders. Hum Psychopharmacol. 2001;16:S7–S19. doi: 10.1002/hup.266. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiol Aging. 2002;23:921–39. doi: 10.1016/s0197-4580(02)00027-1. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McKinnon MC, Yucel K, Nazarov A, MacQueen GM. A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci. 2009;34:41–54. [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KJ, Baran SE, Wright RL, Conrad CD. Chronic stress enhances spatial memory in ovariectomized female rats despite CA3 dendritic retraction: possible involvement of CA1 neurons. Neuroscience. 2005;135:1045–54. doi: 10.1016/j.neuroscience.2005.06.083. Epub 2005 Sep 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KJ, Wilson JO, Harman J, Wright RL, Wieczorek L, Gomez J, Korol DL, Conrad CD. Chronic 17beta-estradiol or cholesterol prevents stress-induced hippocampal CA3 dendritic retraction in ovariectomized female rats: Possible correspondence between CA1 spine properties and spatial acquisition. Hippocampus. 2009;31:31. doi: 10.1002/hipo.20678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KJ, Wilson JO, Harman J, Wright RL, Wieczorek L, Gomez J, Korol DL, Conrad CD. Chronic 17beta-estradiol or cholesterol prevents stress-induced hippocampal CA3 dendritic retraction in ovariectomized female rats: Possible correspondence between CA1 spine properties and spatial acquisition. Hippocampus. 2010;20:768–86. doi: 10.1002/hipo.20678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon SB, Malcangio M. Current challenges in glia-pain biology. Neuron. 2009;64:46–54. doi: 10.1016/j.neuron.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–35. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Mendlewicz J, Kriwin P, Oswald P, Souery D, Alboni S, Brunello N. Shortened onset of action of antidepressants in major depression using acetylsalicylic acid augmentation: a pilot open-label study. Int Clin Psychopharmacol. 2006;21:227–31. doi: 10.1097/00004850-200607000-00005. [DOI] [PubMed] [Google Scholar]
- Miguelez M, Lacasse M, Kentner AC, Rizk I, Fouriezos G, Bielajew C. Short-and long-term effects of interleukin-2 on weight, food intake, and hedonic mechanisms in the rat. Behav Brain Res. 2004;154:311–9. doi: 10.1016/j.bbr.2004.02.022. [DOI] [PubMed] [Google Scholar]
- Miller AH. Letter to the Editor Re: “an inflammatory review of glucocorticoids in the CNS” by Sorrells et al. Brain, Behavior and Immunity 21, 259-272, 2007. Brain Behav Immun. 2007;21:988–9. doi: 10.1016/j.bbi.2007.06.003. author reply 990. Epub 2007 Jul 23. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–41. doi: 10.1016/j.biopsych.2008.11.029. Epub 2009 Jan 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BH, Schultz LE, Gulati A, Cameron MD, Pletcher MT. Genetic regulation of behavioral and neuronal responses to fluoxetine. Neuropsychopharmacology. 2008;33:1312–22. doi: 10.1038/sj.npp.1301497. Epub 2007 Jul 4. [DOI] [PubMed] [Google Scholar]
- Milman A, Rosenberg A, Weizman R, Pick CG. Mild traumatic brain injury induces persistent cognitive deficits and behavioral disturbances in mice. J Neurotrauma. 2005;22:1003–10. doi: 10.1089/neu.2005.22.1003. [DOI] [PubMed] [Google Scholar]
- Modestin J, Furrer R, Malti T. Different traumatic experiences are associated with different pathologies. Psychiatr Q. 2005;76:19–32. doi: 10.1007/s11089-005-5578-y. [DOI] [PubMed] [Google Scholar]
- Mohapel P, Frielingsdorf H, Haggblad J, Zachrisson O, Brundin P. Platelet-derived growth factor (PDGF-BB) and brain-derived neurotrophic factor (BDNF) induce striatal neurogenesis in adult rats with 6-hydroxydopamine lesions. Neuroscience. 2005;132:767–76. doi: 10.1016/j.neuroscience.2004.11.056. [DOI] [PubMed] [Google Scholar]
- Molina-Hernandez M, Tellez-Alcantara NP, Perez-Garcia J, Olivera-Lopez JI, Jaramillo-Jaimes MT. Antidepressant-like actions of minocycline combined with several glutamate antagonists. Prog Neuropsychopharmacol Biol Psychiatry. 2008a;32:380–6. doi: 10.1016/j.pnpbp.2007.09.004. Epub 2007 Sep 14. [DOI] [PubMed] [Google Scholar]
- Molina-Hernandez M, Tellez-Alcantara NP, Perez-Garcia J, Olivera-Lopez JI, Jaramillo-Jaimes MT. Desipramine or glutamate antagonists synergized the antidepressant-like actions of intra-nucleus accumbens infusions of minocycline in male Wistar rats. Prog Neuropsychopharmacol Biol Psychiatry. 2008b;32:1660–6. doi: 10.1016/j.pnpbp.2008.06.010. Epub 2008 Jul 1. [DOI] [PubMed] [Google Scholar]
- Molteni R, Calabrese F, Cattaneo A, Mancini M, Gennarelli M, Racagni G, Riva MA. Acute stress responsiveness of the neurotrophin BDNF in the rat hippocampus is modulated by chronic treatment with the antidepressant duloxetine. Neuropsychopharmacology. 2009;34:1523–32. doi: 10.1038/npp.2008.208. Epub 2008 Nov 19. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–5. doi: 10.1126/science.1088417. Epub 2003 Nov 13. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Harkness KL. Life stress, the “kindling” hypothesis, and the recurrence of depression: considerations from a life stress perspective. Psychol Rev. 2005;112:417–45. doi: 10.1037/0033-295X.112.2.417. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Barrot M, Powell CM, Berton O, Galanis V, Gemelli T, Meuth S, Nagy A, Greene RW, Nestler EJ. Essential role of brain-derived neurotrophic factor in adult hippocampal function2. Proc Natl Acad Sci U S A. 2004;101:10827–32. doi: 10.1073/pnas.0402141101. Epub 2004 Jul 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteggia LM, Luikart B, Barrot M, Theobold D, Malkovska I, Nef S, Parada LF, Nestler EJ. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol Psychiatry. 2007;61:187–97. doi: 10.1016/j.biopsych.2006.03.021. Epub 2006 May 12. [DOI] [PubMed] [Google Scholar]
- Mostert JP, Koch MW, Heerings M, Heersema DJ, De Keyser J. Therapeutic potential of fluoxetine in neurological disorders. CNS Neurosci Ther. 2008;14:153–64. doi: 10.1111/j.1527-3458.2008.00040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller HD, Hanumanthiah KM, Diederich K, Schwab S, Schabitz WR, Sommer C. Brain-derived neurotrophic factor but not forced arm use improves long-term outcome after photothrombotic stroke and transiently upregulates binding densities of excitatory glutamate receptors in the rat brain. Stroke. 2008;39:1012–21. doi: 10.1161/STROKEAHA.107.495069. Epub 2008 Jan 31. [DOI] [PubMed] [Google Scholar]
- Muller N, Schwarz MJ, Dehning S, Douhe A, Cerovecki A, Goldstein-Muller B, Spellmann I, Hetzel G, Maino K, Kleindienst N, Moller HJ, Arolt V, Riedel M. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry. 2006;11:680–4. doi: 10.1038/sj.mp.4001805. Epub 2006 Feb 21. [DOI] [PubMed] [Google Scholar]
- Munhoz CD, Garcia-Bueno B, Madrigal JL, Lepsch LB, Scavone C, Leza JC. Stress-induced neuroinflammation: mechanisms and new pharmacological targets. Braz J Med Biol Res. 2008;41:1037–46. doi: 10.1590/s0100-879x2008001200001. [DOI] [PubMed] [Google Scholar]
- Murakami S, Imbe H, Morikawa Y, Kubo C, Senba E. Chronic stress, as well as acute stress, reduces BDNF mRNA expression in the rat hippocampus but less robustly. Neurosci Res. 2005;53:129–39. doi: 10.1016/j.neures.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci. 2009;10:861–72. doi: 10.1038/nrn2735. Epub 2009 Nov 4. [DOI] [PubMed] [Google Scholar]
- Murray CA, Lynch MA. Evidence that increased hippocampal expression of the cytokine interleukin-1 beta is a common trigger for age- and stress-induced impairments in long-term potentiation. J Neurosci. 1998;18:2974–81. doi: 10.1523/JNEUROSCI.18-08-02974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musch MW, Clarke LL, Mamah D, Gawenis LR, Zhang Z, Ellsworth W, Shalowitz D, Mittal N, Efthimiou P, Alnadjim Z, Hurst SD, Chang EB, Barrett TA. T cell activation causes diarrhea by increasing intestinal permeability and inhibiting epithelial Na+/K+-ATPase. J Clin Invest. 2002;110:1739–47. doi: 10.1172/JCI15695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, Greiner K, Nemeroff CB, Miller AH. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med. 2001;344:961–6. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- Nair A, Bonneau RH. Stress-induced elevation of glucocorticoids increases microglia proliferation through NMDA receptor activation. J Neuroimmunol. 2006;171:72–85. doi: 10.1016/j.jneuroim.2005.09.012. Epub 2005 Nov 8. [DOI] [PubMed] [Google Scholar]
- Nampiaparampil DE. Prevalence of chronic pain after traumatic brain injury: a systematic review. Jama. 2008;300:711–9. doi: 10.1001/jama.300.6.711. [DOI] [PubMed] [Google Scholar]
- Neese SL, Sherill LK, Tan AA, Roosevelt RW, Browning RA, Smith DC, Duke A, Clough RW. Vagus nerve stimulation may protect GABAergic neurons following traumatic brain injury in rats: An immunocytochemical study. Brain Res. 2007;1128:157–63. doi: 10.1016/j.brainres.2006.09.073. Epub 2006 Nov 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB, Mayberg HS, Krahl SE, McNamara J, Frazer A, Henry TR, George MS, Charney DS, Brannan SK. VNS therapy in treatment-resistant depression: clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology. 2006;31:1345–55. doi: 10.1038/sj.npp.1301082. Epub 2006 Apr 19. [DOI] [PubMed] [Google Scholar]
- Nery FG, Monkul ES, Hatch JP, Fonseca M, Zunta-Soares GB, Frey BN, Bowden CL, Soares JC. Celecoxib as an adjunct in the treatment of depressive or mixed episodes of bipolar disorder: a double-blind, randomized, placebo-controlled study. Hum Psychopharmacol. 2008;23:87–94. doi: 10.1002/hup.912. [DOI] [PubMed] [Google Scholar]
- Nesse RM. Is depression an adaptation? Arch Gen Psychiatry. 2000;57:14–20. doi: 10.1001/archpsyc.57.1.14. [DOI] [PubMed] [Google Scholar]
- Nguyen KT, Deak T, Owens SM, Kohno T, Fleshner M, Maier SF. Exposure to acute stress induces brain interleukin-1beta protein in the rat. J Neurosci. 1998;18:2239–46. doi: 10.1523/JNEUROSCI.18-06-02239.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–47. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygren J, Kokaia M, Wieloch T. Decreased expression of brain-derived neurotrophic factor in BDNF(+/−) mice is associated with enhanced recovery of motor performance and increased neuroblast number following experimental stroke. J Neurosci Res. 2006;84:626–31. doi: 10.1002/jnr.20956. [DOI] [PubMed] [Google Scholar]
- Ohira K, Furuta T, Hioki H, Nakamura KC, Kuramoto E, Tanaka Y, Funatsu N, Shimizu K, Oishi T, Hayashi M, Miyakawa T, Kaneko T, Nakamura S. Ischemia-induced neurogenesis of neocortical layer 1 progenitor cells. Nat Neurosci. 2009;13:173–9. doi: 10.1038/nn.2473. [DOI] [PubMed] [Google Scholar]
- Onder G, Pellicciotti F, Gambassi G, Bernabei R. NSAID-related psychiatric adverse events: who is at risk? Drugs. 2004;64:2619–27. doi: 10.2165/00003495-200464230-00001. [DOI] [PubMed] [Google Scholar]
- Oomen CA, Mayer JL, de Kloet ER, Joels M, Lucassen PJ. Brief treatment with the glucocorticoid receptor antagonist mifepristone normalizes the reduction in neurogenesis after chronic stress. Eur J Neurosci. 2007;26:3395–401. doi: 10.1111/j.1460-9568.2007.05972.x. Epub 2007 Dec 4. [DOI] [PubMed] [Google Scholar]
- O'Sullivan JB, Ryan KM, Curtin NM, Harkin A, Connor TJ. Noradrenaline reuptake inhibitors limit neuroinflammation in rat cortex following a systemic inflammatory challenge: implications for depression and neurodegeneration. Int J Neuropsychopharmacol. 2009;12:687–99. doi: 10.1017/S146114570800967X. Epub 2008 Dec 2. [DOI] [PubMed] [Google Scholar]
- Pae CU, Marks DM, Han C, Patkar AA. Does minocycline have antidepressant effect? Biomed Pharmacother. 2008;62:308–11. doi: 10.1016/j.biopha.2007.12.005. Epub 2008 Jan 14. [DOI] [PubMed] [Google Scholar]
- Pandey DK, Yadav SK, Mahesh R, Rajkumar R. Depression-like and anxiety-like behavioural aftermaths of impact accelerated traumatic brain injury in rats: a model of comorbid depression and anxiety? Behav Brain Res. 2009;205:436–42. doi: 10.1016/j.bbr.2009.07.027. Epub 2009 Aug 4. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Neuroscience. Feeling the pain of social loss. Science. 2003;302:237–9. doi: 10.1126/science.1091062. [DOI] [PubMed] [Google Scholar]
- Pardon MC, Roberts RE, Marsden CA, Bianchi M, Latif ML, Duxon MS, Kendall DA. Social threat and novel cage stress-induced sustained extracellular-regulated kinase1/2 (ERK1/2) phosphorylation but differential modulation of brain-derived neurotrophic factor (BDNF) expression in the hippocampus of NMRI mice. Neuroscience. 2005;132:561–74. doi: 10.1016/j.neuroscience.2005.01.033. [DOI] [PubMed] [Google Scholar]
- Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–13. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- Parihar VK, Hattiangady B, Kuruba R, Shuai B, Shetty AK. Predictable chronic mild stress improves mood, hippocampal neurogenesis and memory. Mol Psychiatry. 2009;15:15. doi: 10.1038/mp.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SW, Lee CH, Lee JG, Lee SJ, Kim NR, Kim YH. Differential effects of ziprasidone and haloperidol on immobilization stress-induced mRNA BDNF expression in the hippocampus and neocortex of rats. J Psychiatr Res. 2009;43:274–81. doi: 10.1016/j.jpsychires.2008.05.010. Epub 2008 Jul 25. [DOI] [PubMed] [Google Scholar]
- Paulson JF, Bazemore SD. Prenatal and postpartum depression in fathers and its association with maternal depression: a meta-analysis. Jama. 2010;303:1961–9. doi: 10.1001/jama.2010.605. [DOI] [PubMed] [Google Scholar]
- Paykel ES. Stress and affective disorders in humans. Semin Clin Neuropsychiatry. 2001;6:4–11. doi: 10.1053/scnp.2001.19411. [DOI] [PubMed] [Google Scholar]
- Pechnick RN, Zonis S, Wawrowsky K, Pourmorady J, Chesnokova V. p21Cip1 restricts neuronal proliferation in the subgranular zone of the dentate gyrus of the hippocampus. Proc Natl Acad Sci U S A. 2008;105:1358–63. doi: 10.1073/pnas.0711030105. Epub 2008 Jan 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen ED, Waje-Andreassen U, Vedeler CA, Aamodt G, Mollnes TE. Systemic complement activation following human acute ischaemic stroke. Clin Exp Immunol. 2004;137:117–22. doi: 10.1111/j.1365-2249.2004.02489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pencea V, Bingaman KD, Wiegand SJ, Luskin MB. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci. 2001;21:6706–17. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng CH, Chiou SH, Chen SJ, Chou YC, Ku HH, Cheng CK, Yen CJ, Tsai TH, Chang YL, Kao CL. Neuroprotection by Imipramine against lipopolysaccharide-induced apoptosis in hippocampus-derived neural stem cells mediated by activation of BDNF and the MAPK pathway. Eur Neuropsychopharmacol. 2008;18:128–40. doi: 10.1016/j.euroneuro.2007.05.002. Epub 2007 Jun 12. [DOI] [PubMed] [Google Scholar]
- Perera TD, Coplan JD, Lisanby SH, Lipira CM, Arif M, Carpio C, Spitzer G, Santarelli L, Scharf B, Hen R, Rosoklija G, Sackeim HA, Dwork AJ. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J Neurosci. 2007;27:4894–901. doi: 10.1523/JNEUROSCI.0237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A, Wortwein G, Gruber SH, ElKhoury A, Mathe AA. Nortriptyline mediates behavioral effects without affecting hippocampal cytogenesis in a genetic rat depression model. Neurosci Lett. 2009;451:148–51. doi: 10.1016/j.neulet.2008.12.046. Epub 2008 Dec 27. [DOI] [PubMed] [Google Scholar]
- Pham K, Nacher J, Hof PR, McEwen BS. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci. 2003;17:879–86. doi: 10.1046/j.1460-9568.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- Ploughman M, Windle V, MacLellan CL, White N, Dore JJ, Corbett D. Brain-derived neurotrophic factor contributes to recovery of skilled reaching after focal ischemia in rats. Stroke. 2009;40:1490–5. doi: 10.1161/STROKEAHA.108.531806. Epub 2009 Jan 22. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Zanotti L, Rossi B, Brambilla E, Ottoboni L, Salani G, Martinello M, Cattalini A, Bergami A, Furlan R, Comi G, Constantin G, Martino G. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature. 2005;436:266–71. doi: 10.1038/nature03889. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Zanotti L, Brambilla E, Rovere-Querini P, Capobianco A, Alfaro-Cervello C, Salani G, Cossetti C, Borsellino G, Battistini L, Ponzoni M, Doglioni C, Garcia-Verdugo JM, Comi G, Manfredi AA, Martino G. Immune regulatory neural stem/precursor cells protect from central nervous system autoimmunity by restraining dendritic cell function. PLoS One. 2009;4:e5959. doi: 10.1371/journal.pone.0005959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachal Pugh C, Fleshner M, Watkins LR, Maier SF, Rudy JW. The immune system and memory consolidation: a role for the cytokine IL-1β. Neurosci Biobehav Rev. 2001;25:29–41. doi: 10.1016/s0149-7634(00)00048-8. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Janssen WG, Hof PR, McEwen BS, Morrison JH. Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp Neurol. 2005;196:199–203. doi: 10.1016/j.expneurol.2005.07.008. Epub 2005 Aug 10. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, McEwen BS, Morrison JH. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–20. doi: 10.1093/cercor/bhi104. Epub 2005 May 18. [DOI] [PubMed] [Google Scholar]
- Rage F, Givalois L, Marmigere F, TapiaArancibia L, Arancibia S. Immobilization stress rapidly modulates BDNF mRNA expression in the hypothalamus of adult male rats. Neuroscience. 2002;112:309–18. doi: 10.1016/s0306-4522(02)00072-6. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. Epub 2005 Nov 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramkumar K, Srikumar BN, Shankaranarayana Rao BS, Raju TR. Self-stimulation rewarding experience restores stress-induced CA3 dendritic atrophy, spatial memory deficits and alterations in the levels of neurotransmitters in the hippocampus. Neurochem Res. 2008;33:1651–62. doi: 10.1007/s11064-007-9511-x. Epub 2007 Oct 23. [DOI] [PubMed] [Google Scholar]
- Rantamaki T, Hendolin P, Kankaanpaa A, Mijatovic J, Piepponen P, Domenici E, Chao MV, Mannisto PT, Castren E. Pharmacologically diverse antidepressants rapidly activate brain-derived neurotrophic factor receptor TrkB and induce phospholipase-Cgamma signaling pathways in mouse brain. Neuropsychopharmacology. 2007;32:2152–62. doi: 10.1038/sj.npp.1301345. Epub 2007 Feb 21. [DOI] [PubMed] [Google Scholar]
- Rao U, Chen LA, Bidesi AS, Shad MU, Thomas MA, Hammen CL. Hippocampal changes associated with early-life adversity and vulnerability to depression. Biol Psychiatry. 2009;67:357–6. doi: 10.1016/j.biopsych.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasika S, AlvarezBuylla A, Nottebohm F. BDNF mediates the effects of testosterone on the survival of new neurons in an adult brain. Neuron. 1999;22:53–62. doi: 10.1016/s0896-6273(00)80678-9. [DOI] [PubMed] [Google Scholar]
- Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 2009;32:611–8. doi: 10.1016/j.tins.2009.07.007. Epub 2009 Sep 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds MA, Kirchick HJ, Dahlen JR, Anderberg JM, McPherson PH, Nakamura KK, Laskowitz DT, Valkirs GE, Buechler KF. Early biomarkers of stroke. Clin Chem. 2003;49:1733–9. doi: 10.1373/49.10.1733. [DOI] [PubMed] [Google Scholar]
- Rickhag M, Teilum M, Wieloch T. Rapid and long-term induction of effector immediate early genes (BDNF, Neuritin and Arc) in peri-infarct cortex and dentate gyrus after ischemic injury in rat brain. Brain Res. 2007;1151:203–10. doi: 10.1016/j.brainres.2007.03.005. Epub 2007 Mar 12. [DOI] [PubMed] [Google Scholar]
- Robaeys G, De Bie J, Wichers MC, Bruckers L, Nevens F, Michielsen P, Van Ranst M, Buntinx F. Early prediction of major depression in chronic hepatitis C patients during peg-interferon alpha-2b treatment by assessment of vegetative-depressive symptoms after four weeks. World J Gastroenterol. 2007;13:5736–40. doi: 10.3748/wjg.v13.i43.5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson E, Grace S, Wallington T, Stewart DE. Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen Hosp Psychiatry. 2004;26:289–95. doi: 10.1016/j.genhosppsych.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Le Doux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annu Rev Neurosci. 2009 doi: 10.1146/annurev.neuro.051508.135620. in press. [DOI] [PubMed] [Google Scholar]
- Rook GA. The hygiene hypothesis and the increasing prevalence of chronic inflammatory disorders. Trans R Soc Trop Med Hyg. 2007;101:1072–4. doi: 10.1016/j.trstmh.2007.05.014. Epub 2007 Jul 9. [DOI] [PubMed] [Google Scholar]
- Rook GA, Lowry CA. The hygiene hypothesis and psychiatric disorders. Trends Immunol. 2008;29:150–8. doi: 10.1016/j.it.2008.01.002. Epub 2008 Mar 6. [DOI] [PubMed] [Google Scholar]
- Rook GA. Review series on helminths, immune modulation and the hygiene hypothesis: the broader implications of the hygiene hypothesis. Immunology. 2009;126:3–11. doi: 10.1111/j.1365-2567.2008.03007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbrock H, Koros E, Bloching A, Podhorna J, Borsini F. Effect of chronic intermittent restraint stress on hippocampal expression of marker proteins for synaptic plasticity and progenitor cell proliferation in rats. Brain Res. 2005;1040:55–63. doi: 10.1016/j.brainres.2005.01.065. [DOI] [PubMed] [Google Scholar]
- Rossi C, Angelucci A, Costantin L, Braschi C, Mazzantini M, Babbini F, Fabbri ME, Tessarollo L, Maffei L, Berardi N, Caleo M. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur J Neurosci. 2006;24:1850–6. doi: 10.1111/j.1460-9568.2006.05059.x. Epub 2006 Oct 16. [DOI] [PubMed] [Google Scholar]
- Rost NS, Wolf PA, Kase CS, Kelly-Hayes M, Silbershatz H, Massaro JM, D'Agostino RB, Franzblau C, Wilson PW. Plasma concentration of C-reactive protein and risk of ischemic stroke and transient ischemic attack: the Framingham study. Stroke. 2001;32:2575–9. doi: 10.1161/hs1101.098151. [DOI] [PubMed] [Google Scholar]
- Roumestan C, Michel A, Bichon F, Portet K, Detoc M, Henriquet C, Jaffuel D, Mathieu M. Anti-inflammatory properties of desipramine and fluoxetine. Respir Res. 2007;8:35. doi: 10.1186/1465-9921-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Siefert SE. Clinical issues in considering vagus nerve stimulation for treatment-resistant depression. Exp Neurol. 2009;3:3. doi: 10.1016/j.expneurol.2009.04.015. [DOI] [PubMed] [Google Scholar]
- Ryan LM, Warden DL. Post concussion syndrome. Int Rev Psychiatry. 2003;15:310–6. doi: 10.1080/09540260310001606692. [DOI] [PubMed] [Google Scholar]
- Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E, Agerman K, Haapasalo A, Nawa H, Aloyz R, Ernfors P, Castren E. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003;23:349–57. doi: 10.1523/JNEUROSCI.23-01-00349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–5. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- Sala G, Galimberti G, Canevari C, Raggi ME, Isella V, Facheris M, Appollonio I, Ferrarese C. Peripheral cytokine release in Alzheimer patients: correlation with disease severity. Neurobiol Aging. 2003;24:909–14. doi: 10.1016/s0197-4580(03)00010-1. [DOI] [PubMed] [Google Scholar]
- Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, Bonventre JV, Woolf CJ. Interleukin-1β-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–5. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- Sandi C, Davies HA, Cordero MI, Rodriguez JJ, Popov VI, Stewart MG. Rapid reversal of stress induced loss of synapses in CA3 of rat hippocampus following water maze training. Eur J Neurosci. 2003;17:2447–56. doi: 10.1046/j.1460-9568.2003.02675.x. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–9. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Santos M, Kovari E, Hof PR, Gold G, Bouras C, Giannakopoulos P. The impact of vascular burden on late-life depression. Brain Res Rev. 2009;62:19–32. doi: 10.1016/j.brainresrev.2009.08.003. Epub 2009 Sep 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Stress, Glucocorticoids, and Damage to the Nervous System: The Current State of Confusion. Stress. 1996;1:1–19. doi: 10.3109/10253899609001092. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Is impaired neurogenesis relevant to the affective symptoms of depression? Biol Psychiatry. 2004;56:137–9. doi: 10.1016/j.biopsych.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Sato Y, Chin Y, Kato T, Tanaka Y, Tozuka Y, Mase M, Ageyama N, Ono F, Terao K, Yoshikawa Y, Hisatsune T. White matter activated glial cells produce BDNF in a stroke model of monkeys. Neurosci Res. 2009;65:71–8. doi: 10.1016/j.neures.2009.05.010. Epub 2009 Jun 6. [DOI] [PubMed] [Google Scholar]
- Scaccianoce S, Del Bianco P, Caricasole A, Nicoletti F. and Catalani A. Relationship between learning, stress and hippocampal brain-derived neurotrophic factor. Neuroscience. 2003;121:825–8. doi: 10.1016/s0306-4522(03)00514-1. [DOI] [PubMed] [Google Scholar]
- Schabitz WR, Berger C, Kollmar R, Seitz M, Tanay E, Kiessling M, Schwab S, Sommer C. Effect of brain-derived neurotrophic factor treatment and forced arm use on functional motor recovery after small cortical ischemia. Stroke. 2004;35:992–7. doi: 10.1161/01.STR.0000119754.85848.0D. Epub 2004 Feb 26. [DOI] [PubMed] [Google Scholar]
- Schabitz WR, Steigleder T, Cooper-Kuhn CM, Schwab S, Sommer C, Schneider A, Kuhn HG. Intravenous brain-derived neurotrophic factor enhances poststroke sensorimotor recovery and stimulates neurogenesis. Stroke. 2007;38:2165–72. doi: 10.1161/STROKEAHA.106.477331. Epub 2007 May 17. [DOI] [PubMed] [Google Scholar]
- Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192:348–56. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Schulte-Herbruggen O, Fuchs E, Abumaria N, Ziegler A, Danker-Hopfe H, Hiemke C, Hellweg R. Effects of escitalopram on the regulation of brain-derived neurotrophic factor and nerve growth factor protein levels in a rat model of chronic stress. J Neurosci Res. 2009;87:2551–60. doi: 10.1002/jnr.22080. [DOI] [PubMed] [Google Scholar]
- Segi-Nishida E, Warner-Schmidt JL, Duman RS. Electroconvulsive seizure and VEGF increase the proliferation of neural stem-like cells in rat hippocampus. Proc Natl Acad Sci U S A. 2008;105:11352–7. doi: 10.1073/pnas.0710858105. Epub 2008 Aug 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry. 2008;64:527–32. doi: 10.1016/j.biopsych.2008.05.005. Epub 2008 Jun 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–61. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira M, Licht A, Milman A, Pick CG, Shohami E, Eldar-Finkelman H. Role of glycogen synthase kinase-3beta in early depressive behavior induced by mild traumatic brain injury. Mol Cell Neurosci. 2007;34:571–7. doi: 10.1016/j.mcn.2006.12.006. Epub 2006 Dec 28. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Pieper CF, Barch DM, Welsh-Boehmer K, McKinstry RC, MacFall JR, D'Angelo G, Garcia KS, Gersing K, Wilkins C, Taylor W, Steffens DC, Krishnan RR, Doraiswamy PM. Support for the vascular depression hypothesis in late-life depression: results of a 2-site, prospective, antidepressant treatment trial. Arch Gen Psychiatry. 2010;67:277–85. doi: 10.1001/archgenpsychiatry.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–61. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci. 2001;21:6292–7. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorter E, Tyrer P. Separation of anxiety and depressive disorders: blind alley in psychopharmacology and classification of disease. BMJ. 2003;327:158–60. doi: 10.1136/bmj.327.7407.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, Link BG, Dohrenwend BP, Skodol AE, Stueve A, Mirotznik J. Characterizing life events as risk factors for depression: the role of fateful loss events. J Abnorm Psychol. 1989;98:460–7. doi: 10.1037//0021-843x.98.4.460. [DOI] [PubMed] [Google Scholar]
- Silvestri A, Vitale C, Ferretti F, Onorati D, Fini M, Rosano GM. Plasma levels of inflammatory C-reactive protein and interleukin-6 predict outcome in elderly patients with stroke. J Am Geriatr Soc. 2004;52:1586–7. doi: 10.1111/j.1532-5415.2004.52430_7.x. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–62. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Sirianni RW, Olausson P, Chiu AS, Taylor JR, Saltzman WM. The behavioral and biochemical effects of BDNF containing polymers implanted in the hippocampus of rats. Brain Res. 2010;1321:40–50. doi: 10.1016/j.brainres.2010.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuciak JA, Lewis DR, Wiegand SJ, Lindsay RM. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF) Pharmacol Biochem Behav. 1997;56:131–7. doi: 10.1016/S0091-3057(96)00169-4. [DOI] [PubMed] [Google Scholar]
- Skolnick P, Popik P, Trullas R. Glutamate-based antidepressants: 20 years on. Trends Pharmacol Sci. 2009;30:563–9. doi: 10.1016/j.tips.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Smith CJ, Emsley HC, Gavin CM, Georgiou RF, Vail A, Barberan EM, del Zoppo GJ, Hallenbeck JM, Rothwell NJ, Hopkins SJ, Tyrrell PJ. Peak plasma interleukin-6 and other peripheral markers of inflammation in the first week of ischaemic stroke correlate with brain infarct volume, stroke severity and long-term outcome. BMC Neurol. 2004;4:2. doi: 10.1186/1471-2377-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DC, Modglin AA, Roosevelt RW, Neese SL, Jensen RA, Browning RA, Clough RW. Electrical stimulation of the vagus nerve enhances cognitive and motor recovery following moderate fluid percussion injury in the rat. J Neurotrauma. 2005;22:1485–502. doi: 10.1089/neu.2005.22.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DC, Tan AA, Duke A, Neese SL, Clough RW, Browning RA, Jensen RA. Recovery of function after vagus nerve stimulation initiated 24 hours after fluid percussion brain injury. J Neurotrauma. 2006;23:1549–60. doi: 10.1089/neu.2006.23.1549. [DOI] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995;15:1768–77. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith RP, Taylor CM. Irritability: definition, assessment and associated factors. Br J Psychiatry. 1985;147:127–36. doi: 10.1192/bjp.147.2.127. [DOI] [PubMed] [Google Scholar]
- Sorrells SF, Sapolsky RM. An inflammatory review of glucocorticoid actions in the CNS. Brain Behav Immun. 2007;21:259–72. doi: 10.1016/j.bbi.2006.11.00. Epub 2006 Dec 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa N, Lukoyanov NV, Madeira MD, Almeida OF, Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97:253–66. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- Sparkman NL, Buchanan JB, Heyen JR, Chen J, Beverly JL, Johnson RW. Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers. J Neurosci. 2006;26:10709–16. doi: 10.1523/JNEUROSCI.3376-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spennato G, Zerbib C, Mondadori C, Garcia R. Fluoxetine protects hippocampal plasticity during conditioned fear stress and prevents fear learning potentiation. Psychopharmacology (Berl) 2008;196:583–9. doi: 10.1007/s00213-007-0993-7. Epub 2007 Nov 9. [DOI] [PubMed] [Google Scholar]
- Spijker J, de Graaf R, Bijl RV, Beekman AT, Ormel J, Nolen WA. Duration of major depressive episodes in the general population: results from The Netherlands Mental Health Survey and Incidence Study (NEMESIS) Br J Psychiatry. 2002;181:208–13. doi: 10.1192/bjp.181.3.208. [DOI] [PubMed] [Google Scholar]
- Spitzer C, Barnow S, Gau K, Freyberger HJ, Grabe HJ. Childhood maltreatment in patients with somatization disorder. Aust N Z J Psychiatry. 2008;42:335–41. doi: 10.1080/00048670701881538. [DOI] [PubMed] [Google Scholar]
- Stappenbeck TS, Miyoshi H. The role of stromal stem cells in tissue regeneration and wound repair. Science. 2009;324:1666–9. doi: 10.1126/science.1172687. [DOI] [PubMed] [Google Scholar]
- Steel Z, Chey T, Silove D, Marnane C, Bryant RA, van Ommeren M. Association of torture and other potentially traumatic events with mental health outcomes among populations exposed to mass conflict and displacement: a systematic review and meta-analysis. Jama. 2009;302:537–49. doi: 10.1001/jama.2009.1132. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun. 2007;21:901–12. doi: 10.1016/j.bbi.2007.03.011. Epub 2007 May 1. [DOI] [PubMed] [Google Scholar]
- Stone EA, Lin Y, Quartermain D. A final common pathway for depression? Progress toward a general conceptual framework. Neurosci Biobehav Rev. 2008;32:508–24. doi: 10.1016/j.neubiorev.2007.08.007. Epub 2007 Oct 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nat Neurosci. 2006;9:526–33. doi: 10.1038/nn1668. Epub 2006 Mar 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strike PC, Wardle J, Steptoe A. Mild acute inflammatory stimulation induces transient negative mood. J Psychosom Res. 2004;57:189–94. doi: 10.1016/S0022-3999(03)00569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styron W. Darkness Visible: A Memoir of Madness. Vintage; New York: 1990. [Google Scholar]
- Sugahara H, Akamine M, Kondo T, Fujisawa K, Yoshimasu K, Tokunaga S, Kudo C. Somatic symptoms most often associated with depression in an urban hospital medical setting in Japan. Psychiatry Res. 2004;128:305–11. doi: 10.1016/j.psychres.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Sugama S, Fujita M, Hashimoto M, Conti B. Stress induced morphological microglial activation in the rodent brain: involvement of interleukin-18. Neuroscience. 2007;146:1388–99. doi: 10.1016/j.neuroscience.2007.02.043. Epub 2007 Apr 11. [DOI] [PubMed] [Google Scholar]
- Sugama S, Takenouchi T, Fujita M, Conti B, Hashimoto M. Differential microglial activation between acute stress and lipopolysaccharide treatment. J Neuroimmunol. 2009;207:24–31. doi: 10.1016/j.jneuroim.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Sunanda, Meti BL, Raju TR. Entorhinal cortex lesioning protects hippocampal CA3 neurons from stress-induced damage. Brain Res. 1997;770:302–6. doi: 10.1016/s0006-8993(97)00888-3. [DOI] [PubMed] [Google Scholar]
- Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, Griebel G, Hen R, Belzung C. Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol Psychiatry. 2008;64:293–301. doi: 10.1016/j.biopsych.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Tai YH, Wang YH, Wang JJ, Tao PL, Tung CS, Wong CS. Amitriptyline suppresses neuroinflammation and up-regulates glutamate transporters in morphine-tolerant rats. Pain. 2006;124:77–86. doi: 10.1016/j.pain.2006.03.018. Epub 2006 May 11. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Kato M, Matsuura M, Mobbs D, Suhara T, Okubo Y. When your gain is my pain and your pain is my gain: neural correlates of envy and schadenfreude. Science. 2009;323:937–9. doi: 10.1126/science.1165604. [DOI] [PubMed] [Google Scholar]
- Takeda H, Tsuji M, Yamada T, Masuya J, Matsushita K, Tahara M, Iimori M, Matsumiya T. Caffeic acid attenuates the decrease in cortical BDNF mRNA expression induced by exposure to forced swimming stress in mice. Eur J Pharmacol. 2006;534:115–21. doi: 10.1016/j.ejphar.2006.01.026. Epub 2006 Feb 21. [DOI] [PubMed] [Google Scholar]
- Tenore PL. Psychotherapeutic benefits of opioid agonist therapy. J Addict Dis. 2008;27:49–65. doi: 10.1080/10550880802122646. [DOI] [PubMed] [Google Scholar]
- Thomas AJ, O'Brien JT, Davis S, Ballard C, Barber R, Kalaria RN, Perry RH. Ischemic basis for deep white matter hyperintensities in major depression: a neuropathological study. Arch Gen Psychiatry. 2002;59:785–92. doi: 10.1001/archpsyc.59.9.785. [DOI] [PubMed] [Google Scholar]
- Thomas RM, Hotsenpiller G, Peterson DA. Acute psychosocial stress reduces cell survival in adult hippocampal neurogenesis without altering proliferation. J Neurosci. 2007;27:2734–43. doi: 10.1523/JNEUROSCI.3849-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornhill R, Thornhill NW. Sociobiology and the Social Sciences: The Evolution of Psychological Pain. Texas Tech University Press; 1989. [Google Scholar]
- Tietjen GE, Brandes JL, Peterlin BL, Eloff A, Dafer RM, Stein MR, Drexler E, Martin VT, Hutchinson S, Aurora SK, Recober A, Herial NA, Utley C, White L, Khuder SA. Childhood Maltreatment and Migraine (Part III). Association With Comorbid Pain Conditions. Headache. 2009;21:21. doi: 10.1111/j.1526-4610.2009.01558.x. [DOI] [PubMed] [Google Scholar]
- Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci. 2001;21:2580–8. doi: 10.1523/JNEUROSCI.21-08-02580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torner L, Karg S, Blume A, Kandasamy M, Kuhn HG, Winkler J, Aigner L, Neumann ID. Prolactin prevents chronic stress-induced decrease of adult hippocampal neurogenesis and promotes neuronal fate. J Neurosci. 2009;29:1826–33. doi: 10.1523/JNEUROSCI.3178-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey KJ. Physiology and immunology of the cholinergic anti-inflammatory pathway. J Clin Invest. 2007;117:289–96. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–25. doi: 10.1038/nn1659. Epub 2006 Feb 26. [DOI] [PubMed] [Google Scholar]
- Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med. 2008;358:252–60. doi: 10.1056/NEJMsa065779. [DOI] [PubMed] [Google Scholar]
- Tynan RJ, Naicker S, Hinwood M, Nalivaiko E, Buller KM, Pow DV, Day TA, Walker FR. Chronic stress alters the density and morphology of microglia in a subset of stress-responsive brain regions. Brain Behav Immun. 2010 doi: 10.1016/j.bbi.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, Lalla D, Woolley M, Jahreis A, Zitnik R, Cella D, Krishnan R. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- U.S. National Library of Medicine and the National Institutes of Health Prednisone. 2009. http://www.nlm.nih.gov/medlineplus/druginfo/meds/a601102.html#side-effects MedlinePlus Druginfo. http://www.nlm.nih.gov/medlineplus/druginfo/meds/a601102.html#side-effects.
- Uccelli A, Pistoia V, Moretta L. Mesenchymal stem cells: a new strategy for immunosuppression? Trends Immunol. 2007;28:219–26. doi: 10.1016/j.it.2007.03.001. Epub 2007 Apr 2. [DOI] [PubMed] [Google Scholar]
- Vaidya VA, Terwilliger RM, Duman RS. Role of 5-HT2A receptors in the stress-induced down-regulation of brain-derived neurotrophic factor expression in rat hippocampus. Neurosci Lett. 1999;262:1–4. doi: 10.1016/s0304-3940(99)00006-3. [DOI] [PubMed] [Google Scholar]
- van Donkelaar EL, van den Hove DL, Blokland A, Steinbusch HW, Prickaerts J. Stress-mediated decreases in brain-derived neurotrophic factor as potential confounding factor for acute tryptophan depletion-induced neurochemical effects. Eur Neuropsychopharmacol. 2009;19:812–21. doi: 10.1016/j.euroneuro.2009.06.012. Epub 2009 Jul 28. [DOI] [PubMed] [Google Scholar]
- Van Houdenhove B, Luyten P. Central sensitivity syndromes: stress system failure may explain the whole picture. Semin Arthritis Rheum. 2009;39:218–9. doi: 10.1016/j.semarthrit.2008.08.008. author reply 220-1. Epub 2008 Oct 29. [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–5. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957–66. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- Vollmar P, Haghikia A, Dermietzel R, Faustmann PM. Venlafaxine exhibits an antiinflammatory effect in an inflammatory co-culture model. Int J Neuropsychopharmacol. 2008;11:111–7. doi: 10.1017/S1461145707007729. Epub 2007 Apr 20. [DOI] [PubMed] [Google Scholar]
- Vollmayr B, Faust H, Lewicka S, Henn FA. Brain-derived-neurotrophic-factor (BDNF) stress response in rats bred for learned helplessness. Mol Psychiatry. 2001;6:471–4. 358. doi: 10.1038/sj.mp.4000907. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–8. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–7. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Wager-Smith K. Trigger threshold of neuronal circuits mediating behavioral defenses: the role of episodes of altered sensitivity in mood and sickness. in preparation. 2010 [Google Scholar]
- Wakefield JC, Schmitz MF, First MB, Horwitz AV. Extending the bereavement exclusion for major depression to other losses: evidence from the National Comorbidity Survey. Arch Gen Psychiatry. 2007;64:433–40. doi: 10.1001/archpsyc.64.4.433. [DOI] [PubMed] [Google Scholar]
- Walsh BT, Seidman SN, Sysko R, Gould M. Placebo response in studies of major depression: variable, substantial, and growing. Jama. 2002;287:1840–7. doi: 10.1001/jama.287.14.1840. [DOI] [PubMed] [Google Scholar]
- Wan Y, Xu J, Ma D, Zeng Y, Cibelli M, Maze M. Postoperative Impairment of Cognitive Function in Rats: A Possible Role for Cytokine-mediated Inflammation in the Hippocampus. Anesthesiology. 2007;106:436–443. doi: 10.1097/00000542-200703000-00007. [DOI] [PubMed] [Google Scholar]
- Wang JW, David DJ, Monckton JE, Battaglia F, Hen R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci. 2008;28:1374–84. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Duman RS. VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc Natl Acad Sci U S A. 2007;104:4647–52. doi: 10.1073/pnas.0610282104. Epub 2007 Mar 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, Cameron HA, Daniels DC, McEwen BS. Phenytoin prevents stress- and corticosterone-induced atrophy of CA3 pyramidal neurons. Hippocampus. 1992a;2:431–5. doi: 10.1002/hipo.450020410. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992b;588:341–5. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, Daniels DC, Cameron H, McEwen BS. Tianeptine attenuates stress-induced morphological changes in the hippocampus. Eur J Pharmacol. 1992c;222:157–62. doi: 10.1016/0014-2999(92)90830-w. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001;24:450–5. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- Westenbroek C, Den Boer JA, Veenhuis M, Ter Horst GJ. Chronic stress and social housing differentially affect neurogenesis in male and female rats. Brain Res Bull. 2004;64:303–8. doi: 10.1016/j.brainresbull.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology. 2002;122:1140–56. doi: 10.1053/gast.2002.32392. [DOI] [PubMed] [Google Scholar]
- Wieloch T, Nikolich K. Mechanisms of neural plasticity following brain injury. Curr Opin Neurobiol. 2006;16:258–64. doi: 10.1016/j.conb.2006.05.011. Epub 2006 May 18. [DOI] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci. 2006;26:12387–96. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. Epub 2005 Jul 19. [DOI] [PubMed] [Google Scholar]
- World Health Organization Depression. 2009. http://www.who.int/mental_health/management/depression/definition/en/
- Wright CE, Strike PC, Brydon L, Steptoe A. Acute inflammation and negative mood: mediation by cytokine activation. Brain Behav Immun. 2005;19:345–50. doi: 10.1016/j.bbi.2004.10.003. Epub 2004 Dec 8. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Chopp M. Emerging treatments for traumatic brain injury. Expert Opin Emerg Drugs. 2009;14:67–84. doi: 10.1517/14728210902769601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Qing H, Lu W, Keegan D, Richardson JS, Chlan-Fourney J, Li XM. Quetiapine attenuates the immobilization stress-induced decrease of brain-derived neurotrophic factor expression in rat hippocampus. Neurosci Lett. 2002;321:65–8. doi: 10.1016/s0304-3940(02)00034-4. [DOI] [PubMed] [Google Scholar]
- Xu H, Luo C, Richardson JS, Li XM. Recovery of hippocampal cell proliferation and BDNF levels, both of which are reduced by repeated restraint stress, is accelerated by chronic venlafaxine. Pharmacogenomics J. 2004;4:322–31. doi: 10.1038/sj.tpj.6500265. [DOI] [PubMed] [Google Scholar]
- Xu H, Chen Z, He J, Haimanot S, Li X, Dyck L, Li XM. Synergetic effects of quetiapine and venlafaxine in preventing the chronic restraint stress-induced decrease in cell proliferation and BDNF expression in rat hippocampus. Hippocampus. 2006;16:551–9. doi: 10.1002/hipo.20184. [DOI] [PubMed] [Google Scholar]
- Xu Y, Ku B, Tie L, Yao H, Jiang W, Ma X, Li X. Curcumin reverses the effects of chronic stress on behavior, the HPA axis, BDNF expression and phosphorylation of CREB. Brain Res. 2006;1122:56–64. doi: 10.1016/j.brainres.2006.09.009. Epub 2006 Oct 3. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Ninomiya M, Hernandez Acosta P, Garcia-Verdugo JM, Sunabori T, Sakaguchi M, Adachi K, Kojima T, Hirota Y, Kawase T, Araki N, Abe K, Okano H, Sawamoto K. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 2006;26:6627–36. doi: 10.1523/JNEUROSCI.0149-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanpallewar SU, Fernandes K, Marathe SV, Vadodaria KC, Jhaveri D, Rommelfanger K, Ladiwala U, Jha S, Muthig V, Hein L, Bartlett P, Weinshenker D, Vaidya VA. Alpha2-adrenoceptor blockade accelerates the neurogenic, neurotrophic, and behavioral effects of chronic antidepressant treatment. J Neurosci. 2010;30:1096–109. doi: 10.1523/JNEUROSCI.2309-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yrjanheikki J, Keinanen R, Pellikka M, Hokfelt T, Koistinaho J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci U S A. 1998;95:15769–74. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci U S A. 1999;96:13496–500. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu TS, Zhang G, Liebl DJ, Kernie SG. Traumatic brain injury-induced hippocampal neurogenesis requires activation of early nestin-expressing progenitors. J Neurosci. 2008;28:12901–12. doi: 10.1523/JNEUROSCI.4629-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun SJ, Park HJ, Yeom MJ, Hahm DH, Lee HJ, Lee EH. Effect of electroacupuncture on the stress-induced changes in brain-derived neurotrophic factor expression in rat hippocampus. Neurosci Lett. 2002;318:85–8. doi: 10.1016/s0304-3940(01)02492-2. [DOI] [PubMed] [Google Scholar]
- Yunus MB. Fibromyalgia and overlapping disorders: the unifying concept of central sensitivity syndromes. Semin Arthritis Rheum. 2007;36:339–56. doi: 10.1016/j.semarthrit.2006.12.009. Epub 2007 Mar 13. [DOI] [PubMed] [Google Scholar]
- Zhang RL, LeTourneau Y, Gregg SR, Wang Y, Toh Y, Robin AM, Zhang ZG, Chopp M. Neuroblast division during migration toward the ischemic striatum: a study of dynamic migratory and proliferative characteristics of neuroblasts from the subventricular zone. J Neurosci. 2007;27:3157–62. doi: 10.1523/JNEUROSCI.4969-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–60. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zhou QG, Hu Y, Hua Y, Hu M, Luo CX, Han X, Zhu XJ, Wang B, Xu JS, Zhu DY. Neuronal nitric oxide synthase contributes to chronic stress-induced depression by suppressing hippocampal neurogenesis. J Neurochem. 2007;103:1843–54. doi: 10.1111/j.1471-4159.2007.04914.x. Epub 2007 Sep 13. [DOI] [PubMed] [Google Scholar]
- Ziegelstein RC. Depression in patients recovering from a myocardial infarction. Jama. 2001;286:1621–7. doi: 10.1001/jama.286.13.1621. [DOI] [PubMed] [Google Scholar]
- Zorner B, Wolfer DP, Brandis D, Kretz O, Zacher C, Madani R, Grunwald I, Lipp HP, Klein R, Henn FA, Gass P. Forebrain-specific trkB-receptor knockout mice: behaviorally more hyperactive than “depressive”. Biol Psychiatry. 2003;54:972–82. doi: 10.1016/s0006-3223(03)00418-9. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, McCorkle R, Seligman DA, Schmidt K. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun. 2001;15:199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]
- Zuess J. An Integrative Approach to Depression: Part 1 - Etiology. Complementary Health Practice Review. 2003;8:9–24. [Google Scholar]
- Zuliani G, Ranzini M, Guerra G, Rossi L, Munari MR, Zurlo A, Volpato S, Atti AR, Ble A, Fellin R. Plasma cytokines profile in older subjects with late onset Alzheimer's disease or vascular dementia. J Psychiatr Res. 2007;41:686–93. doi: 10.1016/j.jpsychires.2006.02.008. Epub 2006 Apr 4. [DOI] [PubMed] [Google Scholar]