Abstract
Background
Differentiating psychogenic parkinsonism from neurodegenerative Parkinson's disease (PD) with psychogenic features is a diagnostic challenge.
Case report
We report a detailed longitudinal clinical description of three cases presenting with suspected psychogenic parkinsonism. Dopamine transporter single-photon emission computed tomography (DAT-SPECT) was used as a supplemental diagnostic study and influenced clinical management.
Discussion
DAT-SPECT quantified the integrity of the striatal dopaminergic system in these cases of clinically uncertain parkinsonism and supported clinical decision-making.
Keywords: Psychogenic parkinsonism, Parkinson's disease, dopamine transporter imaging, striatal dopaminergic deficit
Introduction
123I-labled 2β-carbomethoxy-3β-(4-iodophenyl)-N-(3-fluoropropyl)nortropane ([123I]-FP-CIT) single-photon emission computed tomography (SPECT) imaging was approved for clinical use in the United States in 2011 for visualization of striatal dopamine transporter activity to identify presynaptic dopaminergic parkinsonism.1 Although some physicians question its clinical utility,1 dopamine transporter SPECT imaging (DAT-SPECT) is increasingly recognized as having practical relevance.2 To add to the growing number of uses, we describe a specific scenario where management of three parkinsonian patients with psychogenic features was influenced by imaging. While previous work3 addressed the role of DAT-SPECT imaging in evaluating psychogenic parkinsonism, to our knowledge, ours is the first demonstration that this imaging tool markedly influenced clinical management in a cohort of three patients.
Distinguishing “pure” psychogenic parkinsonism from neurodegenerative Parkinson's disease (PD) with concurrent psychogenic features is a diagnostic dilemma. Correct diagnosis is relevant to clinical management in preventing pure psychogenic patients from exposure to unwarranted anti-parkinsonian (anti-PD) medications, while providing adequate treatment to those with neurodegenerative PD complicated by psychogenic overlay. While clinical exam and tremor electrophysiology can document psychogenic features, they generally cannot inform about concomitant neurodegenerative disease. In our cohort, we demonstrate that DAT-SPECT is a useful diagnostic tool that can serve to distinguish pure psychogenic parkinsonism from neurodegenerative PD with psychogenic overlay and thus guide clinical management.
Case report
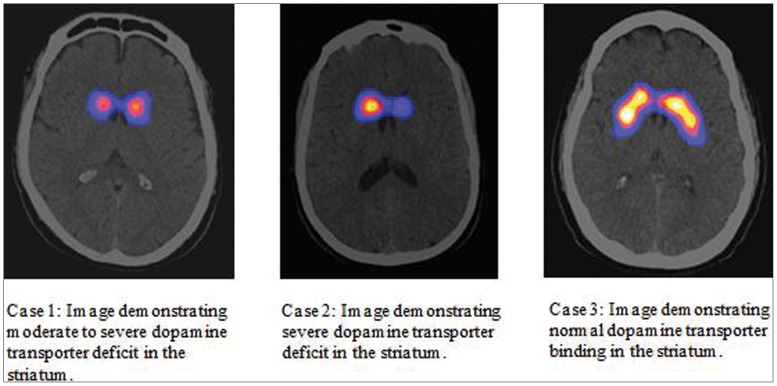
A 39-year-old male (Case 1) was referred for evaluation of left hand rest tremor, rigidity, and poor fine motor coordination. On exam, he had mild amplitude left hand rest and postural tremor with a semi-purposeful appearance. The tremor was distractible, suggestible, and entrainable. There was mild left arm cogwheel rigidity and bradykinesia. Anti-PD medications were started 24 months after symptom onset, with rasagiline 1 mg daily first, then ropinirole 6 mg daily. On medications, he reported minimal improvement with gradual worsening of his left hand tremor and fine motor coordination. Despite reported worsening in left hand function, follow-up examinations demonstrated an ability to complete complex fine motor tasks. While psychogenic features were unequivocally confirmed during examination, underlying neurodegenerative parkinsonism could not be excluded. [123I]-FP-CIT SPECT (Figure 1, Table 1) was obtained and showed moderate-to-severe dopamine transporter deficit. Levodopa therapy was subsequently started with improved symptoms.
Figure 1. [123I]-FP-CIT SPECT scan images.
Case 1, Case 2, and Case 3, respectively. The images were interpreted by a nuclear medicine physician (Z.S.) using established criteria and were quantitatively analyzed using the BRASS software (Hermes Medical Solutions, Stockholm Sweden). The quantification program is objective since it is entirely operator independent.
TABLE 1. Striatal Binding of Cases 1, 2, and 3.
| Striatal Binding | Case 1 | Case 2 | Case 3 | Healthy Controls (mean±SD, n = 7) |
|---|---|---|---|---|
| Right caudate | 1.01 | 1.51 | 1.86 | 1.94±0.56 |
| Left caudate | 1.10 | 0.84 | 1.85 | 1.98±0.43 |
| Right putamen | 0.56 | 0.81 | 1.83 | 1.75±0.38 |
| Left putamen | 0.60 | 0.40 | 1.70 | 1.83±0.41 |
Abbreviation: SD, Standard Deviation.
This parameter was calculated as a specific binding ratio relative to the occipital lobe as a reference tissue. The striatal specific binding ratio is calculated as: (S-O)/O. Where, S is activity in the striatum (caudate or putamen) and O is the activity in the occipital lobe. Any positive number indicates higher binding in the striatum than in the reference region. The reference normal striatal binding values were obtained in seven healthy controls.
A 36-year-old female (Case 2) presented for evaluation of parkinsonism. Four years before presentation, she developed micrographia, right arm tremor, and rigidity and was diagnosed with PD at another institution. On initial evaluation, she was already prescribed daily doses of rasagiline 1 mg, carbidopa/levodopa 350 mg, and ropinirole 2 mg. On exam, she had mild right arm rest/postural hand tremor and mild-to-moderate cogwheel rigidity bilaterally. Her rigidity and tremor were distractible. She had moderate bradykinesia bilaterally with variability. With this presentation, psychogenic parkinsonism was strongly suspected, but underlying neurodegenerative parkinsonism could not be excluded. Her anti-PD medications were continued based on her reported symptomatic relief; however, reported side effects and questions about the variability of benefits created concern of unnecessary drug exposure if she did not have underlying neurodegenerative disease. [123I]-FP-CIT SPECT (Figure 1, Table 1) was obtained and revealed moderate-to-severe dopamine transporter deficit. With this result, her anti-PD medications were titrated to adequate symptomatic control.
A 41-year-old male (Case 3) presented for evaluation of parkinsonism. Initial symptoms included foot dystonia and right arm tremor. He had been diagnosed with parkinsonism 1 year prior and was on 600 mg of carbidopa/levodopa and 800 mg of entacapone daily. On examination, he had semi-purposeful right arm global tremor with variability in frequency. His tremor was distractible and decreased in amplitude when the opposite side of the body was examined. He displayed oppositional rigidity in his lower extremities. There was pseudoakinesia with deliberate slowness on finger-taps. We suspected psychogenic parkinsonism but could not rule out underlying neurodegenerative PD. Diagnosis of dystonic tremor was considered but was lower on the differential due to the prominent elements of parkinsonism with psychogenic features. Diagnostic work-up included [123I]-FP-CIT SPECT (Figure 1, Table 1), which was negative. In light of this result, we started the process of carefully tapering his anti-PD medications. He has been connected with a psychiatrist with expertise in psychogenic movement disorders.
For each case, the images were interpreted by a nuclear medicine physician (Z.S.) using established criteria and were quantitatively analyzed using BRASS software.4,5
Discussion
Neurodegenerative PD with psychogenic overlay can prove a diagnostic quandary. Electrophysiological tremor recording is useful in proving the presence of a psychogenic tremor but cannot unequivocally identify individuals with underlying neurodegenerative PD, especially in non-tremor predominant cases. In contrast, DAT-SPECT can quantify with high sensitivity presynaptic striatal dopaminergic deficit, which is not present in pure psychogenic disease but is expected in cases of neurodegenerative PD even if complicated by a marked psychogenic overlay. While a previous study3 examined DAT-SPECT as a diagnostic tool in psychogenic parkinsonism, we highlight the impact of DAT-SPECT in influencing treatment decisions in the specific scenario where “pure” psychogenic parkinsonism cannot be distinguished from neurodegenerative PD with psychogenic features by clinical exam alone.
Psychogenic parkinsonism accounts for approximately 10% of all cases of psychogenic movement disorders.6 It can mimic idiopathic PD and proves a diagnostic challenge even for experienced movement disorder neurologists. In contrast to idiopathic PD, individuals with psychogenic parkinsonism are more likely to display variability in tremor frequency/rhythm, pseudoakinesia with exaggerated slowness, and oppositional rigidity without true cogwheeling.3 Features of distractibility, suggestibility, and entrainment of tremor are also characteristic.
When diagnostic uncertainty arises in cases of suspected neurodegenerative PD with psychogenic features, treatment decisions may be difficult. Anti-PD medication trials are often attempted but may be difficult to interpret due to the placebo effect and variability of patient response. Prolonged medication trials in patients with “pure” psychogenic parkinsonism are potentially harmful and may unnecessarily increase health care spending. DAT-SPECT showing striatal dopaminergic deficit can support a diagnosis of PD in the right clinical context, thus improving diagnostic confidence and supporting early institution of anti-PD medications, which has been shown to be beneficial in previous studies.7
The diagnostic accuracy of DAT-SPECT has been evaluated. Two multicenter phase III studies serving as the basis for DAT-SPECT approval in the United States were reported so have DAT-SPECT sensitivities of 79% and 97% and specificities of 97% and 98%, respectively.1 Another study of 743 cases found low error rates of DAT-SPECT in PD diagnosis: five false-positives and two false-negatives with a sensitivity and specificity of 99.4% and 98.6%, respectively.8 Notably, the diagnostic accuracy of DAT-SPECT in these studies was determined using clinical diagnosis as the “gold standard.” Without pathological data, the certainty of the clinical diagnosis made in these studies is unknown. The diagnostic challenge in early PD is demonstrated in previous clinical trials.8 For example, in the Earlier versus Later Levodopa Therapy in Parkinson Disease (ELLDOPA) study, 15% of subjects initially diagnosed as PD were later re-classified as subjects without evidence of dopaminergic deficit (SWEDDs) after SPECT imaging.7,8
DAT-SPECT provides quantification of the presynaptic dopaminergic transporter system rather than a specific diagnosis. Diagnostic accuracy is improved by narrowing the clinically developed differential diagnoses to neurodegenerative versus non-neurodegenerative parkinsonian syndromes. A negative DAT-SPECT can support a variety of diagnoses in the right clinical context, including psychogenic parkinsonism, dopa-responsive dystonia associated with parkinsonism, essential tremor, vascular parkinsonism, or drug-induced parkinsonism. On the other hand, a positive DAT-SPECT could indicate a diagnosis of PD, multiple system atrophy (MSA), progressive supranuclear palsy (PSP), or corticobasal degeneration (CBD). Admittedly, DAT-SPECT is unreliable in distinguishing PD from other neurodegenerative parkinsonian disorders. However, in our particular cohort, no significant consideration of Parkinson Plus syndromes could be made on clinical grounds. Additionally, a negative DAT-SPECT does not exclude metabolic causes of dopa-responsive dystonia associated with parkinsonism, such as Guanosine Triphosphate (GTP) cyclohydrolase 1 deficiency. While DAT-SPECT may not be appropriate in every scenario, combining DAT-SPECT results with clinical findings can improve diagnostic accuracy and guide treatment in clinically uncertain cases.
The cost effectiveness of DAT-SPECT factors into the utility of the scan. A review on the economic impact of DAT-SPECT showed reduced overall cost of care in cases of clinically uncertain parkinsonian syndromes.8 In these cases, cost savings were achieved by avoiding unnecessary medications and improving time spent on appropriate management.8 While accounting for the long-term cost benefits of the DAT-SPECT, risks associated with obtaining the scan should also be recognized. De la Fuente-Fernandez1 reported a low radiation effective dose of 3.94 mSv for DAT-SPECT, which is similar to the radiation exposure of a computed tomography (CT) scan of the neck (4 mSv). Estimated cancer risk based on these values ranged from 1 in 5,000 to 7,500 depending on age.1,8 Risk-benefit assessments are key when considering DAT-SPECT as an adjunctive study.
Notably, Case 3 could be classified under the broad category of SWEDDs. The term SWEDDs encompasses a heterogeneous group of diagnoses with the unifying criteria of features of parkinsonism with normal dopaminergic imaging.9 SWEDDs can have similar phenomenological features as in neurodegenerative PD and have been identified in previous PD clinical trials based on normal dopaminergic imaging.10 There is no consistent evidence to suggest SWEDDs require dopaminergic therapy.
The negative DAT-SPECT in Case 3 taken together with the prominent psychogenic features and lack of supportive evidence for alternative diagnoses (drug-induced PD, dystonic tremor), led to the diagnosis of psychogenic parkinsonism. Counseling was provided to Case 3 to carefully explain how the diagnosis of psychogenic parkinsonism was reached (based on the normal DAT-SPECT results in addition to clinical exam features), and discussion ensued regarding the best management, including referral to psychiatric counseling and tapering off anti-PD medications. In all three cases, a critical decision-making point was reached where it was unclear that continuing anti-PD medications was appropriate. This was explained during follow-up visits as the basis for ordering the DAT-SPECT scan, to improve diagnostic accuracy and decide on best medical therapy. In Cases 1 and 2, DAT-SPECT findings were useful in narrowing the differential diagnoses to causes of neurodegenerative parkinsonism, with PD as the most probable diagnosis based on the clinical presentations. Cases 1 and 2 did not have phenotypic features supportive of other neurodegenerative parkinsonian syndromes. DAT-SPECT was clinically meaningful in these cases as it increased diagnostic confidence leading to more aggressive anti-PD management in the first two cases and appropriate referral for psychiatric counseling for the third case.
The emergence of DAT-SPECT introduced a supplemental diagnostic tool that can quantify nigrostriatal dopaminergic system integrity. Consideration of the clinical utility of DAT-SPECT should include its potential impact on treatment decisions in clinically uncertain cases, diagnostic accuracy, cost effectiveness, radiation exposure risk, and on patients' expectations about a timely diagnosis. When applied in our cases of clinically uncertain psychogenic parkinsonism, DAT-SPECT improved diagnostic certainty and helped support treatment decisions.
Footnotes
Funding: None.
Financial disclosure: None
Conflict of Interests: The authors report no conflict of interest.
References
- 1.de la Fuente-Fernandez R. Role of DaTSCAN and clinical diagnosis in Parkinson disease. Neurology. 2012;78:696–701. doi: 10.1212/WNL.0b013e318248e520. [DOI] [PubMed] [Google Scholar]
- 2.Felicio AC, Godeiro-Junior C, Moriyama TS, et al. Degenerative parkinsonism in patients with psychogenic parkinsonism: a dopamine transporter imaging study. Clin Neurol Neurosurg. 2010;112:282–285. doi: 10.1016/j.clineuro.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Benaderette S, Zanotti Fregonara P, Apartis E, et al. Psychogenic parkinsonism: a combination of clinical, electrophysiological, and [(123)I]-FP-CIT SPECT scan explorations improves diagnostic accuracy. Mov Disord. 2006;21:310–317. doi: 10.1002/mds.20720. [DOI] [PubMed] [Google Scholar]
- 4.Seibyl JP. Single-photon emission computed tomography and positron emission tomography evaluations of patients with central motor disorders. Semin Nucl Med. 2008;38:274–286. doi: 10.1053/j.semnuclmed.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Morton RJ, Guy MJ, Clauss R, Hinton PJ, Marshall CA, Clarke EA. Comparison of different methods of DatSCAN quantification. Nucl Med Commun. 2005;26:1139–1146. doi: 10.1097/00006231-200512000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Hallett M. Psychogenic parkinsonism. J Neurol Sci. 2011;310:163–165. doi: 10.1016/j.jns.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fahn S, Oakes D, Shoulson I, et al. Levodopa and the progression of Parkinson's disease. N Engl J Med. 2004;351:2498–2508. doi: 10.1056/NEJMoa033447. [DOI] [PubMed] [Google Scholar]
- 8.Bajaj N, Hauser RA, Grachev ID. Clinical utility of dopamine transporter single photon emission CT (DaT-SPECT) with (123I) ioflupane in diagnosis of parkinsonian syndromes. J Neurol Neurosurg Psychiatry . 2013 doi: 10.1136/jnnp-2012-304436. doi: 10.1136/jnnp-2012-304436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwingenschuh P, Ruge D, Edwards MJ, et al. Distinguishing SWEDDs patients with asymmetric resting tremor from Parkinson's disease: a clinical and electrophysiological study . Mov Disord. 2010;25:560–569. doi: 10.1002/mds.23019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Utiumi MA, Felício AC, Borges CR, et al. Dopamine transporter imaging in clinically unclear cases of parkinsonism and the importance of Scans Without Evidence of Dopaminergic Deficit (SWEDDs) Arq Neuropsiquiatr. 2012;70:667–673. doi: 10.1590/S0004-282X2012000900004. [DOI] [PubMed] [Google Scholar]