Abstract
Rationale
Memantine is a N-methyl-d-aspartic acid receptor (NMDAR) channel blocker that binds to dizocilpine sites and appears well tolerated during chronic use. Published studies suggest NMDAR antagonists prevent development of tolerance to effects of morphine by blocking NMDAR hyperactivation.
Objectives
We sought to compare effects of memantine to those of the more frequently studied dizocilpine and to evaluate memantine as a potential adjunct to modify tolerance to mu-opioid receptor agonists.
Methods
Sprague–Dawley rats were trained to discriminate morphine (3.2 mg/kg) and saline under fixed ratio 15 schedules of food delivery. Potency and maximal stimulus or rate-altering effects of cumulative doses of morphine were examined 30 min after pretreatment with dizocilpine (0.032–0.1 mg/kg) or memantine (5–10 mg/kg) and after chronic treatment with combinations of dizocilpine or memantine and morphine, 10 mg/kg twice daily, for 6 to 14 days. Effects of dizocilpine or memantine on morphine antinociception were examined in a 55 °C water tail-withdrawal assay with drug treatments parallel to those in discrimination studies.
Results
Acutely, memantine attenuated while dizocilpine potentiated the stimulus and antinociceptive effects of morphine. Neither chronic dizocilpine nor memantine blocked tolerance to the stimulus effects of morphine. In contrast, combined-treatment with dizocilpine (0.1 mg/kg) blocked tolerance to antinociceptive effects of lower (0.1∼3.2 mg/kg) but not higher doses of morphine, whereas memantine did not block tolerance.
Conclusions
Memantine and dizocilpine interacted differently with morphine, possibly due to different NMDAR binding profiles. The lack of memantine-induced changes in morphine tolerance suggests memantine may not be a useful adjunct in chronic pain management.
Keywords: Memantine, Dizocilpine, Morphine, Antinociception, Drug discrimination, Tolerance
Introduction
Competitive, noncompetitive and glycine site N-methyl-d-aspartic acid receptor (NMDAR) antagonists block development of tolerance or reverse an established tolerance to antinociceptive effects of mu-opioid receptor (MOR) agonists (for reviews, see Elliott et al. 1995; Herman et al. 1995; Trujillo 1995), at doses that have no effect on pain responsiveness. Such blockade of tolerance is assumed to be mediated through blockade of NMDAR hyperactivation rather than through changes in antinociceptive effects of MOR agonists themselves. Indeed, acute administration of MOR agonists can cause sustained increases in NMDAR-mediated current (Chen and Huang 1991; Martin et al. 1997; Narita et al. 2008), and such potentiation is enhanced after repeated MOR agonist treatment (Kow et al. 2002). MORs and NMDARs are co-localized in several brain regions, including frontal cortex, nucleus accumbens, hippocampus, amygdala, thalamus, periaqueductal gray (PAG), and spinal cord (Commons et al. 1999; Gracy et al. 1997; Mao et al. 1995; Milner and Drake 2001; Narita et al. 2008; Radley et al. 2007; Xie et al. 1992). In these regions, at least three MOR-NMDAR interactions exist: (1) presynaptic modulation of glutamate release (Guo et al. 2005; Hao et al. 2005; Terman et al. 2001); (2) postsynaptic interaction on the same neuron via protein kinase C (Ghelardini et al. 2007; Narita et al. 2008); or (3) disinhibition of postsynaptic NMDAR-containing neurons by reduced presynaptic release of gamma-aminobutyric acid (GABA) from GABAergic terminals (Milner and Drake 2001). Each interaction could lead to hyperactivation of NMDARs and subsequent neural plasticity (Trujillo 2000), yielding the intriguing possibility that NMDAR antagonists may alter development of tolerance and function as adjuvant drugs in chronic pain treatment (Chevlen 2000; Katz 2000; Lossignol et al. 2005), but see (Dudgeon et al. 2007; Galer et al. 2005). However, this possibility is complicated by side-effects of high-affinity NMDAR antagonists, which include ataxia, cognitive impairment and psychotomimetic actions (Rogawski and Wenk 2003).
Memantine is an uncompetitive NMDAR antagonist binding to a site close to the magnesium binding site in the channel (Chen and Lipton 1997; Chen et al. 1992). It has been used clinically for more than 20 years (Parsons et al. 1999) and in 2000 was approved in the USA for symptom management in moderate and severe Alzheimer's disease. Unlike uncompetitive NMDAR antagonists such as dizocilpine, memantine is well tolerated, possibly due to its strong voltage dependency, fast unblocking, and partial trapping kinetics (Danysz and Parsons 2003; Rogawski and Wenk 2003). Because potencies of phencyclidine and dizocilpine to inhibit morphine tolerance correspond to their relative affinities at NMDARs (Trujillo and Akil 1994), memantine, which is also thought to act at NMDARs, might block tolerance to morphine. Therefore, we examined the acute and chronic effects of memantine on tolerance to morphine-induced antinociception and compared these effects with those of the most commonly studied uncompetitive NMDAR antagonist, dizocilpine, in a warm-water tail withdrawal assay.
Chronic treatment with MOR agonists can be accompanied by tolerance to some of their effects (e.g., antinociception) and sensitization to others (e.g., increased velocity of locomotion). Moreover, tolerance to different effects does not necessarily develop in parallel (see Brady and Holtzman 1981; Colpaert et al. 1976; Sannerud and Young 1987). Therefore, NMDAR antagonists might alter tolerance to different effects of MOR agonists differently. To determine whether NMDAR antagonist-induced changes in tolerance to morphine-induced antinociception would predict changes in tolerance to stimulus and rate-altering effects of morphine, we also compared the acute and chronic effects of memantine with those of dizocilpine in rats trained to discriminate 3.2 mg/kg morphine from saline in a two-lever drug discrimination procedure. The timing of acute doses and chronic treatment conditions were identical in both studies, using a treatment regimen previously shown to yield tolerance to both discriminative and antinociceptive effects of morphine (Walker and Young 2001; Young 1991).
Methods and Materials
Subjects
Male Sprague–Dawley rats (N = 90 for antinociception experiments, 45 for stimulus control experiments) were housed individually in suspended stainless steel wire-mesh cages under a 12 h light/dark cycle with food (LabDiet®, PMI Nutrition International, LCC, Brentwood, MO) and water provided ad libitum. All rats were allowed to grow to 330–380 g and then fed 16–18 g of LabDiet® rodent chow daily to maintain the weights. All animal procedures were carried out in compliance with the guidelines of the Texas Tech University Health Sciences Center Institutional Animal Care and Use Committee and the 1996 Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, National Academy of Sciences; http://www.nap.edu/readingroom/books/labrats/).
Antinociceptive effects of morphine
Apparatus
A week before the initiation of studies, rats were trained to lie in rodent restraint tubes (Harvard Apparatus) in an environmentally controlled room. Water with appropriate temperature was made by mixing hot water (80 °C) from a water bath (Model 182, Precision Scientific Inc.) and room temperature tap water in an insulated mug, with temperatures measured by thermocouple (Model BAT-12, Sensortek Inc.). The latency of removing tails from the water bath was measured by digital stopwatch (Fisher Scientific Inc.).
Tail-withdrawal procedure
Antinociceptive effects of morphine were tested in a tail-withdrawal procedure. Briefly, latencies with which rats removed tails from 55 °C water were measured 15 min after each cumulative dose of morphine until the tail remained in the water longer than 15 s (a latency recorded as 15 s), the solubility limit of morphine was reached, or another behavior (i.e., convulsions) interfered with measurement. Other details were as described in (Walker and Young 2001) except that latency in 40 °C water was measured only three times prior to the first presentation of 55 °C water (85% of rats did not remove tails within 15 s on two presentations and therefore qualified for tests), and testing periods lasted 5 min. Morphine tests were conducted no more often than every 7 days. Each rat received at least three morphine tests and one test of repeated saline injections before exposure to any NMDAR antagonist.
Antinociceptive effects of dizocilpine or memantine, alone and in combination with morphine
To test if an NMDAR antagonist itself induced antinociception, separate groups of rats were tested with cumulative doses of dizocilpine or memantine, with doses chosen to increase the total dose by 0.25 or 0.5 log10. To assess effects of NMDAR antagonist pretreatment, different groups of rats received antagonist 30 min before tests (Grass et al. 1996; Kozela et al. 2001), followed by cumulative doses of morphine.
Effects of dizocilpine and memantine on development and expression of tolerance to antinociceptive effects of morphine
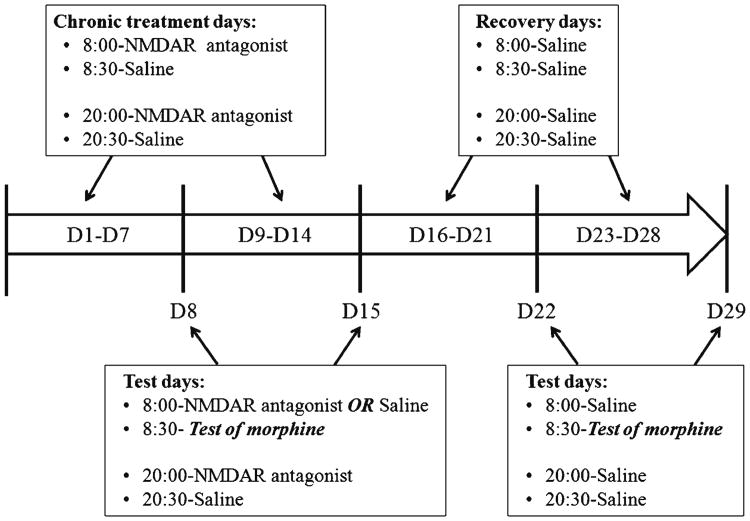
Rats were tested with cumulative doses of morphine, ranked by sensitivity, and assigned to different treatment groups in rank order so that all groups contained the full range of initial sensitivities to morphine. Different groups of rats received different chronic drug treatments, as shown in Table 1, and tests of cumulative doses of morphine were given 12 h after treatment. To assess effects of NMDAR antagonists on expression of tolerance, rats received either saline or an appropriate dose of NMDAR antagonist 30 min before tests. A schematic of the treatment and testing schedule is shown in Fig. 1.
Table 1. Chronic drug treatment regimens.
| Condition | 1 | 2 | 3 |
|---|---|---|---|
| Treatment weeks | |||
| a.m. | NMDAR antgt./SAL | SAL/MS | NMDAR antgt./MS |
| p.m. | NMDAR antgt./SAL | SAL/MS | NMDAR antgt./MS |
| Recovery weeks | |||
| a.m. | SAL/SAL | SAL/SAL | SAL/SAL |
| p.m. | SAL/SAL | SAL/SAL | SAL/SAL |
Treatments were given twice daily at a 10- to 14 h-interval for 1 or 2 weeks. Each treatment comprised two injections, 30 min apart. All groups received saline (SAL) under the same injection regimen for another 2 weeks to assess recovery
NMDAR antgt. NMDAR antagonist (0.032 or 0.1 mg/kg of dizocilpine; 7.5 or 10 mg/kg of memantine), MS 10 mg/kg of morphine
Figure 1.
A schematic example of chronic treatment (Table 1, Condition 1) and testing schedule on a time scale. The white arrow is the time scale. Text in boxes above the white arrow identifies each injection during chronic treatment or recovery. Text in boxes below the white arrow identifies pretreatment on test day, time of test and evening injections. D1 (or other numbers) Day 1 (or other days)
Discriminative stimulus effects of morphine
Apparatus
Experiments were conducted in operant conditioning chambers (Med Associates Inc. St. Albans, VT) housed in ventilated, sound-attenuating cubicles. On one wall of each chamber, two retractable response levers were mounted 6.0 cm above the floor. A stimulus lamp was mounted above each lever, and a recessed food receptacle was located between the levers. A houselight and two inactive nose-poke holes were located on the opposite wall. Food pellets (45 mg, TestDiet™, Richmond, IN) were delivered by a pellet dispenser located outside the chamber.
Drug discrimination procedure
Saline and 3.2 mg/kg morphine were established as discriminative stimulus under a fixed ratio 15 schedule of food delivery using training and testing procedures as described by Walker etal. (1994), except that training sessions consisted of one 10-min timeout and a 15-min ratio component. Tests of morphine alone were conducted until rats displayed consistent morphine sensitivity, as assessed by less than 0.25 log10 change in the morphine dose necessary for stimulus control. Then the following experiments were conducted.
Stimulus effects of dizocilpine or memantine, alone and in combination with morphine
To determine whether NMDAR antagonists mimicked the stimulus effects of morphine, rats were tested with cumulative doses of dizocilpine or memantine. To assess effects of NMDAR antagonists pretreatment, rats received NMDAR antagonist 30 min before a test of morphine.
Effects of dizocilpine or memantine on development and expression of tolerance to stimulus effects of morphine
To assess effects of NMDAR antagonists on development and expression of tolerance, training was suspended and the same rats received chronic drug treatment (Table 1) followed by tests of morphine, accompanied by saline or NMDAR antagonist pretreatment (Fig 1). To minimize the number of rats used, rats received one to four cycles of chronic treatment at 2-month intervals. Treatment order was counterbalanced as much as possible.
Drugs
Morphine sulfate (National Institution on Drug Abuse, Rockville, MD), dizocilpine maleate, and memantine hydrochloride (Sigma-Aldrich) were dissolved in physiological saline so that each subcutaneous injection was administered in a volume of 0.1 ml/100 g body weight into the dorsal flank.
Data analysis
Latencies for tail withdrawal were converted into percent possible maximal effect (MPE) by the formula:
Control latencies were measured at the first 55 °C presentation in each test. Dose–response curves were fitted to data from all rats using the semilogarithmic form of the logistic dose–response equation:
where E is %MPE, Emin and Emax are the minimum and maximum of the sigmoid dose–response curve, A is dose (in milligrams per kilogram), ED50 is the dose causing 50% maximal effect, and h is the Hill slope.
For drug discrimination experiments, the percentage of responses on the morphine-paired lever was fit to sigmoidal dose–response curves using the above equation, in which Emin and Emax were set at zero and 100. Only data from trials in which more than two reinforcers were obtained were included in these curves. Response rates (responses/s) from all trials were used to fit dose–response curves using the following nonlinear regression model (Emin was set at zero):
Data analyses were conducted with GraphPad Prism (version 5 for Windows, GraphPad Software, San Diego, CA). Differences among curves (within-group, before and after treatment) were analyzed by simultaneously fitting curves to the individual dose–response data using above regression models; slopes and logED50 values were compared by F test, with P<0.05. The preferred values were used as control values to calculate tolerance ratios (ED50 (after)/ED50 (before) and 95% confidence intervals for each group, using the built-in EC50 shift model in GraphPad Prism. Differences between groups were indicated by confidence intervals that did not overlap.
Results
Antinociceptive effects of morphine
Antinociceptive effects of dizocilpine or memantine, alone and in combination with morphine
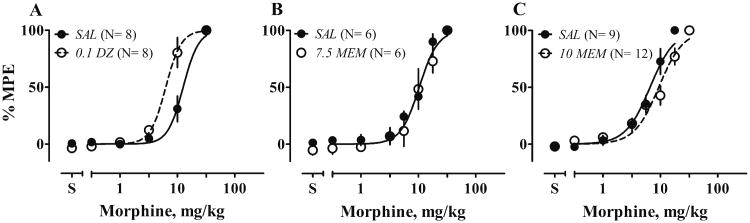
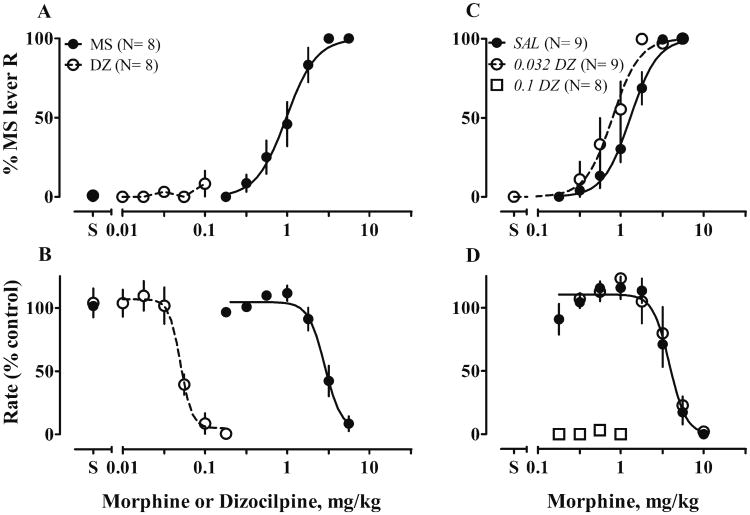
All rats received multiple tests of morphine and of repeated saline injections before any interaction tests. The sensitivity to morphine decreased 2-fold across the first four tests and remained stable thereafter. Repeated saline injections did not induce antinociception (data not shown). Acute pretreatment with 0.1 mg/kg dizocilpine decreased the morphine ED50 about 2-fold (F1, 72 = 42.8, P < 0.0001, Fig. 2a). In contrast, acute pretreatment with 10 mg/kg memantine decreased the Hill slope from 2.7 to 1.7 (F1, 140 = 4.3, P = 0.04) and, when curves were fit with a shared slope of 2.0, increased the ED50 by 1.4-fold (F1, 140 = 8.0, P = 0.005, Fig.2c). Lower doses of memantine did not change sensitivity to morphine (7.5 mg/kg, Fig. 2b; 5 mg/kg, not shown). Dizocilpine or memantine alone, at doses 2 to 3-fold higher than those tested in combination with morphine, did not produce antinociception (data not shown).
Figure 2.
Acute effects of dizocilpine (DZ) or memantine (MEM) on antinociceptive effects of morphine (MS) in a 55 °C water tail-withdrawal assay. Rats received either saline (filled circle) or an appropriate dose of NMDAR (milligrams per kilogram, open circles) 30 min before tests of cumulative doses of MS. Control tests (SAL as pretreatment) were conducted before NMDAR antagonist tests. Each rat served as its own control. Numbers in parentheses are the numbers of rats tested. Ordinates: latency expressed as percent maximal possible effect. Abscissa: cumulative doses of drugs, in milligrams per kilogram. Points and bars: means and SEM. Text in italics identifies the −30-min pretreatment: SAL saline, 0.1 DZ 0.1 mg/kg dizocilpine, 7.5 or 10 MEM 7.5 or 10 mg/kg memantine
Effects of dizocilpine or memantine on development and expression of tolerance to antinociceptive effects of morphine
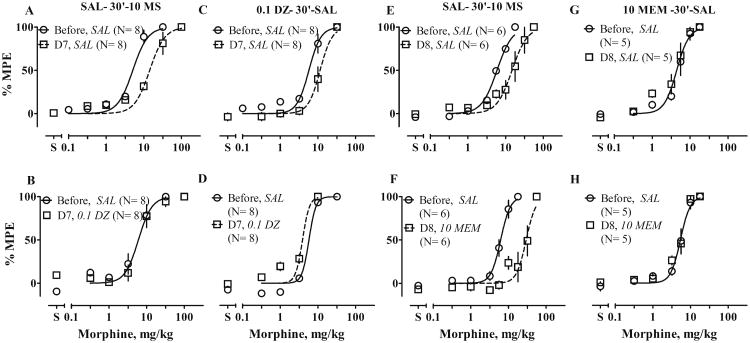
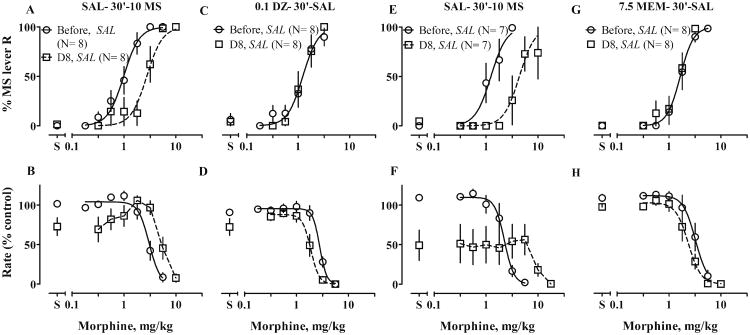
Twice daily (b.i.d.) treatment with morphine for 1 week produced tolerance to morphine-induced antinociception, such that the morphine ED50 increased 2.8-fold in dizocilpine experiments and 2.5-fold in memantine experiments (Fig. 3a, e; Table 2). Two weeks after the end of morphine treatment, ED50 values recovered by 30% (data not shown). To our surprise, chronic treatment with dizocilpine and saline also produced 2-fold tolerance to morphine (Fig. 3c; Table 2), which disappeared 1 week after treatment ended (data not shown). Chronic treatment with memantine (10 mg/kg) did not change sensitivity to morphine (Fig. 3g; Table 2).
Figure 3.
Effects of chronic treatment with 10 mg/kg of morphine (10 MS) or with NMDAR antagonists on antinociceptive effects of MS. Rats received chronic treatments, identified in column titles, b.i.d., for 6 to 7 days and were tested with cumulative doses of MS in a 55 °C water tail-withdrawal assay both before treatment (circles) and 12 h after (squares) the last injection on Day 6 or 7 of treatment; i.e., on Day 7 (D7) or Day 8 (D8). On test days, rats received saline or an appropriate dose of NMDAR antagonist 30 min before cumulative doses of MS. Other details as in Fig. 2. Text in italics identifies the −30-min pretreatment: SAL saline, 0.1 DZ 0.1 mg/kg dizocilpine, 10 MEM 10 mg/kg memantine
Table 2. Effects of dizocilpine (DZ) or memantine (MEM) on tolerance to antinociceptive effects of morphine (MS).
| Treatment | Pretreatment 30 min before test | ED50 (before) | ED50 (after) | F(dfn,dfd) | ED50 (D8)/ED50 (before) | 95% CI | Slope |
|---|---|---|---|---|---|---|---|
| SAL + MS | SAL | 5.1 ± 1.1 | 14.3 ± 1.1b, * | 58.6(1,101) | 2.8 ± 0.37 | 2.2 – 3.5 | 2.2 ± 0.25a |
| 0.1 mg/kg DZ | 6.0 ± 1.1 | 6.0 ± 1.1 | 0.65 (1,85) | 1.1 ± 0.13c | 0.81 – 1.3 | 2.4 ± 0.39 | |
| 0.1 mg/kg DZ + SAL | SAL | 5.7 ± 1.1 | 11.4 ± 1.1b, * | 25.6 (1,85) | 2.0 ± 0.20 | 1.6 – 2.4 | 2.8 ± 0.45 |
| 0.1 mg/kg DZ | 5.8 ± 1.1 | 3.9 ± 1.1b, * | 11.1 (1,69) | 0.67 ± 0.036c | 0.60 – 0.75 | 4.7 ± 0.10 | |
| 0.1 mg/kg DZ + MS | SAL | 2.2 ± 1.1 | 3.0 ± 1.3 | N/A | |||
| 0.1 mg/kg DZ | 5.4 ± 1.2 | 1.9 ± 1.2b, * | 21.8 (1,77) | 0.35 ± 0.066c | 0.21 – 0.48 | 1.8 ± 0.29a | |
| SAL + MS | SAL | 5.9 ± 1.1 | 14.8 ± 1.1b, * | 26.8(1,75) | 2.5 ± 0.29 | 1.9 – 3.1 | 2.1 ± 0.35 |
| 10 mg/kg MEM | 6.4 ± 1.1 | 29.8 ± 1.1b, * | 105.8 (1,81) | 4.7 ± 0.43c | 3.8 – 5.5 | 3.6 ± 0.66 | |
| 10 mg/kg MEM + SAL | SAL | 4.4 ± 1.1 | 4.4 ± 1.1 | 1.8 (1,51) | 0.92 ± 0.091c | 0.73 – 1.1 | 2.6 ± 0.47 |
| 10 mg/kg MEM | 5.4 ± 1.1 | 5.4 ± 1.1b | 0.41 (1,56) | 0.97 ± 0.058 * | 0.85 – 1.1 | 3.3 ± 0.46 | |
| 10 mg/kg MEM + MS | SAL | 6.6 ± 1.1 | 12.4 ± 1.1b, * | 17.5 (1,80) | 1.9 ± 0.21 | 1.5 – 2.3 | 2.2 ± 0.34 |
| 10 mg/kg MEM | 4.8 ± 1.1 | 12.3 ± 1.1b, * | 53.0 (1,87) | 2.6 ± 0.22 | 2.1 – 3.0 | 3.0 ± 0.45 | |
| 7.5 mg/kg MEM + SAL | 10 mg/kg MEM | 8.8 ± 1.1 | 8.8 ± 1.1 | 2.2 (1,95) | 0.92 ± 0.083c | 0.75 – 1.1 | 2.8 ± 0.38 |
| 7.5 mg/kg MEM + MS | SAL | 11.4 ± 1.1 | 28.0 ± 1.1b, * | 45.7 (1,121) | 2.5 ± 0.20 | 2.1 – 2.9 | 2.1 ± 0.25 |
| 10 mg/kg MEM | 10.0 ± 1.1 | 37.0 ± 1.1b, * | 160.0 (1,118) | 3.7 ± 0.25 | 3.2 – 4.2 | 3.1 ± 0.38 |
Values are mean ± SEM of ED50 values for MS (in milligrams per kilogram), obtained in tests of cumulative dose of MS before and after chronic treatment, tolerance ratios (ED50 after/ED50 before and their 95% confidence interval (CI)
N/A the dose–effect functions determined before and after treatment crossed and therefore were not fit with a shared slope. ED50 values and tolerance ratios were not compared
P < 0.01
Group “SAL + MS” pretreated with SAL and group “0.1 mg/kg DZ + MS” pretreated with either SAL or 0.1 mg/kg DZ do not share the same slope. To compare ED50 values, curves in two of these groups were fit with shared slopes
ED50 after values differ from ED50 before values in the same group
Ratios differ from those of “SAL+MS” groups pretreated with saline (SAL; entries set in italics)
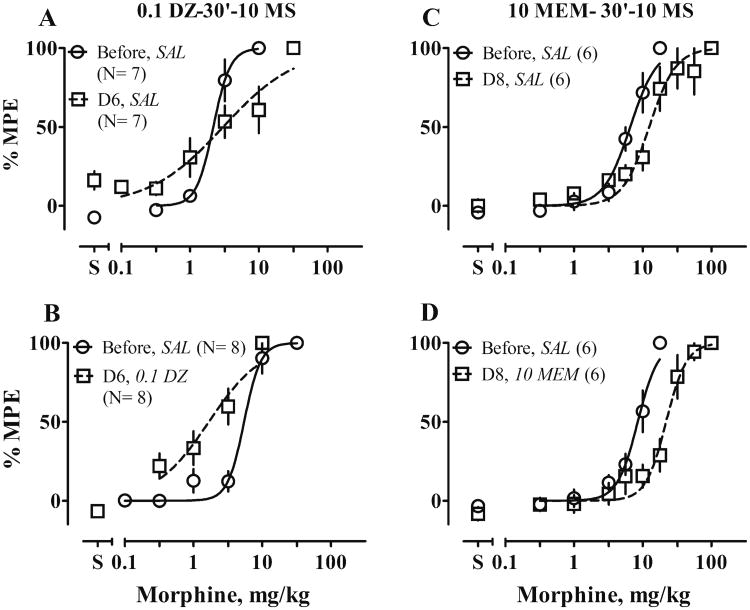
Combined treatment with dizocilpine and morphine changed the slope of the morphine dose–effect function (F1, 66 = 19.7, P < 0.0001; Fig. 4a). As a result, dizocilpine seemed to block tolerance to lower (0.1∼3.2 mg/kg) but not higher doses of morphine. Combined treatment with memantine and morphine did not change tolerance to morphine (Fig. 4c; Table 2).
Figure 4.
Effects of combined treatment with 10 mg/kg of morphine (10 MS) and a NMDAR antagonist on development and expression of tolerance to antinociceptive effects of MS. Rats received chronic treatment, shown in column titles, b.i.d. for 6 to 7 days, and were tested with cumulative doses of MS in a 55 °C water tail-withdrawal assay before treatment and 12 h after the last injection on Day 6 or 7 of treatment; i.e., on Day 7 (D7) or Day 8 (D8). On test days, rats received saline (SAL) or an appropriate dose of NMDAR antagonist (in milligrams per kilogram) 30 min before tests. Left, dizocilpine (DZ)-MS interaction; right, memantine (MEM)-MS interaction. Other details as in Fig. 2. Text in italics identifies the −30-min pretreatment: SAL saline, 0.1 DZ 0.1 mg/kg dizocilpine, 10 MEM 10 mg/kg memantine
To assess the impact of acute pretreatment with dizocilpine or memantine on expression of tolerance, a single dose of either was given before tests of morphine. Tolerance ratios after pretreatment with dizocilpine were smaller than those after pretreatment with saline (Fig. 3a vs. b and c vs. d; Table 2). Thus, 0.1 mg/kg dizocilpine potentiated effects of morphine with or without chronic morphine treatment. Acute pretreatment with dizocilpine blocked expression of tolerance (Fig. 3b; Table 2), whereas dizocilpine given both chronically with morphine and as an acute pretreatment changed the slope of the morphine dose–effect function; analysis with a shared slope (1.8) suggested that acute dizocilpine blocked expression of tolerance (Fig. 4b; Table 2). Pretreatment of 10 mg/kg memantine slightly increased tolerance ratios, consistent with its acute effect in the absence of chronic morphine (Figs. 3f and 4d; Table 2). Notably, however, acute memantine did not increase the ED50 of morphine in rats treated chronically with memantine and saline (Fig. 3h, Table 2), suggesting that rats developed tolerance to memantine itself. To assess this possibility, a lower dose of memantine (7.5 mg/kg) was given chronically, and rats were challenged with 10 mg/kg of memantine before the test of morphine. In support of the suggestion that 10 mg/kg memantine induced tolerance to memantine itself, acute memantine did not increase the ED50 of morphine in rats treated chronically with the lower dose of 7.5 mg/kg and saline. This lower dose of memantine also did not block development of morphine tolerance (Table 2).
Discriminative stimulus effects of morphine
Stimulus effects of dizocilpine or memantine, alone and in combination with morphine
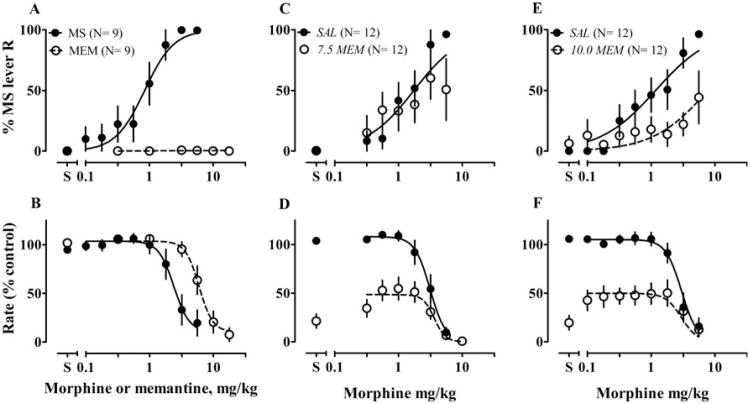
All rats acquired stimulus control; in tests, morphine produced dose-dependent increases in responses on the morphine-appropriate lever and decreases in response rates. Cumulative doses of dizocilpine or memantine evoked responses on only the saline-paired lever and decreased response rates at higher doses (Figs. 5a, b and 6a, b). Pretreatment with 0.032 mg/kg dizocilpine potentiated stimulus effects of morphine (ED50 decreased about 0.6-fold, F1, 123 = 12.3, P = 0.0006; Fig. 5c); a higher dose of dizocilpine abolished responses (Fig. 5d). Acute 10 mg/kg memantine attenuated stimulus effects of morphine (ED50 increased about 6-fold, F1, 129 = 23.9, P < 0.0001, Fig. 6e). Lower doses of memantine (5 mg/kg, data not shown; 7.5 mg/kg, Fig 6c) did not change sensitivity to morphine. At low doses of morphine (0.1–1.8 mg/kg), adding memantine (7.5 or 10 mg/kg) suppressed response rates to 50 % of control rates (Fig. 6d, f).
Figure 5.
Effects on discriminative control of acute dizocilpine, alone and on discriminative stimulus effects of morphine. Left stimulus effects of morphine (MS) or dizocilpine (DZ) alone in an operant discrimination procedure. Rats were trained to discriminate 3.2 mg/kg MS and saline and then tested with cumulative doses of MS (0.1–5.6 mg/kg) or DZ (0.01–0.32 mg/kg). Right, acute effects of DZ on stimulus effects of MS. Either saline or 0.032 mg/kg DZ was given 30 min before tests of cumulative doses of MS. Each rat served as its own control. The numbers in parentheses are numbers of rats tested. Ordinates: Upper, percentage responses on the MS-appropriate lever; lower, response rates expressed as percentage of control rates (the average response rates of 10 saline trials preceding the test). Abscissae: cumulative doses of drugs, in milligrams per kilogram; points and bars, means and SEM. Text in italics identifies the −30-min pretreatment: SAL saline, 0.032 or 0.1 DZ 0.032 or 0.1 mg/kg dizocilpine
Figure 6.
Effects on discriminative control of acute memantine, alone and on discriminative stimulus effects of morphine. Left, stimulus effects of morphine (MS) or memantine (MEM) alone in an operant discrimination procedure. Rats were trained to discriminate 3.2 mg/kg MS and saline, and then were tested with cumulative doses of MS (0.1–5.6 mg/kg) or MEM (0.32–17.8 mg/kg). Center and right, acute effects of MEM on stimulus effects of MS. Either saline or MEM (7.5 or 10 mg/kg) was given 30 min before tests of cumulative doses of MS. Other details as in Fig. 5. Text in italics identifies the −30 min pretreatment: SAL saline, 7.5 or 10 MEM 7.5 or 10 mg/kg memantine
Effects of dizocilpine or memantine on development and expression of tolerance to stimulus effects of morphine
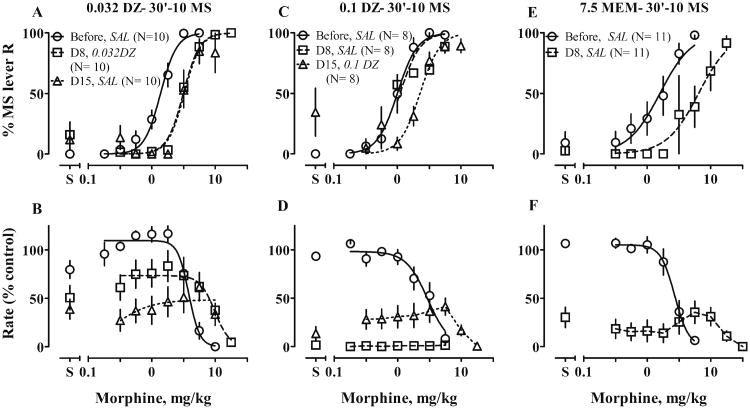
When training was suspended, one-week treatment with saline and morphine induced tolerance to stimulus effects of morphine (Fig. 7a, b, (P < 0.0001), e, f, (P < 0.0001); Table 3). Two weeks after the end of morphine treatment regimens, sensitivity recovered completely (data not shown). Chronic dizocilpine alone did not change potency of morphine (0.1 mg/kg; fig. 7c, d; Table 3). When the same dose was combined with morphine for chronic treatment, only three rats responded after 7 days of treatment (Fig. 8c, d); their ED50 values did not differ from ED50 values before treatment (Table 3). A tolerance ratio could not be calculated because rates were severely decreased. To test whether dizocilpine pretreatment would restore responding and affect expression of tolerance, the same group of rats received chronic morphine treatment for an additional week, followed by acute treatment with 0.1 mg/kg dizocilpine before a second test of morphine. At this test, all rats responded, although at rates less than 50% of control. When analyzed with a shared slope, the morphine ED50 increased about 2.3-fold, suggesting 0.1 mg/kg dizocilpine did not block expression of tolerance (Fig. 8c, d; Table 3). A lower dose of dizocilpine (0.032 mg/kg) exerted less response disruption and did not block development or expression of tolerance (Fig. 8a, b; Table 3).
Figure 7.
Effects of chronic treatment with 10 mg/kg of morphine (10 MS) or with NMDAR antagonists on stimulus effects of morphine (MS). Rats were trained to discriminate 3.2 mg/kg MS and saline and then received chronic treatment, shown in column titles, b.i.d. for 7 days. Tests of cumulative doses of MS were conducted before treatment and 12 h after the last injection on Day 7 of treatment; i.e., on Day 8 (D8). On test days, rats received SAL 30 min before the tests. Other details as in Fig. 5. Text in italics identifies the −30-min pretreatment: SAL saline
Table 3. Effects of dizocilpine (DZ) or memantine (MEM) on tolerance to stimulus effects of morphine (MS).
| Treatment | Pretreatment 30 min before test | ED50 (before) | ED50 (after) | F(dfn,dfd) | ED50 (after)/ED50 (before) | 95% CI | Slope |
|---|---|---|---|---|---|---|---|
| SAL + MS | SAL | 0.99 ± 1.1 | 2.8 ± 1.1a, * | 34.4 (1, 92) | 2.8±0.43 | 2.1–3.5 | 2.9±0.58 |
| 0.1 mg/kg DZ + SAL | SAL | 1.2 ± 1.1 | 1.2 ± 1.1 | 0.00022 (1, 78) | 1.0 ± 0.16b | 0.75 – 1.3 | 2.9 ± 0.61 |
| 0.1 mg/kg DZ + MS | SAL | 1.0 ± 1.1 | 1.0 ± 1.1 | 0.089 (1, 50) | N/A | N/A | 2.8 ± 0.42 |
| 0.1 mg/kg DZ | 1.0 ± 1.1 | 2.3 ± 1.1a, * | 42.5 (1, 91) | 2.3 ± 0.20 | 1.9 – 2.7 | 2.8 ± 0.42 | |
| 0.032 mg/kg DZ + MS | SAL | 1.4 ± 1.1 | 3.3 ± 1.1a, * | 55.1 (1, 106) | 2.4 ± 0.24 | 1.9 – 2.9 | 3.4 ± 0.032 |
| 0.032 mg/kg DZ | 1.4 ± 1.1 | 3.1 ± 1.1a, * | 98.2 (1, 125) | 2.3 ± 0.13 | 2.0 – 2.5 | 3.4 ± 0.032 | |
| SAL + MS | SAL | 1.2 ± 1.1 | 4.4 ± 1.2a, * | 23.6 (1, 53) | 3.5±0.53 | 2.5–4.6 | 2.9±0.82 |
| 7.5 mg/kg MEM + SAL | SAL | 1.6 ± 1.1 | 1.6 ± 1.1 | 0.29 (1, 74) | 0.96 ± 0.10b | 0.75 – 1.2 | 3.4 ± 0.79 |
| 7.5 mg/kg MEM + MS | SAL | 1.6 ± 1.2 | 6.5 ± 1.2a, * | 16.1 (1, 80) | 4.1 ± 0.78 | 2.5 – 5.7 | 1.7 ± 0.41 |
Values are mean ± SEM of ED50 values for MS (in milligrams per kilogram), obtained in tests of cumulative doses of MS before and after chronic treatment, tolerance ratios (ED50 (after)/ED50 (before)), and their 95% confidence interval (CI)
N/A tolerance ratios cannot be calculated
P<0.01
ED50 (after) values differ from ED50 (before) values in the same group
Ratios differ from “SAL+MS” groups pretreated with saline (SAL; entries set in italics)
Figure 8.
Effects of combined treatment with 10 mg/kg of morphine (10 MS) and a NMDAR antagonist on development and expression of tolerance to discriminative stimulus effects of MS. Rats were trained to discriminate 3.2 mg/kg MS and saline and then received chronic treatment, shown in column titles, b.i.d. for 7 or 14 days. Tests of cumulative doses of MS were conducted before treatment and 12 h after the last injections on Day 7 or 14 of treatment; i.e., on Day 8 (D8) and Day 15 (D15). On test days, rats received SAL or an appropriate dose of NMDAR antagonist (in milligrams per kilogram) 30 min before tests. Other details as in Fig. 5. Text in italics identifies the −30-min pretreatment: SAL saline, 0.032 or 0.1 DZ 0.032 or 0.1 mg/kg dizocilpine
At a dose of 10 mg/kg, chronic memantine completely suppressed responses when given either alone or with morphine. We therefore decreased the dose to 7.5 mg/kg, which neither changed the morphine ED50 when given chronically with saline (Fig. 7g, h), nor blocked development of tolerance (P = 0.0001, Fig. 8e; Table 3) when given chronically with morphine. This combination still severely suppressed responses (Fig. 8f).
Discussion
Under the conditions studied, acute memantine attenuated antinociceptive and discriminative stimulus effects of morphine, but chronic memantine did not block the development or expression of tolerance to either effect. In contrast, acute dizocilpine potentiated antinociceptive and stimulus effects of morphine. Chronic dizocilpine blocked development of tolerance to antinociceptive effects of low to moderate doses of morphine, and acute dizocilpine blocked expression of tolerance, but chronic dizocilpine did not block tolerance to stimulus effects of morphine. As discussed below, these different effects of memantine and dizocilpine may reflect differences in NMDAR binding profiles.
Impact of acute pretreatment with dizocilpine or memantine
The acute effects of dizocilpine and memantine on morphine-induced antinociception paralleled their effects on morphine-induced stimulus control. Reported effects of NMDAR antagonists on morphine-induced antinociception are inconsistent, with dizocilpine and memantine reported to enhance (Belozertseva et al. 2000; Bryant et al. 2006; Carlezon et al. 2000; Grass et al. 1996; Kozela et al. 2001), block or attenuate (Craft and Lee 2005; Lutfy et al. 1993; Popik et al. 2000b), or, for memantine, exert no effect (Kozela et al. 2003; Popik et al. 2000b; Redwine and Trujillo 2003). Differences across studies could arise from multiple factors (cf. Bryant et al. 2006). Previous studies used tail-flick assays, whereas the present experiments used a warm water tail-withdrawal assay, in which tail temperature may rise more quickly and in a linear fashion (Le Bars et al. 2001). At equivalent heat intensity, latency may be shorter in a tail-withdrawal assay than in a tail-flick assay. Understanding what, if any, role such differences play in study outcomes requires greater knowledge of controlling relations in the two assays. Moreover, tail-flick and tail-withdrawal are under supraspinal control (Le Bars et al. 2001), and MOR-agonist mediated antinociception in the tail-flick assay involves both spinal and supraspinal sites (Yeung and Rudy 1980a; b). The effects of acute systemic NMDAR antagonists may arise from complex NMDAR-MOR interactions at the same or different sites in pain pathways. When both agents act in the same site (e.g., spinal cord dorsal horn), activation of MORs leads to NMDAR hyperactivation, thereby inducing hyperalgesia (Mao et al. 1995; Mayer et al. 1999). Blocking NMDARs at these common sites could potentiate MOR-mediated antinociception. On the other hand, blocking NMDARs at one site of a pain pathway could attenuate antinociception induced by MOR agonists at a different site. For example, microinjection of dizocilpine into the rostral ventromedial medulla (RVM) reduces antinociception elicited by morphine in the ventrolateral PAG (Bodnar 2000; Spinella et al. 1996). Indeed, this effect of NMDAR antagonists in the RVM is shared by serotonergic antagonists such as methysergide, ritanserin or ICS205930 (Bodnar 2000). Because memantine, unlike dizocilpine, blocks 5HT3 receptors and NMDARs with similar potency (Rogawski and Wenk 2003), opposite effects of systemic dizocilpine and memantine may represent net effects arising from their differential distribution or receptor selectivity in multiple sites of pain pathways. Such MOR-NMDAR interactions may also contribute to their opposite effects on the stimulus effects of morphine. Of interest, brain regions such as the ventral tegmental area (VTA) and PAG are essential to both antinociceptive and stimulus effects of morphine (Bodnar 2000; Inoue et al. 2003; Jacquet 1988; Krivsky et al. 2006; Shoaib and Spanagel 1994).
Psychological mechanisms may also contribute to observed differences. In rats (Carlezon et al. 2000) trained to discriminate the combination of morphine (3.2 mg/kg) and dizocilpine (0.05 mg/kg) from saline, dizocilpine evoked 20∼40% generalization whereas morphine evoked < 20%, suggesting that the combination produced a novel cue that partially generalized to dizocilpine but not morphine. Accordingly, we expected dizocilpine to attenuate the morphine cue. The observed potentiation may have resulted from our use of different relative doses of each drug. Memantine at 10 mg/kg (Fig. 6), but not lower doses (cf. Popik and Danysz 1997) did attenuate stimulus effects of morphine. Alternatively, memantine may produce stimulus effects that differ strongly from those of morphine and mask those of lower (0.1∼3.2 mg/kg) but not higher doses of morphine (Gauvin and Young 1989). A study to characterize stimulus control by memantine, alone and in combination with morphine, would be informative.
Impact of chronic pretreatment with dizocilpine or memantine
Memantine and dizocilpine also differed in chronic effects. Dizocilpine is commonly reported to block tolerance to antinociceptive of low doses of morphine (for reviews, see Elliott et al. 1995; Trujillo 1995). One recent study that used a warm water tail-withdrawal assay, however, reported that dizocilpine facilitates development of tolerance to antinociceptive effects of 7.5 mg/kg morphine (Bryant et al. 2006). This study also reported that chronic dizocilpine alone induces tolerance to morphine-induced antinociception, similar to our results (Fig. 3c). In contrast to dizocilpine, memantine had no effects on tolerance to antinociceptive effects of morphine. Effects of memantine have been studied only in mice, using a tail-flick test (Belozertseva and Bespalov 1998; Popik et al. 2000a). In both studies, memantine blocked development of tolerance. The differences of these results and ours may have arisen from the afore-mentioned factors (e.g., nociception test) or from differences in the metrics used to assess tolerance; i.e., use of changes of effects of a single dose of morphine as opposed to changes in ED50 values. When, as in our study, dose–response curves differ in slope (e.g., morphine alone and morphine + dizocilpine, Fig. 4a), the interaction demonstrates that, if changes in the effects of single doses of morphine are used to assess tolerance, the choice of morphine dose may decide the observed effects of the NMDAR antagonist. Thus, using changes in ED50 as the measure of tolerance may provide a better estimate of the effects of NMDAR antagonists. In addition, Allen and Dykstra (2000) found that the competitive NMDAR antagonist LY293558 (0.3 mg/kg) blocks tolerance of a smaller magnitude but only reduces tolerance of a larger magnitude. Similarly, in our study dizocilpine blocked tolerance to low doses of morphine but not higher doses. Additionally, in the current study, unlike many earlier studies, rats were not naïve to high doses of morphine and had demonstrated decreased sensitivity to morphine prior to chronic treatment, yielding a smaller magnitude of tolerance following chronic morphine treatment. However, this difference in tolerance magnitude is unlikely to have caused the lack of memantine effect. Indeed, if memantine does block tolerance to morphine-induced antinociception, its effects should have appeared under conditions that produced only a smaller magnitude of tolerance.
The observed differences in effects of chronic dizocilpine and memantine could result from details of their biophysical interactions with NMDARs. Compared to dizocilpine, memantine has faster association/dissociation rates from NMDAR, its blockade is more voltage-dependent, and it is only partially trapped in the channel (for details, see Rogawski and Wenk 2003). These unique binding properties allow memantine to leave the channel quickly and therefore allow maintenance of ongoing synaptic activities. Dizocilpine, on the other hand, completely shuts down the channel and blocks all synaptic activities. If development of tolerance to antinociceptive effects of MOR agonists is due to hyperactivation of NMDAR by MOR agonists and subsequent neural plasticity (Trujillo 2000), the fact that memantine can spare synaptic activities required for neural plasticity may underlie its lack of effect on tolerance.
Neither dizocilpine nor memantine blocked tolerance to stimulus effects of morphine. In a similar study by Bespalov and et al. (1999), chronic treatment with the combination of 0.1 mg/kg dizocilpine and 20 mg/kg morphine, which had smaller effects on response rate than our combination, also did not block tolerance to stimulus effects of morphine. It is possible that the neuronal plasticity mediated by NMDARs has a higher threshold for induction in the discriminative stimulus assay than in the nociception assay. As a result, the same treatment regimen may induce a greater magnitude of activation of NMDARs in nociception assays than in discrimination assays. A theoretical characteristic of uncompetitive NMDAR antagonists is use dependence, such that the higher level of activation of NMDARs, the higher the magnitude of antagonist-induced blockade (Huettner and Bean 1988; Rogawski and Wenk 2003). Such use dependence may explain why the same dose of dizocilpine blocked development of tolerance to morphine-induced antinociception but not tolerance to stimulus effects of morphine.
Summary
Contrary to what we expected, memantine and dizocilpine exerted opposite acute effects on antinociceptive and discriminative stimulus effects of morphine. Their effects on tolerance to morphine-induced antinociceptive and stimulus effects also differed. The different binding profiles of dizocilpine and memantine at NMDARs and in various CNS sites may determine their different acute and chronic interactions with morphine. Moreover, memantine may not be a beneficial adjunct to MOR agonists in chronic pain management, due to a lack of effect on tolerance to morphine antinociception accompanied by disruptive side effects (combinations of morphine and high doses of memantine severely decreased operant responses). Indeed, clinical studies assessing effects of combination of dextromethorphan, another clinically available low affinity NMDA channel blocker, and MOR agonists in chronic pain (cancer pain, neuropathic, and other non-cancer pain) treatment have yielded conflicting results (Chevlen 2000; Fisher et al. 2000; Katz 2000; Weinbroum et al. 2000). More recent double-blinded, parallel group or placebo-controlled studies have shown that the combination does not add benefit in terms of efficacy or safety in patients with chronic cancer pain (Dudgeon et al. 2007) or non-malignant, non-neuropathic pain (Galer et al. 2005). Further work is required to predict the effectiveness of NMDAR antagonists in altering MOR agonist tolerance in clinical pain patients.
Acknowledgments
This research was supported by US Public Health Service Grant DA03796.
Footnotes
No author reports actual or potential conflicts of interest.
Contributor Information
Yukun Chen, Department of Pharmacology and Neuroscience, Texas Tech University Health Sciences Center, Lubbock, TX 79430, USA.
Marianne Evola, Department of Pharmacology and Neuroscience, Texas Tech University Health Sciences Center, Lubbock, TX 79430, USA.
Alice M. Young, Email: alice.young@ttu.edu, Department of Pharmacology and Neuroscience, Texas Tech University Health Sciences Center, Lubbock, TX 79430, USA; Department of Psychology, Texas Tech University, Lubbock, TX 79409-1075, USA.
References
- Allen RM, Dykstra LA. Role of morphine maintenance dose in the development of tolerance and its attenuation by an NMDA receptor antagonist. Psychopharmacology (Berl) 2000;148:59–65. doi: 10.1007/s002130050025. [DOI] [PubMed] [Google Scholar]
- Belozertseva IV, Bespalov AY. Effects of NMDA receptor channel blockers, dizocilpine and memantine, on the development of opiate analgesic tolerance induced by repeated morphine exposures or social defeats in mice. Naunyn Schmiedebergs Arch Pharmacol. 1998;358:270–274. doi: 10.1007/pl00005252. [DOI] [PubMed] [Google Scholar]
- Belozertseva IV, Dravolina OA, Neznanova ON, Danysz W, Bespalov AY. Antinociceptive activity of combination of morphine and NMDA receptor antagonists depends on the inter-injection interval. Eur J Pharmacol. 2000;396:77–83. doi: 10.1016/s0014-2999(00)00184-9. [DOI] [PubMed] [Google Scholar]
- Bespalov AY, Balster RL, Beardsley PM. N-Methyl-d-aspartate receptor antagonists and the development of tolerance to the discriminative stimulus effects of morphine in rats. J Pharmacol Exp Ther. 1999;290:20–27. [PubMed] [Google Scholar]
- Bodnar RJ. Supraspinal circuitry mediating opioid antinociception: antagonist and synergy studies in multiple sites. J Biomed Sci. 2000;7:181–194. doi: 10.1007/BF02255465. [DOI] [PubMed] [Google Scholar]
- Brady LS, Holtzman SG. Locomotor activity in morphine-dependent and post-dependent rats. Pharmacol Biochem Behav. 1981;14:361–370. doi: 10.1016/0091-3057(81)90403-2. [DOI] [PubMed] [Google Scholar]
- Bryant CD, Eitan S, Sinchak K, Fanselow MS, Evans CJ. NMDA receptor antagonism disrupts the development of morphine analgesic tolerance in male, but not female C57BL/6 J mice. Am J Physiol Regul Integr Comp Physiol. 2006;291:R315–R326. doi: 10.1152/ajpregu.00831.2005. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Kosten TA, Nestler EJ. Behavioral interactions caused by combined administration of morphine and MK-801 in rats. Psychopharmacology (Berl) 2000;151:261–272. doi: 10.1007/s002130000462. [DOI] [PubMed] [Google Scholar]
- Chen L, Huang LY. Sustained potentiation of NMDA receptor-mediated glutamate responses through activation of protein kinase C by a mu opioid. Neuron. 1991;7:319–326. doi: 10.1016/0896-6273(91)90270-a. [DOI] [PubMed] [Google Scholar]
- Chen HS, Lipton SA. Mechanism of memantine block of NMDA-activated channels in rat retinal ganglion cells: uncompetitive antagonism. J Physiol. 1997;499(Pt 1):27–46. doi: 10.1113/jphysiol.1997.sp021909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HS, Pellegrini JW, Aggarwal SK, Lei SZ, Warach S, Jensen FE, Lipton SA. Open-channel block of N-methyl-d-aspartate (NMDA) responses by memantine: therapeutic advantage against NMDA receptor-mediated neurotoxicity. J Neurosci. 1992;12:4427–4436. doi: 10.1523/JNEUROSCI.12-11-04427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevlen E. Morphine with dextromethorphan: conversion from other opioid analgesics. J Pain Symptom Manage. 2000;19:S42–S49. doi: 10.1016/s0885-3924(99)00130-x. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Kuyps JJ, Niemegeers CJ, Janssen PA. Discriminative stimulus properties of fentanyl and morphine: tolerance and dependence. Pharmacol Biochem Behav. 1976;5:401–408. doi: 10.1016/0091-3057(76)90103-9. [DOI] [PubMed] [Google Scholar]
- Commons KG, van Bockstaele EJ, Pfaff DW. Frequent colocalization of mu opioid and NMDA-type glutamate receptors at postsynaptic sites in periaqueductal gray neurons. J Comp Neurol. 1999;408:549–559. [PubMed] [Google Scholar]
- Craft RM, Lee DA. NMDA antagonist modulation of morphine antinociception in female vs. male rats. Pharmacol Biochem Behav. 2005;80:639–649. doi: 10.1016/j.pbb.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Danysz W, Parsons CG. The NMDA receptor antagonist memantine as a symptomatological and neuroprotective treatment for Alzheimer's disease: preclinical evidence. Int J Geriatr Psychiatry. 2003;18:S23–S32. doi: 10.1002/gps.938. [DOI] [PubMed] [Google Scholar]
- Dudgeon DJ, Bruera E, Gagnon B, Watanabe SM, Allan SJ, Warr DG, MacDonald SM, Savage C, Tu D, Pater JL. A phase III randomized, double-blind, placebo-controlled study evaluating dextromethorphan plus slow-release morphine for chronic cancer pain relief in terminally ill patients. J Pain Symptom Manage. 2007;33:365–371. doi: 10.1016/j.jpainsymman.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Elliott K, Kest B, Man A, Kao B, Inturrisi CE. N-methyl-d-aspartate (NMDA) receptors, mu and kappa opioid tolerance, and perspectives on new analgesic drug development. Neuropsychopharmacology. 1995;13:347–356. doi: 10.1016/0893-133X(95)00083-P. [DOI] [PubMed] [Google Scholar]
- Fisher K, Coderre TJ, Hagen NA. Targeting the N-methyl-d-aspartate receptor for chronic pain management. Preclinical animal studies, recent clinical experience and future research directions. J Pain Symptom Manage. 2000;20:358–373. doi: 10.1016/s0885-3924(00)00213-x. [DOI] [PubMed] [Google Scholar]
- Galer BS, Lee D, Ma T, Nagle B, Schlagheck TG. MorphiDex (morphine sulfate/dextromethorphan hydrobromide combination) in the treatment of chronic pain: three multicenter, randomized, double-blind, controlled clinical trials fail to demonstrate enhanced opioid analgesia or reduction in tolerance. Pain. 2005;115:284–295. doi: 10.1016/j.pain.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Gauvin DV, Young AM. Evidence for perceptual masking of the discriminative morphine stimulus. Psychopharmacology (Berl) 1989;98:212–221. doi: 10.1007/BF00444694. [DOI] [PubMed] [Google Scholar]
- Ghelardini C, Galeotti N, Vivoli E, Norcini M, Zhu W, Stefano GB, Guarna M, Bianchi E. Molecular interaction in the mouse PAG between NMDA and opioid receptors in morphine-induced acute thermal nociception. J Neurochem. 2008;105:91–100. doi: 10.1111/j.1471-4159.2007.05117.x. [DOI] [PubMed] [Google Scholar]
- Gracy KN, Svingos AL, Pickel VM. Dual ultrastructural localization of mu-opioid receptors and NMDA-type glutamate receptors in the shell of the rat nucleus accumbens. J Neurosci. 1997;17:4839–4848. doi: 10.1523/JNEUROSCI.17-12-04839.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grass S, Hoffmann O, Xu XJ, Wiesenfeld-Hallin Z. N-methyl-d-aspartate receptor antagonists potentiate morphine's antinociceptive effect in the rat. Acta Physiol Scand. 1996;158:269–273. doi: 10.1046/j.1365-201X.1996.566309000.x. [DOI] [PubMed] [Google Scholar]
- Guo M, Xu NJ, Li YT, Yang JY, Wu CF, Pei G. Morphine modulates glutamate release in the hippocampal CA1 area in mice. Neurosci Lett. 2005;381:12–15. doi: 10.1016/j.neulet.2005.01.071. [DOI] [PubMed] [Google Scholar]
- Hao Y, Yang JY, Guo M, Wu CF, Wu MF. Morphine decreases extracellular levels of glutamate in the anterior cingulate cortex: an in vivo microdialysis study in freely moving rats. Brain Res. 2005;1040:191–196. doi: 10.1016/j.brainres.2005.01.072. [DOI] [PubMed] [Google Scholar]
- Herman BH, Vocci F, Bridge P. The effects of NMDA receptor antagonists and nitric oxide synthase inhibitors on opioid tolerance and withdrawal. Medication development issues for opiate addiction. Neuropsychopharmacology. 1995;13:269–293. doi: 10.1016/0893-133X(95)00140-9. [DOI] [PubMed] [Google Scholar]
- Huettner JE, Bean BP. Block of N-methyl-d-aspartate-activated current by the anticonvulsant MK-801: selective binding to open channels. Proc Natl Acad Sci U S A. 1988;85:1307–1311. doi: 10.1073/pnas.85.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Mishina M, Ueda H. Locus-specific rescue of GluRepsilon1 NMDA receptors in mutant mice identifies the brain regions important for morphine tolerance and dependence. J Neurosci. 2003;23:6529–6536. doi: 10.1523/JNEUROSCI.23-16-06529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquet YF. The NMDA receptor: central role in pain inhibition in rat periaqueductal gray. Eur J Pharmacol. 1988;154:271–276. doi: 10.1016/0014-2999(88)90201-4. [DOI] [PubMed] [Google Scholar]
- Katz NP. MorphiDex (MS:DM) double-blind, multiple-dose studies in chronic pain patients. J Pain Symptom Manage. 2000;19:S37–S41. doi: 10.1016/s0885-3924(99)00129-3. [DOI] [PubMed] [Google Scholar]
- Kow LM, Commons KG, Ogawa S, Pfaff DW. Potentiation of the excitatory action of NMDA in ventrolateral periaqueductal gray by the mu-opioid receptor agonist, DAMGO. Brain Res. 2002;935:87–102. doi: 10.1016/s0006-8993(02)02532-5. [DOI] [PubMed] [Google Scholar]
- Kozela E, Danysz W, Popik P. Uncompetitive NMDA receptor antagonists potentiate morphine antinociception recorded from the tail but not from the hind paw in rats. Eur J Pharmacol. 2001;423:17–26. doi: 10.1016/s0014-2999(01)01084-6. [DOI] [PubMed] [Google Scholar]
- Kozela E, Pilc A, Popik P. Inhibitory effects of MPEP, an mGluR5 antagonist, andmemantine, an N-methyl-d-aspartate receptor antagonist, on morphine antinociceptive tolerance in mice. Psychopharmacology (Berl) 2003;165:245–251. doi: 10.1007/s00213-002-1287-8. [DOI] [PubMed] [Google Scholar]
- Krivsky JA, Stoffel EC, Sumner JE, Inman BC, Craft RM. Role of ventral tegmental area, periaqueductal gray and parabrachial nucleus in the discriminative stimulus effects of morphine in the rat. Behav Pharmacol. 2006;17:259–270. doi: 10.1097/00008877-200605000-00007. [DOI] [PubMed] [Google Scholar]
- Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev. 2001;53:597–652. [PubMed] [Google Scholar]
- Lossignol DA, Obiols-Portis M, Body JJ. Successful use of ketamine for intractable cancer pain. Support Care Cancer. 2005;13:188–193. doi: 10.1007/s00520-004-0684-4. [DOI] [PubMed] [Google Scholar]
- Lutfy K, Hurlbut DE, Weber E. Blockade of morphine-induced analgesia and tolerance in mice by MK-801. Brain Res. 1993;616:83–88. doi: 10.1016/0006-8993(93)90195-s. [DOI] [PubMed] [Google Scholar]
- Mao J, Price DD, Mayer DJ. Mechanisms of hyperalgesia and morphine tolerance: a current view of their possible interactions. Pain. 1995;62:259–274. doi: 10.1016/0304-3959(95)00073-2. [DOI] [PubMed] [Google Scholar]
- Martin G, Nie Z, Siggins GR. mu-Opioid receptors modulate NMDA receptor-mediated responses in nucleus accumbens neurons. J Neurosci. 1997;17:11–22. doi: 10.1523/JNEUROSCI.17-01-00011.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer DJ, Mao J, Holt J, Price DD. Cellular mechanisms of neuropathic pain, morphine tolerance, and their interactions. Proc Natl Acad Sci U S A. 1999;96:7731–7736. doi: 10.1073/pnas.96.14.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, Drake CT. Ultrastructural evidence for presynaptic mu opioid receptor modulation of synaptic plasticity in NMDA-receptor-containing dendrites in the dentate gyrus. Brain Res Bull. 2001;54:131–140. doi: 10.1016/s0361-9230(00)00415-9. [DOI] [PubMed] [Google Scholar]
- Narita M, Hashimoto K, Amano T, Narita M, Niikura K, Nakamura A, Suzuki T. Post-synaptic action of morphine on glutamatergic neuronal transmission related to the descending antinociceptive pathway in the rat thalamus. J Neurochem. 2008;104:469–478. doi: 10.1111/j.1471-4159.2007.05059.x. [DOI] [PubMed] [Google Scholar]
- Parsons CG, Danysz W, Quack G. Memantine is a clinically well tolerated N-methyl-d-aspartate (NMDA) receptor antagonist—a review of preclinical data. Neuropharmacology. 1999;38:735–767. doi: 10.1016/s0028-3908(99)00019-2. [DOI] [PubMed] [Google Scholar]
- Popik P, Danysz W. Inhibition of reinforcing effects of morphine and motivational aspects of naloxone-precipitated opioid withdrawal by N-methyl-d-aspartate receptor antagonist, memantine. J Pharmacol Exp Ther. 1997;280:854–865. [PubMed] [Google Scholar]
- Popik P, Kozela E, Danysz W. Clinically available NMDA receptor antagonists memantine and dextromethorphan reverse existing tolerance to the antinociceptive effects of morphine in mice. Naunyn Schmiedebergs Arch Pharmacol. 2000a;361:425–432. doi: 10.1007/s002109900205. [DOI] [PubMed] [Google Scholar]
- Popik P, Kozela E, Pilc A. Selective agonist of group II glutamate metabotropic receptors, LY354740, inhibits tolerance to analgesic effects of morphine in mice. Br J Pharmacol. 2000b;130:1425–1431. doi: 10.1038/sj.bjp.0703438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Farb CR, He Y, Janssen WG, Rodrigues SM, Johnson LR, Hof PR, LeDoux JE, Morrison JH. Distribution of NMDA and AMPA receptor subunits at thalamo-amygdaloid dendritic spines. Brain Res. 2007;1134:87–94. doi: 10.1016/j.brainres.2006.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redwine KE, Trujillo KA. Effects of NMDA receptor antagonists on acute mu-opioid analgesia in the rat. Pharmacol Biochem Behav. 2003;76:361–372. doi: 10.1016/j.pbb.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Wenk GL. The neuropharmacological basis for the use of memantine in the treatment of Alzheimer's disease. CNS Drug Rev. 2003;9:275–308. doi: 10.1111/j.1527-3458.2003.tb00254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sannerud CA, Young AM. Environmental modification of tolerance to morphine discriminative stimulus properties in rats. Psychopharmacology (Berl) 1987;93:59–68. doi: 10.1007/BF02439587. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Spanagel R. Mesolimbic sites mediate the discriminative stimulus effects of morphine. Eur J Pharmacol. 1994;252:69–75. doi: 10.1016/0014-2999(94)90576-2. [DOI] [PubMed] [Google Scholar]
- Spinella M, Cooper ML, Bodnar RJ. Excitatory amino acid antagonists in the rostral ventromedial medulla inhibit mesencephalic morphine analgesia in rats. Pain. 1996;64:545–552. doi: 10.1016/0304-3959(95)00192-1. [DOI] [PubMed] [Google Scholar]
- Terman GW, Eastman CL, Chavkin C. Mu opiates inhibit long-term potentiation induction in the spinal cord slice. J Neurophysiol. 2001;85:485–494. doi: 10.1152/jn.2001.85.2.485. [DOI] [PubMed] [Google Scholar]
- Trujillo KA. Effects of noncompetitive N-methyl-d-aspartate receptor antagonists on opiate tolerance and physical dependence. Neuropsychopharmacology. 1995;13:301–307. doi: 10.1016/0893-133X(95)00088-U. [DOI] [PubMed] [Google Scholar]
- Trujillo KA. Are NMDA receptors involved in opiate-induced neural and behavioral plasticity? A review of preclinical studies. Psychopharmacology (Berl) 2000;151:121–141. doi: 10.1007/s002130000416. [DOI] [PubMed] [Google Scholar]
- Trujillo KA, Akil H. Inhibition of opiate tolerance by non-competitive N-methyl-d-aspartate receptor antagonists. Brain Res. 1994;633:178–188. doi: 10.1016/0006-8993(94)91538-5. [DOI] [PubMed] [Google Scholar]
- Walker EA, Young AM. Differential tolerance to antinociceptive effects of mu opioids during repeated treatment with etonitazene, morphine, or buprenorphine in rats. Psychopharmacology (Berl) 2001;154:131–142. doi: 10.1007/s002130000620. [DOI] [PubMed] [Google Scholar]
- Walker EA, Makhay MM, House JD, Young AM. In vivo apparent pA2 analysis for naltrexone antagonism of discriminative stimulus and analgesic effects of opiate agonists in rats. J Pharmacol Exp Ther. 1994;271:959–968. [PubMed] [Google Scholar]
- Weinbroum AA, Rudick V, Paret G, Ben-Abraham R. The role of dextromethorphan in pain control. Can J Anaesth. 2000;47:585–596. doi: 10.1007/BF03018952. [DOI] [PubMed] [Google Scholar]
- Xie CW, Morrisett RA, Lewis DV. Mu opioid receptor-mediated modulation of synaptic currents in dentate granule cells of rat hippocampus. J Neurophysiol. 1992;68:1113–1120. doi: 10.1152/jn.1992.68.4.1113. [DOI] [PubMed] [Google Scholar]
- Yeung JC, Rudy TA. Multiplicative interaction between narcotic agonisms expressed at spinal and supraspinal sites of antinociceptive action as revealed by concurrent intrathecal and intracerebroventricular injections of morphine. J Pharmacol Exp Ther. 1980a;215:633–642. [PubMed] [Google Scholar]
- Yeung JC, Rudy TA. Sites of antinociceptive action of systemically injected morphine: involvement of supraspinal loci as revealed by intracerebroventricular injection of naloxone. J Pharmacol Exp Ther. 1980b;215:626–632. [PubMed] [Google Scholar]
- Young AM. NIDA Res Monogr. 116. 1991. Tolerance to drugs acting as discriminative stimuli; pp. 197–211. [PubMed] [Google Scholar]