Abstract
The molybdenum cofactor (Moco) is a redox cofactor found in all kingdoms of life and its biosynthesis is essential for survival of many organisms including humans. The first step of Moco biosynthesis is a unique transformation of GTP into cyclic pyranopterin monophosphate (cPMP). In bacteria, MoaA and MoaC catalyze this transformation, although the specific functions of these enzymes were not fully understood. Here, we report the first isolation and structural characterization of a product of MoaA. This molecule was isolated under anaerobic conditions from a solution of MoaA incubated with GTP, SAM and sodium dithionite in the absence of MoaC. Structural characterization by chemical derivatization, MS, and NMR spectroscopy, suggested the structure of this molecule to be (8S)-3′,8-cyclo-7,8-dihydroguanosine 5′-triphosphate (3′,8-cH2GTP). The isolated 3′,8-cH2GTP was converted to cPMP by MoaC or its human homolog, MOCS1B, with high specificities (Km < 0.060 μM and 0.79 ±0.24 μM for MoaC and MOCS1B, respectively), suggesting the physiological relevance of 3′,8-cH2GTP. These observations, in combination with some mechanistic studies of MoaA, unambiguously demonstrates that MoaA catalyzes a unique radical C-C bond formation reaction, and that, in contrast to previous proposals, MoaC plays a major role in the complex rearrangement to generate the pyranopterin ring.
Introduction
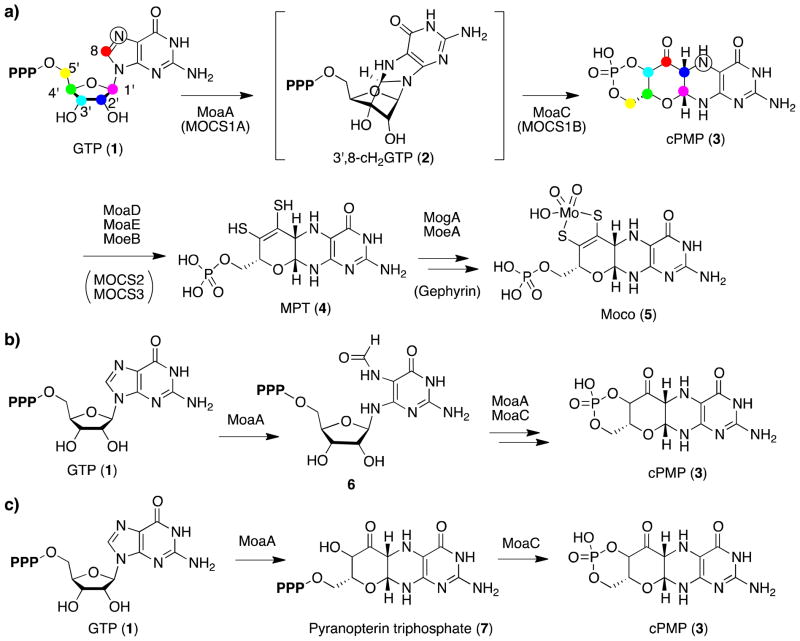
Molybdenum cofactor (Moco, Figure 1a; 5) is a redox cofactor found in almost all organisms1,2. Moco-dependent enzymes play central roles in many biologically important processes such as purine and sulfur catabolism in mammals, anaerobic respiration in bacteria, and nitrate assimilation in plants2. In humans, Moco deficiency results in the pleiotropic loss of all molybdenum enzyme activities, causing neurological abnormalities and early childhood death3,4. Unlike many other cofactors, Moco cannot be taken up as a nutrient, and thus requires de novo biosynthesis. Moco biosynthesis (Figure 1a) is thought to be conserved among all organisms5,6 and initiated by the conversion of GTP (1) into cPMP (3)7–9. cPMP is then converted to Moco by the introductions of two sulfur atoms10–12 and a molybdate13–16.
Figure 1.
(a) Moco biosynthetic pathway in bacteria and humans. The human enzymes are indicated in parenthesis. The symbols on GTP and cPMP indicate the source of the carbon and nitrogen atoms in cPMP as determined by isotope labeling studies7,17. P designates a phosphate group. 3′,8-cH2GTP is shown in brackets, as it had not been identified prior to this report. (b) Previous proposal for the functions of MoaA and MoaC with 2-amino-5-formylamino-6-ribofuranosylamino- 4-pyrimidinone triphosphate (6) as an intermediate29,33.(c) Previous proposal for the functions of MoaA and MoaC by Begley et al.30. Pyranopterin triphosphate 7 was proposed as the product of MoaA.
Previous isotope tracer experiments indicated that the conversion of GTP to cPMP proceeds through the insertion of C-8 of guanine between C-2′ and C-3′ of ribose (Figure 1a)7,17. This contrasts with the biosynthesis of pterin rings in other GTP derived cofactors such as folate and flavins, in which C-8 of GTP is released as a formate during the reaction catalyzed by GTP cyclohydrolases (Figure S1a)18,19. Thus, the retention of GTP C-8 in Moco biosynthesis indicated a novel mechanism of pterin ring formation.
Two enzymes, MoaA and MoaC in bacteria, are responsible for the conversion of GTP to cPMP7,9,20,21. Bioinformatic analysis suggested that MoaA belongs to the radical SAM (S-adenosyl-L-methionine) superfamily22. Enzymes in this superfamily catalyze the reductive cleavage of SAM using an oxygen-labile [4Fe-4S] cluster as a reductant, and transiently generate a 5′-deoxyadenosyl radical (5′-dA•), which then abstracts a H-atom from substrate to initiate radical reactions23. The classification of MoaA as a radical SAM enzyme was supported by an in vitro activity assay of MoaA in the presence of MoaC, in which the conversion of GTP into cPMP requires the presence of SAM9. The X-ray crystal structures of Staphylococcus aureus MoaA9 revealed the binding of SAM to a [4Fe-4S] cluster, in a fashion similar to other radical SAM enzymes24,25. The structure also revealed the presence of an additional C-terminal [4Fe-4S] cluster. This C-terminal [4Fe-4S] cluster was shown to bind various purine nucleoside 5′-triphosphates including GTP in crystal structures26 as well as in solution based on electron-nuclear double resonance (ENDOR) spectroscopy27. Together with the reported binding constant (0.29 μM)26, GTP was proposed as a substrate of MoaA.
Many radical SAM enzymes are known to catalyze complex rearrangement reactions23,28. MoaA has also been considered to catalyze the majority, if not all, of the complex rearrangement of GTP to form the pyranopterin ring of cPMP5,6,29,30. Schindelin and Hänzelmann first proposed that, in the absence of MoaC, purified MoaA catalyzes a conversion of GTP to a molecule with a 6-hydroxy-2,4,5-triaminopyrimidine partial structure based on the chemical derivatization to dimethylpterin (DMPT)26. Unfortunately, no data was presented. This observation was analogous to those for folate biosynthesis, where the imidazole moiety of guanine base in GTP is first hydrolyzed to an 2-amino-5-formylamino-6-ribofuranosylamino-4-pyrimidinone triphosphate (Figure S1a)31, which may be chemically derivatized to DMPT (Figure S1b). This analogy has prompted speculations about the reaction catalyzed by MoaA29,32, which consider 2-amino-5-formylamino-6-ribofuranosylamino-4-pyrimidinone triphosphate (6) as an intermediate or a product of the MoaA catalyzed reaction (Figure 1b).
Recently, more definitive proposal for the product of MoaA was made by Begley et al.30. In this report, the authors performed LCMS analysis of small molecules produced after incubation of MoaA with GTP, SAM and dithionite in the absence of MoaC, and observed a molecule with a light absorption at 320 nm with a mass signal at m/z = 524 [M+H]+ in the presence of GTP, SAM and sodium dithionite. Reactions using series of deuterated GTP suggested that a deuterium at the 3′ position of GTP is transferred to 5′-deoxyadenosine (5′-dA) produced in this assay. However, the observed putative MoaA product was not isolated for further structural characterization, and the relation of this observation to the earlier one by Schindelin and Hänzelmann26 was not discussed. It is currently unknown whether the putative MoaA product could serve as a substrate of MoaC, and be converted to cPMP. Nevertheless, based on these observations, the authors proposed that MoaA catalyzes a conversion of GTP into pyranopterin triphosphate (7, Figure 1c)30.
The role of MoaC, on the other hand, has been significantly underappreciated. MoaC does not show significant amino acid sequence similarities to functionally characterized proteins. Currently, structures of MoaC from four different organisms have been reported32–35. A putative ligand binding site was proposed based on the conservation of amino acid residues and their relative positions in a crystal structure34. A structure of MoaC from Thermus thermophilus in combination with isothermal titration calorimetry experiments suggested that nucleotide triphosphate may bind to the putative ligand binding site33. In the recent publication by Begley et al.30, MoaC was proposed to be responsible only for the formation of the cyclic phosphate (Figure 1c), based on their premise that the MoaA product is pyranopterin triphosphate. However, uncertainty remains about the relevance of this proposal due to the limited characterization of the MoaA reaction product.
In the current study, we report the isolation and detailed characterization of the MoaA reaction product, which unambiguously delineated the individual reactions catalyzed by MoaA and MoaC. The isolation of the MoaA reaction product was achieved under anaerobic conditions with careful control of pH. Structural characterization by chemical derivatization, MS, and NMR spectroscopy established the structure of this molecule as (8S)-3′,8-cyclo-7,8-dihydroguanosine 5′-triphosphate (3′,8-cH2GTP, 2). The relevance of the isolated 3′,8-cH2GTP as a physiological Moco biosynthetic intermediate was demonstrated by steady state kinetic analysis of MoaC and its human homolog, MOCS1B. MoaC and MOCS1B converted 3′,8-cH2GTP to cPMP with Km values of < 0.06 μM and 0.79 μM, respectively. Additional studies on the stoichiometry of the MoaA reaction in combination with an isotope tracer experiment suggest that the conversion of GTP to 3′,8-cH2GTP proceeds through H-abstraction from the 3′ position by consuming a stoichiometric amount of SAM. The observations presented here in sum unequivocally delineate the individual functions of MoaA and MoaC, and provide insights into the biosynthesis of cPMP. The current identification of 3′,8-cH2GTP as a substrate of MoaC is a sharp contrast to previous proposals29,30, in which MoaC was thought to have minimal or no function in the formation of the pyranopterin structure. These results provide basis for future mechanistic studies of reactions catalyzed by these two enzymes.
Materials and Methods
QAE A25 Sephadex resin, Guanosine 5′-triphosphate (GTP), [U-13C5,15N10]GTP, S-adenosyl-L-methionine (SAM), 5′-deoxyadesnosine (5′-dA), dithiothreitol (DTT), sodium dithionite, 2,3-butanedione, and DMPT were purchased from Sigma-Aldrich. DEAE sepharose FF resin was from GE Healthcare. [3-2H]Ribose was from Omicron Biochemicals Inc. Chemically competent E. coli DH5α and BL21(DE3) cells, and all PCR primers were from Invitrogen. pET expression plasmids were from Novagen. Calf-intestine alkaline phosphatase (CIAP, 20 U/μL) was from NEB. UV-vis absorption spectra were determined using a U-3900 UV-VIS ratio recording double-beam spectrometer (HITACHI) or Nanodrop 1000 (Thermo Scientific). Non-linear least square fitting of kinetic data was carried out using KaleidaGraph software (Synergy Software, Reading, PA). Anaerobic experiments were carried out in a UNIlab workstation glove box (MBaun, Stratham, NH) maintained at 10 ± 2 °C with O2 concentration < 0.1 ppm. All anaerobic solutions were degassed on a Schlenk line, and equilibrated in the glove box atmosphere for > 12 h. All plastic devices used for anaerobic use were evacuated for > 12 h in the glove box antechamber before bringing into the glove box. All DNA sequences were confirmed by Eton Bioscience Inc. PCR was carried out using PfuUltraII polymerase (Stratagene) according to the manufacturer’s protocol. All HPLC experiments were performed on a Hitachi L-2130 Pump equipped with an L-2455 diode array detector, an L-2485 fluorescence detector, an L-2200 autosampler and an ODS Hypersil C18 column (Thermo Scientific) housed in an L-2300 column oven maintained at 40 °C. Staphylococcus aureus MoaA and MoaC were expressed and purified by following published protocols9 with minor modification as described in the Supporting Methods. The expression plasmids for SUF proteins36 and MOCS1B37 were kindly provided by Dr. Herman Schindelin. The 5-methylthioribose kinase expression plasmid38 was a generous gift from Dr. John Gerlt.
Stepwise Assay of MoaA and MoaC
The stepwise MoaA/MoaC assays were carried out under anaerobic conditions. First, MoaA (5 μM) was incubated with GTP (1 mM), SAM (1 mM), and sodium dithionite (1 mM) in 200 μL of assay buffer (50 mM Tris-HCl pH 7.6, 1 mM MgCl2, 2 mM DTT and 0.3 M NaCl). The reaction was initiated by the addition of MoaA, and incubated for 60 min at 25 °C, at which point MoaA was removed by ultrafiltration (Amicon Ultra 30 K, Millipore). To an aliquot (80 μL) of the resulting small molecule fraction, 10 μL of 50 μM MoaC was added and incubated for 60 minutes at 25 °C. The reaction was stopped by an addition of 10 μL of 25% (w/v) TCA. cPMP formed during the incubation with MoaC was oxidized to compound Z and quantified by HPLC following the published protocol7. Briefly, a solution (10 μL) containing 1% (w/v) I2 and 2% (w/v) KI was added to the quenched reaction mixture, and incubated for 20 min at room temperature. After removal of precipitation by centrifugation, an aliquot (10 μL) of the supernatant was injected to HPLC. The chromatography was performed by an isocratic elution with 0.1% TFA in H2O with a flow rate of 1 mL/min, and monitored by fluorescence (Ex. 367 nm, Em. 450 nm). Under these conditions, the void volume was 1.5 min and compound Z was eluted at 2.7 min. The authentic compound Z standard was isolated from ΔMoeB E. coli strain by following the reported protocol8.
Detection of the MoaA product as DMPT
MoaA (5 μM) was incubated with GTP (1 mM), SAM (1 mM), and sodium dithionite (1 mM) in assay buffer (0.5 mL). The reaction was initiated by the addition of MoaA, and incubated at 25 °C for a specified time. An aliquot (80 μL) was removed at each time point and mixed with 10 μL of 0.5 M HCl to quench the reaction. The resulting mixture was incubated at 95 °C for 5 min to facilitate the hydrolysis, followed by an addition of 6 μL 1 M NaOH to adjust the pH to 8.5. The resulting solution was combined with 25 μL of 0.66% (v/v) 2,3-butanedione solution in 0.9 M Tris-HCl, pH 8.5, and incubated at 95 °C for 45 min. After removal of precipitation by centrifugation, an aliquot (10 μL) of the supernatant was injected to HPLC.The chromatography was performed by an isocratic elution with 92.5% 20 mM sodium acetate pH 6.0, 7.5% MeOH with a flow rate of 1 mL/min, and monitored by fluorescence (Ex. 365 nm, Em. 445 nm). Under these conditions, the void volume was 1.5 min and DMPT was eluted at 5.6 min.
3′,8-cH2GTP Stability Test
The temperature and pH stabilities were assessed by incubating 3′,8-cH2GTP (50 μM) solution in anaerobic buffer (15 mM ammonium formate pH 3.5, 50 mM Tris-HCl pH 7.6, or 15 mM ammonium bicarbonate pH 9.0) in the glovebox at 10 or 22 °C.To test for oxygen sensitivity, 3′,8-cH2GTP (50 μM) solution was taken out of the glovebox, and exposed to air by rigorous pipetting. For each reaction, an aliquot (10 μL) was removed at specified time point and incubated with MoaC in 80 μL of assay buffer (50 mM Tris-HCl, pH 7.6, 0.3 M NaCl, 1 mM MgCl2, and 2 mM DTT). The reaction was quenched with 10 μL 25% TCA, and cPMP was quantified as described above for the stepwise assay.
Isolation of 3′,8-cH2GTP
The MoaA reaction and subsequent purification of 3′,8-cH2GTP were carried out under strict anaerobic conditions (< 0.1 ppm O2). The large scale MoaA reaction for the isolation of 3′,8-cH2GTP was carried out with MoaA (200 μM), SAM (1 mM), sodium dithionite (1 mM), and GTP or [U-13C5,15N10]GTP (200 μM) in 50 mM Tris-HCl pH 7.6 (200 mL) at 22 °C for 60 minutes. These reactions typically produced 12–20 μmol of 3′,8-cH2GTP. Subsequent purification was carried out at 10 °C. The MoaA reaction mixture was first passed through an ultrafiltration membrane (Amicon YM-30, Millipore) to remove the protein, and the resulting filtrate was applied to a QAE A25 Sephadex (250 mL, bicarbonate form) column. The column was washed with 750 mL of H2O, and elution was performed by a linear gradient (600 x 600 mL) of 200 – 800 mM ammonium bicarbonate, pH 9.1. To identify the fractions containing 3′,8-cH2GTP, an aliquot (10 μL) of each fraction was mixed with 80 μL of MoaC (20 μM) in assay buffer, and incubated for 30 min at 25 °C. The resulting solutions were analyzed for cPMP as described above for the stepwise assay. The fractions containing 3′,8-cH2GTP were combined, lyophilized, re-dissolved in water and applied to a DEAE sepharose FF (15 mL, bicarbonate form) column. The column was washed with 50 mL of 100 mM ammonium bicarbonate, pH 9.1, and eluted by a linear gradient (300 x 300 mL) of 100 – 200 mM ammonium bicarbonate, pH 9.1. The fractions containing 3′,8-cH2GTP were identified by UV-vis absorption spectroscopy and the MoaC activity assay described above.After removal of the solvent by lyophilization, the purified 3′,8-cH2GTP was characterized by NMR and ESI-TOF-MS.
Phosphate quantitation
Phosphate quantitation was carried out based on the published protocol8,39. Purified 3′,8-cH2GTP (4 pmol) was incubated with CIP (5 units, each unit is defined as the amount of enzyme that hydrolyzes 1 nmol p-nitrophenylphosphate per 1 min at 37 °C) for 1 hour at 37 °C in 100 μL of 50 mM Tris-HCl pH 7.9 containing 100 mM NaCl, 10 mM MgCl2 and 1 mM DTT. The resulting solution was then mixed with 0.233 mL of 1.4 %(w/v) ascorbic acid, 0.36 % (w/v) ammonium molybdate tetrahydrate in 0.86 M H2SO4. After an hour of incubation at 37 °C, the phosphate concentration was determined based on the absorption of the phosphomolybdate complex at 820 nm (ε820 nm = 26.0 mM-1 cm-1)39.
Determination of the molecular weight of 3′,8-cH2GTP
Purified 3′,8-cH2GTP (100 μM) in anaerobic water was prepared in the glovebox. An aliquot (10 μL) of the sample was injected into the ESI-TOF MS instrument (Agilent 6224) operated in the negative ion mode. The typical mass accuracy of the instrument was 5 ppm. For the characterization of theobserved MS signal, 3′,8-cH2GTP (100 μM) was incubated with MoaC (50 μM) in 400 μL of 300 mM ammonium bicarbonate buffer pH 9.1 for 20 min at 25 °C.After removal of MoaC by ultrafiltration (Amicon YM-10, Millipore), the solution was lyophilized and the residue was dissolved in 400 μL of anaerobic water. The resulting solution was analyzed by ESI-TOF MS as described for 3′,8-cH2GTP.
NMR measurements of purified 3′,8-cH2GTP
All NMR spectra were recorded on 500, 600, or 800 MHz Varian Inova NMR spectrometers operated with VNMRJ 3.1 software, and analyzed by the ACD/NMR processor (ACD/Labs). The samples were dissolved in anaerobic deuterium oxide (Sigma-Aldrich, 99.9 atom% enriched), loaded in 3 mm NMR tubes (Wilmad LabGlass) in the glovebox, and sealed with butyl rubber septa (Sigma-Aldrich). NMR measurements were carried out for 24–48 h at a sample temperature of 6 °C. No decomposition of 3′,8-cH2GTP was observed during the NMR measurements based on a comparison of 1H NMR spectra at the beginning and the end of the measurements. Chemical shifts are reported in δ based on an internal standard, 4,4-dimethyl-4-silapentane-1-sulfonic acid (DSS, δCH3 = 0.00) as reference. Maleic acid (δCH = 6.0, Sigma-Aldrich) was used as an internal standard for signal quantitation.
MoaA/C coupled assay
Enzyme activity assays were performed at 25 °C under anaerobic conditions by incubating MoaA (0.5 μM) and MoaC (5 μM) with GTP (1 mM), SAM (1 mM) and sodium dithionite (1 mM) in assay buffer. The reaction was initiated by the addition of MoaA. At each time point, an aliquot (90 μL) was removed and mixed with 10 μL 25% (w/v) TCA to quench the reaction. cPMP was quantified as described above for the stepwise assay.
Preparation of MOCS1B expression plasmid
The MOCS1B gene in the pQE-MOCS1B plasmid originally prepared by Hänzelmann et al.37 was subcloned into pET-28b to improve protein expression. NdeI restriction site was introduced by PCR at the 5′ end of the MOCS1B gene in the pQE-MOCS1B plasmid using primers MOCS1B-f (CAT CAC CAT CAC CAT ATG ATG AGT TTC TC) and MOCS1B-r (GAG AAA CTC ATC ATA TGG TGA TGG TGA TG). After confirmation of the DNA sequence, the resulting plasmid was digested with NdeI and SalI and the MOCS1B gene fragment was subcloned into the corresponding site of pET28b to obtain pET-MOCS1B.
Expression of MOCS1B
E. coli BL21(DE3) cells were co-transformed with pET-MOCS1B and grown overnight on LB agar plates. All growths were carried out in the presence of Kanamycin (50 mg/L). A single colony was picked and grown in LB medium (5 mL) to saturation (~16 h). Two mL of this solution was diluted into 200 mL LB in a 500 mL baffled flask and incubated at 37 °C until growth reached saturation. A portion of this culture (10 mL) was then used to inoculate 1.5 L of 2YT medium in a 2.8 L baffled flask and grown at 37 °C until an OD600 reached 0.6–0.8, at which time the temperature was lowered to 15 °C and MOCS1B expression was induced with 0.3 mM IPTG. The culture was continued for additional 20 hours until saturation, and cells were harvested by centrifugation, frozen in liquid N2 and stored at -80 °C. Typically, 10 g of wet cell paste/L were obtained.
Purification of MOCS1B
The cell paste (10 g) of E. coli BL21(DE3) expressing MOCS1B was suspended in 50 ml buffer A ( 50 mM Tris-HCl, pH 9.0, 0.3 M NaCl, 10% glycerol) supplemented with 1% Triton-X and a protease inhibitor cocktail (Calbiochem # 539132). The cell suspension was homogenized, and lysed by two passages through a French pressure cell operating at 14,000 psi. After removal of cell debris by centrifugation (20,000 g, 20 min, 4 °C), DNA was precipitated by dropwise addition of 0.2 volumes ofbuffer A containing 8% (w/v) streptomycin sulfate. The mixture was stirred for an additional 15 min, and the precipitated DNA was removed by centrifugation (20,000 g, 20 min, 4 °C). Solid (NH4)2SO4 (0.23 g per mL) was then added over 15 min to 40% saturation. The solution was stirred for an additional 20 min and the precipitated protein was isolated by centrifugation (20,000 g, 20 min, 4 °C). The protein pellet was dissolved in a minimal volume of buffer A supplemented with 1% Triton-X, 0.5 mM phenylmethanesulfonylfluoride (PMSF) and 20 mM imidazole, and applied to a Ni-NTA column (10 ml). The column was then washed with 10 volumes of the same buffer, followed by another 10 volumes of buffer A containing 20 mM imidazole. MOCS1B was subsequently eluted with 500 mM imidazole in buffer A.Fractions containing MOCS1B were identified by SDS-PAGE, combined and exchanged into buffer A on a Sephadex G-25 column. The resulting MOCS1B was concentrated by ultrafiltration (Amicon Ultra 3 K, Millipore) to 0.2 mM, flash frozen in liquid nitrogen and stored at -80 °C. The concentration of MOCS1B was determined based on UV absorption at 280 nm using an extinction coefficient (ε280nm = 15.9 mM-1 cm-1) determined by the Edelhoch’s method40. To characterize the three MOCS1B polypeptides, each polypeptide was purified on SDS-PAGE and digested with L-1-tosylamido-2-phenylethyl chloromethyl ketone treated Porcin trypsin (Promega). The resulting tryptic peptides were analyzed by LC-MS at the Duke proteomics core facility.
Activity Assays of MoaC and MOCS1B
MoaC (0.1 μM) or MOCS1B (0.5 μM) was anaerobically incubated with 3′,8-cH2GTP at specified concentrations in assay buffer at 25 °C. The reaction was initiated by the addition of MoaC or MOCS1B, and an aliquot (90 μL) was removed at each time point and mixed with 10 μL of 25% (w/v) TCA to quench the reaction.cPMP was quantified as described above for the stepwise assay.
Quantitation of 5′-dA
Enzyme reactions were performed and quenched as described for the MoaA/C coupled assay except that 20 μM MoaA and 40 μM MoaC were used. After the TCA quenching, the solution was clarified by centrifugation, and an aliquot (70 μL) of the supernatant was injected to HPLC. Chromatography was performed by a linear gradient of 0–15% MeOH in 27 mM KH2PO4 pH 4.5 (25 min, 2 mL/min), and monitored by UV absorption at 256 nm.
Preparation of [3′-2H]GTP
Enzymatic synthesis of [3′-2H]guanosine
[3-2H]Ribose (1 mM, > 95% enriched) was incubated at 27 °C with ATP (2 mM), guanine (2 mM), 5-methylthioribose kinase38 (3.5 μM) and purine nucleoside phosphorylase (Sigma-Aldrich, 3.5 μM) in 200 mL of 10 mM Tris-HCl pH 7.6 containing 2.5 mM MgCl2 and 2.5 mM DTT. After 2 h of incubation at 27 °C, the solution was adjusted to pH 3 with 1 M HCl, and loaded on to a DOWEX 50W-X8 column (2 x 10 cm, ammonium form).The column was washed with 15 mM ammonium formate pH 3.0, and eluted with 300 mM ammonium formate pH 9.0.Fractions containing guanosine were identified by UV-vis absorption spectrum and TLC. Guanosine was typically eluted after 7 – 10 CV of elution. The fractions containing guanosine were combined, and the solvent was removed by lyophilization to yield 100 μmol of [3′-2H]guanosine (94 ± 3 % atom 2H).1H-NMR (500 MHz, D2O) δ 7.95 (s, 1 H), 5.86 (d, J = 7.3 Hz, 1 H), 4.62 (m, 1H), 4.18 (br, 1 H), 3.84 (dd, J = 2.9, 12.7 Hz, 1H), 3.77 (dd, J = 3.9, 12.7 Hz, 1H); HRMS (ESI-TOF) m/z [M+H]+ calcd. for C10H13DN5O5 285.106; found 285.107.
Phosphorylation of [3′-2H]guanosine
Phosphorylation of guanosine was performed by the Ludwig’s method41. Briefly, [3′-2H]guanosine (100 μmol) was dried by co-evaporation with dry pyridine, and dissolved in dry (MeO)3PO (1.5 mL). To this solution, POCl3 (130 μmol) was added, and the mixture was stirred on ice for 2 h. Then, Bis-tri-n-butylammonium pyrophosphate (500 μmol) in anhydrous DMF (2 mL) and Bu3N (100 μL) was added under rigorous stirring. After 10 min, 7 mL of 1 Mtriethylammonium bicarbonate pH 7.5 was poured into the solution and stirred for 15 min. The resulting mixture was diluted by 5-fold with water, and applied to QAE A25 Sephadex (Sigma, 4 x 20 cm, bicarbonate form). The column was washed with 0.2 M ammonium bicarbonate pH 8.0 (100 mL), and the elution was performed with a linear gradient (450 x 450 mL) of 0.2 – 1 M ammonium bicarbonate pH 8.0. GTP was eluted between 0.55 – 0.7 M ammonium bicarbonate, judged by UV-vis absorption spectrum. Lyophilization of these fractions yielded 24 μmol of [3′-2H]GTP (94 ± 3 % atom 2H).1H-NMR (500 MHz, D2O) δ 7.92 (s, 1 H), 5.71 (d, J = 5.9 Hz, 1 H), 4.56 (d, J = 5.9 Hz, 1 H), 4.15 (br, 1 H), 4.04 (m, 2 H); 31P-NMR (160 MHz, D2O) δ −8.04 (br), −11.26 (d, J = 19 Hz), −22.45 (br); HRMS (ESI-TOF) m/z [M-H]− calcd. for C10H14DN5O14P3 522.990; found 522.991.
MoaA reaction with [3′-2H]GTP and isolation of [5′-2H]5′-dA
[3′-2H]GTP (98 μM) was anaerobically incubated with MoaA (50 μM), MoaC (100 μM), SAM (1 mM), and sodium dithionite (1 mM) in assay buffer for 1 hour at 25 °C.Proteins were removed by ultrafiltration (Amicon Ultra 30 K, Millipore), and the resulting filtrate was applied to a C18 RP Silica 90 column (Sigma, 2 x 8 cm) equilibrated in water. Elution was performed using a linear gradient (300 x 300 mL) with 0–30% MeOH in water. 5′-dA was eluted at 15% MeOH judged by UV-vis absorption at 256 nm. After removal of the solvent by rotary evaporator, the sample was further purified by HPLC using conditions identical to those described above for the quantitation of 5′-dA except that 30 mM ammonium formate pH 4.5 was used as the aqueous solvent. The resulting sample was lyophilized and characterized by 1H NMR, and 2H NMR spectroscopies and ESI-TOF-MS.
Results
Investigation for an intermediate between GTP and cPMP
While small molecules produced by MoaA were previously proposed to serve as a substrate of MoaC30, the conversion of this molecule to cPMP by MoaC in the absence of MoaA has never been demonstrated. Considering the complexity of the conversion of GTP into cPMP, and enzyme-catalyzed radical reactions in general28,42, the absence of such demonstration leaves significant ambiguity about the relevancy of the observations to Moco biosynthesis. Thus, we carried out stepwise activity assays using Staphylococcus aureus MoaA and MoaC. In these assays, GTP (1 mM) was first incubated with MoaA (5 μM), SAM (1 mM) and sodium dithionite (1 mM) in the absence of MoaC. The resulting small molecules were separated from MoaA by ultrafiltration, and subsequently incubated with MoaC (5.6 μM). Any cPMP present in the resulting reaction solution was quantified by HPLC analysis after a conversion to its fluorescent derivative, compound Z (Figure 2a; 8), following the previously established protocol8. Figure 2a shows the results of HPLC analysis that shows a peak co-migrating with compound Z. Quantitation of the observed HPLC peak using an authentic standard suggested formation of 3.6 ± 0.3 μM compound Z, substoichiometric to MoaA. Control reactions lacking GTP or SAM did not yield compound Z. These observations are consistent with the presence of a small molecule intermediate that is produced by MoaA, and converted to cPMP by MoaC.
Figure 2. Activity assays of MoaA.

(a) HPLC analysis of stepwise MoaA/MoaC activity assay (Ex. 367 nm, Em. 450 nm). In the complete reaction, GTP (1 mM), SAM (1 mM) and sodium dithionite (1 mM) were incubated with MoaA (5 μM) for 60 min at 25 °C, followed by removal of MoaA by ultrafiltration and incubation with MoaC (15 μM) for 60 min at 25 °C. cPMP was converted tocompound Z (8), and detected by HPLC. Also shown are the chromatograms for the compound Z standard, and control reactions without GTP or SAM. (b) HPLC analysis of MoaA activity assay (Ex. 365 nm, Em. 445 nm). In the complete condition, MoaA (5 μM), was incubated with GTP (1 mM), SAM (1 mM) and sodium dithionite (1 mM) at 25 °C for 60 min. The reaction product was subjected to acid hydrolysis, followed by incubation with 2,3-butanedione, and analyzed for the fluorescent DMPT (9). Also shown are the chromatograms for the DMPT standard, and for control reactions lacking GTP, SAM, dithionite, or MoaA. A small amount of DMPT (~10% of the complete condition) was formed in the GTP negative control due to the co-purification of GTP with MoaA. (c) Time-course of 3′,8-cH2GTP formation determined after conversion to DMPT (filled circles) or compound Z (open circle). The conditions for the MoaA reaction and the derivatization of the product to DMPT are identical to (b). The amount of compound Z at 60 min is based on the quantitation of the HPLC peak in (a). Each point is an average of three replicates, and the error bars are calculated based on the standard deviation.
Chemical derivatization of the MoaA reaction product
For the biosynthesis of other pterins and flavins, nucleotide biosynthetic intermediates with a 6-hydroxy-2,4,5-triaminopyrimidine partial structure have been reported (see Figure S1a for the structural formula)31,43. These nucleotides have been characterized by derivatization of a hydrolytically released 6-hydroxy-2,4,5-triaminopyrimidine to the highly fluorescent DMPT (9, see Figure S1b for the chemical equation for the derivatization).Schindelin et al. briefly mentioned in their publication26 that the MoaA reaction product can be converted to DMPT, although no data were presented. We thus attempted a similar derivatization to investigate whether the MoaA product contains an acid-labile 6-hydroxy-2,4,5-triaminopyrimidine partial structure. For this purpose, enzyme activity assays were performed with MoaA (5 μM), GTP (1 mM), SAM (1 mM) and sodium dithionite (1 mM) under anaerobic conditions in the absence of MoaC. The reaction was stopped by the addition of hydrochloric acid, and the resulting solution was incubated at 95 °C to facilitate hydrolysis, followed by incubation with 2,3-butanedione at pH 8. Figure 2b shows the HPLC analysis of this reaction, which reveals the formation of DMPT. Omission of any of the reaction components resulted in significant decrease of DMPT. The time course assay (Figure 2c) revealed that increasing amounts of DMPT were formed with prolonged incubation time (Figure 2c). The concentration of DMPT observed in this assay at the 60 min time point (3.8 ± 0.2 μM) was substoichiometric to that of MoaA (5 μM), and within errors to the amount of cPMP observed in the stepwise assay (3.6 ± 0.3 μM, compare filled and open circles at 60 min time point in Figure 2c). Together with the results of the stepwise assays, these preliminary characterizations prompted us to attempt isolation of 3′,8-cH2GTP.
Isolation of 3′,8-cH2GTP
The isolation of 3′,8-cH2GTP was a significant challenge primarily due to its limited stability. As shown in Figure 3a, decomposition of 3′,8-cH2GTP was observed under aerobic conditions or under acidic pH. This limited stability required all the purifications to be carried out in an anaerobic glove box (O2 concentration < 0.1 ppm) maintained at 10 °C using ammonium bicarbonate buffer at pH 9.1. Under these conditions, no decomposition was observed at least for 3 h (Figure 3a).
Figure 3.
(a) Stability of 3′,8-cH2GTP under different conditions. Solution containing 3′,8-cH2GTP was incubated for 10 - 180 min under specified conditions. At each time point, an aliquot was removed and 3′,8-cH2GTP was quantified by HPLC after its conversion to compound Z. Each point is an average of three replicates, and the error bars are calculated based on the standard deviation.(b) UV-vis absorption spectrum of 3′,8-cH2GTP (bold trace) (60 μM). The thin traces are spectra after exposure to air at 22 °C for the specified time.
MoaA reaction conditions were chosen to maximize the production of 3′,8-cH2GTP, as well as the percentage conversion of GTP to 3′,8-cH2GTP.It was important to consume as much GTP as possible in the MoaA reaction because of the close separation between GTP and 3′,8-cH2GTP during purification with anion exchange resin. The reaction is limited by the substoichiometric turnover of MoaA. The maximum turnover observed after 60 min of incubation at 25 °C was 0.5 ± 0.2 when stoichiometric or excess amount of GTP relative to MoaA was used (Figure 2c). The use of substoichiometric amount of GTP to MoaA significantly lowered the yield. Thus, for the isolation of 3′,8-cH2GTP, GTP concentration was kept stoichiometric relative to MoaA.
Purification conditions also required careful optimization to avoid decomposition. Successful purification was achieved by the use of two anion exchange columns. A strong anion exchange (QAE sepharose) resin was used as the first step of purification to process a large volume of sample and to separate 3′,8-cH2GTP and GTP from the other components in the MoaA assay mixture. A weak anion exchange (DEAE sepharose) resin was used to separate GTP from 3′,8-cH2GTP (see Figure S3 for the chromatograms). Typically, 10 μmol of 3′,8-cH2GTP was isolated from 200 mL of enzyme reaction mixture containing 0.2 mM MoaA, 0.2 mM GTP, 1.0 mM SAM and 1.0 mM sodium dithionite. The isolated 3′,8-cH2GTP had a unique absorption feature at 322 nm (Figure 3b, bold trace). This absorption feature also exhibited oxygen sensitivity and diminished upon exposure to air (Figure 3b, thin traces), consistent with the oxygen sensitivity of 3′,8-cH2GTP observed by the biochemical characterization (Figure 3a).
The isolated 3′,8-cH2GTP was also analyzed for the number of phosphates in the molecule, as its close migration with GTP on the anion exchange resin suggested the presence of a triphosphate group. The use of a colorimetric phosphate assay39 after a phosphatase treatment revealed 2.93 ± 0.03 phosphate groups per molecule. This result is consistent with 3′,8-cH2GTP being a triphosphate compound.
Molecular weight determination of 3′,8-cH2GTP
An ESI-TOF-MS analysis of isolated 3′,8-cH2GTP revealed m/z = 521.984 ± 0.001 [M-H]− (Figure S4a), consistent with the molecular formula of C10H15N5O14P3 (calcd. m/z = 521.983 [M-H]−). The observed mass was also consistent with GTP, which migrates close to 3′,8-cH2GTP during the anion exchange chromatographies (see Figure S3), and occasionally contaminated 3′,8-cH2GpTP. Thus, it was important to verify that the observed signal was indeed associated with 3′,8-cH2GTP rather than contaminating GTP. To distinguish 3′,8-cH2GTP from GTP, purified 3′,8-cH2GTP was incubated with MoaC and analyzed by MS, which exhibited no MS signal at m/z = 521.984, and instead exhibited a signal at m/z = 362.052 ± 0.001 [M-H]− (Figure S4b) corresponding to a hydrate form of cPMP (m/z [M-H]− calcd. for C10H13N5O8P, 362.051). These findings suggest that the observed MS signal was indeed associated with the intact 3′,8-cH2GTP, but not contaminating GTP.
Structural characterization of 3′,8-cH2GTP by NMR spectroscopy
Further structural characterization of 3′,8-cH2GTP was carried out using NMR spectroscopy. Figure 4a shows the 1H NMR spectrum of purified 3′,8-cH2GTP with six signals between 4.1 – 5.3 ppm. To confirm that these signals are associated with 3′,8-cH2GTP, the observed 1H NMR signals were quantified by comparing the integrals of the observed signals with that of a known concentration of maleic acid as an internal standard. The concentration of 0.59 ± 0.14 mM was determined by this analysis, which agreed well with the concentration (0.67 ± 0.03 mM) biochemically determined after its conversion to compound Z.
Figure 4. NMR structural characterization of 3′,8-cH2GTP.
(a) 1H NMR (600 MHz, D2O) and (b) 1H-decoupled 13C NMR (200 MHz, D2O) spectra of 3′,8-cH2GTP and [U-13C10,15N5]3′,8-cH2GTP, respectively. No signal associated with 3′,8-cH2GTP was observed outside the shown chemical shift range. The intensity of the H-1′ signal in (a) was decreased due to the water suppression at 4.95 ppm. The insets in (b) shows magnified view of the signals for C-3′, C-4′ and C-8. (c) 13C-13C COSY spectrum of [U-13C10,15N5]3′,8-cH2GTP. The assignments for the observed correlations are indicated. The chemical shift region for C-1′ - C-5′, and C-8 is shown. The spectrum with full chemical shift range is available in Figure S6. (d) 13C-13C COSY (bold), and selected 1H-13C, and 1H-15N HMBC (single-headed arrows) correlations, and NOE (a double-headed arrow). A complete list of the observed HMBC correlations is available in Table 1. P designates a phosphate group.
The 13C NMR spectrum (Figure 4b) was recorded on 3′,8-cH2GTP uniformly labeled with 13C and 15N, prepared from [U-13C10,15N5]GTP. Four carbon signals were found at 111, 154, 156, and 160 ppm, suggesting the presence of four sp2 carbons. The major multiplicities of the 13C NMR signals (Figure 4b) are derived from 1J13C-13C coupling. The analysis of the multiplicities (Figure 4b and Table 1) in combination with the 13C-13C COSY correlations (Figure S6, all 2D NMR spectra are available in the supplementary information) established the connectivities among the signals at 111, 156, and 160 ppm. Together with the comparison with the chemical shifts reported for various purines andpyrimidines (Table S1)44,45, we assigned the four sp2 carbon signals to the carbons in the 6-hydroxy-2,4,5-triaminopyrimidine moiety of 3′,8-cH2GTP.
Table 1.
Summary of NMR data for 3′,8-cH2GTP
| No. | 13C δ(ppm) (m, JCC(Hz)) | 1H δ(ppm) (m, JHH(Hz)) | 1H-1H COSY | 13C-13C COSY | 1H-13C HMBC | 1H-15N HMBC | NOESY |
|---|---|---|---|---|---|---|---|
| 2 | 153.6, (s) | ||||||
| 4 | 156.1, (d, 83.1) | C-5, C-6 | |||||
| 5 | 110.8 (t, 76.0) | C-4, C-6 | |||||
| 6 | 160.0 (d, 68.0) | C-4, C-5 | H-8 | ||||
| 8 | 75.6 (d, 42.5) | 5.20 (s) | C-3′ | H-1′, H-4′ | N-9 | H-5a | |
| 1′ | 90.8 (d, 40.0) | 5.02 (s) | C-2′ | H-2′, H-8 | N-9 | ||
| 2′ | 73.0 (t, 40.0) | 4.14 (s) | C-1′, C-3′ | H-8 | |||
| 3′ | 80.0 (q, 39.9) | C-8, C-2′, C-4′ | H-1′, H-2′, H-4′ | ||||
| 4′ | 75.9 (t, 42.0) | 4.48 (dd, 3.9, 7.3) | H-5′a, H-5′b | C-3′, C-5′ | H-8, H-1′, H-2′, H-5′ | ||
| 5′a | 63.7 (d, 44.9) | 4.25 (dta, 11.3, 6.2) | H-4′, H-5′b | C-4′ | H-8 | ||
| 5′b | 4.39 (dta, 11.3, 5.3) | H-4′, H-5′a |
The doublet feature is derived from a 3J1H-1H to the geminal 1H, and the triplet feature is from a 3J1H-1H to H-4′ and a 3J31P-1H to 31P of the phosphate group.
The remaining 13C NMR signals were found between 63.7 and 90.8 ppm with observed 1J13C-13C coupling constants of ~40 Hz (Figure 4b and Table 1), suggesting that they are all sp3 carbons46. The analysis of the multiplicities in combination with the 13C-13C COSY correlations (Figure 4c) unambiguously determined the C-C connectivities as shown in Figure 4d. The most notable is the presence of C-3′ as a tertiary carbon that appeared as a quartet signal in the 13C NMR spectrum (see the inset of Figure 4b). The connectivity of C-3′ to C-8 in addition to C-2′and C-4′ was established by the 13C-13C COSY correlations (Figure 4c and 4d). This feature distinguishes this molecule from GTP, cPMP, or molecules that have been previously proposed as MoaA products; 2-amino-5-formylamino-6-ribofuranosylamino-4-pyrimidinone triphosphate (6) and pyranopterin triphosphate (7)29,30, because none of these molecules has a tertiary carbon. This analysis suggested that during the MoaA reaction, the C3′-C8 bond is formed, but the pyranopterin structure is not yet established.
Further NMR analysis provided basis for the complex multi-ring structure around C-8. 1H-13C HMQC (Figure S7), multiplicity of the 13C NMR signal (Figure 4b) and the 13C-13C COSY correlation (Figure 4c) suggested that C-8 is a methine primary carbon. The 1H-13C HMBC correlations from H-8 to C-1′, and from H-1′ to C-8 (Figure 4d) suggested a connection between C-8 and C-1′ through a heteroatom. The 1H-15N HMBC correlations to the same N from H-1′ and from H-8 (Figure 4d) suggested that the bridging heteroatom is N. The second heteroatom on C-8 would be either N or O. The reported chemical shift values for methine primary carbons covalently bound to an N and an O are 84–97 ppm31,47–49, whereas those with two Ns are 71–86 ppm48,50–53. Thus, the chemical shift observed for C-8 (75.6 ppm) is most consistent with the presence of the second N on C-8.
The connections of C-1′ and C-8 to nitrogen atoms suggested the connections to 6-hydroxy-2,4,5-triaminopyrimidine because all nitrogen atoms are associated with the 6-hydroxy-2,4,5-triaminopyrimidine based on the molecular formula determined by MS. The connection to 6-hydroxy-2,4,5-triaminopyrimidine is supported by the observation of a weak 1H-13C HMBC correlation from H-8 to C-6 (Figures 4d, and S9). While several different orientations for 6-hydroxy-2,4,5-triaminopyrimidine are consistent with the NMR data (see Figure S11 for possible structures), the nitrogen bridging C-8 and C-1′ is likely N-9, considering that the previous isotope tracer experiments indicated that the N9-C1′ bond is maintained during the transformation of GTP into cPMP7,17. The second N on C-8 was assigned as N-7, considering that C8-N7 bond is present in GTP, and there is no evidence suggesting that the other Ns are involved in the transformation of GTP to cPMP. Based on these analyses, we propose the orientation of the 6-hydroxy-2,4,5-triaminopyrimidine connection as shown in Figure 4d.
The structures of the remaining part of the molecule were based on the following analysis. The furanose ring was based on the 1H-13C HMBC correlations from H-1′ to C-4′ (Figure 4d). The position of the triphosphate group was based on the 3JHP = 5 – 6 Hz observed for the 1H NMR signals for H-5′a and H-5′b (Figure 4a, and Table 1), which are comparable to those reported for various nucleotides54,55. The hydroxyl groups at the 2′ and 3′ positions were based on the NMR chemical shifts observed for H-2′, C-2′, and C-3′. The stereochemistry at C-1′, C-2′, C-3′, and C-4′ is based on GTP and cPMP56. The absence of an apparent J coupling between H-1′ and H-2′ (3JH1′H2′ < 4 Hz) is consistent, based on the Karplus relations57, with the calculated dihedral angle of 59 ° between H1′-C1′-C2′-H2′ (see Figure S12 for the energy minimized conformation of 3′,8-cH2GTP). The stereochemistry at C-8 is based on NOE observed between H-8 and H-5′a (Figure 4c; a double headed arrow).
Based on all the analysis described above, we propose the structure of the isolated molecule as (8S)-3′,8-cyclo-7,8-dihydroguanosine 5′-triphosphate (3′,8-cH2GTP, Figure 4c). The proposed structure is consistent with all the NMR data (Table 1), as well as MS, phosphate assay and chemical derivatization to DMPT described above.
The relevance of 3′,8-cH2GTP to Moco biosynthesis
The physiological relevance of 3′,8-cH2GTP as an intermediate of Moco biosynthesis was further investigated by steady state kinetics of the MoaA and MoaC catalyzed reactions. The kinetic parameters for the MoaC catalyzed conversion of 3′,8-cH2GTP to cPMP were determined using purified 3′,8-cH2GTP as the substrate. The kinetic parameters for the MoaA catalyzed reaction were determined by a coupled assay in the presence of a 10-fold excess of MoaC to ensure efficient conversion of 3′,8-cH2GTP to cPMP. In both assays, cPMP was converted to compound Z and quantified by HPLC. The results are summarized in Table 2. The kcat of MoaC was ~4-fold greater than that of MoaA determined by this method, and the Km of MoaC for 3′,8-cH2GTP was < 0.06 μM, the lower limit of detection. Km of MoaA for GTP was found to be 1.4 ± 0.2 μM, which may be compared to the previously reported binding constant of 0.29 ± 0.11 μM determined by an equilibrium dialysis26. These observations support the conclusion that 3′,8-cH2GTP is likely the physiological substrate of MoaC, and the biosynthetic intermediate between GTP and cPMP during Moco biosynthesis.
Table 2.
Steady state kinetic parameters for MoaA, MoaC, and MOCS1B.
| Enzyme | Substrate | Km (μM) | kcat (min−1) | kcat/Km (μM−1 min−1) |
|---|---|---|---|---|
| MoaA | GTP | 1.4 ± 0.2 | 0.045 ± 0.003 | 0.032 ± 0.012 |
| SAM | 4.1 ± 1.3 | 0.043 ± 0.004 | 0.011 ± 0.003 | |
| MoaC | 3′,8-cH2GTP | < 0.060 | 0.17 ± 0.026 | > 2.8 |
| MOCS1Ba | 3′,8-cH2GTP | 0.79 ± 0.24 | 0.092 ± 0.020 | 0.12 ± 0.08 |
Purified MOCS1B was a complex of a full length peptide (26 kDa) with two N-terminally truncated peptides (18 and 16 kDa). See Figure S14 for details.
The relevance of 3′,8-cH2GTP as an intermediate in human Moco biosynthesis was investigated by the characterization of a human homolog of MoaC.Previous gene complementation studies suggested that the human proteins, MOCS1A and MOCS1B, have functions that correspond to those of MoaA and MoaC, respectively37. In human cells, MOCS1B is expressed as an N-terminal fusion with a catalytically inactive MOCS1A20,37. Since we have not been successful in obtaining the fusion protein, the stand-alone MOCS1B domain was expressed as an N-terminal His-tag protein, and purified by Ni-affinity chromatography. SDS-PAGE and MS analysis of the purified protein revealed that MOCS1B (26 kDa) was co-purified with two other shorter MOCS1B peptides (16 and 18 kDa) truncated at the N-terminus (see Figure S14 for details of characterization). The ratio of the truncated MOCS1B to the full length MOCS1B was not altered by the use of protease inhibitor cocktails or further purification by anion exchange, size exclusion and phenyl sepharose column chromatography. Because the truncation occurred only in the poorly conserved N-terminal region of MOCS1B (see Figure S14c for a multiplesequence alignment), we used this MOCS1B for further characterization. The HPLC analysis of the product of the MOCS1B activity assay with 3′,8-cH2GTP as the substrate revealed that cPMP was formed with the kinetic parameters of Km = 0.79 ± 0.24 μM, and kcat = 0.092 ± 0.020 min−1, which are somewhat less efficient than, but comparable to Km and kcat determined for MoaC (Table 2). While these kinetic parameters could be affected by the observed truncation at the N-terminus and/or the lack of the MOCS1A domain, the results show that 3′,8-cH2GTP is a substrate for MOCS1B, and thus suggest that 3′,8-cH2GTP may be a biosynthetic intermediate in human Moco biosynthesis as well.
Stoichiometry of the MoaA reaction and the position of H-atom abstraction
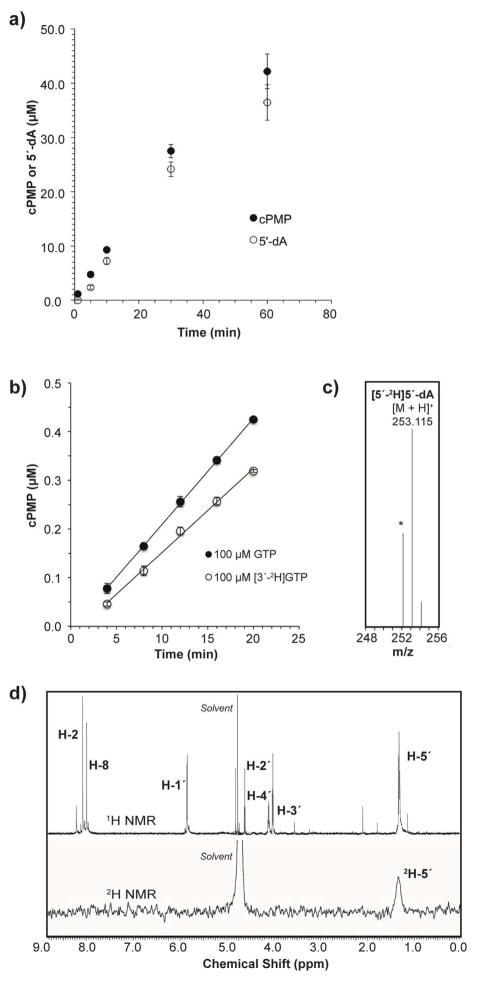
Since MoaA is a member of the radical SAM superfamily, the conversion of GTP to 3′,8-cH2GTP is likely initiated by a radical formation at C-3′ by 5′-dA• formed by reductive cleavage of SAM, followed by the attack of C-3′ to C-8. In the reactions catalyzed by radical SAM enzymes, SAM may be consumed as a co-substrate, or regenerated and used as a co-catalyst23. Since the conversion of GTP into 3′,8-cH2GTP could take place either of the two mechanisms, we quantified the amount of 5′-dA and cPMP formed in the MoaA/MoaC coupled assay. A 2-fold excess of MoaC was used relative to MoaA to ensure complete conversion of 3′,8-cH2GTP to cPMP. HPLC quantitation of 5′-dA suggested that, under this condition, stoichiometric amounts of 5′-dA and 3′,8-cH2GTP were formed (Figure 5a).
Figure 5. Mechanistic studies on the MoaA catalyzed H-atom abstraction.<.
br>(a) Stoichiometry of formation of 5′-dA (open circles) and cPMP (filled circles) in the MoaA/MoaC coupled assay. MoaA (20 μM) was incubated with GTP (1 mM), SAM (1 mM) and sodium dithionite (1 mM) in the presence of MoaC (40 μM) at 25 °C. cPMP was quantified by HPLC after its conversion to compound Z. Each point is an average of 3–6 replicates, and the error bars are calculated based on the standard deviation. (b) Rate of cPMP formation from GTP or [3′-2H]GTP by MoaA and MoaC. MoaA (0.5 μM) was incubated with GTP or [3′-2H]GTP (0.1 mM) in the presence of sodium dithionite (1 mM), SAM (1mM) and MoaC (5 μM) at 25°C. cPMP was quantified by HPLC after its conversion to compound Z. Each point is an average of four replicates, and the error bars are calculated based on the standard deviation. (c) ESI-TOF-MS and (d) 1H NMR (600 MHz in D2O) and 2H NMR (76.75 MHz in H2O) spectra of 5′-dA isolated from the reaction in the presence of MoaA (50 μM), MoaC (100 μM), SAM (1 mM), [3′-2H]GTP (98 μM, 94 ± 3 % atom 2H) and sodium dithionite (1 mM). The signal indicated with (*) is non-deuterated 5′-dA (m/z [M+H]+ calcd. for C10H14N5O3 252.110; found 252.111) presumably derived from non-labeled GTP (see main text).
The determination of the stoichiometry of SAM cleavage for radical SAM enzymes has been frequently obscured by abortive production of 5′-dA58. While our data is consistent with stoichiometric formation of 5′-dA, it is important to show that the observed 5′-dA is indeed the product of H-atom abstraction from GTP, but not a product of abortive SAM cleavage. Thus, we investigated the transfer of a deuterium atom on GTP into 5′-dA. Based on the structure of 3′,8-cH2GTP, the reaction most likely proceeds through H-abstraction from the 3′ position. Thus, [3′-2H]GTP was chemoenzymatically prepared from [3-2H]ribose, and used to investigate a deuterium transfer from [3′-2H]GTP to 5′-dA. When [3′-2H]GTP was used as a substrate, a small but significant kinetic isotope effect of VH/VD = 1.28 ± 0.05 was observed (Figure 5b), which is comparable to a value previously reported for another radical SAM enzyme, BtrN (VH/VD = 1.3 ± 0.1)59.
To investigate the H-3′ transfer from GTP to 5′-dA, 5′-dA was isolated from a large scale MoaA/MoaC coupled reaction mixture, where [3′-2H]GTP (98 μM) was incubated with MoaA (50 μM), MoaC (100 μM), SAM (1 mM), and sodium dithionite (1 mM) at 25 °C for 60 min. HPLC analysis of this assay solution revealed formation of 48 ± 2 μM of 5′-dA and 47 ± 2 μM of cPMP. 5′-dA was isolated using reverse phase silica-gel column chromatography and HPLC, and characterized by MS (Figure 5c), and 1H and 2H NMR spectroscopies (Figure 5d). These analysis revealed incorporation of a single deuterium atom in the 5′-methyl group of 5′-dA, consistent with a recent observation by Begley’s group30. Under the conditions we investigated, ~30% of 5′-dA was formed with no deuterium incorporation (Figure 5c). We attribute this at least partially to the incomplete labeling of [3′-2H]GTP (94 ± 3% atom 2H), and the presence of non-labeled GTP copurified with MoaA. The amount of co-purified GTP was determined as 16 ± 1% that of MoaA by HPLC after its release from MoaA by acid denaturing. Together with the ~50% conversion of [3′-2H]GTP to cPMP, these observations suggest that majority of the non-labeled 5′-dA formed in this reaction was originated from the non-labeled GTP in the sample. Thus, our observation is consistent with a mechanism, in which MoaA catalyzes H-abstraction from the 3′ position of GTP by stoichiometric consumption of SAM.
Discussion
This report describes the isolation and structural characterization of 3′,8-cH2GTP, a missing intermediate in Moco biosynthesis. Our stepwise assay indicated that there is a small molecule that is produced by MoaA in the presence of GTP, SAM and dithionite, and that may serve as a substrate of MoaC. This compound was isolated from the in vitro MoaA assay mixture using two sequential anion exchange column chromatographies under strict anaerobic conditions, and structurally characterized by chemical derivatization, MS and NMR spectroscopy. The physiological relevance of the isolated 3′,8-cH2GTP was investigated by the steady state kinetic analysis of MoaC or its human homolog, MOCS1B. In these analyses, both enzymes catalyze the conversion of 3′,8-cH2GTP to cPMP with high specificities (Km values of < 0.060 and 0.79 μM, respectively). Although the observed turnover rate for MoaA (kcat = 0.053 min−1), and MoaC (kcat = 0.17 min−1) were slow, they are typical for enzymes in cofactor biosynthesis60,61, including other steps of Moco biosynthesis62, which may be attributed to the small amount of cofactors required in the cells. Thus, our data presented here suggest that 3′,8-cH2GTP may represent the physiologically relevant intermediate of Moco biosynthesis.
The MoaA reaction product has been described previously. Schindelin and Hänzelmann26 first stated in their publication that in the reaction of MoaA with SAM and GTP without MoaC they observed the formation of small molecule that may be converted to DMPT. Unfortunately, no data were presented. Our analysis in this report provides strong evidence that MoaA product indeed has an acid labile 6-hydroxy-2,4,5-triaminopyrimidine moiety that may be converted to DMPT. More recently, Begley et al. reported the observation of a molecule with a light absorption feature at 320 nm in the LCMS analysis of the reaction mixture of MoaA incubated with GTP, SAM and dithionite. The observation of a mass signal at m/z = 524 [M+H]+ at the same retention time led the authors to propose this molecule as pyranopterin triphosphate (7, Figure 1c). While this conclusion is distinct from ours, the reported light absorption feature and the mass signal are also consistent with 3′,8-cH2GTP. In addition, Begley et al. mentioned that the observed molecule was oxygen sensitive, which is also acharacteristic of 3′,8-cH2GTP. On the other hand, our NMR data are inconsistent with pyranopterin triphosphate. Thus, we conclude that the previously described putative MoaA products26,30 were indeed 3′,8-cH2GTP.
It is noteworthy that Begley et al. also considered 3′,8-cH2GTP as an intermediate of cPMP biosynthesis, but suggested to exist only transiently during the MoaA catalyzed conversion of GTP to pyranopterin triphosphate30. This proposal appears to be based mostly on their observation of the deuterium transfer from the 3′ position of GTP to 5′-dA, and their proposal for the structure of MoaA as pyranopterin triphosphate, which we now believe to be a mis-assignment. While their deuterium tracer experiments support the formation of a radical at C-3′, prior to our work, to our knowledge, there was no precedent for the reaction between a C-3′ centered radical and C-8 in purine nucleosides or nucleotides. In addition, the radical formation at C-3′ could also be consistent with the previous proposals with 6 as an intermediate (Figure 1b)29,33 if the H-3′ abstraction was to take place after the formation of 6. There are precedents for formyl group transfer reactions by radical mechanisms63. Our isolation and characterization of 3′,8-cH2GTP as a MoaA product, provide a strong evidence for the function of MoaA to catalyze the conversion of GTP to 3′,8-cH2GTP. As 3′,8-cH2GTP can be chemically derivatized to DMPT, our model is also consistent with the observations by Schindelin and Hänzelmann26.
Our identification of 3′,8-cH2GTP as the product of MoaA as well as the substrate of MoaC is a sharp contrast to the previous consensus in the field, where MoaA was considered largely responsible for the formation of the pyranopterin ring, and a relatively minor role was considered for MoaC29,30. Our observations suggest that MoaC plays a major role in the formation of pyranopterin ring. The reactions catalyzed by MoaA and MoaC may be compared to the conversion of GTP into 7,8-dihydroneopterin triphosphate by GTP cyclohydrolase I in folate biosynthesis (see Figure S1a). The first step of the GTP cyclohydrolases I reaction is the hydrolysis of the imidazole moiety of the guanine base using a water activated on Zn2+ in the active site31,64. This hydrolysis triggers the ensuing complex Amadori rearrangement18,65–67 that takes place in the same active site68,69. Our results suggest that Moco biosynthesis is also initiated by modification of GTP at the C-8 position, but to a distinct molecule, 3′,8-cH2GTP. In contrast to GTP cyclohydrolases I, MoaA is incapable of catalyzing the rearrangement reactions, presumably due to the lack of appropriate amino acid residues in the active-site. Instead, 3′,8-cH2GTP is transferred to MoaC where a complex rearrangement reaction proceeds. In the following paragraphs, we will discuss the mechanisms of the reactions catalyzed by each of these two enzymes.
The observation of 3′,8-cH2GTP formed from GTP by a radical SAM enzyme, MoaA, indicated to us that the reaction would proceed through a H-atom abstraction from the 3′ position. This is consistent with the deuterium tracer experiments by Begley et al.30. In reactions catalyzed by radical SAM enzymes, SAM may be used catalytically or stoichiometrically. However, the demonstration of the stoichiometry for radical SAM catalyzed reactions is frequently complicated by abortive cleavage of SAM, where SAM is reductively cleaved to methionine and 5′-dA regardless of product formation. The abortive SAM cleavage has been reported for many radical SAM enzymes to various degrees58,70–74. A notable example is the recent report on QueE by Bandarian et al.70. QueE catalyzes a complex rearrangement of 7-carboxy-7-deazaguanine to 6-carboxy-5,6,7,8-tetrahydropterin during deazapurine biosynthesis. Although SAM is used catalytically in this reaction, detectable amount of 5′-dA was formed and contained deuterium atoms that originated from substrate. Thus, although previous studies indicated a transfer of a deuterium atom at the 3′ position of GTP to 5′-dA30, the lack of knowledge about the stoichiometry of the reaction left significant ambiguity about how SAM is used in the MoaA reaction. Our observation of the stoichiometric formation of 5′-dA and cPMP in the MoaA/MoaC coupled assay, in combination with the demonstration of the stoichiometric transfer of a deuterium atom at the 3′ position of GTP to the methyl group of 5′-dA, is consistent with the stoichiometric consumption of SAM and H-atom abstraction from the 3′ position.
Based on the observations described above and our structural characterization of 3′,8-cH2GTP, in combination with chemical precedents and the reported structures of MoaA, we propose a mechanism for the MoaA catalyzed reaction (Figure 6). The deuterium tracer experiments of ours and Begley et al.30 suggested that the reaction is initiated by the abstraction of H-3′ by 5′-dA•, generated by the reductive cleavage of SAM using the N-terminal 4Fe-4S cluster as a reductant. The resulting C-3′ radical (10) then attacks C-8 and generates an 3′,8-cycloGTP aminyl radical intermediate 11, which is then converted to 3′,8-cH2GTP. While the aminyl radical 11 could be reduced by a re-abstraction of H-atom from 5′-dA, our observation of the stoichiometric formation of 5′-dA in the MoaA/MoaC coupled assay relative to cPMP disfavors such mechanism. Thus, another electron source is required. One possible reductant is the C-terminal [4Fe-4S] cluster that binds GTP26,27. A reduction of 5′,8-cyclo-deoxyadenosine aminyl radical by reduced methylviologen (E° = -0.45 V)75 has been reported. Thus, considering the reported redox potentials of related [4Fe-4S] clusters (E° = -400 ~ -600 V)76,77, the C-terminal [4Fe-4S] cluster may carry out the reduction of the aminyl radical. The redox role of the C-terminal [4Fe-4S] cluster may be probed by EPR spectroscopy36, and are currently being investigated in our lab.
Figure 6.

Proposed mechanism for the MoaA catalyzed conversion of GTP to 3′,8-cH2GTP.
The reduction of the aminyl radical 11 may also be facilitated by the three conserved Arg residues, R17, R266 and R268 (numbering based on S. aureus MoaA), which are 3.2 – 4.6 Å from N-7 of GTP in the crystal structure26. Mutations in these residues have been found in Moco-deficient patients78. MoaA variants with mutations in these residues still bind GTP to a various degree, but do not produce cPMP when assayed with MoaC26. These Arg residues may be assisting the aminyl radical reduction by electrostatic stabilization of the anionic charge on the base, or by providing a H+. Controlled movements of protons and electrons are often critical for redox reactions in enzyme catalysis79.
Another important feature of the active-site of MoaA is the presence of conserved hydrophobic amino acid residues, V167, I194 and I253, surrounding 2′-OH and 3′-OH of GTP (Figure 7)26. No amino acid residues are in H-bonding interaction distance from 2′-OH and 3′-OH, which is inconsistent with the earlier proposal where MoaA catalyzes complex rearrangement reactions that require general acid/base catalysis at 3′-OH30. On the other hand, the structural feature of MoaA active site is consistent with our proposal for the function of MoaA to catalyze the conversion of GTP to 3′,8-cH2GTP, in which no general acid/base catalysis is required.
Figure 7. A model of the MoaA active site.

SAM was modeled into the X-ray crystal structure of MoaA in complex with GTP26 (PDB ID: 2FB3) based on the structural alignment with the X-ray crystal structure of MoaA in complex with SAM9 (PDB ID: 1TV8). Oxygens are shown in red, nitrogens in blue, sulfurs in yellow, phosphorous in orange, and irons in brown.
The hydrophobic environment around 2′-OH and 3′-OH of GTP may also be important for directing the reaction of C-3′ centered radical with C-8 by preventing side reactions. To our knowledge, the reaction of C-3′ radical with C-8 of purine nucleoside/nucleotide is unprecedented28,42,80. Radical formation at C-3′ in DNA and RNA results in a formation of 3′-keto-2′-deoxyribonucleotides or a C3′-C4′ bond cleavage by complex mechanisms80. Reactions of the C-3′ nucleotide radical in enzymes have been extensively studied for ribonucleotide reductase (RNR)81,82, where the radical formation on C-3′ facilitates dissociation of 2′-OH. Mechanistic studies on RNR and related synthetic model systems suggest that the dissociation of 2′-OH requires general acid/base catalysts at 2′-OH and 3′-OH82. The difference in the active-site structures of MoaA and RNR that both generates a radical at C-3′, but produce distinct products, may indicate the mechanism by which enzymes control radical reactions. The roles of the MoaA active site amino acid residues are currently under investigation.
The following complex rearrangement reactions are catalyzed by MoaC. While MoaC was previously known to be essential for the biosynthesis of cPMP7,9,20,21, the lack of understanding about the structure of the substrate of MoaC precluded identification of the exact role of this enzyme during the conversion of GTP into cPMP. Thus, our finding that 3′,8-cH2GTP may be the physiological substrate provides a means to study the mechanism of the complex rearrangement reaction catalyzed by MoaC.
The structures of MoaC32–35,83 or previous in vitro characterization of MoaC in the presence of MoaA9 did not reveal any complex cofactor or metals in MoaC. Thus, the MoaC reaction appears to take place via general acid/base catalysis using amino acid side chain of MoaC. Our characterization of 3′,8-cH2GTP suggested susceptibility of this molecule to acid hydrolysis. Thus, the transformation of GTP into 3′,8-cH2GTP by MoaA may be sufficient to make this molecule susceptible to complex rearrangement by MoaC presumably using general acid/base catalysts. While several different mechanisms may be possible, Figure 8 shows a possible mechanism for the conversion of 3′,8-cH2GTP to cPMP by MoaC most consistent with the currently available chemical and enzymological precedents. The reaction is likely initiated by hydrolysis of the constrained pyrrolidine and imidazoline rings of 3′,8-cH2GTP to an aldehyde 12, based on the susceptibility of 3′,8-cH2GTP to acid hydrolysis during derivatization to DMPT (Figure 2b). Acid hydrolysis of various aminal molecules has been well documented84. The aldehyde 12 is then converted to a hexose 13. The mechanism of this reaction could be either a retroaldol-aldol rearrangement initiated by the deprotonation at 2′-OH, or an α-ketol rearrangement initiated by the deprotonation at 3′-OH. A similar rearrangement reaction has been reported for 1-deoxy-D-xylulose-5-phosphate reductoisomerase85. The hexose 13 is then converted to cPMP by formation of the pterin ring and the cyclic phosphate. Considering that MoaC binds nucleoside triphosphate stronger than di- and monophosphate33, the cyclic phosphate formation may be the last or close to the last step of the catalysis. Studies to further narrow the possible mechanisms are currently underway.
Figure 8. Possible mechanism of the MoaC catalyzed conversion of 3′,8-cH2GTP to cPMP.

P designates a phosphate group.
Conclusion
This work describes the isolation and characterization of 3′,8-cH2GTP, an intermediate in Moco biosynthesis, which had previously eluded structural characterization. Our finding defines the reactions catalyzed by MoaA and MoaC, and provides insights into their catalytic mechanisms. In contrast to the previous notion in the field, our results suggests a complex mechanism for the MoaC catalyzed reaction.
Supplementary Material
Acknowledgments
This work was supported by start-up funds from the Duke University Medical Center (to K. Y.), and in part by National Institutes of Health (NIH) Grant P30-CA014236 (to A.A.R.). We acknowledge the Duke University NMR Spectroscopy Center shared resource for spectrometer time.Instrumentation in the Center was purchased with support from NIH, NSF, HHMI and the North Carolina Biotechnology Center. We would like to thank George R. Dubay (Duke University, Department of Chemistry) for assistance on the MS measurements. We acknowledge the Duke proteomics core facility for the MS analysis of MOCS1B. We acknowledge J. A. Gerlt (University of Illinois at Urbana-Champaign) for providing an expression plasmid for 5-methylthioribose kinase, and Petra Hänzelmann and Hermann Schindelin (University of Würzburg) for providing expression plasmids for the MOCS1B and SUF proteins. We thank Dr. JoAnne Stubbe (MIT, Departments of Chemistry and Biology) for critical reading of the manuscript.
Footnotes
SUPPORTING INFORMATION. Detailed protocol for expression and purification of Staphylococcus aureus MoaA and MoaC; 6-hydroxy-2,4,5-triaminopyrimidine nucleotides in the pterin and flavin biosynthesis; SDS-PAGE and UV-vis spectra of MoaA, and MoaC; Time course of the 3′,8-cH2GTP stability assay; Chromatograms of 3′,8-cH2GTP purification; MS characterization of 3′,8-cH2GTP; Energy minimized conformation of the nucleoside moiety of 3′,8-cH2GTP; 2D NMR spectra of 3′,8-cH2GTP; Steady state kinetic analysis of MoaA, MoaC, and MOCS1B; MS characterization of MOCS1B; MoaA assay with [3′-2H]GTP; Possible mechanism of conversion of 3′,8-cH2GTP to cPMP by MoaC; 13C NMR Summary for purine and pyrimidine bases; NMR data for 3′,8-cH2GTP. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Hille R, Nishino T, Bittner F. Coord Chem Rev. 2011;255:1179–1205. doi: 10.1016/j.ccr.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hille R. Chem Rev. 1996;96:2757–2816. doi: 10.1021/cr950061t. [DOI] [PubMed] [Google Scholar]
- 3.Johnson JL, Waud WR, Rajagopalan KV, Duran M, Beemer FA, Wadman SK. Proc Natl Acad Sci U S A. 1980;77:3715–3719. doi: 10.1073/pnas.77.6.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen HJ, Fridovich I, Rajagopalan KV. J Biol Chem. 1971;246:374–382. [PubMed] [Google Scholar]
- 5.Mendel RR, Schwarz G. Coord Chem Rev. 2011;255:1145–1158. [Google Scholar]
- 6.Leimkuhler S, Wuebbens MM, Rajagopalan KV. Coord Chem Rev. 2011;255:1129–1144. doi: 10.1016/j.ccr.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wuebbens MM, Rajagopalan KV. J Biol Chem. 1995;270:1082–1087. doi: 10.1074/jbc.270.3.1082. [DOI] [PubMed] [Google Scholar]
- 8.Wuebbens MM, Rajagopalan KV. J Biol Chem. 1993;268:13493–13498. [PubMed] [Google Scholar]
- 9.Hänzelmann P, Schindelin H. Proc Natl Acad Sci U S A. 2004;101:12870–12875. doi: 10.1073/pnas.0404624101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pitterle DM, Rajagopalan KV. J Bacteriol. 1989;171:3373–3378. doi: 10.1128/jb.171.6.3373-3378.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pitterle DM, Johnson JL, Rajagopalan KV. J Biol Chem. 1993;268:13506–13509. [PubMed] [Google Scholar]
- 12.Gutzke G, Fischer B, Mendel RR, Schwarz G. J Biol Chem. 2001;276:36268–36274. doi: 10.1074/jbc.M105321200. [DOI] [PubMed] [Google Scholar]
- 13.Nichols JD, Rajagopalan KV. J Biol Chem. 2005;280:7817–7822. doi: 10.1074/jbc.M413783200. [DOI] [PubMed] [Google Scholar]
- 14.Nichols J, Rajagopalan KV. J Biol Chem. 2002;277:24995–25000. doi: 10.1074/jbc.M203238200. [DOI] [PubMed] [Google Scholar]
- 15.Kuper J, Llamas A, Hecht HJ, Mendel RR, Schwarz G. Nature. 2004;430:803–806. doi: 10.1038/nature02681. [DOI] [PubMed] [Google Scholar]
- 16.Llamas A, Mendel RR, Schwarz G. J Biol Chem. 2004;279:55241–55246. doi: 10.1074/jbc.M409862200. [DOI] [PubMed] [Google Scholar]
- 17.Rieder C, Eisenreich W, O’Brien J, Richter G, Gotze E, Boyle P, Blanchard S, Bacher A, Simon H. Eur J Biochem. 1998;255:24–36. doi: 10.1046/j.1432-1327.1998.2550024.x. [DOI] [PubMed] [Google Scholar]
- 18.Burg AW, Brown GM. J Biol Chem. 1968;243:2349–2358. [PubMed] [Google Scholar]
- 19.Fischer M, Bacher A. Chembiochem. 2011;12:670–680. doi: 10.1002/cbic.201000681. [DOI] [PubMed] [Google Scholar]
- 20.Reiss J, Cohen N, Dorche C, Mandel H, Mendel RR, Stallmeyer B, Zabot MT, Dierks T. Nat Genet. 1998;20:51–53. doi: 10.1038/1706. [DOI] [PubMed] [Google Scholar]
- 21.Reiss J, Christensen E, Kurlemann G, Zabot MT, Dorche C. Hum Genet. 1998;103:639–644. doi: 10.1007/s004390050884. [DOI] [PubMed] [Google Scholar]
- 22.Sofia HJ, Chen G, Hetzler BG, Reyes-Spindola JF, Miller NE. Nucleic Acids Res. 2001;29:1097–1106. doi: 10.1093/nar/29.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frey PA, Hegeman AD, Ruzicka FJ. Crit Rev Biochem Mol Biol. 2008;43:63–88. doi: 10.1080/10409230701829169. [DOI] [PubMed] [Google Scholar]
- 24.Walsby CJ, Hong W, Broderick WE, Cheek J, Ortillo D, Broderick JB, Hoffman BM. J Am Chem Soc. 2002;124:3143–3151. doi: 10.1021/ja012034s. [DOI] [PubMed] [Google Scholar]
- 25.Vey JL, Drennan CL. Chem Rev. 2011;111:2487–2506. doi: 10.1021/cr9002616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hänzelmann P, Schindelin H. Proc Natl Acad Sci U S A. 2006;103:6829–6834. doi: 10.1073/pnas.0510711103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lees NS, Hänzelmann P, Hernandez HL, Subramanian S, Schindelin H, Johnson MK, Hoffman BM. J Am Chem Soc. 2009;131:9184–9185. doi: 10.1021/ja903978u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Q, Liu W. J Biol Chem. 2011;286:30245–30252. doi: 10.1074/jbc.R111.272690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iobbi-Nivol C, Leimkuhler S. Biochim Biophys Acta. 2012 doi: 10.1016/j.bbabio.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Mehta AP, Hanes JW, Abdelwahed SH, Hilmey DG, Hänzelmann P, Begley TP. Biochemistry. 2013;52:1134–1136. doi: 10.1021/bi3016026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bracher A, Fischer M, Eisenreich W, Ritz H, Schramek N, Boyle P, Gentili P, Huber R, Nar H, Auerbach G, Bacher A. J Biol Chem. 1999;274:16727–16735. doi: 10.1074/jbc.274.24.16727. [DOI] [PubMed] [Google Scholar]
- 32.Srivastava VK, Srivastava S, Arora A, Pratap JV. PLoS One. 2013;8:e58333. doi: 10.1371/journal.pone.0058333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanaujia SP, Jeyakanthan J, Nakagawa N, Balasubramaniam S, Shinkai A, Kuramitsu S, Yokoyama S, Sekar K. Acta Crystallogr D Biol Crystallogr. 2010;66:821–833. doi: 10.1107/S0907444910019074. [DOI] [PubMed] [Google Scholar]
- 34.Wuebbens MM, Liu MT, Rajagopalan K, Schindelin H. Structure. 2000;8:709–718. doi: 10.1016/s0969-2126(00)00157-x. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida H, Yamada M, Kuramitsu S, Kamitori S. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2008;64:589–592. doi: 10.1107/S174430910801590X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hänzelmann P, Hernandez HL, Menzel C, Garcia-Serres R, Huynh BH, Johnson MK, Mendel RR, Schindelin H. J Biol Chem. 2004;279:34721–34732. doi: 10.1074/jbc.M313398200. [DOI] [PubMed] [Google Scholar]
- 37.Hänzelmann P, Schwarz G, Mendel RR. J Biol Chem. 2002;277:18303–18312. doi: 10.1074/jbc.M200947200. [DOI] [PubMed] [Google Scholar]
- 38.Imker HJ, Fedorov AA, Fedorov EV, Almo SC, Gerlt JA. Biochemistry. 2007;46:4077–4089. doi: 10.1021/bi7000483. [DOI] [PubMed] [Google Scholar]
- 39.Ames BN. Methods Enzymol. 1966;8:115–118. [Google Scholar]
- 40.Edelhoch H. Biochemistry. 1967;6:1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- 41.Ludwig J. Acta Biochim Biophys. 1981;16:131–133. [PubMed] [Google Scholar]
- 42.Stubbe J, van der Donk WA. Chem Rev. 1998;98:705–762. doi: 10.1021/cr9400875. [DOI] [PubMed] [Google Scholar]
- 43.Bacher A, Lingens F. J Biol Chem. 1971;246:7018–7022. [PubMed] [Google Scholar]
- 44.Cho BP. Magn Reson Chem. 1993;31:1048–1053. [Google Scholar]
- 45.Spectral Database for Organic Compounds. National Institute of Advanced Industrial Science and Technology; [accessed on Jan 16, 2013]. http://riodb01.ibase.aist.go.jp/sdbs/ [Google Scholar]
- 46.Weigert FJ, Roberts JD. J Am Chem Soc. 1972;94:6021–6025. doi: 10.1021/ja00772a014. [DOI] [PubMed] [Google Scholar]
- 47.Hochlowski JE, Andres WW, Theriault RJ, Jackson M, McAlpine JB. J Antibiot (Tokyo) 1987;40:145–148. doi: 10.7164/antibiotics.40.145. [DOI] [PubMed] [Google Scholar]
- 48.Tsunakawa M, Kamei H, Konishi M, Miyaki T, Oki T, Kawaguchi H. J Antibiot. 1988;41:1366–1373. doi: 10.7164/antibiotics.41.1366. [DOI] [PubMed] [Google Scholar]
- 49.Nyerges M, Rudas M, Bitter I, Toke L, Szantay C. Tetrahedron. 1997;53:3269–3280. [Google Scholar]
- 50.Gao X, Chooi YH, Ames BD, Wang P, Walsh CT, Tang Y. J Am Chem Soc. 2011;133:2729–2741. doi: 10.1021/ja1101085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buttachon S, Chandrapatya A, Manoch L, Silva A, Gales L, Bruyere C, Kiss R, Kijjoa A. Tetrahedron. 2012;68:3253–3262. [Google Scholar]
- 52.Dachriyanus, Sargent MV, Wahyuni FS. Aust J Chem. 2000;53:159–160. [Google Scholar]
- 53.Numata A, Takahashi C, Ito Y, Takada T, Kawai K, Usami Y, Matsumura E, Imachi M, Ito T, Hasegawa T. Tetrahedron Letters. 1993;34:2355–2358. [Google Scholar]
- 54.Lee CH, Ezra FS, Kondo NS, Sarma RH, Danyluk SS. Biochemistry. 1976;15:3627–3638. doi: 10.1021/bi00661a034. [DOI] [PubMed] [Google Scholar]
- 55.Tsuboi M, Takahash S, Kyogoku Y, Hayatsu H, Ukita T, Kainosho M. Science. 1969;166:1504. doi: 10.1126/science.166.3912.1504. [DOI] [PubMed] [Google Scholar]
- 56.Daniels JN, Wuebbens MM, Rajagopalan KV, Schindelin H. Biochemistry. 2008;47:615–626. doi: 10.1021/bi701734g. [DOI] [PubMed] [Google Scholar]
- 57.Karplus M. J Chem Phys. 1959;30:11–15. [Google Scholar]
- 58.Chatterjee A, Li Y, Zhang Y, Grove TL, Lee M, Krebs C, Booker SJ, Begley TP, Ealick SE. Nat Chem Biol. 2008;4:758–765. doi: 10.1038/nchembio.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yokoyama K, Numakura M, Kudo F, Ohmori D, Eguchi T. J Am Chem Soc. 2007;129:15147–15155. doi: 10.1021/ja072481t. [DOI] [PubMed] [Google Scholar]
- 60.Challand MR, Martins FT, Roach PL. J Biol Chem. 2010;285:5240–5248. doi: 10.1074/jbc.M109.056606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bracher A, Schramek N, Bacher A. Biochemistry. 2001;40:7896–7902. doi: 10.1021/bi010322v. [DOI] [PubMed] [Google Scholar]
- 62.Wuebbens MM, Rajagopalan KV. J Biol Chem. 2003;278:14523–14532. doi: 10.1074/jbc.M300453200. [DOI] [PubMed] [Google Scholar]
- 63.Jung ME, Choe SWT. Tetrahedron Letters. 1993;30:6247–6250. [Google Scholar]
- 64.Auerbach G, Herrmann A, Bracher A, Bader G, Gutlich M, Fischer M, Neukamm M, Garrido-Franco M, Richardson J, Nar H, Huber R, Bacher A. Proc Natl Acad Sci U S A. 2000;97:13567–13572. doi: 10.1073/pnas.240463497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bracher A, Eisenreich W, Schramek N, Ritz H, Gotze E, Herrmann A, Gutlich M, Bacher A. J Biol Chem. 1998;273:28132–28141. doi: 10.1074/jbc.273.43.28132. [DOI] [PubMed] [Google Scholar]
- 66.Shiota T, Palumbo MP, Tsai L. J Biol Chem. 1967;242:1961–1969. [PubMed] [Google Scholar]
- 67.Weygand F, Wacker H, Dahms G, Schliep HJ, Waldschmidt M, Simon H. Angew Chem Int Edit. 1961;73:402. [Google Scholar]
- 68.Nar H, Huber R, Meining W, Schmid C, Weinkauf S, Bacher A. Structure. 1995;3:459–466. doi: 10.1016/s0969-2126(01)00179-4. [DOI] [PubMed] [Google Scholar]
- 69.Nar H, Huber R, Auerbach G, Fischer M, Hosl C, Ritz H, Bracher A, Meining W, Eberhardt S, Bacher A. Proc Natl Acad Sci U S A. 1995;92:12120–12125. doi: 10.1073/pnas.92.26.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McCarty RM, Krebs C, Bandarian V. Biochemistry. 2013;52:188–198. doi: 10.1021/bi301156w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grove TL, Lee KH, St Clair J, Krebs C, Booker SJ. Biochemistry. 2008;47:7523–7538. doi: 10.1021/bi8004297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Benjdia A, Leprince J, Sandstrom C, Vaudry H, Berteau O. J Am Chem Soc. 2009;131:8348–8349. doi: 10.1021/ja901571p. [DOI] [PubMed] [Google Scholar]
- 73.Padovani D, Thomas F, Trautwein AX, Mulliez E, Fontecave M. Biochemistry. 2001;40:6713–6719. doi: 10.1021/bi002936q. [DOI] [PubMed] [Google Scholar]
- 74.Cicchillo RM, Iwig DF, Jones AD, Nesbitt NM, Baleanu-Gogonea C, Souder MG, Tu L, Booker SJ. Biochemistry. 2004;43:6378–6386. doi: 10.1021/bi049528x. [DOI] [PubMed] [Google Scholar]
- 75.Chatgilialoglu C, Guerra M, Mulazzani QG. J Am Chem Soc. 2003;125:3839–3848. doi: 10.1021/ja029374d. [DOI] [PubMed] [Google Scholar]
- 76.Wang SC, Frey PA. Biochemistry. 2007;46:12889–12895. doi: 10.1021/bi701745h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hinckley GT, Frey PA. Biochemistry. 2006;45:3219–3225. doi: 10.1021/bi0519497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reiss J, Johnson JL. Hum Mutat. 2003;21:569–576. doi: 10.1002/humu.10223. [DOI] [PubMed] [Google Scholar]
- 79.Reece SY, Hodgkiss JM, Stubbe J, Nocera DG. Philos Trans R Soc Lond B Biol Sci. 2006;361:1351–1364. doi: 10.1098/rstb.2006.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dedon PC. Chem Res Toxicol. 2008;21:206–219. doi: 10.1021/tx700283c. [DOI] [PubMed] [Google Scholar]
- 81.Stubbe J, Ackles D. J Biol Chem. 1980;255:8027–8030. [PubMed] [Google Scholar]
- 82.Cotruvo JA, Stubbe J. Annu Rev Biochem. 2011;80:733–767. doi: 10.1146/annurev-biochem-061408-095817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Srivastava S, Srivastava VK, Arora A, Pratap JV. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2012;68:687–691. doi: 10.1107/S174430911201665X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alexakis A, Mangeney P, Lensen N, Tranchier JP, Gosmini R, Raussou S. Pure Appl Chem. 1996;68:531–534. [Google Scholar]
- 85.Munos JW, Pu X, Mansoorabadi SO, Kim HJ, Liu HW. J Am Chem Soc. 2009;131:2048–2049. doi: 10.1021/ja807987h. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.