Abstract
Recent studies demonstrate that rapid translocation of the acid sphingomyelinase (ASM), a lysosomal hydrolase, to the outer leaflet of the cell membrane and concomitant release of ceramide constitute a common cellular signaling cascade to various stimuli including CD95 ligation, UV-irradiation, bacterial and viral infections. Reactive oxygen species (ROS) were shown to play a crucial role in regulating this signaling cascade at least for some bacterial infections and UV-irradiation. However, the precise role of ROS for regulation of ASM is unknown. Here, by confocal microscopy and flow cytometry analysis, we demonstrate that hydrogen peroxide (H2O2), a primary form of ROS in mammalian cells, induces very rapid translocation of ASM and formation of ceramide-enriched membrane platforms in the plasma membrane of Jurkat T cells. In parallel, H2O2 triggers lysosome trafficking and fusion with the plasma membrane, i.e. lysosome exocytosis, as detected by exposure of a lysosome-associated protein, LAMP1. Depletion of intracellular Ca2+ by cell permeable EGTA-AM inhibits H2O2-induced lysosome exocytosis, ASM translocation and formation of ceramide-enriched platforms. Pharmacological inhibition or genetic deficiency of ASM did not affect H2O2-induced lysosome exocytosis. These results indicate that ROS-induced membrane translocation of ASM is mediated by exocytosis of lysosomes, which is dependent on intracellular Ca2+ release.
Keywords: Ceramide, lysosomes, acid sphingomyelinase, vesicle, fusion
Introduction
Acid sphingomyelinase (ASM) is a hydrolase that degrades sphingomyelin to ceramide and phosphocholine [[1–3]. ASM localizes in conventional lysosomes or in specialized lysosomal compartments named secretory lysosomes, which store newly synthesized secretory proteins [[4]. In humans, deficiency of ASM activity results in the lysosomal storage disorders Niemann-Pick disease types A and B (NPDA and NPDB) [[5]. In NPDA, ASM activity is completely absent and lipid-laden cells are prominent in the central nervous system and throughout the reticuloendothelial system, leading to death in early childhood (less than 3 years) [[4]. In NPDB, low levels of residual ASM activity prevent major neurological pathology, allowing survival into late childhood and adulthood. However, type B NPD individuals develop progressive reticuloendothelial system disease. Moreover, type B patients also have progressive pulmonary dysfunction and frequent respiratory infections that may lead to death [6–8].
ASM is activated in response to a variety of stimuli including CD95, DR5 ligation, UV-irradiation, bacterial and viral infections [6–10]. The precise mechanism for ASM activation remains elusive. In many cases, ASM activation is initiated by rapid translocation of the ASM to the outer leaflet of the cell membrane [9, 11]]. ASM targets membrane sphingolipids-enriched microdomains, which contains around 70% of total membrane sphingomyelin [12]]. ASM then hydrolyzes sphingomyelin and releases ceramide, a hydrophobic sphingolipid that spontaneously forms ceramide-enriched microdomains that fuse to larger ceramide-enriched domains [[9, 13]]. Ceramide-enriched membrane platforms serve as important signaling platforms [9, 13]]. However, the mechanisms that mediate initial ASM activation require definition. Here, we focus on early events of ASM activation, i.e. how ASM externalization is regulated.
Oxidative stress refers to the imbalance of enhanced production of reactive oxygen species (ROS) and/or impaired function of the antioxidant system. ROS include superoxide anions (O2·−), hydroxyl radicals, and hydrogen peroxide (H2O2). O2·−, once generated, rapidly dismutates into H2O2 at low pH or catalyzed by superoxide dismutase (SOD). H2O2 can be further converted into highly reactive hydroxyl radicals via iron-catalyzed Fenton reaction under pathological conditions [14]. Since ROS are rapidly produced, short-lived and diffusible, ROS have been proposed as second messengers to mediate cellular responses to many stimuli such as cancer chemotherapeutic agents, UV, ionizing radiation, and TNF [6, 7, 15, 16]. Many of these stimuli also activate ASM and release ceramide in a variety of mammalian cells. Recent studies suggest that ROS generation is an upstream signal to trigger ASM translocation and activation. In U937 cells, ROS scavengers block UV-irradiation-induced ASM translocation, activation and ceramide-enriched membrane platform formation [6]. In splenocytes, TRAIL-induced ASM activation was blocked by ROS scavengers and antioxidants [17]. In macrophages, Pseudomonas aeruginosa infection results in ASM translocation and activation, ceramide release and formation of ceramide-enriched platforms, which are blocked by ROS scavengers and inhibitors of NADPH oxidase, a primary ROS generating enzyme [18]. In neutrophils, ROS regulate ASM-derived ceramide production, CD95 clustering and neutrophil apoptosis [19]. However, it is unknown how ROS regulate ASM activity and whether ROS are involved in ASM externalization.
Here, we demonstrate that H2O2, a primary form of ROS in mammalian cells, induces rapid translocation of ASM and formation of ceramide-enriched membrane platforms in the plasma membrane. In parallel, H2O2 triggers lysosome trafficking and fusion with the plasma membrane, i.e. lysosome exocytosis. Depletion of intracellular Ca2+ inhibits H2O2-induced lysosome exocytosis, ASM translocation and formation of ceramide-enriched membrane platforms. Thus, our data indicate that ROS promotes membrane lysosome trafficking of ASM, which is dependent on intracellular Ca2+ release.
Methods
Cells culture
Jurkat T cells (clone E6) were from ATCC. All cells were cultured in phenol red-free RPMI-1640 supplemented with 10% fetal calf serum (FCS), 10 mM HEPES (pH 7.4), 2 mM L-glutamine, 1 mM sodium pyruvate, 100 uM nonessential amino acids, 100 U/mL penicillin, 100 μg/mL streptomycin (all Life Technologies) and 50μM beta-mercaptoethanol.
Flow cytometry
To detect surface ASM or lysosome-associated protein 1 (LAMP1) by flow cytometry, cells were stimulated with H2O2 (1 mM) at 37°C, washed with ice-cold PBS supplemented with 1% FCS and 0.1% NaN3 and blocked for 20 min with the same buffer. Cells were washed and Fc receptors were neutralized by incubation with an irrelevant rabbit IgG (20 μg/mL, Sigma) for 45 min prior to addition of the primary antibodies. Cells were washed again and incubated for 45 min with 1 μg/mL of a polyclonal anti-ASM antibody or a monoclonal PE-conjugated anti-LAMP1 antibodies (both from Santa Cruz Biotech. Inc.) in PBS, 1% FCS and 0.1% NaN3. For analysis of ASM, cells were re-washed and labeled for 45 min with 1 μg/mL of F(ab′)2 fragments of Cy3-coupled donkey anti-goat antibodies (Jackson ImmunoResearch Inc.), washed in PBS and subjected to flow cytometry analysis using a BD-Calibur (BD Biosciences). For LAMP1 staining, cells were re-washed in PBS and directly subjected to flow cytometry.
Fluorescence microscopy
Lymphocytes were washed in HEPES/Saline (H/S, 132 mM NaCl, 20 mM HEPES (pH 7.4), 5 mM KCl, 1 mM CaCl2, 0.7 mM MgCl2 and 0.8 mM MgSO4), resuspended in the same buffer, rested for 10 min at 37°C and stimulated with H2O2 for indicated time periods. The stimulation was stopped by addition of 1 mL ice-cold PBS with 1% FCS, cells were then washed in PBS and fixed in 1% PFA (w/v) in PBS (pH 7.3) for 15 min. Samples were washed in PBS and blocked with 1% FCS in PBS, 1% NaN3 for 20 min to reduce unspecific antibody binding. Cells were then washed in PBS and incubated for 45 min at 4°C with mouse anti-ceramide antibodies (1: 50 dilution, Glycobiotech) and/or goat anti-ASM antibodies (500 ng/mL) in PBS, 1% FCS and 0.1% NaN3. Control samples were incubated with an irrelevant murine IgM, respectively (both from Sigma). Samples were washed three times in PBS and stained for 45 min with Cy3-coupled F(ab′)2 fragments of anti-mouse IgM antibodies and/or FITC-labeled F(ab′)2 fragments of donkey anti-goat antibodies (all from Jackson ImmunoResearch Inc.) in PBS, 1% FCS and 0.1% NaN3. Cells were again washed and immobilized on glass cover slips coated with 1% (v/v) poly-L-lysine for 10 min. The cover slips were embedded in Moviol and viewed with a Leica TCS NT scanning confocal microscope. Clustering was defined as one or several intense spots of fluorescence on the cell surface, whereas unstimulated cells displayed a homogenous distribution of the fluorescence throughout the membrane. In each experiment, the presence or absence of clustering in samples of 200 cells was scored by two independent observers. The results are given as percentage of cells showing a cluster.
Isolation of detergent resistant membranes (DRMs)
Sphingolipids-enriched microdomains are biophysically characterized as DRM fractions, which have lower density compared with detergent soluble membrane fractions. The DRM fractions were isolated by sucrose gradient centrifugation as described previously [9]. Briefly, 107 cells were lysed in 0.6 mL MES buffer (25 mM morpholinoethane sulfonic acid, 150 mM NaCl, 1 mM EDTA, pH = 6.5) with a cocktail of protease inhibitors (Roche) and 1% Triton X-100. Cell extracts were homogenized by 5 passages through a 25G needle and incubated on ice for 30 min. 0.6 mL homogenates were then adjusted with 60% sucrose to 40% sucrose and overlaid with 1.8 mL 35% sucrose and then 1.8 mL 5% sucrose. Samples were centrifuged at 100,000 × g for 18 h at 4°C (rotor: MLS50, Beckman). The gradient was divided into 6 fractions and 0.9 mL/fraction from top to bottom. Fractions 1–3 were mixed and designated as light fractions (enriched in DRMs). Heavy fractions 4–6 were mixed and designated as heavy fractions (none-DRMs).
Immunoblot analyses
106 cells were lysed in Laemmli sample buffer (2% SDS, 10% glycerol, 5% 2-mercaptoethanol, 60 mM Tris/HCl, 0.001% Bromophenol Blue, pH 6.8). Samples were sonicated for 15–20 sec and boiled for 5 min at 95°C. The protein samples were separated by 10% SDS–PAGE and transferred to nitrocellulose membranes. The blots were blocked in 4% BSA in Tris-buffered Saline-supplemented with 0.1% Tween 20 and incubated overnight at 4°C with anti-ASM or anti-Actin antibodies (both in 1:1000 dilution in 4% BSA/PBS and from Santa Cruz Biotech. Inc.). To determine the purity of DRM fractions after sucrose gradient isolation, 20 μl sample of light and heavy fractions (as described above) were applied for SDS-PAGE and the presence of flotillin-1, a marker protein for DRM fractions, was examined using anti-flotillin-1 antibodies (Santa Cruz Biotech. Inc., 1:1000 dilution in 4% BSA/PBS). Blots were labeled with appropriate alkaline phosphatase-coupled secondary antibodies (Santa Cruz Biotechnology Inc.) and developed using the Tropix system (Bedford Inc.).
Acid sphingomyelinase activity assay
Activity of the acid sphingomyelinase in light and heavy fractions was measured as previously described [17]. Briefly, after sucrose gradient isolation, 100 μl sample from light or heavy fractions were mixed with 100 μl reaction buffer containing 100 mM sodium acetate (pH 5.0), 0.2% Triton X-100 and a cocktail of protease (Roche) and phosphatase inhibitors (Sigma). The reaction mixture was incubated with 0.02 μCi [14C]-sphingomyelin for 30 min at 37°C. [14C]-sphingomyelin as a substrate for ASM was dried prior to use and resuspended in 50 mM sodium acetate (pH 5.0), 0.2% Triton X-100 followed by 10 min bath sonication to promote the formation of micelles. The reaction was stopped by addition of 1 mL of CHCl3:CH3OH (v/v). Phases were separated by 5 min centrifugation at 14,000 rpm and an aliquot of the aqueous phase was applied for liquid scintillation counting. Hydrolysis of [14C]-sphingomyelin by ASM results in release of [14C]-choline chloride into the aqueous phase, whereas ceramide and intact [14C]-sphingomyelin remain in the organic phase. Therefore, the release of [14C]-choline chloride (pmol/107 cells/h) serves to determine the activity of the ASM.
Isolation of murine spleen T-cells
Murine spleen T cells were obtained from sphingomyelin phosphodiesterase 1-deficient (Smpd1−/−) mice lacking Asm activity or normal wild-type mice as previously described [7]. Briefly, single cell suspension of splenocytes was obtained by mechanical disruption of the spleen, followed by Ficoll gradient centrifugation (Histopaque, Sigma) to remove erythrocytes and cellular debris. Untouched murine spleen T-cells were enriched by depleting splenocytes with MHC II MicroBeads by MACS (Miltenyi Biotec. Inc.). The cells were maintained in RPMI-1640 supplemented as described above. Smpd1−/− mice (on a C57BL/6 background) were kindly provided by Dr. R. Kolesnick (Memorial Sloan-Kettering Cancer Center, NY, USA); syngenic wild-type mice were from the same heterozygous breeding.
Results
ROS induce ASM translocation, platform formation in Jurkat Cell
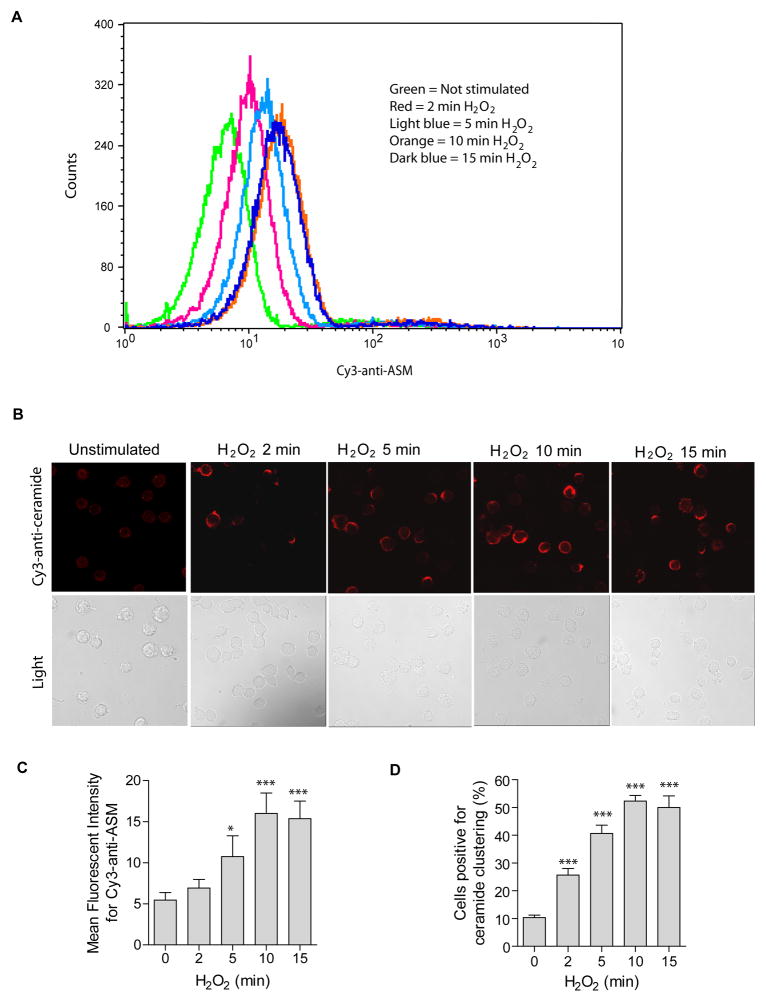
We first investigated whether hydrogen peroxide (H2O2) induces ASM externalization in the membrane of human Jurkat T cells. The results reveal a very rapid externalization of ASM (Fig. 1A) and ceramide clustering in the membrane of human Jurkat T cells (Fig. 1B) upon treatment with H2O2. Quantitative analysis shows that ~50% of all cells formed ceramide-enriched membrane platforms within 10 min treatment (Fig. 1C). Since the optimum response of ASM externalization was obtained at 10 min, the same treatment was used in all experiments of the present study if not otherwise mentioned.
Fig. 1. Oxidative stress induces ASM externalization and formation of ceramide-enriched membrane platforms.
(A) Oxidative stress by H2O2 (1 mM) induces a translocation of ASM onto the cell surface. Surface ASM was detected by flow cytometry analysis of cells incubated with Cy3-labeled goat anti-ASM antibodies. Shown is a representative flow cytometry analysis from four independent experiments. (B) H2O2 (1 mM)-induced ASM translocation correlates with rapid clustering of ceramide in the membrane and formation of ceramide-enriched membrane platforms. Representative confocal fluorescence microscopy and light images from three independent experiments are shown. (C) Displayed are the summarized data showing the mean fluorescence intensity for Cy3-anti-ASM staining from four independent experiments. (D) The quantitative analysis of the data from four independent experiments, which each included analysis of 200 cells/time point, shows the formation of ceramide-enriched membrane platforms in a large proportion of the cells. Displayed is the percentage of cells with a ceramide-enriched membrane platform at indicated time points. Panel C and D show the mean ± S.D. Significant differences between treated and non-treated controls were determined by t-test and are indicated by *** (P<0.001, t-test) or * (P<0.05, t-test), respectively.
ROS-induced ASM translocation and activation in ceramide-enriched lipid raft microdomains
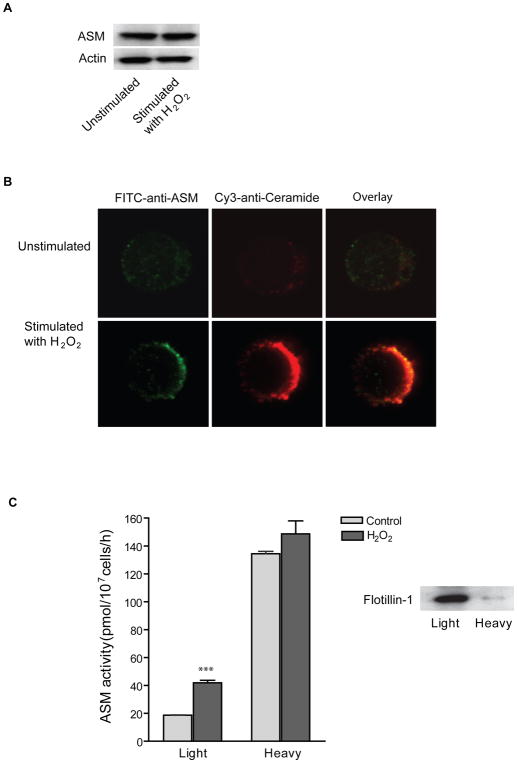
As shown in Fig. 2A, stimulation with H2O2 had no effect on ASM expression level in Jurkat T cells. To investigate the spatial relation between ASM and ceramide, Jurkat cells were stimulated with H2O2 and live cells were stained with FITC-labeled anti-ASM and Cy3-labeled anti-ceramide antibodies. Confocal microscopy revealed that ASM co-localized in ceramide-enriched membrane platforms after H2O2 stimulation (Fig. 2B). Further, we determined the activity of ASM in isolated sphingolipids-enriched microdomains after subcellular fractionation across a sucrose density gradient. H2O2 increased ASM activity in isolated DRMs (light fractions) whereas only minor increase was detected in non-DRM fractions (heavy fractions) (Fig. 2C). The efficiency of DRM isolation was indicated by detection of flotillin-1, which was primarily present in light membrane fractions (Fig. 2C inset). We decided to detect the ASM by enzyme assays, since these assays are more sensitive than western blots and, most important, at present the molecular size of the ASM form in the heavy fraction is not clear. Thus, at present they are the only way to unambiguously prove the presence or absence of the ASM in LRs.
Fig. 2. Oxidative stress induces ASM translocation and activation of ASM onto membrane lipid rafts.
(A) Western blot analysis of ASM and Actin in Jurkat cells stimulated with or without H2O2 (10 min, 1 mM). The experiments were repeated three times with similar results. (B) Confocal microscopy of Jurkat cells revealed a translocation of ASM onto the surface of the cell membrane and a co-localization of ASM with ceramide-enriched membrane platforms. Shown is a representative results from three independent experiments. (C) ASM activity in DRM fractions is increased upon H2O2 stimulation. Low density membrane fractions were collected and designated as DRMs (Light). High density membrane fractions were collected in parallel and designated as none-DRM fraction (Heavy). The DRM maker protein flotillin-1 was detected by Western blot analysis. Shown is the mean ± S.D. of three independent experiments. Significant differences between treated and non-treated controls were determined by t-test and are indicated by ***, respectively (P<0.001, t-test).
ROS induce lysosomal trafficking and fusion (exocytosis)
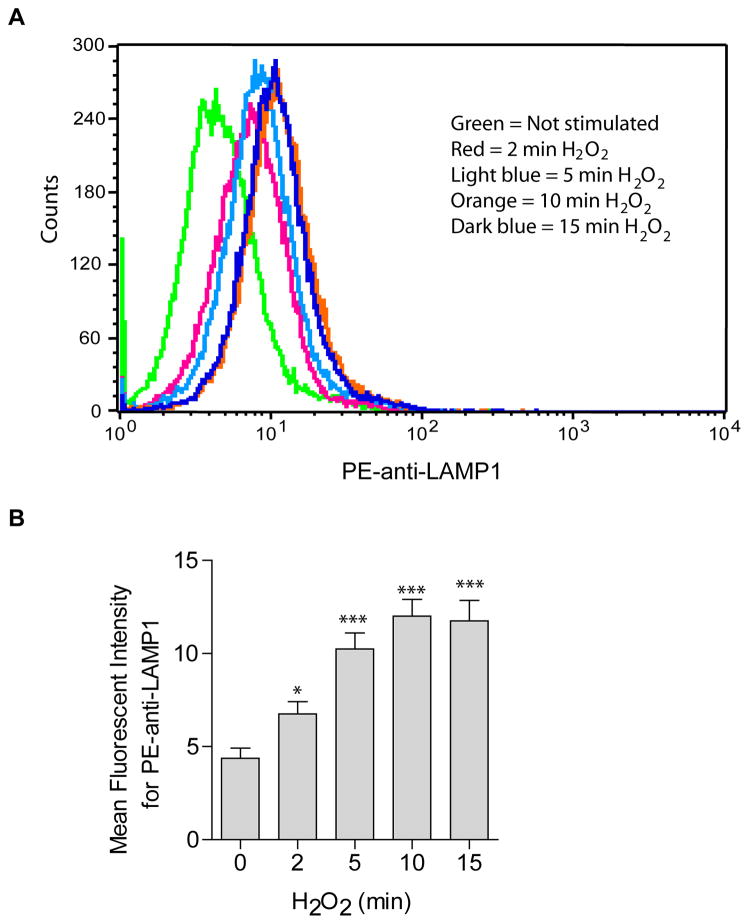
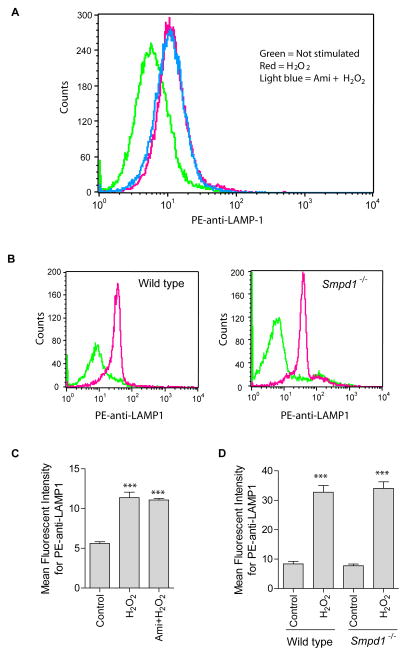
To test whether ASM-translocation onto the cell membrane is mediated by lysosomal fusion with the plasma membrane, cells were treated with H2O2 and surface staining was performed in live cells at 4°C with PE-conjugated anti-LAMP1, a lysosomal marker protein. Cells were analyzed by flow cytometry. The results reveal a time-dependent increase in membrane staining for LAMP1 upon H2O2 treatment (Fig. 3). Plasma membrane exposure of LAMP1 exposure was maximized at around 10 min H2O2 stimulation.
Fig. 3. H2O2induces lysosome excytosis.
The translocation of LAMP1 onto the cell surface served as marker for lysosome fusion with the plasma membrane and was detected by flow cytometry analysis using PE-conjugated goat anti-LAMP1. (A) Shown is a representative flow cytometry analysis from four independent experiments. (B) Summarized data showing the mean fluorescence intensity for PE-anti-LAMP1 staining (n=4). Shown are the mean ± SD. Significant differences between treated and non-treated controls were determined by t-test and are indicated by *** (P<0.001, t-test) or * (P<0.05, t-test), respectively.
ROS-induced lysosomal trafficking and fusion with and ASM translocation to plasma membrane are Ca2+-dependent
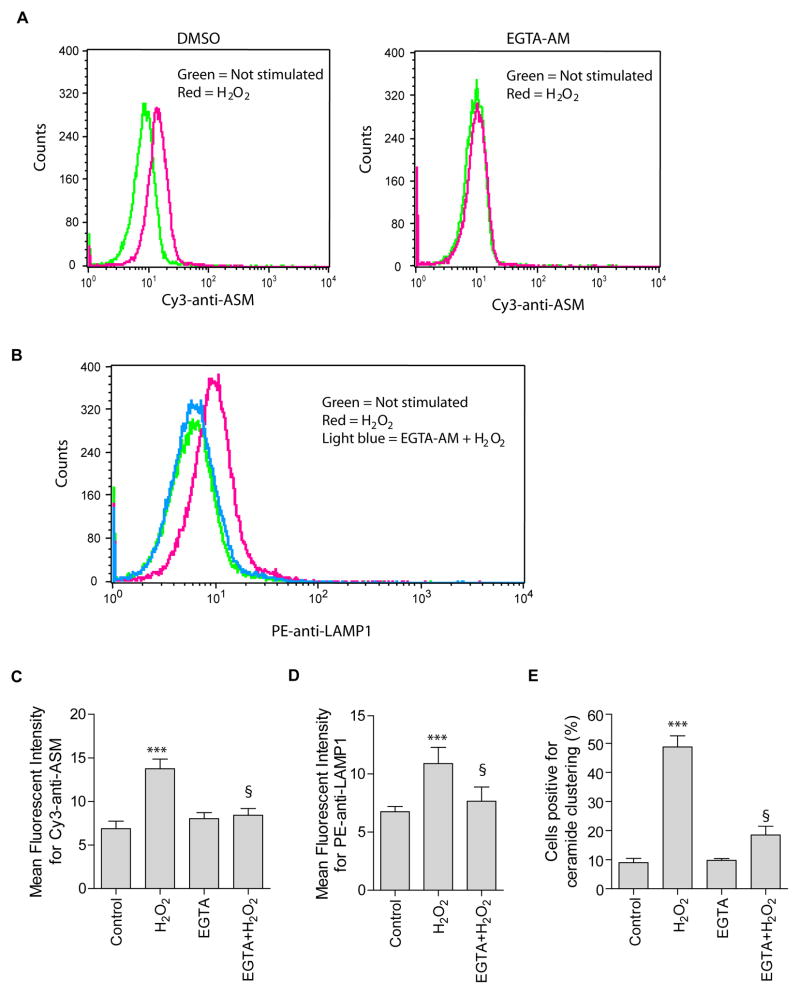
Previous studies have indicated that the increase in [Ca2+]i triggers lysosome exocytosis [20]. H2O2 triggers a rapid and transient increase in [Ca2+]i in Jurkat T cells [21]. Thus, we tested the hypothesis that ROS-induced a transient increase in [Ca2+]i that is involved in the lysosomal fusion with and ASM externalization to the plasma membrane. To examine if extracellular Ca2+ is involved in the increase in [Ca2+]i, cells were incubated with PBS buffer lacking Ca2+. The absence of extracellular Ca2+ had no effect on H2O2-induced ASM externalization (Fig. 4A left panel). Further, to deplete intracellular Ca2+, cells were incubated in PBS buffer in the presence of cell membrane permeable Ca2+ chelator EGTA-AM (100 μM). In contrast, chelation of intracellular Ca2+ by EGTA-AM abolished ASM externalization (Fig. 4A right panel). EGTA-AM also inhibited surface exposure of LAMP1 (Fig. 4B) and formation of ceramide-enriched membrane platforms (Fig. 4C). These data suggest that Ca2+-dependent lysosome trafficking is an important mechanism for translocation of ASM to the membrane.
Fig. 4. H2O2-induced lysosomal excytosis and ASM externalization are Ca2+-dependent.
(A, B) Jurkat cells were stimulate with or without H2O2 (1 mM, 10 min) in the absence (DMSO) or presence of EGTA-AM (100 μM) in a Ca2+-free PBS buffer. H2O2-induced ASM externalization and lysosomal excytosis were detected by flow cytometry analysis using Cy3-conjugated anti-ASM (A) and PE-conjugated goat anti-LAMP1 (B). Shown is a representative flow cytometry analysis from four independent experiments. (C) and (D) summarized data of four independent studies and show the mean fluorescence intensity for Cy3-anti-ASM or PE-anti-LAMP1 staining. (E) Attenuation of H2O2-induced ceramide-enriched membrane platforms by EGTA-AM. Displayed is the percentages of cells with a ceramide-enriched membrane platform in the cells treated with 1 mM H2O2 for 10 min (n = 4). Panel C–E show the mean ± S.D. Significant differences were determined by t-test (***, H2O2 vs. Control, P<0.001; §, EGTA+H2O2 vs. H2O2, P<0.001).
Effect of ASM inhibition and deficiency on lysosome exocytosis
To address whether ASM is critical for lysosome exocytosis, we performed experiments in spleen T cells freshly isolated from wild type and Smpd1−/− mice lacking Asm activity. In addition, we inhibitied ASM by treatment with amitriptyline, a known pharmacological blocker of ASM. Inhibition of ASM activity by treatment of Jurkat cells with amitriptyline (10 μM) did not affect H2O2-induced LAMP1 translocation (Fig. 5A). Likewise, Asm deficiency did not alter H2O2-induced LAMP1 translocation in freshly isolated spleen T cells (Fig. 5B). These data indicate that lysosome trafficking is independent of ASM activity upon H2O2 stimulation.
Fig. 5. Effect of ASM inhibition or deficiency on H2O2-induced lysosomal exocytosis.
(A) Shown is a representative flow cytometry analysis diagram using PE-conjugated goat anti-LAMP1 antibodies in Jurkat cells with or without ASM inhibitor amitriptyline (10 μM) (n = 4). (B) Spleen T cells were isolated from wild-type and Asm deficient (Smpd1−/−) mice, stimulated without (green) or with H2O2 (red) and stained with PE-conjugated goat anti-LAMP1 antibodies. Shown are representative flow cytometry analyses from 3 independent experiments. Summarized data from 3 independent studies show the mean fluorescence intensity for PE-anti-LAMP1 staining in Jurkat cells (C) or spleen T cells (D). Displayed is the mean ± S.D. Significant differences between treated and non-treated controls were determined by t-test and are indicated by *** (P<0.001, t-test).
Discussion
Here, we show that oxidative stress triggers intracellular Ca2+-dependent lysosome trafficking to and fusion with the plasma membrane, i.e. lysosome exocytosis, that results in surface expression of the ASM. Depletion of intracellular Ca2+ abolished ASM externalization and concomitant formation of ceramide-enriched membrane platforms. In addition, ROS induced lysosome exocytosis in the absence of ASM activity. These data suggest a novel role for Ca2+-dependent lysosome exocytosis in oxidative stress-induced ASM externalization, activation and formation of ceramide-enriched membrane platforms.
Our data suggest that lysosome exocytosis mediates ASM externalization. Several recent studies support a role of lysosome exocytosis in ASM externalization. In cytotoxic T lymphocytes, exocytosis of secretory granules, the only lysosome type organelles present in the cells, trafficks Asm to the plasma membrane [22]. In endothelial cells, disruption of lysosome functions by glycyl-L-phenylalanine 2-naphthylamide, a lysosome-disrupting cathepsin C substrate, abolished CD95 ligation-induced ASM translocation [23]. Upon stimulation, lysosomes, especially secretory lysosomes, can traffick and fuse with plasma membrane and release its acid hydrolases to the extracellular milieu [24]. Fusion of lysosomes with the plasma membrane results in the exposure of ASM on the outer leaflet of the cell membrane [25]. We previously demonstrated that ASM externalization is required to induce ceramide-mediated death receptor clustering, DISC formation and apoptosis initiation [9, 26]. Here, we found inhibition of lysosome exocytosis also abolished the clustering of membrane ceramides. Thus, these data support the view that ROS induce lysosome trafficking of ASM, most likely by secretory lysosomes, to plasma membrane leading to ceramide release and formation of ceramide-enriched membrane platforms.
The present data provide evidence that ROS-induced lysosome exocytosis is Ca2+-dependent. Several studies showed that lysosomes may traffick to and fuse with the plasma membrane in response to an increase in [Ca2+]i [20, 27] and lysosome exocytosis is regulated by the Ca2+ sensor synaptotagmin-VII, which restricts both the kinetics and the extent of Ca2+-dependent fusion [25]. In T cells, Ca2+ signaling initiated by TCR activation and ionomycin, a Ca2+ ionophore, has been implicated in lysosome exocytosis [22]. However, the role of Ca2+ in ROS-induced lysosome exocytosis and ASM externalization is undefined. [Ca2+]i can be elevated via influx of extracellular Ca2+ through membrane Ca2+ channels or mobilization of intracellular Ca2+ release from Ca2+ stores such as sarco-endoplamic reticula and mitochondria [28]. In the present study, intracellular chelation of Ca2+ by cell-permeable EGTA-AM but not extracellular Ca2+ removal abolished ROS-induced lysosome exocytosis indicating that increase in [Ca2+]i is primarily originated from intracellular Ca2+ stores. Most interestingly, ASM externalization and formation of ceramide-enriched platform were also blocked by EGTA-AM indicating that increase in [Ca2+]i serves as driving force for lysosome exocytosis resulting in ASM externalization and consequent ASM activation and ceramide release.
Some stress and bacterial stimuli such as UV-irradiation and Pseudomonas aeruginosa induce ROS-dependent plasma membrane trafficking of ASM as well as increase in [Ca2+]i [6, 8, 29, 30]. In this respect, our findings suggest a novel role of ROS as secondary messengers to these stimuli to trigger Ca2+-dependent lysosome trafficking of ASM resulting in formation of ceramide-enriched membrane platforms. However, activation of death receptors including CD95 and DR4/5 induces ROS-dependent ASM trafficking without alteration in [Ca2+]i suggesting that trafficking of ASM by ROS can be also independent of Ca2+ [17, 31]. The precise mechanism for Ca2+-independent ASM externalization is not well defined, which may involve PKCδ. ROS activate PKCδ, which then may interact with ASM-containing vesicles and translocate ASM to plasma membrane [11, 32].
Another important conclusion from this study is that ROS-induced lysosome exocytosis is independent of ASM expression and activity. This is consistent with a recent study showing TCR-triggered fusion of lysosome-type lytic granules is not altered in Asm-deficient cytotoxic T lymphocytes compared to wild-type cells, while in contrast, Asm deficiency results in impaired expulsion of vesicular contents [22]. The role of Asm and ceramide have also been investigated in other fusion events. Asm is required for fusion of late phagosomes with lysosomes since transfer of lysosomal fluid phase markers and lysosomal proteases cathepsin B, D and L into bacteria containing phagosomes are severely impaired in Asm-deficient macrophages [33]. Moreover, ceramide has been shown to trigger macroautophagy, another form of cell death and is probably involved in late formation of autophagolysosomes [34]. Thus, it seems that ASM activity is required for intracellular fusion processes, while lysosome exocytosis is independent of ASM activity.
The present study did not attempt to explore whether ceramide derived from other pathways such as de novo ceramide synthesis plays a role in ROS-induced lysosome exocytosis. De novo ceramide synthesis occurs primarily in the endoplasmic reticulum, where newly synthesized ceramide is transported to the Golgi apparatus via vesicular trafficking or by the action of the ceramide transfer protein. In the Golgi apparatus, ceramides are converted to other sphingolipids (i.e. sphingomyelin or glycosphingolipids) [35]. Previous studies have demonstrated that ceramide release via sphingomyelin hydrolysis is a much faster event than the de novo ceramide synthesis [36, 37]. In this regard, the present study revealed a very rapid lysosome exocytosis triggered by ROS (2 min). Therefore, our data support the view that the location and time period required for de novo ceramide synthesis may not favor its role in membrane fusion process and thereby mediate rapid signaling events. Nonetheless, the precise role of de novo ceramide synthesis in lysosome exocytosis requires future investigation.
In conclusion, we propose that Ca2+-dependent lysosome trafficking of ASM mediates ASM externalization under oxidative stress. The implication of our findings may also explain the action of ROS as a secondary messenger mediating ASM externalization, activation, ceramide release and formation of ceramide-enriched platforms in response to external stimuli, at least, for UV-irradiation and some bacterial infections.
Acknowledgments
This study was funded by DFG grant Gu 335–16/2 and NIH grant 2RO1HLO75316–05 to EG.
Abbreviations used are
- ASM
acid sphingomyelinase
- DRM
detergent resistant membranes
- H2O2
hydrogen peroxidase
- LAMP1
lysosome associated protein 1
- NADA and NADB
Niemann-Pick disease type A and B
- O2.·−
superoxide anions
- SMPD1
sphingomyelin phosphodiesterase 1
- ROS
reactive oxygen species
- SOD
superoxide dismutase
Footnotes
Author contribution
XL and YZ performed the experiments. EG and YZ designed the project and wrote the manuscript.
References
- 1.Ferlinz K, Hurwitz R, Vielhaber G, Suzuki K, Sandhoff K. Occurrence of two molecular forms of human acid sphingomyelinase. Biochem J. 1994;301 (Pt 3):855–862. doi: 10.1042/bj3010855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levade T, Jaffrezou JP. Signalling sphingomyelinases: Which, where, how and why? Biochim Biophys Acta. 1999;1438:1–17. doi: 10.1016/s1388-1981(99)00038-4. [DOI] [PubMed] [Google Scholar]
- 3.Huynh KK, Eskelinen EL, Scott CC, Malevanets A, Saftig P, Grinstein S. Lamp proteins are required for fusion of lysosomes with phagosomes. Embo J. 2007;26:313–324. doi: 10.1038/sj.emboj.7601511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGovern MM, Pohl-Worgall T, Deckelbaum RJ, Simpson W, Mendelson D, Desnick RJ, Schuchman EH, Wasserstein MP. Lipid abnormalities in children with types a and b niemann pick disease. J Pediatr. 2004;145:77–81. doi: 10.1016/j.jpeds.2004.02.048. [DOI] [PubMed] [Google Scholar]
- 5.McGovern MM, Aron A, Brodie SE, Desnick RJ, Wasserstein MP. Natural history of type a niemann-pick disease: Possible endpoints for therapeutic trials. Neurology. 2006;66:228–232. doi: 10.1212/01.wnl.0000194208.08904.0c. [DOI] [PubMed] [Google Scholar]
- 6.Charruyer A, Grazide S, Bezombes C, Muller S, Laurent G, Jaffrezou JP. Uv-c light induces raft-associated acid sphingomyelinase and jnk activation and translocation independently on a nuclear signal. J Biol Chem. 2005;280:19196–19204. doi: 10.1074/jbc.M412867200. [DOI] [PubMed] [Google Scholar]
- 7.Dumitru CA, Carpinteiro A, Trarbach T, Hengge UR, Gulbins E. Doxorubicin enhances trail-induced cell death via ceramide-enriched membrane platforms. Apoptosis. 2007;12:1533–1541. doi: 10.1007/s10495-007-0081-9. [DOI] [PubMed] [Google Scholar]
- 8.Grassme H, Jendrossek V, Riehle A, von Kurthy G, Berger J, Schwarz H, Weller M, Kolesnick R, Gulbins E. Host defense against pseudomonas aeruginosa requires ceramide-rich membrane rafts. Nat Med. 2003;9:322–330. doi: 10.1038/nm823. [DOI] [PubMed] [Google Scholar]
- 9.Grassme H, Jekle A, Riehle A, Schwarz H, Berger J, Sandhoff K, Kolesnick R, Gulbins E. Cd95 signaling via ceramide-rich membrane rafts. J Biol Chem. 2001;276:20589–20596. doi: 10.1074/jbc.M101207200. [DOI] [PubMed] [Google Scholar]
- 10.Grassme H, Riehle A, Wilker B, Gulbins E. Rhinoviruses infect human epithelial cells via ceramide-enriched membrane platforms. J Biol Chem. 2005;280:26256–26262. doi: 10.1074/jbc.M500835200. [DOI] [PubMed] [Google Scholar]
- 11.Zeidan YH, Hannun YA. Activation of acid sphingomyelinase by protein kinase cdelta-mediated phosphorylation. J Biol Chem. 2007;282:11549–11561. doi: 10.1074/jbc.M609424200. [DOI] [PubMed] [Google Scholar]
- 12.Smart EJ, Graf GA, McNiven MA, Sessa WC, Engelman JA, Scherer PE, Okamoto T, Lisanti MP. Caveolins, liquid-ordered domains, and signal transduction. Mol Cell Biol. 1999;19:7289–7304. doi: 10.1128/mcb.19.11.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cremesti AE, Goni FM, Kolesnick R. Role of sphingomyelinase and ceramide in modulating rafts: Do biophysical properties determine biologic outcome? FEBS Lett. 2002;531:47–53. doi: 10.1016/s0014-5793(02)03489-0. [DOI] [PubMed] [Google Scholar]
- 14.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen HM, Pervaiz S. Tnf receptor superfamily-induced cell death: Redox-dependent execution. Faseb J. 2006;20:1589–1598. doi: 10.1096/fj.05-5603rev. [DOI] [PubMed] [Google Scholar]
- 16.Gius D, Spitz DR. Redox signaling in cancer biology. Antioxid Redox Signal. 2006;8:1249–1252. doi: 10.1089/ars.2006.8.1249. [DOI] [PubMed] [Google Scholar]
- 17.Dumitru CA, Gulbins E. Trail activates acid sphingomyelinase via a redox mechanism and releases ceramide to trigger apoptosis. Oncogene. 2006;25:5612–5625. doi: 10.1038/sj.onc.1209568. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Li X, Carpinteiro A, Gulbins E. Acid sphingomyelinase amplifies redox signaling in pseudomonas aeruginosa-induced macrophage apoptosis. J Immunol. 2008;181:4247–4254. doi: 10.4049/jimmunol.181.6.4247. [DOI] [PubMed] [Google Scholar]
- 19.Scheel-Toellner D, Wang K, Craddock R, Webb PR, McGettrick HM, Assi LK, Parkes N, Clough LE, Gulbins E, Salmon M, Lord JM. Reactive oxygen species limit neutrophil life span by activating death receptor signaling. Blood. 2004;104:2557–2564. doi: 10.1182/blood-2004-01-0191. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez A, Webster P, Ortego J, Andrews NW. Lysosomes behave as ca2+-regulated exocytic vesicles in fibroblasts and epithelial cells. J Cell Biol. 1997;137:93–104. doi: 10.1083/jcb.137.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sen CK, Roy S, Packer L. Involvement of intracellular ca2+ in oxidant-induced nf-kappa b activation. FEBS Lett. 1996;385:58–62. doi: 10.1016/0014-5793(96)00346-8. [DOI] [PubMed] [Google Scholar]
- 22.Herz J, Pardo J, Kashkar H, Schramm M, Kuzmenkina E, Bos E, Wiegmann K, Wallich R, Peters PJ, Herzig S, Schmelzer E, Kronke M, Simon MM, Utermohlen O. Acid sphingomyelinase is a key regulator of cytotoxic granule secretion by primary t lymphocytes. Nat Immunol. 2009;10:761–768. doi: 10.1038/ni.1757. [DOI] [PubMed] [Google Scholar]
- 23.Jin S, Zhang Y, Yi F, Li PL. Critical role of lipid raft redox signaling platforms in endostatin-induced coronary endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2008;28:485–490. doi: 10.1161/ATVBAHA.107.159772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luzio JP, Pryor PR, Bright NA. Lysosomes: Fusion and function. Nat Rev Mol Cell Biol. 2007;8:622–632. doi: 10.1038/nrm2217. [DOI] [PubMed] [Google Scholar]
- 25.Rao SK, Huynh C, Proux-Gillardeaux V, Galli T, Andrews NW. Identification of snares involved in synaptotagmin vii-regulated lysosomal exocytosis. J Biol Chem. 2004;279:20471–20479. doi: 10.1074/jbc.M400798200. [DOI] [PubMed] [Google Scholar]
- 26.Grassme H, Cremesti A, Kolesnick R, Gulbins E. Ceramide-mediated clustering is required for cd95-disc formation. Oncogene. 2003;22:5457–5470. doi: 10.1038/sj.onc.1206540. [DOI] [PubMed] [Google Scholar]
- 27.Martinez I, Chakrabarti S, Hellevik T, Morehead J, Fowler K, Andrews NW. Synaptotagmin vii regulates ca(2+)-dependent exocytosis of lysosomes in fibroblasts. J Cell Biol. 2000;148:1141–1149. doi: 10.1083/jcb.148.6.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gardner P. Calcium and t lymphocyte activation. Cell. 1989;59:15–20. doi: 10.1016/0092-8674(89)90865-9. [DOI] [PubMed] [Google Scholar]
- 29.Ratner AJ, Bryan R, Weber A, Nguyen S, Barnes D, Pitt A, Gelber S, Cheung A, Prince A. Cystic fibrosis pathogens activate ca2+-dependent mitogen-activated protein kinase signaling pathways in airway epithelial cells. J Biol Chem. 2001;276:19267–19275. doi: 10.1074/jbc.M007703200. [DOI] [PubMed] [Google Scholar]
- 30.Lao Y, Chang DC. Mobilization of ca2+ from endoplasmic reticulum to mitochondria plays a positive role in the early stage of uv- or tnfalpha-induced apoptosis. Biochem Biophys Res Commun. 2008;373:42–47. doi: 10.1016/j.bbrc.2008.05.172. [DOI] [PubMed] [Google Scholar]
- 31.Lepple-Wienhues A, Belka C, Laun T, Jekle A, Walter B, Wieland U, Welz M, Heil L, Kun J, Busch G, Weller M, Bamberg M, Gulbins E, Lang F. Stimulation of cd95 (fas) blocks t lymphocyte calcium channels through sphingomyelinase and sphingolipids. Proc Natl Acad Sci U S A. 1999;96:13795–13800. doi: 10.1073/pnas.96.24.13795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brodie C, Blumberg PM. Regulation of cell apoptosis by protein kinase c delta. Apoptosis. 2003;8:19–27. doi: 10.1023/a:1021640817208. [DOI] [PubMed] [Google Scholar]
- 33.Schramm M, Herz J, Haas A, Kronke M, Utermohlen O. Acid sphingomyelinase is required for efficient phago-lysosomal fusion. Cell Microbiol. 2008;10:1839–1853. doi: 10.1111/j.1462-5822.2008.01169.x. [DOI] [PubMed] [Google Scholar]
- 34.Pattingre S, Bauvy C, Levade T, Levine B, Codogno P. Ceramide-induced autophagy: To junk or to protect cells? Autophagy. 2009;5:558–560. doi: 10.4161/auto.5.4.8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 36.Seumois G, Fillet M, Gillet L, Faccinetto C, Desmet C, Francois C, Dewals B, Oury C, Vanderplasschen A, Lekeux P, Bureau F. De novo c16- and c24-ceramide generation contributes to spontaneous neutrophil apoptosis. J Leukoc Biol. 2007;81:1477–1486. doi: 10.1189/jlb.0806529. [DOI] [PubMed] [Google Scholar]
- 37.Dbaibo GS, Kfoury Y, Darwiche N, Panjarian S, Kozhaya L, Nasr R, Abdallah M, Hermine O, El-Sabban M, de The H, Bazarbachi A. Arsenic trioxide induces accumulation of cytotoxic levels of ceramide in acute promyelocytic leukemia and adult t-cell leukemia/lymphoma cells through de novo ceramide synthesis and inhibition of glucosylceramide synthase activity. Haematologica. 2007;92:753–762. doi: 10.3324/haematol.10968. [DOI] [PubMed] [Google Scholar]