Abstract
The developments of medicine always follow innovations in science and technology. In the past decade, such innovations have made cancer-related targeted therapies possible. In general, the term “targeted therapy” has been used in reference to cellular and molecular level oriented therapies. However, improvements in the delivery and planning of traditional radiation therapy have also provided cancer patients more options for “targeted” treatment, notably stereotactic radiosurgery (SRS) and stereotactic body radiotherapy (SBRT). In this review, the progress and controversies of SRS and SBRT are discussed to show the role of stereotactic radiation therapy in the ever evolving multidisciplinary care of cancer patients.
Keywords: Targeted therapy, stereotactic radiosurgery, stereotactic body radiotherapy, dose density, radiation therapy, time-adjusted biologically effective dose
The publication of “The evolution of phase I trials in cancer medicine” has generated much interest in the oncology community[1]. This report has an excellent summary on the complexity and difficulty in oncology targeted therapeutic drug development.
Targeted Therapy in the Radiation Oncology Field
Most drugs used as targeted therapies are delivered through a bifaceted targeted delivery mechanism. Primary targeting involves the delivery of compounds to target organs such as through the blood or the lymph circulation system, or the direct delivery to tumor through mechanical approaches. The secondary targeting is the unique mechanism of the compound either in finding the target cell (e.g., the antibody aspect of rituximab targeting CD20+ cells) or in altering molecular or biochemical reactions that ultimately damage cancer cells [e.g., vascular epithelial growth factor (VEGF) inhibitors such as bevacizumab altering blood supply to tumors]. Unlike this type of targeted therapy, radiation therapy is in some ways more straightforward. The only targeting process is the precise and accurate delivery of ionizing radiation to the site of interest.
Evolving Controversy of Using Traditional Radiobiology to Guide “Targeted” Radiotherapy
The role of radiation therapy in cancer management has evolved over time. As a local therapy, radiation can be used in the definitive, neoadjuvant, and adjuvant settings, often in conjunction with chemotherapy. In all instances, the treatment strategy balances the need to deliver a potentially curative dose of radiation while attempting to minimize acute and late toxicities. It is difficult to evaluate the extent of cell destruction caused by external beam radiation treatment (EBRT), the most common radiotherapy. It is also difficult to standardize the radiation dose and schedule for different malignant histologies and tumor locations. To address the issue of radiation dose standardization using conventional fractionation (1.8–2 Gy/fraction), older calculation methods such as the linear quadratic (LQ) model were developed[2]. Using cell killing information collected in vitro, the LQ model assumes there are two components of radiation-induced cell destruction—one component proportional to dose and one proportional to the square of the dose[3]. The limitation of the LQ model became evident when higher-dose-per-fraction treatment strategies, such as GammaKnife stereotactic radiosurgery (SRS) and CyberKnife stereotactic body radiotherapy (SBRT), were developed. With the SRS and SBRT techniques, single doses are 10 times higher than doses routinely delivered with conventional radiation. Using these SRS and SBRT techniques, high doses can be given in 1 to 5 fractions with acceptable toxicities to organs at risk. The application of the LQ model for low-dose conventional fractions may not have the same consequences as the use of the model for SRS or SBRT. More specifically, the LQ equation possibly overestimates cell destruction, and it may not properly describe the cell survival curve for the high doses used in SRS or SBRT[3]. Equations calculating the biological effectiveness of different dose fractionations based on tumor histology have not yet been reported.
Comparing studies of SRS and SBRT can also be challenging. Studies may report equivalent prescription doses; however, differences in fractionation schedules can result in a substantial difference in the biologically effective dose (BED). BED can serve as a useful parameter for comparing the potency of two different fractionation schedules[4]. SBRT for early-stage prostate cancer is a good example for the usefulness of BED. A phase I/II study from Stanford University was designed using 36.25 Gy in 5 fractions for a low-risk group of patients. The 36.25 Gy dose was calculated using the BED equation. At the median follow-up time of 5 years, the toxicities and efficacy as measured by controlling biochemical failure were encouraging[5]. Nevertheless, the controversy over BED remains. Since it was first reported in 1989, BED has been modified to optimize its usage in dose-escalation studies, concurrent chemo-radiotherapy, brachytherapy, high-LET particle beams and radionuclide-targeted therapy, and to quantify treatments using ionizing radiation. In 2003, an overall treatment time factor was proposed, creating time-adjusted BED (tBED). Recently, dose density (a reduction in overall treatment time and increase in overall dose) and its validation were reported in a meta-analysis in lung cancer[6]. Notably, a 1-Gy increase in tBED correlates with a 3% increase in lung cancer patient survival, suggesting a relationship between tBED and survival. Further studies is needed to validate this finding. Application of tBED in other types of cancer also requires further study.
The understanding of radiobiology has also progressed. Traditionally, four “R” principals have been used to describe the responses of cells to irradiation: repopulation, redistribution, reoxygenation, and repair. At the cellular and molecular levels, the progress of understanding of the mechanism for different doses has been made to some extend. In terms of the cellular and molecular mechanisms of action for different doses of radiation are still not well understood. For ablative doses used in SRS and SBRT, endothelial cell apoptosis and changes in vasculature have been reported to play a role in cytotoxicity[7]. Although molecular changes in response to ablative doses have been reported[8], much remains to be explored to further understand how cells are killed.
Progress in Target-oriented Radiosurgery
Advances in radiation technology have made treating smaller and hard-to-target tumors possible while reducing radiation doses to organs at risk. SRS and SBRT require accuracy in delivery of high-dose radiation, patient immobilization, target localization, maneuvers to either limit or compensate for target movement (tracking software), and the use of stereotaxy. SRS and SBRT can be completed in 1 to 5 fractions. These techniques provide an option for patients who refuse surgery, have inoperable disease, or have previously underwent radiotherapy but have local recurrence. Using lung cancer as an example, the progress in software (planning system) and hardware (delivery system) for dosimetry is obvious (Figure 1).
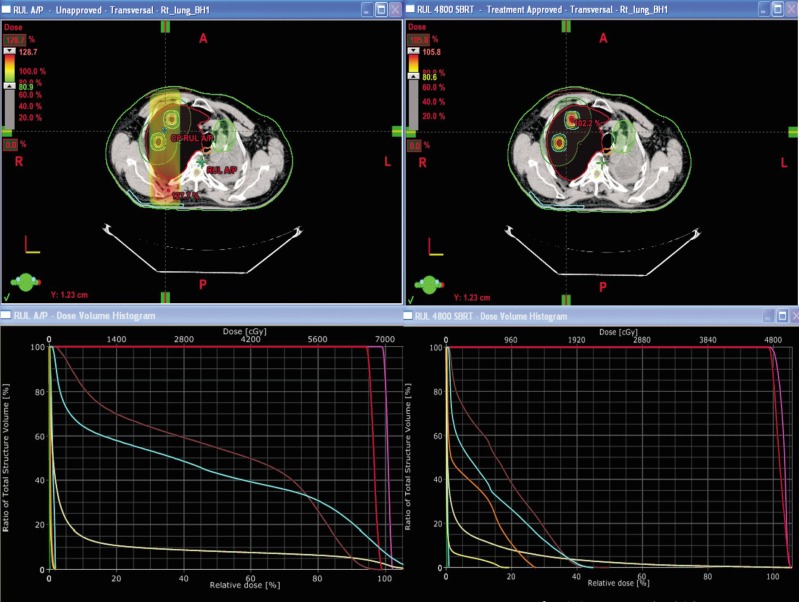
Figure 1. Comparison of conventional AP/PA two-beam plan vs. stereotactic body radiotherapy (SBRT) seven-beam plan for an 83-year-old lung cancer patient.
The patient, who previously underwent radiation treatment in the left upper lung, presented with two new primary lesions on the contralateral side. The upper panels show the transverse view of dose distribution, and the lower panels show the dose volume histogram (DVH). The left panels show the conventional plan, and the right panels show the SBRT plan. Both plans spared constrained left upper lung, but the conventional plan will cause significant toxicity to the skin and ribs. The SBRT plan has the advantage of delivering 1/3 of the V20 of the conventional plan (V20: 3.2% for the SBRT plan vs. 9.6% for the conventional plan).
Because of the high-dose radiation typically used in SRS and SBRT, it is critically important to ensure accuracy in delivery to the intended target. This requires rigorous quality control and assurance measures for treatment planning and delivery. Tumor sites tend to move (e.g., respiratory movement) within the body between fractionated treatments, causing difficulties in immobilizing the target tumor. Therefore, tumor-tracking techniques will continue to play an integral role in these techniques. Considerations for the selection of appropriate treatment candidates include prior radiation history of the affected tissues, treatment volume, organ function, capacity for recovery, number of disease sites, and other individual cancer-related factors[9].
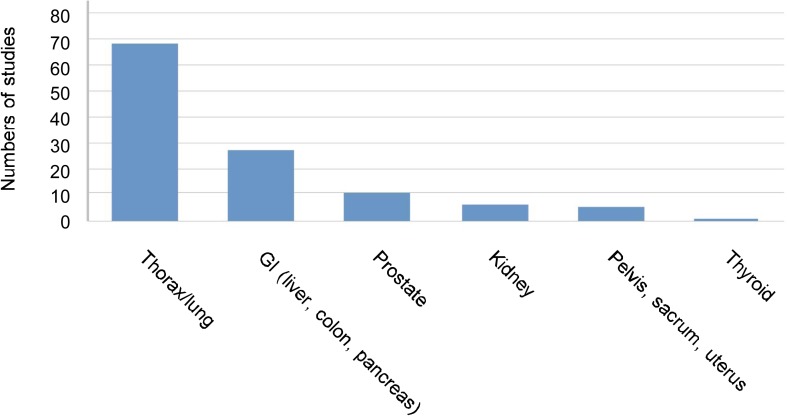
Clinical data on SRS and SBRT have become more prevalent in the literature. A summary of SBRT studies since 2000 is provided in Figure 2. The bulk of these studies focus on tumors of the lung/thorax (68 studies)[3], while fewer focus on tumors of the gastrointestinal tract (pancreas, liver, and colon; 27 studies) and other sites (uterus, pelvis, sacrum, kidney, prostate, and thyroid; <10 studies per site). Ten studies included multiple treatment sites. Collectively, the clinical data suggests that SRS and SBRT have the potential to improve clinical outcomes for cancer patients. For example, recent studies show that target-oriented radiosurgery may provide survival benefits compared with conventional radiation treatment for early-stage lung cancer patients who are candidates for radiation therapy (Figure 3). Currently, there are approximately 400 facilities equipped to perform SRS and SBRT in the United States.
Figure 2. Different sites of cancer have been studied using SBRT technique.
We performed a literature search on Medline using the key words “SBRT” and “cancer type.” The search was limited to patients who were medically inoperable or refused surgery. The search also limited to papers published in English between 2000 and 2009. The studies about thoracic/lung cancer account for the majority of the papers reviewed in the present study.
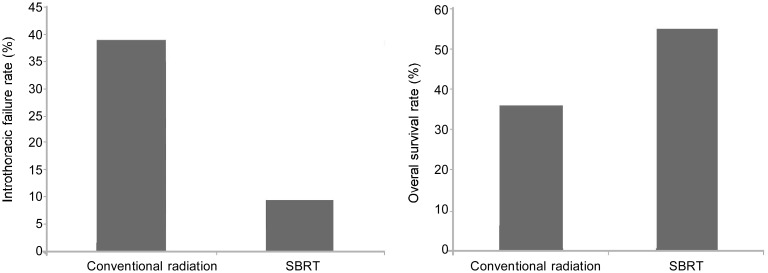
Figure 3. SBRT potentially improves survival for patients with early-stage lung cancer compared with conventional radiotherapy.
We compared data from a retrospective study from a single institute[11] and the RTOG 0236 study protocol[12] to determine the effect of conventional radiation vs. SBRT on lung cancer. In the retrospective study, conventional radiation was typically delivered using 1.8–2.75 Gy per fraction to a total dose of 54–70 Gy in 5–7 weeks. All patients had medically operable disease but underwent conventional radiation instead. The 3-year survival rate after conventional radiation was 36%, and the intrathoracic failure rate was 39%[11]. In the RTOG 0236 study, SBRT was typically delivered using 10–18 Gy per fraction to a total dose of 48–60 Gy in 3–5 fractions. All patients did not undergo surgery due to poor medical conditions. The dose actually delivered was closer to 54 Gy in 3 fractions of 18 Gy. The 3-year survival rate was 55% for non-surgical patients, and the local failure rate 9.4%[12].
Clinical Applications of “Targeted” Therapy and Phase III Studies
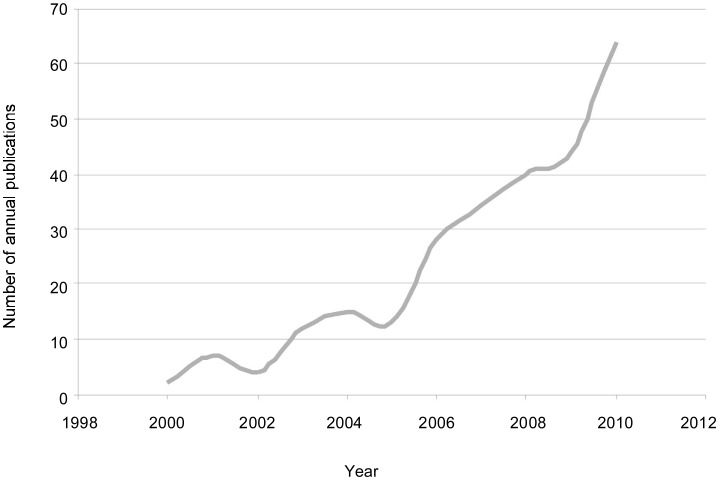
The controversy of using new innovations as standard treatments without phase III study validation was raised as early as 1996[10]. Radiation oncologists who favor technological innovation felt strongly that randomized trials were not unnecessary, the gain from the more highly conformal beams were self-evident. However, the question is not the magnitude of the gain but whether the gain is achievable. The reality is that widespread clinical practice using new techniques prior to their validation is already occurring. Searching Medline using key words “CyberKnife” and “cancer” and inputting a year of interest, we found that the number of clinical reports on CyberKnife treatment for cancer has increased over the past decade (Figure 4). However, with the exception of SBRT for lung cancers, there is no sufficient evidence that SRS and SBRT are superior to conventional radiation therapy in prolonging disease-free or overall survival. Thus, the 10-fold increase in literature reports for CyberKnife treatment within the past 10 years is likely because of the practical availability of SRS and SBRT techniques, rather than on the availability of supporting evidence of their effects. To date, few reports comparing the different techniques have been published to help settle this controversy. Nevertheless, additional phase I/II data are anticipated to support the need for phase III trials.
Figure 4. The number of published studies on CyberKnife treatment for cancer markedly increased over the past decade.
We performed a literature search on Medline using the key words “CyberKnife” and “cancer.” The search was limited to papers published in English between 2000 and 2010. Over this period, the number of published papers increased significantly.
Conclusions
In the future, the challenging task of optimizing cancer care will be multifactorial, with goals of improving quality of care by reducing toxicities, reducing physiological stress by prolonging disease-free survival, and ultimately increasing overall survival. Innovative approaches for “targeted” therapy in radiation oncology, like those in drug development, are expected to facilitate this task. Nevertheless, such innovations should be required to meet the standard of proof-of-benefit in a randomized prospective clinical trial before being introduced into widespread clinical practice.
Acknowledgments
The authors wish to thank all members of Radiation Oncology Department at Zangmeister Cancer Center and Columbus CyberKnife, Mount Carmel St Anns Hospital who contributed to the discussion of this publication, particular for Eric Willis and Ken Notter for their valuable assistance.
References
- 1.Tolcher AW. The evolution of phase I trails in cancer medicine: a critical review of the last decade. Chin J Cancer. 2011;30:815–818. doi: 10.5732/cjc.011.10133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fowler JF. 21 years of biologically effective dose. Br J Radiol. 2010;83:554–568. doi: 10.1259/bjr/31372149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang BK, Timmerman RD. Stereotactic body radiation therapy: a comprehensive review. Am J Clin Oncol. 2007;30:637–644. doi: 10.1097/COC.0b013e3180ca7cb1. [DOI] [PubMed] [Google Scholar]
- 4.Kavanagh BD, Timmerman RD. Stereotactic radiosurgery and stereotactic body radiation therapy: an overview of technical considerations and clinical applications. Hematol Oncol Clin North Am. 2006;20:87–95. doi: 10.1016/j.hoc.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 5.King C. Stereotactic body radiotherapy for prostate cancer: current results of a phase II trial. Front Radiat Ther Oncol. 2011;43:428–437. doi: 10.1159/000322507. [DOI] [PubMed] [Google Scholar]
- 6.Machtay M, Bae K, Movsas B, et al. Higher biologically effective dose of radiotherapy is associated with improved outcomes for locally advanced non-small cell lung carcinoma treated with chemoradiation: an analysis of the radiation therapy oncology group. Int J Radiat Oncol Biol Phys. 2012;82:425–434. doi: 10.1016/j.ijrobp.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 8.Zeng M, Narayanan L, Xu XS, et al. Ionizing radiation-induced apoptosis via separate Pms2- and p53-dependent pathways. Cancer Res. 2000;60:4889–4893. [PubMed] [Google Scholar]
- 9.Meyer JL, Verhey L, Xia P, et al. New technologies in the radiotherapy clinic. Front Radiat Ther Oncol. 2007;40:1–17. doi: 10.1159/000106025. [DOI] [PubMed] [Google Scholar]
- 10.Rosenthal DI, Glatstein E. We've got a treatment, but what's the disease? Or a brief history of hypofractionation and its relationship to stereotactic radiosurgery. Oncologist. 1996;1:1–7. [PubMed] [Google Scholar]
- 11.Haffty BG, Goldberg NB, Gerstley J, et al. Results of radical radiation therapy in clinical stage I, technically operable non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 1988;15:69–73. doi: 10.1016/0360-3016(88)90348-3. [DOI] [PubMed] [Google Scholar]
- 12.Timmerman R, Paulus RB, Galvin J. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]