Abstract
Gemcitabine has high activity against nasopharyngeal carcinoma (NPC). The level of ribonucleotide reductase subunit M1 (RRM1) expression is closely related to the efficacy of gemcitabine on non-small cell lung cancer and pancreatic cancer. However, the expression of RRM1 and its association with sensitivity to gemcitabine-based chemotherapy in advanced NPC is not known. In this study, we retrospectively collected 48 formalin-fixed, paraffin-embedded NPC tissues to evaluate the expression of RRM1 using immunohistochemistry. All patients were diagnosed and treated with gemcitabine-based chemotherapy at Sun Yat-sen University Cancer Center. RRM1 expression was positive in 17 (35%) patients. RRM1 expression was not associated with sex, age, performance status, WHO histological type, number of distant metastases, previous treatment, or cycles of gemcitabine-based chemotherapy (P > 0.05). The progression-free survival of the RRM1-positive group was shorter than that of the RRM1-negative group (5 months vs. 7 months, P = 0.036), and the response rate of the RRM1-positive group was somewhat lower than that of the RRM1-negative group (51.6% vs. 35.3%, P = 0.278). There was no significant difference in median survival between the RRM1-positive and RRM1-negative groups (22 months vs. 19 months, P = 0.540). Our results show that RRM1-negative expression is related with longer progression-free survival in advanced NPC patients treated with gemcitabine-based regimens.
Keywords: Gemcitabine, immunohistochemistry, nasopharyngeal carcinoma, RRM1
Nasopharyngeal carcinoma (NPC) is an endemic cancer in Southeast Asia[1],[2]. Approximately 75% of patients with NPC are diagnosed at stage III or IV. Chemoradiotherapy and palliative chemotherapy are the standard treatments for locally advanced and metastatic NPC, respectively[2]–[6]. Despite recent improvements in treatment strategies, chemotherapy alone could control recurrent or metastatic disease for a long term in only a small proportion of patients [7]. The ability to identify patients that will benefit from a chemotherapeutic agent is crucial in establishing individualized treatment. With the developments in pharmacogenomics, molecular biological studies have already been useful tools for customizing treatment [8],[9]. To date, Epstein-Barr virus (EBV) antibodies and EBV DNA have commonly been used as biomarkers in early detection, monitoring, and prognostic prediction for NPC patients. Although some other molecular biomarkers for treatment response monitoring are being studied, few have been fully confirmed in clinical practice to aid treatment decisions[10]–[14].
Ribonucleotide reductase is a key enzyme for the conversion of ribonucleotide diphosphate to deoxyribonucleotide diphosphate. In humans, ribonucleotide reductase contains two subunits, M1 (RRM1) and M2 (RRM2). RRM1 is the key enzyme in the production of deoxyribonucleotides. It is also the binding site for nucleotides and allosteric regulators, and it is involved in the suppression of tumor proliferation, invasiveness, and metastasis [15]. Gemcitabine is an analog of deoxycytidine. Intracellularly, gemcitabine is converted into active diphosphate (dFdCDP) and triphosphate (dFdCTP) nucleosides by deoxycytidine kinase. dFdCTP is incorporated into the DNA chain, which leads to termination of chain elongation. dFdCDP inhibits RRM1, which results in inhibition of DNA synthesis. Gemcitabine has been widely used to treat solid tumors like non-small cell lung cancer (NSCLC), bladder cancer, pancreatic cancer, and metastatic breast cancer. Gemcitabine also has high activity against NPC and has been approved to treat it [16]–[19]. Some preclinical data revealed that high expression of RRM1 is associated with resistance to gemcitabine [20]–[23]. Moreover, many clinical studies have reported an association between RRM1 expression level and tumor response in NSCLC and pancreatic cancer patients treated with gemcitabine-based chemotherapy [22],[23]. However, the relationship between RRM1 expression level and gemcitabine activity in advanced NPC has not been addressed.
In this study, we used immunohistochemistry (IHC) to evaluate RRM1 expression in cases of advanced NPC treated with gemcitabine-based chemotherapy. Our goal was to explore the association of RRM1 expression with clinical outcomes.
Materials and Methods
Patients
Forty-eight patients with advanced NPC, who were treated with gemcitabine-based regimens between December 2001 and February 2011 at Sun Yat-sen University Cancer Center, were included in our study. All tumor biopsy specimens were obtained from the nasopharynx, except for three samples, which were from the cervical lymph nodes. Patient information included age, gender, histopathologic type, performance status, metastases, previous treatment, date of treatment with gemcitabine, cycles of gemcitabine-based chemotherapy, date of progression, and date of death/last follow-up. All patients were required to have no less than a measurable tumor lesion and were treated with gemcitabine-based chemotherapy for a minimum of two cycles, either alone or in combination. Performance status ≤ 2 was also allowed. Signed informed consent was obtained from each patient. The study was approved by the Research Ethics Committee of the Sun Yat-sen University Cancer Center.
Study design
All patients underwent pretreatment assessments, including a complete medical history, physical examination, appropriate imaging examinations (chest radiograph or computed tomography of the thorax and upper abdomen, and magnetic resonance imaging of the nasopharynx), hematologic and biochemical tests. Bone scan was performed for patients with symptoms suggestive of bone metastases. Hematologic and biochemical tests were repeated at the beginning of each treatment cycle. Patients underwent gemcitabine-based chemotherapy: gemcitabine (1000 mg/m2) on days 1 and 8 plus cisplatin (80 mg/m2) or carboplatin (area under curve of 5) on day 1, every 3 weeks; gemcitabine (1000 mg/m2) on days 1 and 8 plus vinorelbine (25 mg/m2) on days 1 and 8, every 3 weeks; gemcitabine (1000 mg/m2) on days 1 and 8 plus docetaxel (75 mg/m2) on day 1, every 3 weeks; gemcitabine (1000 mg/m2) on days 1 and 8 plus xeloda (2500 mg/m2) from day 1 to day 14, every 3 weeks; or intravenous gemcitabine (1000 mg/m2) alone once a week, every 4 weeks. Treatment was continued until disease progression or unacceptable toxic effects were observed. As the shape of nasopharyngeal neoplasm was irregular, tumor response was classified according to standard WHO response criteria[24] and was evaluated after every two cycles of treatment. The response rate was defined as the total number of patients who achieved complete response (CR) and partial response (PR) divided by the number of all evaluable patients. Overall survival (OS) was measured from the beginning of chemotherapy to the date of death or last follow-up. Progression-free survival (PFS) was defined as the duration from the beginning of chemotherapy to the date of tumor progression or death.
Immunohistochemistry
Immunohistochemical (IHC) staining was performed using monoclonal mouse anti-human RRM1 (Abnova, Taiwan). The detailed operation flow was similar to that published by Wang et al.[25]. Sections of 4-µm thick were cut from each of the 48 paraffin-embedded tumor tissues and then routinely deparaffinized and rehydrated. For antigen retrieval, sections were heated in a microwave oven for 30 min in citrate buffer solution (0.01 mmol/L, pH 6.0) and cooled at room temperature for 20 min in phosphate-buffered saline (PBS). After blocking the endogenous peroxidase activity with 0.3% hydrogen peroxidase in PBS for 30 min, the sections were treated with primary antibodies diluted at 1:100 in PBS (containing 1% bovine serum albumin and 0.1% sodium azide, pH 7.4) for 60 min at room temperature. Subsequently, the sections were rinsed in PBS three times and incubated in biotinylated secondary antibodies. After incubation, sections were washed again with PBS and then visualized using diaminobenzidine. Finally, Mayer's hematoxylin was used to counterstain the sections, which were then dehydrated and mounted. For a negative control, distilled water was used in place of primary antibodies during the IHC staining process. For a positive control, sections of formalin-fixed, paraffin-embedded human placenta were used.
RRM1 expression level was assessed based on proportion. The percentage of positive tumor cytoplasm was evaluated for each specimen in five fields at x400 magnification. Two pathologists with no knowledge of the patients' outcome assessed protein expression independently. A specimen in which more than 10% of cells had positive cytoplasm staining was considered positive[25].
Statistical analysis
The relationship between RRM1 expression level, clinicopathologic variables, and response to chemotherapy were evaluated using the Chi-square (χ2) test or Fisher's exact test. The Kaplan-Meier method was used to estimate OS and PFS. The differences in survival between patients with distinct clinicopathologic characteristics were compared with the log-rank test. Independent prognostic factors for PFS were estimated in multivariate Cox proportional hazards regression analysis. The significant factors in univariate analysis were contained in the multivariate model. All statistical analyses were performed using Statistical Program for Social Sciences (SPSS) version 16.0 software. P values were two-sided and considered significant if <0.05.
Results
Patient characteristics
The median age of the 48 patients was 47 years (range, 26–77 years), with 38 (79%) men and 10 (21%) women. All patients had stage IV cancer, Eastern Cooperative Oncology Group performance status (ECOG PS) 0–1, and distant metastasis. All were classified as having WHO Type 2B (undifferentiated non-keratinizing carcinoma) NPC. Thirty-three patients were previously treated with radiotherapy. The median number of cycles of gemcitabine-based chemotherapy and previous chemotherapy were 4 (range, 2–11) and 6 (range, 0–16), respectively. In total, 13 patients previously underwent one regimen of chemotherapy, and 31 patients underwent no less than two regimens. All patients underwent therapy consisting of gemcitabine in combination with platinum (24 patients), vinorelbine (5 patients), docetaxel (3 patients), or xeloda (5 patients), or intravenous gemcitabine monotherapy (11 patients). The clinical characteristics of the 48 patients are shown in Table 1.
Table 1. Clinical characteristics of 48 patients with nasopharyngeal carcinoma (NPC).
| Characteristic | No. of patients (%) |
| Gender | |
| Men | 38 (79) |
| Women | 10 (21) |
| Age (years) | |
| ≥ 47 | 22 (46) |
| < 47 | 26 (54) |
| ECOG performance status | |
| 0 | 28 (58) |
| 1 | 20 (42) |
| Prior radiotherapy | |
| Yes | 33 (69) |
| No | 15 (31) |
| Prior chemotherapy cycles | |
| ≤ 6 | 29 (60) |
| > 6 | 19 (40) |
| Number of prior regimens | |
| 1 | 13 (27) |
| ≥ 2 | 31 (65) |
| Number of distant metastases | |
| 1 | 26 (54) |
| ≥ 2 | 22 (46) |
| Gemcitabine cycles | |
| ≤ 4 | 33 (69) |
| > 4 | 15 (31) |
ECOG, Eastern Cooperative Oncology Group.
Association of clinicopathologic features with RRM1 expression
Representative examples of immunohistochemistry with monoclonal mouse anti-human RRM1 are shown in Figure 1. Of the 48 patients, RRM1 expression was positive in 17 (35%) patients and negative in 31 (65%) patients. The level of RRM1 expression was not associated with any clinicopathologic features. There were no significant differences in gender (P = 0.975), age (P = 0.632), performance status (P =0.202), number of distant metastases (P =0.464), cycles of gemcitabine-based chemotherapy (P = 0.839), prior radiotherapy (P = 0.272), prior chemotherapy cycles (P = 0.867), and number of prior regimens (P = 0.339) between the two groups (Table 2).
Figure 1. Immunohistochemical staining of ribonucleotide reductase subunit M1 (RRM1) in tumor samples from 48 patients with nasopharyngeal carcinoma (NPC).
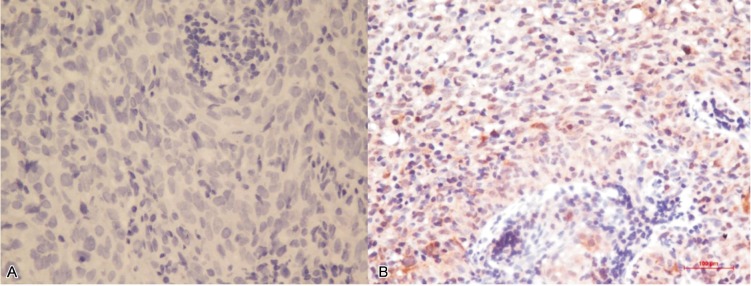
A, representative image of an RRM1-negative sample shows no brown granules in the cytoplasm of tumor cells (400×). B, representative image of an RRM1-positive sample shows brown granules in the cytoplasm of tumor cells (400×).
Table 2. Association between RRM1 expression and clinicopathologic features of 48 NPC patients.
| Characteristic | RRM1–negative | RRM1-positive | χ2 test P value |
| Gender | 0.975 | ||
| Men | 24 | 14 | |
| Women | 7 | 3 | |
| Age (years) | 0.632 | ||
| ≥ 47 | 15 | 7 | |
| < 47 | 16 | 10 | |
| ECOG performance status | 0.202 | ||
| 0 | 16 | 12 | |
| 1 | 15 | 5 | |
| Prior radiotherapy | 0.272 | ||
| Yes | 23 | 10 | |
| No | 8 | 7 | |
| Prior chemotherapy cycles | 0.867 | ||
| ≤ 6 | 19 | 10 | |
| > 6 | 12 | 7 | |
| Number of prior chemotherapy regimens | 0.339 | ||
| 1 | 10 | 3 | |
| ≥ 2 | 18 | 13 | |
| Number of distant metastases | 0.464 | ||
| 1 | 18 | 8 | |
| ≥ 2 | 13 | 9 | |
| Gemcitabine cycles | 0.839 | ||
| ≤ 4 | 21 | 12 | |
| > 4 | 10 | 5 |
Footnotes as in Table 1
Association of RRM1 expression and responses to chemotherapy
All 48 patients underwent at least two cycles of gemcitabine-based chemotherapy. CR was observed in 1 (2%) patient, PR in 21 (44%) patients, SD in 21 (44%) patients, and PD in 5 (10%) patients. The response rate of the RRM1-positive group was slightly lower than that of the RRM1-negative group (51.6% vs. 35.3%, P = 0.278) (Table 3).
Table 3. Association between RRM1 expression and responses to chemotherapy in 48 NPC patients.
| Response | RRM1-negative (n = 31) | RRM1-positive (n = 17) | P value |
| Complete response (case) | 0 | 1 | |
| Partial response (case) | 16 | 5 | |
| Stable disease (case) | 11 | 10 | |
| Progressive disease (case) | 4 | 1 | |
| Response rate (%) | 16 | 6 | 0.278 |
Response rate includes complete response and partial response.
Association of RRM1 expression and clinical outcomes
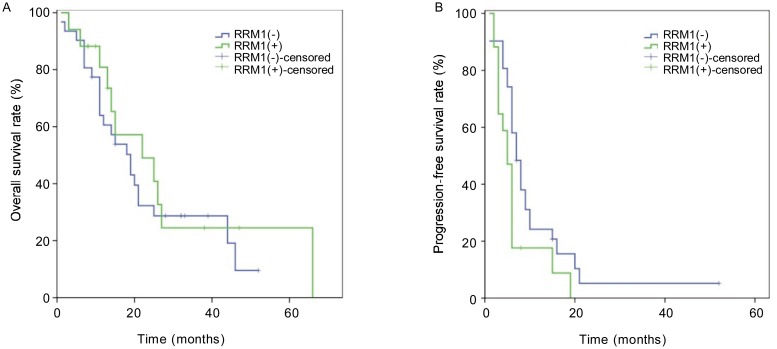
At the time of analyzing the level of RRM1 expression and patient clinical outcome (October 2011), the median follow-up time was 32 months. In total, 34 patients died, 6 were alive, and 8 were lost to follow-up. The median OS and PFS of all patients was 19 months (95% CI, 13.0–24.9 months) and 6 months (95% CI, 5.3–6.7 months), respectively. In the RRM1-negative and RRM1-positive arms, the median survival was 19 months and 22 months, respectively, but this difference was not significant (P = 0.540) (Figure 2A). In contrast, the median PFS was 7 months in the RRM1-negative arm and 5 months in the RRM1-positive arm, and this difference was significant (P = 0.036) (Figure 2B).
Figure 2. Kaplan-Meier survival curves of 17 RRM1-positive and 31 RRM1-negative patients with NPC.
A, RRM1-positive expression is not associated with poor overall survival of patients with NPC (22 months vs. 19 months, P = 0.540); B, RRM1-positive expression is associated with poor progression-free survival in patients with NPC (5 months vs. 7 months, P = 0.036).
Gender, age, performance status, number of distant metastases, cycles of gemcitabine-based chemotherapy, previous treatment, and expression of RRM1 were included in the univariate analysis for PFS. Among these factors, the expression of RRM1 and the number of distant metastases were significant (P < 0.05). The multivariate analysis showed that the expression of RRM1 (hazard ratio, 2.147; 95% CI, 1.097–4.200; P = 0.026) and the number of distant metastases (hazard ratio, 1.859; 95% CI, 1.225–2.842; P = 0.004) were independent prognostic factors for PFS.
Discussion
Our current study indicated that the level of RRM1 expression as determined by IHC staining was significantly associated with the PFS of patients with advanced NPC treated by gemcitabine. The results also indicate that testing RRM1 expression level may be useful for selecting patients with advanced NPC who will benefit from gemcitabine-based chemotherapy.
To our knowledge, primary or secondary drug resistance is the most common factor for treatment failure and is a key hurdle in anticancer treatment. In clinical practice, interindividual responses to the same chemotherapeutic agents or regimens vary. Pharmacogenomics studies have shown that clinical variables (e.g., age, gender, diet, drug-drug interactions) and genetic factors (e.g., drug metabolism, distribution, genetic polymorphism of related receptors) play a critical role in treatment responses [26],[27]. Therefore, the determination of a molecular index is important in establishing personalized treatments and improving curative effects. Gemcitabine is a cell cycle-specific antimetabolite that is converted into two active metabolites that inhibit DNA synthesis. Preclinical and clinical studies on NSCLC and pancreatic cancer have shown that RRM1 is a cellular target for gemcitabine and that the level of RRM1 expression is associated with clinical outcomes [22],[28],[29]. Given these results, we investigated the expression of RRM1 and its association with sensitivity to gemcitabine-based chemotherapy to provide the theoretical basis for gemcitabine-based individualized treatment in advanced NPC.
In the current study, we found no association of RRM1 expression with age, sex, performance status, or histological type, which was accordant with prior results in NSCLC[30]–[32]. Rosell et al.[29] estimated RRM1 mRNA expression in advanced NSCLC treated with gemcitabine and cisplatin and found that patients in the low RRM1 expression arm had significant advantages in OS, PFS, and response rate compared with patients in the high RRM1 expression arm. Other prior clinical studies also revealed that patients with negative RRM1 expression benefit significantly from gemcitabine-based regimens[29],[31],[32]. The most significant finding of our study was the predictive value of low RRM1 expression in NPC patients treated with gemcitabine-based chemotherapy. In the present study, 65% of patients were RRM1-negative and showed longer median PFS than did RRM1-positive patients (7 months vs. 5 months, P = 0.036). Moreover, multivariate analysis indicated that the RRM1 expression may be a useful prognostic factor for advanced NPC patients treated with gemcitabine-based chemotherapy.
Nevertheless, we found no association between RRM1 expression level and OS or response rate. There are several possible explanations for this contradiction. First, our study included a small sample size, making it difficult to achieve expected results. Second, this was a retrospective analysis with confounding factors that could affect the accuracy of the results. For example, the status of patients who were lost to follow-up, the response to prior therapy, and the subsequent therapy after finishing gemcitabine-based chemotherapy were high impact factors in our results. Third, the interaction of chemotherapy combinations may also play a role in the conflicting results. Rosell et al.[29] investigated the relationship between RRM1 mRNA expression and the efficacy of gemcitabine combined with platinum on advanced NSCLC and showed increased survival in patients with low RRM1 expression. However, there was no significant difference in OS between the low and high expression groups when vinorelbine was added to the combination or combined with gemcitabine only. In our study, 5 patients were treated with gemcitabine plus vinorelbine, 3 with gemcitabine plus docetaxel, and 5 with gemcitabine plus xeloda. In view of the study by Rosell et al.[29], the addition of docetaxel or xeloda to gemcitabine may produce a similar effect as vinorelbine.
Despite these limitations, this is the first study, as is known to us, to evaluate the relationship between the level of RRM1 and sensitivity to gemcitabine-based chemotherapy in advanced NPC. Furthermore, our study validated positive expression of RRM1 as an independent factor of poor prognosis.
Conclusion
Our findings indicate that negative RRM1 expression as determined using IHC was consistent with better PFS in advanced NPC patients treated with gemcitabine-based regimens. However, perspective studies are still needed to confirm that RRM1 expression level can be used to guide treatment choice in large patient cohorts.
References
- 1.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Chan AT, Teo PM, Johnson PJ. Nasopharyngeal carcinoma. Ann Oncol. 2002;13:1007–1015. doi: 10.1093/annonc/mdf179. [DOI] [PubMed] [Google Scholar]
- 3.O'Sullivan B. Nasopharynx cancer: therapeutic value of chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2007;69:S118–S221. doi: 10.1016/j.ijrobp.2007.04.085. [DOI] [PubMed] [Google Scholar]
- 4.Baujat B, Audry H, Bourhis J, et al. Chemotherapy in locally advanced nasopharyngeal carcinoma: an individual patient data meta-analysis of eight randomized trials and 1753 patients. Int J Radiat Oncol Biol Phys. 2006;64:47–56. doi: 10.1016/j.ijrobp.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 5.Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol. 1998;16:1310–1317. doi: 10.1200/JCO.1998.16.4.1310. [DOI] [PubMed] [Google Scholar]
- 6.Chan AT, Felip E. Nasopharyngeal cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20:123–125. doi: 10.1093/annonc/mdp150. [DOI] [PubMed] [Google Scholar]
- 7.Fandi A, Bachouchi M, Azli N, et al. Long-term disease-free survivors in metastatic undifferentiated carcinoma of nasopharyngeal type. J Clin Oncol. 2000;18:1324–1330. doi: 10.1200/JCO.2000.18.6.1324. [DOI] [PubMed] [Google Scholar]
- 8.Rosell R, Cecere F, Santarpia M, et al. Predicting the outcome of chemotherapy for lung cancer. Curr Opin Pharm. 2006;6:323–331. doi: 10.1016/j.coph.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Crinò L, Foglietta J, Hamzaj A. Lung cancer. J Thorac Oncol. 2007;2:24–26. doi: 10.1097/01.JTO.0000268637.10332.e3. [DOI] [PubMed] [Google Scholar]
- 10.Shi X, Yuan X, Tao D, et al. Analysis of DNA ploidy, cell cycle and Ki67 antigen in nasopharyngeal carcinoma by flow cytometry. J Huazhong Univ Sci Technolog Med Sci. 2005;25:198–201. doi: 10.1007/BF02873576. [DOI] [PubMed] [Google Scholar]
- 11.Zhao GQ, Xu Y, Wang Q. Significance of serum vascular endothelial growth factor test before radiotherapy in patients with nasopharyngeal carcinoma. Zhong Xi Yi Jie He Xue Bao. 2005;3:274–277. doi: 10.3736/jcim20050408. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 12.Mai HQ, Zeng ZY, Zhang CQ, et al. Elevated plasma big ET-1 is associated with distant failure in patients with advanced-stage nasopharyngeal carcinoma. Cancer. 2006;106:1548–1553. doi: 10.1002/cncr.21790. [DOI] [PubMed] [Google Scholar]
- 13.Doustjalali SR, Yusof R, Govindasamy GK, et al. Patients with nasopharyngeal carcinoma demonstrate enhanced serum and tissue ceruloplasmin expression. J Med Invest. 2006;53:20–28. doi: 10.2152/jmi.53.20. [DOI] [PubMed] [Google Scholar]
- 14.Cho WC, Yip TT, Yip C, et al. Identification of serum amyloid a protein as a potentially useful biomarker to monitor relapse of nasopharyngeal cancer by serum proteomic profiling. Clin Cancer Res. 2004;10:43–52. doi: 10.1158/1078-0432.ccr-0413-3. [DOI] [PubMed] [Google Scholar]
- 15.Fan H, Huang A, Villegas C, et al. The R1 component of mammalian ribonucleotide reductase has malignancy-suppressing activity as demonstrated by gene transfer experiments. Proc Natl Acad Sci USA. 1997;94:13181–13186. doi: 10.1073/pnas.94.24.13181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L, Zhang Y, Huang PY, et al. Phase II clinical study of gemcitabine in the treatment of patients with advanced nasopharyngeal carcinoma after the failure of platinum-based chemotherapy. Cancer Chemother Pharmacol. 2008;61:33–38. doi: 10.1007/s00280-007-0441-8. [DOI] [PubMed] [Google Scholar]
- 17.Ma BB, Tannock IF, Pond GR, et al. Chemotherapy with gemcitabine-containing regimens for locally recurrent or metastatic nasopharyngeal carcinoma. Cancer. 2002;95:2516–2523. doi: 10.1002/cncr.10995. [DOI] [PubMed] [Google Scholar]
- 18.Ngan RK, Yiu HH, Lau WH, et al. Combination gemcitabine and cisplatin chemotherapy for metastatic or recurrent nasopharyngeal carcinoma: report of a phase II study. Ann Oncol. 2002;13:1252–1258. doi: 10.1093/annonc/mdf200. [DOI] [PubMed] [Google Scholar]
- 19.Foo KF, Tan EH, Leong SS, et al. Gemcitabine in metastatic nasopharyngeal carcinoma of the undifferentiated type. Ann Oncol. 2002;13:150–156. doi: 10.1093/annonc/mdf002. [DOI] [PubMed] [Google Scholar]
- 20.Davidson JD, Ma L, Flagella M, et al. An increase in the expression of ribonucleotide reductase large subunit 1 is associated with gemcitabine resistance in non-small cell lung cancer cell lines. Cancer Res. 2004;64:3761–3766. doi: 10.1158/0008-5472.CAN-03-3363. [DOI] [PubMed] [Google Scholar]
- 21.Bergman AM, Eijk PP, Ruiz VHV, et al. In vivo induction of resistance to gemcitabine results in increased expression of ribonucleotide reductase subunit M1 as the major determinant. Cancer Res. 2005;65:9510–9516. doi: 10.1158/0008-5472.CAN-05-0989. [DOI] [PubMed] [Google Scholar]
- 22.Bepler G, Kusmartseva I, Sharma S, et al. RRM1 modulated in vitro and in vivo efficacy of gemcitabine and platinum in non-small-cell lung cancer. J Clin Oncol. 2006;24:4731–4737. doi: 10.1200/JCO.2006.06.1101. [DOI] [PubMed] [Google Scholar]
- 23.Nakahira S, Nakamori S, Tsujie M, et al. Involvement of ribonucleotide reductase M1 subunit overexpression in gemcitabine resistance of human pancreatic cancer. Int J Cancer. 2007;120:1355–1363. doi: 10.1002/ijc.22390. [DOI] [PubMed] [Google Scholar]
- 24.Green S, Weiss GR. Southwest Oncology Group standard response criteria, endpoint definitions and toxicity criteria. Invest New Drugs. 1992;10:239–253. doi: 10.1007/BF00944177. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Zhao J, Yang L, et al. Positive expression of ERCC1 predicts a poorer platinum-based treatment outcome in Chinese patients with advanced non-small-cell lung cancer. Med Oncol. 2010;27:484–490. doi: 10.1007/s12032-009-9239-3. [DOI] [PubMed] [Google Scholar]
- 26.Evans WE, Relling MV. Pharmacogenomics: translating functional genomics into rational therapeutics. Science. 1999;286:487–491. doi: 10.1126/science.286.5439.487. [DOI] [PubMed] [Google Scholar]
- 27.Evans WE, McLeod HL. Pharmacogenomics—drug disposition, drug targets, and side effects. N Engl J Med. 2003;348:538–549. doi: 10.1056/NEJMra020526. [DOI] [PubMed] [Google Scholar]
- 28.Nakahira S, Nakamori S, Tsujie M, et al. Involvement of ribonucleotide reductase M1 subunit overexpression in gemcitabine resistance of human pancreatic cancer. Int J Cancer. 2007;120:1355–1363. doi: 10.1002/ijc.22390. [DOI] [PubMed] [Google Scholar]
- 29.Rosell R, Danenberg KD, Alberola V, et al. Ribonucleotide reductase messenger RNA expression and survival in gemcitabine/cisplatin-treated advanced non-small cell lung cancer patients. Clin Cancer Res. 2004;10:1318–1325. doi: 10.1158/1078-0432.ccr-03-0156. [DOI] [PubMed] [Google Scholar]
- 30.Simon G, Sharma A, Li X, et al. Feasibility and efficacy of molecular analysis-directed individualized therapy in advanced non-small-cell lung cancer. J Clin Oncol. 2007;25:2741–2746. doi: 10.1200/JCO.2006.08.2099. [DOI] [PubMed] [Google Scholar]
- 31.Ceppi P, Volante M, Novello S, et al. ERCC1 and RRM1 gene expressions but not EGFR are predictive of shorter survival in advanced non-small-cell lung cancer treated with cisplatin and gemcitabine. Ann Oncol. 2006;17:1818–1825. doi: 10.1093/annonc/mdl300. [DOI] [PubMed] [Google Scholar]
- 32.Bepler G, Sharma S, Cantor A, et al. RRM1 and PTEN as prognostic parameters for overall and disease-free survival in patients with non-small-cell lung cancer. J Clin Oncol. 2004;22:1878–1885. doi: 10.1200/JCO.2004.12.002. [DOI] [PubMed] [Google Scholar]