Abstract
Currently, image-based 3-dimentional (3D) planning brachytherapy allows for a better assessment of gross tumor volume (GTV) and the definition and delineation of target volume in cervix cancer. In this study, we investigated the feasibility of our novel computed tomography (CT)-guided free-hand high-dose-rate interstitial brachytherapy (HDRISBT) technique for cervical cancer by evaluating the dosimetry and preliminary clinical outcome of this approach. Dose-volume histogram (DVH) parameters were analyzed according to the Gynecological GEC-ESTRO Working Group recommendations for image-based 3D treatment in cervical cancer. Twenty cervical cancer patients who underwent CT-guided free-hand HDRISBT between March 2009 and June 2010 were studied. With a median of 5 (range, 4–7) implanted needles for each patient, the median dose of brachytherapy alone delivered to 90% of the target volume (D90) was 45 (range, 33–54) Gyα/β10 for high-risk clinical target volume (HR-CTV) and 30 (range, 20–36) Gyα/β10 for intermediate-risk clinical target volume (IR-CTV). The percentage of the CTV covered by the prescribed dose (V100) of HR-CTV with brachytherapy alone was 81.9%–99.2% (median, 96.7%). With an additional dose of external beam radiotherapy (EBRT), the median D90 was 94 (range, 83–104) Gyα/β10 for HR-CTV and 77 (range, 70–87) Gyα/β10 for IR-CTV; the median dose delivered to 100% of the target volume (D100) was 75 (range, 66–84) Gyα/β10 for HR-CTV and 65 (range, 57–73) Gyα/β10 for IR-CTV. The minimum dose to the most irradiated 2 cc volume (D2cc) was 73–96 (median, 83) Gyα/β3 for the bladder, 64–98 (median, 73) Gyα/β3 for the rectum, and 52–69 (median, 61) Gyα/β3 for the sigmoid colon. After a median follow-up of 15 months (range, 3–24 months), two patients experienced local failure, and 1 showed internal iliac nodal metastasis. Despite the relatively small number of needles used, CT-guided HDRISBT for cervical cancer showed favorable DVH parameters and clinical outcome.
Keywords: Cervical carcinoma, radiotherapy, high-dose-rate, brachytherapy, dose-volume histogram
A combination of external beam radiotherapy (EBRT), brachytherapy, and chemotherapy is the standard treatment for advanced cervical cancer[1]. Conventional Manchester System-based intracavitary brachytherapy (ICBT) results in the unnecessary irradiation of normal tissues, especially in patients with small tumor masses. Image-based brachytherapy enables radiation oncologists to precisely prescribe radiation doses for tumors, without increasing unnecessary irradiation to normal tissues, which may reduce normal tissue morbidity. However, image-based ICBT has been limited in treating tumors that are large or laterally extended over the Manchester System-specified point-A. The radiation source dwell time of the three applicators (tandem and two ovoids) should be increased, which means that the normal tissue morbidity may increase. With improved tumor coverage and lower doses delivered to organs at risk (OARs), interstitial brachytherapy (ISBT) can provide good local control without serious complications[2]–[4].
Historically, various interstitial needle placement techniques have been developed for performing ISBT[5]–[7]. In the current study, a novel computed tomography (CT)-guided free-hand ISBT approach using plastic flexible needle applicators was developed. To evaluate the feasibility of our CT-guided ISBT, dose-volume parameters were analyzed according to the recommendations of the Gynecological GEC-ESTRO Working Group for image-based treatment planning in cervical cancer brachytherapy[8],[9].
Methods and Materials
Patient selection
Between April 2009 and July 2010, cervical cancer patients who had bulky parametrial extension, bulky primary disease, extensive paravaginal or distal vaginal involvement, which were unlikely to be encompassed sufficiently by intracavitary application, underwent interstitial implantation in the Department of Radiology, Sun Yat-sen University Cancer Center. Clinical staging included physical examination, tissue biopsy, magnetic resonance imaging (MRI) of the pelvis, and chest radiography. Written informed consent was obtained from each patient before treatment.
Treatment strategy
All patients underwent EBRT to the entire pelvis using a four-field box technique with CT-based treatment planning. Extended field radiation was delivered for patients with para-aortic nodal metastasis. An additional boost irradiation was delivered to pelvic lymph node metastases to achieve a total dose of 60 Gy. The patients underwent ISBT following EBRT. Concurrent platinum-based chemotherapy was administered when appropriate.
Applicator implantation
Needle implantation was performed under epidural anesthesia in the CT scan room with the perineum prepped and draped. An intravenous analgesia pump was used for postoperative pain management. The patient was placed in the dorsal lithotomy position, and pelvic examination was performed to assess the dimensions of the tumor, the degree of tumor extension, and its proximity to other pelvic organs. A Foley catheter (Well Lead Medical Co., Guangzhou, China) was inserted, and 7 mL of sterile water diluted with 1 mL of contrast medium (Lopamiro, Shanghai, China) was injected into the catheter balloon. A sterile speculum was inserted into the vagina to expose the vagina and tumor. If the endocervical canal was patent, a single flexible plastic tube applicator with a curved metallic obturator was inserted into the uterine cavity. Subsequently, transvaginal plastic needles with metallic obturators were inserted into the tumor to a depth of 1–2 cm, as determined by the initial pelvic examination. CT-guided imaging was used for subsequent needle implantation. An oral contrast agent was administered on the day before the implantation procedure to accurately delineate the rectum. CT scans were performed with Philips Medical Systems (Cleveland, USA) to demonstrate where and how far the previously positioned needles needed to be inserted and whether more needles were required in a supine position. The needles were inserted stepwise, followed by a CT scan to guide insertion throughout the procedure. When necessary, transperineal needles were inserted to maximize the tumor coverage rate. These needles were fixed using a button stopper, which was sutured to the perineum. When satisfactory tumor coverage had been achieved, button stoppers fixed to plastic tubes and needles were attached to the cervix. The vagina was packed with iodoform gauze to prevent needle movement. To minimize the effects of fluctuating bladder size on dose distribution[10], 300 mL of sterile saline was injected into the bladder through a Foley catheter. This process was repeated before the delivery of each fraction. After injection, a series of sliced 5-mm CT images from the top of the pelvic brim to the lower edge of the ischial tuberosity were obtained with the patient in the supine position. CT images were transferred to the Plato system (Patient Selection System v3.4.0, Nucletron Systems, Veenendaal, the Netherlands) for treatment planning. The ISBT application characteristics were estimated by analyzing the CT simulation planning images. The depth of needle insertion was equal to the distance between the tissue surface and needle tips on axial CT images.
Contouring and brachytherapy planning
For all patients, gross tumor volume (GTV), high-risk clinical target volume (HR-CTV), and intermediate-risk clinical target volume (IR-CTV) were assessed according to the GYN GEC-ESTRO recommendations[8]. To delineate these target volumes, MR images that were obtained at the time of diagnosis were used as the reference. The GTV was determined by a radiation oncologist as the macroscopic extent of the tumor at the time of ISBT. The HR-CTV included the entire cervix, the GTV, and the presumed extracervical tumor extension at the time of EBRT. The IR-CTV encompassed either tumor extension at diagnosis or a margin (0.5–1 cm) around the HR-CTV. The delineation of OARs was defined as a wall thickness of 2 mm for the bladder and 4 mm for the rectum and sigmoid colon.
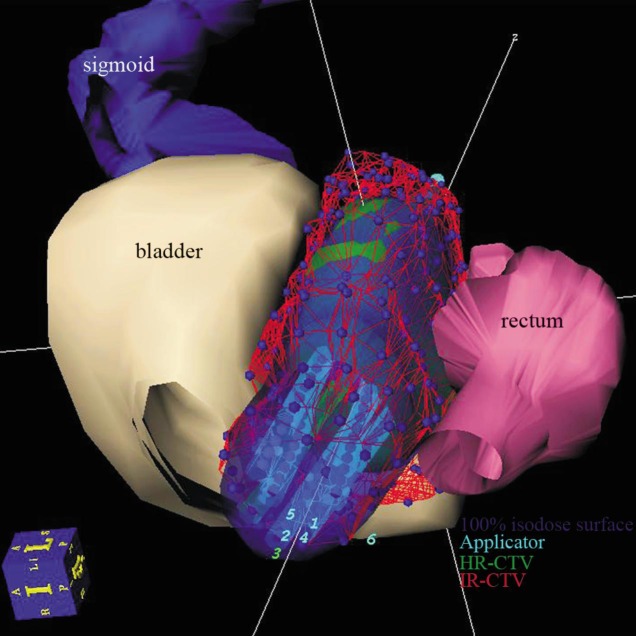
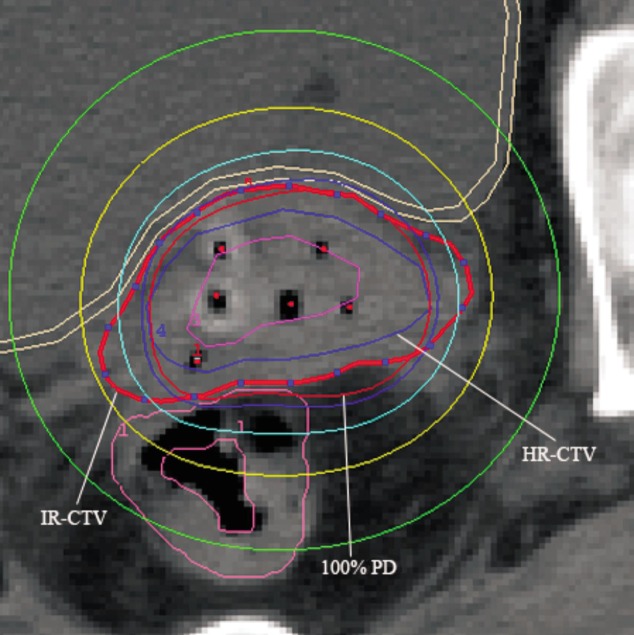
Once the target and OAR volumes were delineated, the catheters were reconstructed by marking the implanted needles on each slice (Figure 1). The treatment plan was generated using a step size of 2.5 mm, with the radiation dose prescribed at the periphery of the target volume and not prescribed to a particular point. The 100% isodose surface of the prescription dose was normalized to cover the HR-CTV (Figure 2). Dwell positions and dwell weights were manually modified after geometric and dose point optimization until dose-volume constraints were optimally matched.
Figure 1. A three-dimensional image of organs at risk (OARs) and target tissues in a 59-year-old patient with stage IIIb cervical squamous cell carcinoma, as defined on CT scans.
Six needles were implanted in this patient, and the 100% isodose surface adequately covered the high-risk clinical target volume (HR-CTV) while avoiding the inappropriate cover of OARs.
Figure 2. The dose-distribution curves of the patient from Figure 1.
IR-CTV, intermediate-risk clinical target volume; 100% PD, 100% prescribed dose; HR-CTV, high-risk clinical target volume. The HR-CTV was adequately covered by the 100% prescribed isodose surface.
A total dose of 32 Gy by 8 fractions was prescribed to all patients, who were treated twice daily using HDR Ir-192 brachytherapy over 4 days (minimum 6-hour intervals between fractions). Implant needle tubes were removed immediately after the treatment was completed. Routine antibiotics were prescribed during the treatment period. The patient was rescanned using the same protocol as the initial CT planning scan 48 h later to determine the position of the applicator and reposition or calculate another treatment plan for the last four fractions if necessary.
DVHs were constructed for target tumor tissues and neighboring organs, including the bladder, rectum, and sigmoid colon. The brachytherapy dose, the minimum dose to the most irradiated 1 cubic centimeter (D1cc) and D2cc of the bladder, sigmoid colon, and rectum, the dose received by at least 90% of the volume (D90), the minimal target dose (D100), and the volume covered by the prescription dose (V100) of the tumor, were calculated by the DVH evaluation function of the PLATO system using a sample number of 10 000 points. Dose-volume constraints were applied as 75 Gyα/β3 (radiation dose unit with an α/β ratio of 3) for the sigmoid colon and rectum and 90 Gyα/β3 for the bladder[9]. The high dose rates (HDRs) were converted to equivalent 2 Gy doses (EQD2) using a linear quadratic formula to determine the biologically equivalent dose (BED). An α/β ratio of 3 was used for normal tissues (α/β3), and 10 was used for tumor tissue (α/β10).
Follow-up
Follow-up was calculated from the start of radiotherapy. Patients were evaluated for local response, complications, and distant metastasis during routine monthly follow-up for the first 6 months, quarterly up to 2 years, and semi-annually thereafter. These patients were followed up until December 11, 2011. At each visit, a clinical examination was performed, and if necessary, CT/MRI scans were performed to assess the disease status. A PET scan was also performed if there was a suspicion of metastasis on clinical/radiologic examination. For the assessment of late toxicity, Radiation Therapy Oncology Group criteria were used.
Results
Basic patient characteristics
Clinical data of 20 patients with pathologically proven cervical cancer treated by ISBT were included in this analysis. The median patient age was 51 (range, 27–61) years. Eighteen patients had cervical squamous cell carcinoma, and 2 had cervical adenocarcinoma. The FIGO classification for local tumor stage revealed that 11 patients were at stage IIb and 9 were at stage IIIb. Ten patients had regional lymph node involvement, and 2 of them had para-aortic lymph node involvement. A median EBRT dose of 50 Gy (range, 46–50 Gy) was prescribed to the 20 patients. The median overall treatment duration was 47 days (range, 33–57 days).
Details of the ISBT
A total of 108 needles were implanted into the 20 patients, with a median number of 5 (range, 4–7) needles per patient, Overall, the procedure lasted between 45 and 120 min (median, 60 min), which included needle insertion and treatment planning; needle insertion was the most time-consuming factor. A median displacement of 4.5 mm (range, -4 to 6 mm) was observed in 11 of the 54 implanted needles in 48 h. Needle penetration of the uterus occurred in 2 patients; no sigmoid colon or bladder wall penetration occurred.
DVH parameters
The median volumes of HR-CTV and IR-CTV were 40 cc (range, 16–89 cc) and 100 cc (range, 46–113 cc), respectively. The median D90 and D100 to the HR-CTV were 45 (range, 33–54) Gyα/β10 and 26 (range, 20–35) Gyα/β10 with brachytherapy alone. For IR-CTV, the median D90 and D100 with brachytherapy alone were 30 (range, 20–37) Gyα/β10 and 16 (range, 7–22) Gyα/β10, respectively. The median V100 of HR-CTV was 96.7% (range, 81.9%–99.2%) with brachytherapy alone. When the EBRT dose was added to the HDR brachytherapy, the median D90 and D100 of HR-CTV was 94 (range, 83–104) Gyα/β10 and 75 (range, 66–84) Gyα/β10, respectively. The median D90 and D100 of IR-CTV was 77 (range, 70–87) Gyα/β10 and 65 (range, 57–72) Gyα/β10, respectively. Table 1 outlines the details of DVH parameters to target volume.
Table 1. Volume and dose of radiation to target tissue.
| Parameter | HR-CTV | IR-CTV |
| Volume (cc) | 40 (16–89) | 100 (46–113) |
| V100 (%, ISBT alone) | 96.7 (81.9–99.2) | |
| D90 (Gyα/β10, ISBT alone) | 45 (33–54) | 30 (20–37) |
| D100 (Gyα/β10, ISBT alone) | 26 (20–35) | 16 (7–22) |
| D90 (Gyα/β10, ISBT+EBRT) | 94 (83–104) | 77 (70–87) |
| D100 (Gyα/β10, ISBT+EBRT) | 75 (66–84) | 65 (57–72) |
HR-CTV, high-risk clinical target volume; IR-CTV, intermediate-risk clinical target volume; ISBT, interstitial brachytherapy; EBRT, external beam radiotherapy; D100, the minimal target dose; D90, dose received by at least 90% of the volume; V100, the volume treated with the prescription dose. All values are presented as median (range).
Table 2 lists DVH parameters to OARs. The median volumes of the bladder, rectum, and sigmoid colon were 59 cc (range, 37–76 cc), 43 cc (range, 33–60 cc), and 25 cc (range, 9–43 cc), respectively. The D2cc and D1cc of the bladder was 83 (range, 73–96) Gyα/β3 and 86 (range, 75–104) Gyα/β3, respectively. For the rectum, the median dose of D2cc and D1cc was 73 (range, 64–98) Gyα/β3 and 76 (range, 68–107) Gyα/β3, respectively. The median sigmoid D2cc and D1cc were 61 (range, 52–69) Gyα/β3 and 74 (range, 53–73) Gyα/β3, respectively.
Table 2. Volume and dose of radiation to organs at risk (OARs).
| OAR | Volume (cc) | D2cc (Gyα/β3) | D1cc (Gyα/β3) |
| Bladder | 59 (37–76) | 83 (73–96) | 86 (75–104) |
| Rectum | 43 (33–60) | 73 (64–98) | 76 (68–107) |
| Sigmoid colon | 25 (9–43) | 61 (52–69) | 74 (53–73) |
D2cc, dose to the most irradiated 2 cc; D1cc, dose to the most irradiated 1 cc. All values are presented as median (range).
Clinical outcome and complications
After radiotherapy, 18 patients experienced complete response, and 2 experienced partial response. Local failure occurred in 2 patients (10%) and nodal metastasis without local failure was observed in 1 patient (5%) during follow-up (median, 17 months; range, 14–30 months). All patients have survived as of this writing. Of the 2 local failures, 1 patient with FIGO stage MB squamous cell carcinoma who had undergone uterine artery embolization before radiation for cervical bleeding developed local recurrence at the junction of the cervix and uterus 7 months after treatment (70 Gyα/β10 to D100 HR-CTV; 57 Gyα/β10 to D100 IR-CTV). Another patient with FIGO stage IIB adenocarcinoma had persistent local disease (69 Gyα/β10 to D100 HR-CTV; 59 Gyα/β10 to D100 IR-CTV). The patient developed right internal iliac nodal relapse and was on palliative chemotherapy; however, it is locally controlled. The actuarial 1-year overall survival (OS), progression-free survival (PFS), and local control rates were 100%, 85% (3 events), and 90% (2 events), respectively.
Grade 1 or 2 acute radiation-related toxicity (diarrhea) was observed in 2 patients. The most severe complication was Grade 3 diarrhea and bloody stool in 1 patient. Needle perforation of the bladder, rectum, or sigmoid colon was not observed.
Discussion
Recently, the use of 3D treatment planning systems has increased in most radiotherapy facilities. This method allows for a better assessment of GTV and the definition and delineation of CTV compared with traditional approaches. This method also allows for optimizing dose distribution based on CTV[11]. A variety of applicator systems have been applied to enable spatial dose distribution to conform to CTV and facilitate dose reduction to normal tissues. ISBT has been approved as the most effective technique in terms of providing satisfactory local control and fewer serious complications[6],[7],[12],[13].
In ISBT, ultrasonography, CT, and MRI have been used to guide needle insertion. Sharma et al.[5] and Stock et al.[14] reported transrectal ultrasound (TRUS) in guiding applicator implantation. Although ultrasonography provided a real-time image of pelvic structures and needle movement in needle insertion, shadows from those needles closest to the ultrasound transducer may interfere with insertion. CT and MRI enabled more accurate placement because they deliver enhanced visualization of the anatomic structures. With superior contrast resolution, MRI can distinguish tumor tissue from the normal uterus and cervix and can define parametrial and vaginal infiltration more clearly than CT. However, MRI-compatible applicators are expensive and can only be used at selected institutions because of applicator and software constraints, as well as its longer scan time[10]. Although CT scanning is effective in guiding needle implantation and treatment planning, traditionally used metal applicators can cause artifacts on CT images, which makes distinguishing tumor tissues from normal tissues difficult. The flexible plastic needles in our study eliminated artifacts on CT images, allowing for a clear visualization of needle implantation and easy contouring of target tissues and OARs.
Currently, the most frequently used applicator systems have fixed intervals between the holes and fixed hole direction, which potentially restricts the point of implantation and the direction of the needles during placement. The fixed direction of applicators may make it difficult to avoid critical normal structures. Additionally, to adequately cover the tumor, more needles are needed. Sharma et al.[5] and Stock et al.[14] have both reported using a median of 18 needles during template-based interstitial implantation. Additionally, transperineal interstitial implantation in template-based systems results in long distances between the needle insertion points and the target tissue[6],[7],[14]. CT-guided transvaginally free-hand needle implantation, which has no direction or interval restrictions, may have the potential to achieve precise tumor coverage with fewer needles and short distances between needle insertion points.
Adequate coverage of the tumor volume is imperative because insufficient radiation increases the risk of pelvic relapse. Yoshida et al.[6] reported the results of a template-based ISBT study. Their prescribed dose to HR-CTV was 70 Gyα/β10, and this prescription achieved a lower radiation dose to OARs (with a median D2cc of 62 Gyα/β3 to the bladder and 65.9 Gyα/β3 to the rectum), which was lower than our ISBT results. However, this prescription also resulted in a lower radiation dose to HR-CTV and reduced local control rate, and they reported a median D90 of 80.6 Gyα/β10 and D100 of 61.4 Gyα/β10 for HR-CTV, with a local control rate of 83% (median follow-up, 18 months; range, 9–33 months). Compared with a median D90, of 94 Gyα/β10 and a median D100 of 75 Gyα/β10 for HR-CTV and a local control rate of 90% in our study (median follow-up, 17 months; range, 14–30 months), our results appear to be better. Aakila et al.[7] reported the feasibility and acute toxicity in real-time intraoperative MRI-guided ISBT for vaginal recurrence of endometrial cancer in 10 patients. Using their template-based ISBT, the median D2cc for the bladder, rectum, and sigmoid colon were 75.4 (range, 42–146) Gyα/β3, 70.3 (range, 43–109) Gyα/β3, and 56.1 (range, 15–117) Gyα/β3, respectively, which concur with our results. The morbidity in their study was manifested by just 1 patient who suffered interstitial radiation-related Grade 3 toxicity. Our interstitial radiation-related morbidity rate was not worse than that in their study. This result suggested that CT-guided transvaginally free-hand needle implantation could achieve similarly precise tumor coverage as template-based ISBT without increasing unnecessary irradiation to normal tissues, despite the use of fewer needles (median, 5; range, 4 to 7) in our technique.
Traditionally, brachytherapy is performed over at least 2 weeks[11]. For brachytherapy with longer fraction intervals, the tumor volume may change drastically, potentially necessitating needle re-implantation and treatment re-planning. This may have implications in terms of patient discomfort and potentially increase the burden on resources. In our clinical practice, needle implantation was only performed at the first fraction, and the treatment plan calculated on this implantation was used for all fractions. Patients were ideally treated with 8 fractions of 4 Gy twice a day over 4 days, which required insertion early in the week. This regimen may potentially minimize toxicity and improve local control in our study.
Changes in rectal and bladder fullness can affect dose distributions. Bladder distension reduces the median dose in the bladder wall, which may reduce treatment-related complications[15]. Hoskin et al.[16] suggested that brachytherapy should be undertaken with a bladder volume of at least 100 mL, which reduced the amount of the small bowel included in the irradiated area with no increase in the bladder dose. Needle applicator displacement between fractions may also affect the dose distribution. Mikami et al.[17] reported a median displacement of 2 mm (range, 9–14 mm) at 45 h in template-based brachytherapy and recommended that the applicator position be checked during treatment using CT scanning. In the current study, 300 mL of sterile saline was injected into the bladder prior to each fraction; patients were re-scanned 48 h after the first simulation scan, and a second treatment plan was calculated if necessary. Our results showed a median displacement of 4.5 mm (range, -4 to 6 mm) in 11 of the 54 implanted needles. A protocol that allows for treatment re-planning and ensures a constant bladder volume may reduce the risk of displacement and its consequences.
The current study, which investigated the use of a CT-guided free-hand needle placement technique in 20 patients with cervical cancer, suggests that CT guidance reduces the risk of needle injury and perforation of pelvic structures. In addition, precise target volume-based dose distribution reduced the minimum dose delivered to the most irradiated volumes of OARs. As a result, there was only one case of Grade 3 toxicity. The limitation of this study was the small number of recruited patients and the short follow-up. Further studies with a larger number of patients and a longer follow-up are needed to assess survival rates and late toxicity of this methodology.
Conclusion
This preliminary study, which investigated the technique of CT-guided free-hand ISBT using a relatively small number of needles, proved to be feasible and effective. DVH results of our study were not inferior to results from template-based studies. Further studies are needed to clarify the indications for image-based free-hand ISBT and to determine long-term safety and efficacy.
References
- 1.Nag S, Erickson B, Thomadsen B, et al. The American Brachytherapy Society recommendations for high-dose-rate brachytherapy for carcinoma of the cervix. Int J Radiat Oncol Biol Phys. 2000;48:201–211. doi: 10.1016/s0360-3016(00)00497-1. [DOI] [PubMed] [Google Scholar]
- 2.Sitathanee C, Pairatchvet V, Narkwong L, et al. High-dose-rate interstitial brachytherapy in the management of carcinoma of the uterine cervix and other gynecologic malignancies. J Med Assoc Thai. 2005;88:1045–1050. [PubMed] [Google Scholar]
- 3.Hsu IC, Speight J, Hai J, et al. A comparison between tandem and ovoids and interstitial gynecologic template brachytherapy dosimetry using a hypothetical computer model. Int J Radiat Oncol Biol Phys. 2002;52:538–543. doi: 10.1016/s0360-3016(01)02691-8. [DOI] [PubMed] [Google Scholar]
- 4.Weitmann HD, Knocke TH, Waldhausl C, et al. Ultrasound-guided interstitial brachytherapy in the treatment of advanced vaginal recurrences from cervical and endometrial carcinoma. Strahlenther Onkol. 2006;182:86–95. doi: 10.1007/s00066-006-1420-4. [DOI] [PubMed] [Google Scholar]
- 5.Sharma DN, Rath GK, Thulkar S, et al. Use of transrectal ultrasound for high dose rate interstitial brachytherapy for patients of carcinoma of uterine cervix. J Gynecol Oncol. 2010;21:12–17. doi: 10.3802/jgo.2010.21.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshida K, Yamazaki H, Takenaka T, et al. A dose-volume analysis of magnetic resonance imaging-aided high-dose-rate image-based interstitial brachytherapy for uterine cervical cancer. Int J Radiat Oncol Biol Phys. 2010;77:765–772. doi: 10.1016/j.ijrobp.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 7.Viswanathan AN, Cormack R, Holloway CL, et al. Magnetic resonance-guided interstitial therapy for vaginal recurrence of endometrial cancer. Int J Radiat Oncol Biol Phys. 2006;66:91–99. doi: 10.1016/j.ijrobp.2006.04.037. [DOI] [PubMed] [Google Scholar]
- 8.Haie-Meder C, Potter R, Van Limbergen E, et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (I): concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol. 2005;74:235–245. doi: 10.1016/j.radonc.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 9.Potter R, Haie-Meder C, Van Limbergen E, et al. Recommendations from Gynaecological (GYN) GEC ESTRO working group (II): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy—3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol. 2006;78:67–77. doi: 10.1016/j.radonc.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Nag S, Cardenes H, Chang S, et al. Proposed guidelines for image-based intracavitary brachytherapy for cervical carcinoma: report from Image-Guided Brachytherapy Working Group. Int J Radiat Oncol Biol Phys. 2004;60:1160–1172. doi: 10.1016/j.ijrobp.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 11.Kirisits C, Potter R, Lang S, et al. Dose and volume parameters for MRI-based treatment planning in intracavitary brachytherapy for cervical cancer. Int J Radiat Oncol Biol Phys. 2005;62:901–911. doi: 10.1016/j.ijrobp.2005.02.040. [DOI] [PubMed] [Google Scholar]
- 12.Jurgenliemk-Schulz IM, Tersteeg RJ, Roesink JM, et al. MRI-guided treatment-planning optimisation in intracavitary or combined intracavitary/interstitial PDR brachytherapy using tandem ovoid applicators in locally advanced cervical cancer. Radiother Oncol. 2009;93:322–330. doi: 10.1016/j.radonc.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Kirisits C, Lang S, Dimopoulos J, et al. The Vienna applicator for combined intracavitary and interstitial brachytherapy of cervical cancer: design, application, treatment planning, and dosimetric results. Int J Radiat Oncol Biol Phys. 2006;65:624–630. doi: 10.1016/j.ijrobp.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 14.Stock RG, Chan K, Terk M, et al. A new technique for performing Syed-Neblett template interstitial implants for gynecologic malignancies using transrectal-ultrasound guidance. Int J Radiat Oncol Biol Phys. 1997;37:819–825. doi: 10.1016/s0360-3016(96)00558-5. [DOI] [PubMed] [Google Scholar]
- 15.Sun LM, Huang HY, Huang EY, et al. A prospective study to assess the bladder distension effects on dosimetry in intracavitary brachytherapy of cervical cancer via computed tomography-assisted techniques. Radiother Oncol. 2005;77:77–82. doi: 10.1016/j.radonc.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Hoskin PJ, Vidler K. Vaginal vault brachytherapy: the effect of varying bladder volumes on normal tissue dosimetry. Br J Radiol. 2000;73:864–866. doi: 10.1259/bjr.73.872.11026862. [DOI] [PubMed] [Google Scholar]
- 17.Mikami M, Yoshida K, Takenaka T, et al. Daily computed tomography measurement of needle applicator displacement during high-dose-rate interstitial brachytherapy for previously untreated uterine cervical cancer. Brachytherapy. 2011;10:318–324. doi: 10.1016/j.brachy.2010.11.006. [DOI] [PubMed] [Google Scholar]