Abstract
Multidrug resistance proteins (MRPs) are members of the C family of a group of proteins named ATP-binding cassette (ABC) transporters. These ABC transporters together form the largest branch of proteins within the human body. The MRP family comprises of 13 members, of which MRP1 to MRP9 are the major transporters indicated to cause multidrug resistance in tumor cells by extruding anticancer drugs out of the cell. They are mainly lipophilic anionic transporters and are reported to transport free or conjugates of glutathione (GSH), glucuronate, or sulphate. In addition, MRP1 to MRP3 can transport neutral organic drugs in free form in the presence of free GSH. Collectively, MRPs can transport drugs that differ structurally and mechanistically, including natural anticancer drugs, nucleoside analogs, antimetabolites, and tyrosine kinase inhibitors. Many of these MRPs transport physiologically important anions such as leukotriene C4, bilirubin glucuronide, and cyclic nucleotides. This review focuses mainly on the physiological functions, cellular resistance characteristics, and probable in vivo role of MRP1 to MRP9.
Keywords: Multidrug resistance protein (MRP), multidrug resistance (MDR), ABC transporter, chemotherapy
Chemotherapy is one of the major treatment modalities available for cancer patients. Unfortunately, during the course of treatment, cancer cells develop resistance to functionally and structurally different anticancer drugs by either acquired (due to host factors) or intrinsic (due to genetic or epigenetic) mechanisms[1],[2]. This phenomenon of resistance to different classes of anticancer drugs by cancer cells is termed multidrug resistance (MDR). This pervasive and insidious clinical problem eventually leads to cancer relapse and death among patients. The mechanisms of MDR have been intensively studied, although not all mechanisms producing MDR have been elucidated. The detailed mechanisms that cancer cells utilize or develop to evade chemotherapy are complex and have been described in detail in several recent reviews[3]–[5]. One of the most important mechanisms underlying MDR is overexpression of adenosine triphosphate (ATP)-binding cassette (ABC) transporters, which efflux a wide spectrum of anticancer drugs against the concentration gradient using ATP-driven energy.
The ABC transporter family, representing the largest family of transmembrane proteins, comprises 49 transporters that are further subdivided into seven subfamilies, ABC-A to -G, based on sequence similarities[6]. Of them the major ABC transporters involved in MDR development are ABC subfamily B member 1 [(ABCB1/P-glycoprotein (P-gp)], ABC subfamily G member 2 [ABCG2, also known as breast cancer resistance protein (BCRP)/mitoxantrone resistance protein (MXR)/placenta-specific ABC protein (ABCP)], and ABC subfamily C member 1 (ABCC1/MRP1)[6],[7]. This review will provide in-depth details about the MRPs involved in conferring MDR in cancer cells.
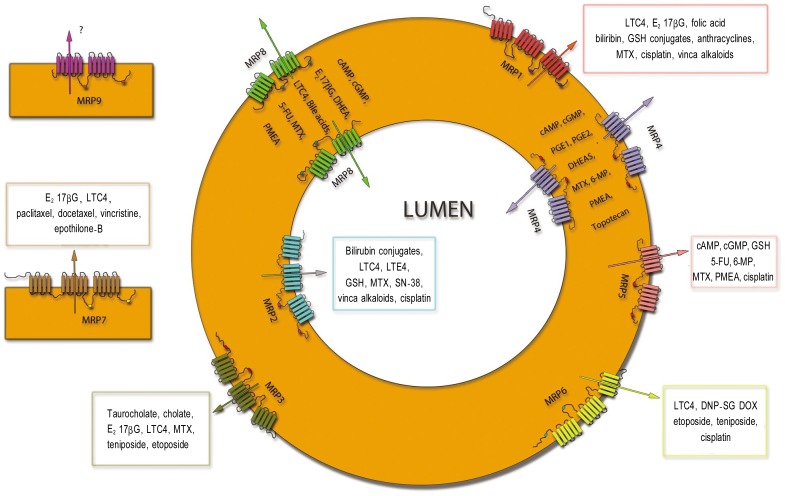
The MRP subfamily, the C subset of the ABC transporter superfamily, is composed of thirteen members, and nine of these are primarily involved in MDR (Table 1)[8]. Based on functional characterization, localization, and cloning studies, these nine MRPs have been established as ATP-dependent efflux transporters for endogenous substances and xenobiotics. The other three members of the MRP subfamily, namely ABCC7/cystic fibrosis transmembrane conductance regulator (CFTR), ABCC8/sufonylurea receptor 1 (SUR1), and ABCC9/SUR2, are not involved in conferring MDR. ABCC7 is a regulated chloride channel, whereas ABCC8 and ABCC9 are intracellular ATP sensors and regulate the specific K+ channel permeability[9]. On the basis of structural topology, the nine main MRPs can be divided into two groups. One has a typical ABC transporter structure and is composed of two membrane spanning domains (MSD) with nucleotide binding domains (NBD1 and NBD2) in between (Figure 1). These can be referred to as “short MRPs” and include MRP4, MRP5, MRP8, and MRP9 (ABCC4, 5, 11 and 13, respectively). The other group, which includes MRP1, 2, 3, 6 and 7 (ABCC1, 2, 3, 6 and 7, respectively), have an additional MSD (MSD0) and are referred as “long MRPs”[10]–[12].
Table 1. Summary of MRP members involved in MDR.
| MRP member | Alternative name[188] | Amino acid identity with MRP1 (%)[63] | Physiological substrate[63] | Tissue distribution[188] |
| MRP1 | ABCC1 | 100 | LTC4, E1S, E217βG, folate | Ubiquitous |
| MRP2 | ABCC2, cMOAT, cMRP | 50 | LTC4, E1S, E217βG | Liver, kidney, gut |
| MRP3 | ABCC3, MOAT-D, cMOAT-2 | 58 | LTC4, E217βG, cholylglycine | Liver, adrenals, pancreas, kidney, gut |
| MRP4 | ABCC4, MOAT-B | 41 | cAMP, cGMP, LTC4, PGE2, folate, urate | Prostate, lung, muscle, pancreas, testis, ovary, bladder, gallbladder |
| MRP5 | ABCC5, MOAT-C, pABC11 | 38 | cAMP, cGMP, folate, 2′-deoxyuridine 5′-monophosphate | Ubiquitous |
| MRP6 | ABCC6, MOAT-E, MLP-1, ARA | 46 | LTC4, S-glutathionyl N-ethylmaleimide | Liver, kidney |
| MRP7 | ABCC10 | 35 | LTC4, E217βG | Pancreas, testis, colon, spinal cord, tonsils, lung, trachea, skin |
| MRP8 | ABCC11 | 33 | DHEAS, LTC4, E217βG, cAMP, cGMP, cholylglycine, folate | Breast, ovary, lung, testis, kidney, liver, colon, and brain |
| MRP9 | ABCC12 | 36 | ? | Breast, testis, brain, skeletal muscle, ovary |
MRP, multidrug resistance protein; MDR, multidrug resistance; MLP-1, MRP-like protein 1; ARA, anthracycline resistance associated. The question mark (?) indicates that information is not available.
Figure 1. The pictorial depiction shows the topography and the location of both the short- (MRP4, MRP5, MRP6, MRP8 and MRP9) and long-form (MRP1, MRP2, MRP3, and MRP7) members of the MRP subfamily.
Each of these transporters pumps out a variety of endogenous and xenobiotic substrates. These transporters are mainly present either apically or basolateraly; however, the localization of MRP7 and MRP9 is still unknown. The substrates of MRP9, the last member to be cloned among the MRP subfamily, are still unknown and are open for discussion. MRP, multidrug resistance protein.
MRP1/ABCC1
MRP1 was first discovered in an anthracycline-resistant cell line HL60/Adr which was the first major ABC transporter other than P-gp. This protein was shown to have a molecular weight of 190 kDa[13]–[16], and Cole et al.[10] subsequently isolated the cDNA from a human lung cancer H69AR cell line. The protein was named multidrug resistance protein 1 (MRP1). Further studies showed MRP1 was present on the basolateral surface of the epithelial membrane and was involved in ATP-dependent efflux of xenobiotics across the cell membrane[17],[18] (Figure 1).
MRP1 is reported to be widely expressed in various tissues including lung, testis, kidney, skeletal and cardiac muscles, placenta, and macrophages[11],[19]. It has also been found to be predominantly localized to blood-tissue barriers, such as the basolateral membrane of the choroid plexus cells of the blood-cerebrospinal fluid barrier, the bronchial epithelium[20],[21], and the apical syncytiotrophoblast membrane of the placenta[22]. MRP1 and P-gp share only 15% amino acid sequence identity and posses some distinct features. Structurally, MRP1 differs from P-gp in that it has an extra MSD, named MSD0, with five transmembrane (TM) helices, and two other MSDs, each having six TM helices with two NBDs in between (Figure 1)[23],[24]. MSD0, does not play a role in trafficking to the plasma membrane or efflux activity, but it is required for efficient retention of MRP1 at the cell surface[17].
In spite of the modest degree of amino acid sequence identity with P-gp, MRP1 has a significant overlapping resistance profile with P-gp. The resistance profile, characterized with the help of transfected cell lines, established that MRP1 confers resistance to a wide range of anticancer drugs such as anthracyclines, vinca alkaloids, epipodophyllotoxins, camptothecins, methotrexate (MTX), saquinavir, and mitoxantrone (MX); however, distinct from P-gp, it does not confer resistance to taxanes, an important component of the P-gp resistance profile (Table 2)[25]–[30]. Studies with fibroblast cell lines from Mrp1 knockout mice show a similar resistance pattern[31]–[33], along with modest sensitization to taxanes and MX. So far, miniscule data are available regarding the involvement of MRP1 in conferring resistance against taxanes and MX. Some newer classes of targeted anticancer drugs, such as tyrosine kinase inhibitors (TKIs, e.g. imatinib), also succumb to MRP1-mediated resistance[33].
Table 2. Summary of MRP members involved in MDR.
| Anticancer drugs | MRP1 | MRP2 | MRP3 | MRP4 | MRP5 | MRP6 | MRP7 | MRP8 | MRP9 | |
| Antimetabolites | 6-mercaptopurine | - | - | - | + | + | - | - | - | ? |
| 6-thioguanine | - | - | - | + | + | - | - | - | ? | |
| 5-fluorouracil | - | - | - | - | + | - | - | + | ? | |
| Methotrexate | + | + | + | + | + | - | - | - | ? | |
| Antibiotics | Daunorubicine | + | - | - | - | - | + | - | - | ? |
| Doxorubicine | + | + | - | - | - | + | - | - | ? | |
| Epirubicine | + | + | - | - | - | + | - | - | ? | |
| Actinomycine D | - | - | - | - | - | + | - | - | ? | |
| Mitoxantrone | - | + | - | - | - | - | - | - | ? | |
| Platinum drug | Cisplatin | - | + | - | - | + | + | - | - | ? |
| Taxanes | Paclitaxel | - | + | - | - | - | - | + | - | ? |
| Docetaxel | - | + | - | - | - | - | + | - | ? | |
| Vinca alkaloids | Vincristine | + | + | - | - | - | - | + | - | ? |
| Vinblastine | + | + | - | - | - | - | + | - | ? | |
| Epipodophyllotoxins | Etoposide | + | + | + | - | - | + | - | - | ? |
| Teniposide | - | - | + | - | - | + | - | - | ? | |
| Camptothecins | Irinotecan | + | - | - | + | - | - | - | - | ? |
| Topotecan | - | + | - | + | - | - | - | - | ? | |
| SN-38 | + | - | - | + | - | - | - | - | ? | |
| Tyrosine kinase inhibitors | Imatinib | + | - | - | - | - | - | - | - | ? |
| Gefitinib | + | - | - | - | - | - | - | - | ? | |
| Miscellaneous | Epothilone B | - | - | - | - | - | - | + | - | ? |
Though MRP1 has an overlapping resistance profile with P-gp, the physiological substrate profile differs significantly. While substrates for P-gp are neutral or mildly positive lipophilic compounds, membrane vesicle transport studies established MRP1 as a lipophilic anionic transporter that can transport glutathione conjugates, such as leukotriene C4 (LTC4) and dinitrophenyl-S-glutathione (DNP-SG)[34],[35]. It can also transport glucuronate conjugates (e.g., E217β G), dianionic bile salts, and sulfate conjugates[36]–[38]. A study using an Mrp1 knockout mouse model also confirmed that LTC4 is indeed a physiological substrate of MRP1 (Table 1)[38].
MRP1 is a basolateral transporter whose activity results in the movement of compounds into tissues that lie beneath the basement membrane[39]. Transport of glutathione and glucuronate conjugates by MRP1 is of interest because they represent phase II metabolism and cellular detoxification. Efflux pumps involved in cellular export have been referred to as GS-X pumps in the case of glutathione (GSH) conjugates[40], and MRP1 has widespread expression and glutathione conjugate efflux characteristic, which indicates MRP1 as GS-X pump[41]. This feature of MRP1 explains the transport capacity of MRP1 for MTX, an organic anion, and arsenite, which can form complex with GSH molecules[42]. In addition, vinca alkaloids and anthracyclines, to which MRP1 confers resistance, are weak organic bases and do not conjugate with acidic ligands in human cells. Hence, resistance to these compounds by MRP1 was unclear. However, recent studies indicate that these drugs are probably co-transported with GSH and that cellular depletion of GSH decreases MRP1-mediated resistance to these drugs. In addition, similar results have been reported in vesicular transport assays of vincristine and daunorubicin[4],[42]–[46]. The detailed transport mechanism for GSH by MRP1 has been postulated and reviewed by Kruh et al.[18].
Clinically, MRP1 levels are elevated in numerous cancer types, such as non-small cell lung cancer (NSCLC)[20],[47], breast cancer, and prostate cancer[47], and they are also related to accelerated relapse in breast cancer[48]. MRP1 expression has been reported in several solid and hematological cancers. Negative correlation between MRP1 expression and response to treatment has also been found. Such studies have been reviewed in detail elsewhere[11],[18],[49],[50]. However, there is no definite consensus drawn with respect to the role of MRP1 in acquired resistance or in prognosis.
MRP2/ABCC2
Mrp2, the second member of the MRP subfamily of ABC transporter, was first cloned from rat hepatocyte and was named as a hepatocellular canalicular multiple organic anion transporter (cMOAT)[51]. MRP2 shares 49% amino acid identity with MRP1 but it has a different expression pattern. While MRP1 is widely expressed in many tissues, MRP2 is mainly expressed in the apical (canalicular) hepatocyte plasma membrane, small intestine, and renal proximal tubules (Table 1)[52]–[54]. MRP2 mRNA is present in the peripheral nerves, gallbladder, placental trophoblasts, and CD4+ lymphocytes[22],[55],[56].
Because MRP2 handles a range of conjugates similar to that of MRP1, it was believed to confer resistance to similar anticancer drugs as well. This hypothesis was formulated based on an experiment in which an antisense MRP2 RNA construct was introduced into human hepatocellular carcinoma HepG2 cells, resulting in enhanced sensitivity to several anticancer drugs such as cisplatin, vincristine, doxorubicin, and the camptothecin derivatives CPT-11 and SN-38[57]. Evers et al.[58] later showed that MRP2 could transport vinblastine in polarized Madin Darby canine kidney epithelial (MDCK) cells, suggesting a potential role for MRP2 in vinblastine resistance. In addition, MRP2-transfected cells also conferred resistance to MTX[59], cisplatin, etoposide, doxorubicin, and epirubicin[60]. The resistance capacity of MRP2 to cisplatin is quite interesting because MRP1 does not confer resistance to cisplatin (Table 2)[25],[27]. However, this phenomenon is convincible, as cisplatin is well known to form toxic GSH complexes in the cells[61].
MRP2 and MRP1 have very similar substrate specificities and mediate transport of some hydrophobic compounds in the presence of GSH, though with different affinities[62]. MRP2 has a transport facility for organic anions including sulfate, glucuronide, and GSH conjugates (Table 1)[63]–[65]. Furthermore, MRP2 is involved in the biliary elimination of certain endogenous conjugates, such as LTC4 and conjugated bilirubins[63],[65] Though these conjugated metabolic complexes are thought to be detoxified, their accumulation may result in reformation of active compounds either spontaneously or by enzymatic hydrolysis.
Mutations within human MRP2 result in an inactive MRP2 protein in the canalicular membrane as observed in Dubin-Johnson syndrome (DJS), a hereditary disorder with modest elevation of serum conjugated albumin[52],[66],[67]. Eisai hyperbilirubinuria rats (EHBRs) and Groninger Yellow transporter rat strains are deficient in Mrp2 and are perfect models to study human DJS[67]–[69].
MRP2 expression has been reported in several human tumor cell lines of lung, gastric, renal, and colorectal cancers[70]. Moreover, few cisplatin- and doxorubicin-resistant cell lines have shown overexpression of MRP2 mRNA[55],[71]. Recent reports by Korita et al.[72] suggest that efficacy of cisplatin-based chemotherapy in patients with hepatocellular carcinoma depends upon MRP2 expression level.
MRP3/ABCC3
The MRP3 protein localizes in the basolateral membrane domain of polarized cells. It was first identified in human and rat hepatocytes, mediating efflux of organic anions into sinusoidal blood. Among the MRPs with known coding sequences, MRP3 shares the highest degree of structural similarity with MRP1 (58% amino acid identity)[73],[74]. MRP3 expression is found in adrenal glands, kidney, small intestine, colon, pancreas, and gallbladder, and with a lower expression in the lungs, spleen, stomach, and tonsils[55],[75]–[77].
Although MRP3 has high structural similarity with MRP1, the affinity of MRP3 for conjugates is considerably lower than that of MRP1. Its drug resistance capabilities are also as extensive as neither MRP1 nor MRP2. The narrow and limited drug resistance profile of MRP3 to epipodophyllotoxins, vincristine, and MTX was reported previously in studies using MRP3-transfected cells [74],[78]. Resistance potency to etoposide and vincristine is quite low compared to MRP1 (Table 2). In contrast to MRP1 and MRP2, MRP3 does not need GSH to transport natural products [79]. In addition, Kool et al.[74] reported an unchanged level of GSH in MRP3-transfected cells. These findings may explain why MRP3 has limited resistance properties— because it has a lower affinity for amphipathic anions and GSH. In recent reports, significant accumulation of etoposide glucuronide in the liver in Mrp2−/−/Mrp3−/− mice was described, but neither single knockout showed this phenomenon, indicating an alternative pathway provided by Mrp2 and Mrp3 for hepatic elimination of etoposide glucuronide[80].
Elevated Mrp3 expression has been reported in cholestatic rat liver[69],[75] and cholestatic human liver[81], as well as in patients with DJS who lack functional MRP2 in the liver canalicular membranes. This suggests that basolateral MRP3 expression in hepatocytes may allow efflux of organic anions from the liver into the blood upon blockade of bile secretion, and that MRP3 is a back-up system for amphipathic anions in cholestatic conditions. Another study revealed Mrp3 as an alternative exporter of bile acids and glucuronides from cholestatic hepatocytes, but the pump was not involved in the enterohepatic circulation of bile acids in Mrp3 knockout mice models[82]. Membrane vesicles, prepared from MRP3-transfected HEK293 cells, were reported to transport LTC4, DNP-SG, and E217β G, prototypical MRP1 substrates, with low affinity[83]. MRP3 confers resistance to and transport capacity for MTX[74],[84]. Increased expression of MRP3 has been reported in human hepatocellular carcinomas[85], primary ovarian cancer[86], and adult acute lymphoblastic leukemia (ALL)[87].
MRP4/ABCC4
MRP4, the fourth member of the MRP subfamily of ABC transporters[88],[89], is one of the shortest members, encoding 1325 amino acids. The gene was discovered in 1996 in a T-lymphoid cell line[74], and it is located on chromosome 13q32.1[55],[88],[90]–[92]. Structurally, MRP4 is composed of a typical ABC transporter core consisting of two NBDs and two MSDs, each MSD with six TMDs (Figure 1)[11],[93],[94]. In addition, the TM6 subunit of MRP4 was found to be conserved among all species[11].
With its dual localizations in the apical (the renal proximal tubule cells and the luminal side of brain capillary endothelium) and basolateral membranes (the prostate tubuloacinar cells, hepatocytes, and choroid plexus epithelium), MRP4 differs from other MRPs that are either located apically or basolaterally[95]. A sequence-based tag against human MRP4 transcript revealed that MRP4 mRNA is present in all tissues except the bone marrow, thymus, vascular endothelium, and soft tissues[93]. MRP4 can pump out diverse endogenous and xenobiotic organic anionic compounds along with their phase II metabloites, thereby conferring resistance to various cytotoxic compounds and, in turn, protecting crucial tissues against them[11],[93].
MRP4 has a wide range of substrate specificity, including antiviral (adefovir, tenofovir, ganciclovir), antibiotic (chephalosporins), cardiovascular (loop diuretics, thiazides, angiotensin II receptor antagonists), and cytotoxic drugs [MTX, 6-thioguanine (6-TG), 6-mercaptopurine (6-MP), topotecan][96]–[98]. Cancer cells selected with a nucleotide analog, 9-(2-phosphonylmethoxy-ethyl) adenine (PMEA), were found to overexpress MRP4 (Table 2)[89]. Subsequently, MRP4 was found to confer resistance to a wide range of base, nucleotide, and nucleoside analogs[99]–[104]. Plant polyphenols, resveratrol, and quercetin are newer additions to the list of substrates for this MRP[105]. In addition, it was seen that with the established nucleoside substrates of MRP4, such as 6-TG and PMEA, only the monophosphate form of the nucleoside analogues and not the diphosphate or triphosphate forms are transported by the pump. This could be due to the fact that nucleoside monophosphates are organic anions[103],[104].
Uptake studies performed on MRP4-enriched isolated membrane vesicles and efflux experiments on MRP4-transfected cells have made it possible to identify various physiological substrates for the transporter[88]. Among the first substrates identified are cAMP and cGMP (Table 1)[101]; however, cAMP and cGMP levels remain relatively unaffected on a whole cell level[92],[106]. Still, evidence suggests that cyclic nucleotide signaling is highly compartmentalized and that MRP4 was responsible for their regulation at a microdomain level and not at a whole cell level[95]. The correlation of MRP4 with cyclic nucleotides was studied in the gut epithelium, wherein a functional and physical coupling of MRP4 with a CFTR chloride channel was observed via a scaffolding protein. This resulted in an efflux of cAMP via the MRP4 transporter[95]. This finding was interesting because Mrp4−/−mice were more susceptible to CFTR-mediated diarrhea[95]. In addition, because high levels of MRP4 were found in the apical membrane of the proximal tubules in the nephron, MRP4 could possibly play a critical role of regulating the cAMP and cGMP levels in urine, in turn affecting the levels of water and salt homeostasis. However, there are no direct data to support this hypothesis in vitro[106],[107]. Furthermore, in vivo data are also not promising, with up- and down-regulation of MRP4 in rats showing no correlation with the excretion rates of nucleotides[108],[109]. Another study involving MRP4 inside-out membrane vesicles showed that MRP4 is responsible for efflux of second messenger cGMP from erythrocytes[101],[110]–[114]. However, there was no in vivo study to support these findings, hence the physiological relevance of cGMP efflux by MRP4 from erythrocytes is questionable[104],[110],[111]. Nevertheless, the MRP4 membrane vesicle study showed that MRP4 could limit base/nucleoside analog accumulation in erythrocytes, which could affect the ability of the erythrocytes to function as carriers of xenobiotics like 6−TG and 6−MP[115].
Through the same MRP4 inside-out vesicle-mediated uptake studies, bile salts and urates (products of human purine) are identified to be physiological substrates of MRP4[98],[116],[117]. However, very low levels of bile salt and urate transport are observed with MRP4, making generation of Mrp4 knockout mice necessary for further analysis of their transport. It was reported that fernesol X−activated receptor (Fxr)−knockout mice, which had lower levels of the major canalicular bile salt export pump (BSEP/ABCB11), had increased Mrp4 mRNA levels[118]. Later on, similar results were reported in rats where relatively low levels of Mrp4 protein in the liver was increased significantly under hepatic stress[109],[119],[120]. In addition to its role in the liver, MRP4 also significantly impacts various other tissues where it is expressed, such as vascular smooth muscle, intestine, and blood cells[119]. MRP4 is also known to transport leukotrienes and prostanoids[119]. Apart from the up−regulation of MRP4 through long−term exposure to nucleoside−based drugs, MRP4 is also reported to be up−regulated in patients with neuroblastoma. However, no conclusive evidence has been provided to link drug resistance with MRP4 activity to date[120]–[122].
MRP5/ABCC5
MRP5 is encoded by a gene located on the 3q27 chromosome and contains 1437 amino acids[123]. Similar to MRP4, the identification of MRP5 was mainly based on expression sequence tag data analysis followed by cDNA fragment cloning[55],[90]. MRP5 is widely expressed, with the highest levels occurring in the heart, brain, lungs, and skeletal muscles[55],[124],[125]. Structurally, MRP5 resembles MRP4 in that it lacks MSD0. The two proteins differ at the NH2 terminus, where MRP5 has 95 extra amino acids compare to 1325 amino acid of MRP4[123]–[125]. The function of this additional segment has still not been elucidated[123]. Membrane localization of MRP5 in MRP5−transfected polarized MDCKII cells was found to be in the basolateral membrane[126]. However, the transporter was located intracellularly, with only minor expression in the plasma membrane, in HEK293 cells[126]. Similar to MRP4, MRP5 can also transport cGMP and thus reduce its intracellular availability. Because of its widespread expression, MRP5 may affect the nitric oxide/cGMP pathway, which could ultimately lead to irregularities in muscle contractions[127],[128]. Indeed, expression of MRP5 in the heart affected the muscle tone and contractility of cardiomyocytes[129],[130]. Due to the abundant presence of Mrp5 in the brain pyramidal neurones and astrocytes, which are the centers for cell signaling in the brain, it reduces the intracellular cGMP levels, which results in inhibition of the Na+/H+ exchanger in rat astrocytes, leading to a decrease intracellular pH value[131]–[133]. MRP5 expression in the capillary endothelial cells of various tissues such as the heart and brain has protective and barrier functions[129],[134]. In addition, MRP5 expression was reported in the basal membrane of syncytiotrophoblasts and around the fetus. Given that fact of its presence in the monocytes obtained from the peripheral whole blood cells in leukemic patients, MRP5 may play a role in the development of resistance to cancer chemotherapy[135].
PMEA, one of the important components of the antiretroviral therapy, is an acyclic nucleoside prototype, which is a potent inhibitor of HIV reverse transcriptase. To investigate the mechanism of resistance to antiviral drugs, Robbins et al.[136] developed a PMEA-resistant cell line and found that resistance to PMEA was mainly due to an increase in drug efflux, suggesting with a high possibility that a transporter was involved in this process. Schuetz et al.[89] followed up this work and found ABC transporters, mainly MRP4, to be responsible for the efflux. Because MRP4 and MRP5 have structural similarities, Wijnholds et al.[126] investigated if they share similar substrates, and through an assay involving a radiolabeled hydrophobic PMEA precursor, they discovered that MRP5 serves as an active transporter of the drug. Subsequently, the same group demonstrated via similar studies that MRP5 confers resistance to 6-MP and 6-TG and their analogs[126], but these results were not reproducible[103]. 5-Flourouracil (5-FU) is an antimetabolite that very closely resembles nucleosides, making it possible that 5-FU could be a substrate of MRP5. This hypothesis was later confirmed[137], making 5-FU the only antimetabolite to be transported by MRP5. However, these results must be further validated to reduce controversy[125].
Physiologically, MRP5 is known to transport cAMP and cGMP, as was first demonstrated by Jedlitschky et al.[138] who used inside-out membrane vesicles obtained from human erythrocytes to show that MRP5 had a higher affinity for cGMP than that for cAMP (Table 1)[138]. cGMP transport via MRP5 was blocked by inhibitors such as probenecid, zaprinast, trequinsin, and sildenafil, indicating that the transport was due to an amphiphilic anionic transporter[89],[111],[139]. This is important because cGMP is the main mediator of nitric oxide (NO) and natriuretic peptides−mediated signaling, which control muscle relaxation, neutrophil degranulation, and platelet aggregation[140]. When cGMP competes with other substrates of MRP5 for transport, the half−maximal concentration of cGMP was 1 mmol/L, which was high for cellular levels[103]. Hence, researchers believe MRP5 to be an overflow pump with a very low affinity for cGMP, decreasing the levels of cGMP when it is overly synthesized[106]. Inhibitors for the pump have been previously reported by Jedlitschky et al.[138], but the results seemed controversial because other laboratories failed to replicate these results.
MRP6/ABCC6
MRP6 consists of three MSDs with five, six, and six TMDs, respectively, as well as two conserved NBDs. Due to its structural similarities with MRP1, it has been classified among the C subfamily of the ABC transporters. MRP1 and MRP6 both share almost 41% structural similarity[141],[142]. Mrp6 was first cloned in rat liver[69], and later it was cloned in humans and mice[143]–[145]. Mutations within the MRP6 gene have been associated with genetic abnormalities of the autosomally inherited connective tissue disorder called pseudoxanthoma elasticum (PXE). To date, 90 distinct disease-causing mutations have been identified and reported in 31 exons of MRP6[146]–[152].
PXE has been characterized by dystrophic elastic fibers in the skin, retina, and large blood vessels, leading to baggy skin, loss of vision, and calcification of blood vessels[146],[153]. The association of MRP6 with this disorder was unexpected because MRP6 was found to be localized mainly in the liver and kidney and with low and even undetectable levels in other tissues[141],[143],[154],[155]. The localization of MRP6 was previously quite controversial, as rat Mrp6 was localized on the basolateral and canalicular plasma membrane of hepatocytes[144] whereas human MRP6 was present only on the basolateral membrane of hepatocytes[156]. Human and mouse kidney proximal tubules in MRP6−transfected MDCKII epithelial cells showed a basolateral manifestation of MRP6[145],[157]. These findings provided new insight into the role of MRP6 in PXE, indicating that the disease could be a result of the absence of a substance that is normally excreted from the liver or kidney in the blood and is involved in tissue homeostasis[158]. Human MRP6 transcripts were later detected in the skin, blood vessels, and retina with the help of reverse transcription−PCR. This was also confirmed via mouse immunohistochemical experiments wherein Mrp6 transcripts were found to be expressed in the skin, retina, and aorta [145],[146].
Substrate analysis studies of MRP6 showed that it is a lipophilic anionic pump, and that the substrates of MRP6 include drugs such as cyclopentapeptide BQ123[144]. Studies with MRP6-transfected Chinese hamster ovary (CHO) cell lines indicated that MRP6 functions as a drug efflux pump[159] and that MRP6 is capable of conferring very low levels of cellular resistance to etoposide and teniposide. In addition, studies suggest that MRP6 confers low levels of resistance to anthracyclines and cisplatin[159].
MRP6 can transport GSH conjugates such as LTC4 and n-ethylmaleimide-glutathione; however, it failed to pump out any glucuronate conjugates such as E217βG [159],[160]. These results indicate that MRP6 is a lipophilic anionic transporter and also that mutations involved in PXE may lead to a loss in activity in transporting these substrates.
MRP7/ABCC10
Similar to MRP1, MRP2, MRP3, and MRP6, MRP7 has three MSDs and two NBDs (Figure 1). Hopper et al.[161] used reverse transcription-PCR to analyze MRP7 transcript expression and reported a low level in the skin, testis, spleen, stomach, colon, kidney, heart, and brain[161]. However, another group discovered that MRP7 transcript expression was highest in the pancreas, followed by the liver, placenta, lungs, kidneys, brain, ovaries, lymph nodes, spleen, heart, leukocytes, and colon[162]. Kao et al.[163] discovered a splice variant of MRP7 that is truncated at its NH2 terminus and has a 15-amino acid deletion between MSD2 and NBD2. MRP7 is a lipophilic anion transporter whose physiological functions are so far unknown. However, one group found a potential role in the suppression of natural killer (NK)-mediated lysis[164]. To date, factors regulating MRP7 expression are unknown. However, MRP7 induction was found in doxorubicin-treated MCF7 cells[162].
Following the discovery of MRP7, Hopper-Borge et al.[97] analyzed the drug resistance profile of MRP7 using MRP7-transfected HEK293 cells. Similar to other MRPs, MRP7 can also confer resistance to several natural product anticancer drugs. A high level of resistance was observed against docetaxel, a microtubule stabilizing agent, whereas a moderate level of resistance was observed against paclitaxel[97]. In addition, MRP7 also confers resistance to microtubule destabilizing vinca alkaloids such as vincristine and vinblastine[97]. Recently, it was discovered that MRP7 might also be associated with vinorelbine resistance in NSCLC[165],[166]. Resistance to taxanes by MRP7 is unique, as no other MRP member confers resistance to paclitaxel[166]. In addition, Hopper-Borge et al.[167] found that MRP7 can also confer resistance to nucleoside-based agents such as the anticancer drugs (Ara-C and gemcitabine) and the antiviral drugs (2′,3′-dideoxycytidine and PMEA) [167]. Another microtubule-stabilizing agent, epothilone B, was also identified as a substrate for MRP7 (Table 2) in the same study[167]. In a separate study, ectopic expression of Mrp7 was observed in mouse embryo fibroblasts deficient in P-gp and Mrp1[167]. The same group also reported that MRP7 has a broad resistance profile for natural product agents, conferring high levels of resistance to docetaxel (46-fold), paclitaxel (116-fold), SN-38 (65-fold), daunorubicin (7.5-fold), etoposide (11-fold), and vincristine (56-fold)[167]. In addition, buthionine sulfoximine did not have any effect on MRP7-mediated resistance to docetaxel or Ara-C, suggesting that MRP7 transport does not involve GSH[167]. In an in vitro study, mouse fibroblast cells from Mrp7 knockout mice were sensitive to several natural anticancer drugs such as docetaxel, paclitaxel, vincristine, and Ara-C, confirming the previously characterized resistance profile of MRP7[97]. These cells also showed increased levels of drug accumulation relative to wild-type controls. In the same study, Mrp7 knockout mice exhibited higher lethality associated neutropenia and marked bone marrow toxicity upon treatment with paclitaxel, indicating that Mrp7 is indispensable for health and viability. Taken together, these results show that MRP7 is an endogenous resistance factor for taxanes, vinca alkaloid anticancer drugs, and nucleoside analogs[168]. In contrast, taccalonolides, another class of natural product microtubule stabilizers, do not succumb to P-gp- or MRP7-mediated resistance[169]. Studies investigating MRP7 expression in lung, breast, and ovarian tumor specimens would be interesting because this protein confers resistance to paclitaxel and vincristine, which are the mainstays of treatment for these particular cancers.
In a transport study involving membrane vesicles from MRP7-transfected HEK293 cells, E217βG, a prototypical substrate of many MRPs, was identified as a substrate of MRP7, with a Km value of 57.8 µmol/L. As E217βG is a glucuronide conjugate, MRP7 might be involved in phase III detoxification. Chen et al.[170] found that MRP7 had modest activity in transporting LTC4 but did not transport glycocholic acid, taurocholic acid, MTX, folic acid, cAMP, or cGMP, which are substrates of other MRP family members (Table 1). They also determined that the biochemical features of MRP7 matched the core features of other MRPs capable of transporting lipophilic anions, though MRP7 had limited substrate selectivity[170]. Furthermore, they observed that the transport of E217βG was competitively inhibited by amphiphiles, such as LTC4, glycolithocholate 3-sulfate, and MK571, as well as lipophilic agents, such as cyclosporine A[170]. This supports the notion that the MRP7 substrate binding pocket has sites for anionic and lipophilic moieties.
MRP7 expression has been reported in salivary gland adenocarcinoma and NSCLC[165],[171],[172]. In addition, MRP7 transcripts have been detected in the HepG2 liver cancer cell line and two prostate cancer cell lines (CWR22Rv1 and TSU−PR1) [173], as well as in breast, lung, colon, prostate, ovarian, and pancreatic tumor specimens[162]. However, information about MRP7 expression in tumors is still limited.
MRP8/ABCC11
MRP8 is a newly found member of the MRP family. The MRP8 gene contains 29 exons and encodes a protein predicted to contain 1382 amino acids[174],[175] that is structurally similar to MRP4 and MRP5, with 2 MSDs, 2 NBDs, and 12 TMDs. Sequence comparisons done between MRP8 and the other family members indicate its close resemblance with MRP51[174],[176]. Bera et al.[174] was the first to discover MRP8 with the help of a gene prediction program and expressed sequence tag (EST) database mining. MRP8 is widely expressed within the human body, with the highest levels occurring in the liver, brain, placenta, breasts, and testes (Table 1)[174],[175]. This widespread expression pattern is purportedly due to MRP8 spliceoforms[175]. MRP8 is known to play a role in the human central and the peripheral nervous system such that the expression of MRP8 is associated with the efflux of dehydroepiandrosterone 3-sulfate (DHEAS), a neuromodulatory steroid[177].
The resistance profile of MRP8 was determined using pig kidney epithelial (LLC-PK1) cells, which ectopically expressed the pump. Studies done using inside-out membrane vesicles showed that MRP8 does not share the same substrates as MRP5, despite their structural similarities. Instead, MRP8 has a similar substrate specificity to MRP4, as both transporters can pump DHEAS, estrone-3-sulfate (E1S), folates, monoanionic bile acids, and GSH and glucuronate conjugates[96],[100],[101]. Nevertheless, there are some marked differences among the two transporters. MRP4 is strictly dependent upon GSH and can transport prostaglandins, PGE1, and PGE2[116],[178]. In contrast, MRP8 transports monoanionic bile acids in the absence of GSH, and it is unable to transport PGE1 and PGE2[179]. MRP8 can confer resistance to PMEA, 2′,3′−dideoxycytidine, 5−FU, MTX, and Ara−C (Table 2)[180]. Transport analysis using MRP8 membrane vesicles showed that MRP8 can transport a wide range of compounds, including nucleotide analogs; lipophilic anions such as natural and synthetic glutathione conjugates LTC4 and DNP−SG; E217βG; monoanionic bile acids glycocholate and taurocholate; steroid sulfates such as DHEAS and E1S; folic acid; and MTX (Table 1)[179]. Under basal and stimulated conditions, expression of MRP8 resulted in decreased intracellular concentrations and increased extracellular concentration of cAMP and cGMP[177].
The results from various studies describing the substrates and expression pattern of MRP8 suggest that it could play a major role in maintaining the normal body functions. With its ability to transport monoanionic bile acids and its expression in the liver, MRP8 may affect homeostasis within hepatocytes[179]. In addition, with the ability to transport glucuronidated and sulfated steroids like E217βG, DHEAS, and E1S, and with its expression in areas such as the breasts, testes, and prostate, MRP8 could play a crucial role in determining how these hormone-regulated tissues would respond to sex steroids[179]. Clinically, MRP8 is reported to be highly expressed in breast cancer patients[174]. In addition, overexpression of MRP8 is reportedly significantly related to lower overall survival in acute myelogenous leukemia (AML) patients, implicating it as a possible biomarker[181]; however, MRP8 expression in normal tissues still needs to be established. Indeed, more studies are required to establish a link between MRP8 expression and the possible clinical significance in MDR[181].
MRP9/ABCC12
MRP9 is the last member within the MRP family to be cloned. There will likely be no further cloning because the sequence is complete, and the last member of the family, ABCC13, is reported to be a pseudogene[182]–[184]. MRP9 is located in close proximity to MRP8 in a head-to-tail orientation at chromosomal region 16q12.1[184]. This locus is implicated as a potential candidate gene(s) for paroxysmal kinesigenic choreoathetosis (PKC), a disorder involving abnormal involuntary movements [184] and infantile convulsions with paroxysmal choreoathetosis (ICCA)[91],[176]. The MRP9 sequence, which is similar to MRP8 sequence, shares 44% identity and 55% sequence similarity with MRP5 sequence [125],[185]. MRP9 also contains 2 MSD and 12 TM helices; however, some researchers have suggested that it has only 1 NBD and 8 TM helices[185]. MRP9 encodes a protein of 1359 amino acids that does not undergo N-glycosylation[185]. According to reports, there are two MRP9 transcripts, one 4.5 kb in length that is expressed in breast cancer, normal breasts, and the testis, and the other 1.3 kb in length that is expressed in the brain, skeletal muscle, and ovaries[185]. However, a recent study showed only full-length Mrp9 in testicular germ cells and mouse sperm[186]. No report has been published about the substrate profile of MRP9[185]. Using membrane vesicles prepared from insect Sf9 cells, no transport was observed for cGMP, cAMP, MTX, GSH, glycocholic acid, taurocholic acid, DHEAS, or E217βG[187].
Conclusions
MRPs have variable tissue distributions, cellular localizations, and pharmacological and physiological functions (Table 1). The uniqueness of MRPs is that they confer resistance to a range of anticancer drugs that is broader than the range of drugs handled by P-gp, the first and the most widely studied ABC transporter. MRP1 and MRP2 confer resistance to natural anticancer drugs such as vinca alkaloids and MTX (antifolate), which are hydrophobic. MRP3 confers resistance to MTX and epipodophylotoxins. The most intriguing feature of MRPs 1-3 is that they provide a transport facility for compounds (drugs, xenobiotics, or physiological substrates) conjugated with GSH, glucuronide or sulfate. MRP4 and MRP5 confer resistance to nucleobase and nucleoside analogs such as PMEA, 6-MP, and 6-TG. MRP8 confers resistance to PMEA but not to 6-MP or 6-TG. MRP7 confers resistance to almost every category of drugs, ranging from natural anticancer drugs to nucleoside analogs and epothilone B. Extensive studies performed on these transporters revealed that they are expressed in tumor tissues. This makes them a prime suspect in the development of MDR apart from P-gp and BCRP. However, there are still insufficient data from which to derive a definite conclusion about MRP expression and the development of clinical MDR. Further studies are required to confirm the role of individual MRP members and target them to confront MDR.
Acknowledgments
This work was supported in part by grants from NIH R15 No. 1R15CA143701 (to Z.S. Chen) and St. John's University Seed Grant No. 579-1110-7002 (Z.S. Chen). K. Sodani and A. Patel thank the Teaching fellowship from Department of Pharmaceutical Sciences, College of Pharmacy and Allied Health Professions, St. John's University.
References
- 1.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Center MM, Ward E, et al. Cancer occurrence. Methods Mol Biol. 2009;471:3–29. doi: 10.1007/978-1-59745-416-2_1. [DOI] [PubMed] [Google Scholar]
- 3.Gottesman MM. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 4.Loe DW, Deeley RG, Cole SP. Characterization of vincristine transport by the M (r) 190 000 multidrug resistance protein (MRP): evidence for cotransport with reduced glutathione. Cancer Res. 1998;58:5130–5136. [PubMed] [Google Scholar]
- 5.Redmond KM, Wilson TR, Johnston PG, et al. Resistance mechanisms to cancer chemotherapy. Front Biosci. 2008;13:5138–5154. doi: 10.2741/3070. [DOI] [PubMed] [Google Scholar]
- 6.Dean M, Rzhetsky A, Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001;11:1156–1166. doi: 10.1101/gr.184901. [DOI] [PubMed] [Google Scholar]
- 7.Dean M, Annilo T. Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Annu Rev Genomics Hum Genet. 2005;6:123–142. doi: 10.1146/annurev.genom.6.080604.162122. [DOI] [PubMed] [Google Scholar]
- 8.Dean M, Allikmets R. Complete characterization of the human ABC gene family. J Bioenerg Biomembr. 2001;33:475–479. doi: 10.1023/a:1012823120935. [DOI] [PubMed] [Google Scholar]
- 9.Keppler D. Multidrug resistance proteins (MRPs, ABCCs): importance for pathophysiology and drug therapy. Handb Exp Pharmacol. 2011;201:299–323. doi: 10.1007/978-3-642-14541-4_8. [DOI] [PubMed] [Google Scholar]
- 10.Cole SP, Bhardwaj G, Gerlach JH, et al. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992;258:1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- 11.Deeley RG, Westlake C, Cole SP. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol Rev. 2006;86:849–899. doi: 10.1152/physrev.00035.2005. [DOI] [PubMed] [Google Scholar]
- 12.Hollenstein K, Dawson RJ, Locher KP. Structure and mechanism of ABC transporter proteins. Curr Opin Struct Biol. 2007;17:412–418. doi: 10.1016/j.sbi.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Marquardt D, McCrone S, Center MS. Mechanisms of multidrug resistance in HL60 cells: detection of resistance-associated proteins with antibodies against synthetic peptides that correspond to the deduced sequence of P-glycoprotein. Cancer Res. 1990;50:1426–1430. [PubMed] [Google Scholar]
- 14.Marsh W, Sicheri D, Center MS. Isolation and characterization of adriamycin-resistant HL-60 cells which are not defective in the initial intracellular accumulation of drug. Cancer Res. 1986;46:4053–4057. [PubMed] [Google Scholar]
- 15.Marsh W, Center MS. Adriamycin resistance in HL60 cells and accompanying modification of a surface membrane protein contained in drug-sensitive cells. Cancer Res. 1987;47:5080–5086. [PubMed] [Google Scholar]
- 16.McGrath T, Latoud C, Arnold ST, et al. Mechanisms of multidrug resistance in HL60 cells. Analysis of resistance associated membrane proteins and levels of MDR gene expression. Biochem Pharmacol. 1989;38:3611–3619. doi: 10.1016/0006-2952(89)90134-2. [DOI] [PubMed] [Google Scholar]
- 17.Bakos E, Evers R, Szakacs G, et al. Functional multidrug resistance protein (MRP1) lacking the N-terminal transmembrane domain. J Biol Chem. 1998;273:32167–32175. doi: 10.1074/jbc.273.48.32167. [DOI] [PubMed] [Google Scholar]
- 18.Kruh GD, Belinsky MG. The MRP family of drug efflux pumps. Oncogene. 2003;22:7537–7552. doi: 10.1038/sj.onc.1206953. [DOI] [PubMed] [Google Scholar]
- 19.Flens MJ, Zaman GJ, van der Valk P, et al. Tissue distribution of the multidrug resistance protein. Am J Pathol. 1996;148:1237–1247. [PMC free article] [PubMed] [Google Scholar]
- 20.Wright SR, Boag AH, Valdimarsson G, et al. Immunohistochemical detection of multidrug resistance protein in human lung cancer and normal lung. Clin Cancer Res. 1998;4:2279–2289. [PubMed] [Google Scholar]
- 21.Brechot JM, Hurbain I, Fajac A, et al. Different pattern of MRP localization in ciliated and basal cells from human bronchial epithelium. J Histochem Cytochem. 1998;46:513–517. doi: 10.1177/002215549804600411. [DOI] [PubMed] [Google Scholar]
- 22.St-Pierre MV, Serrano MA, Macias RI, et al. Expression of members of the multidrug resistance protein family in human term placenta. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1495–R1503. doi: 10.1152/ajpregu.2000.279.4.R1495. [DOI] [PubMed] [Google Scholar]
- 23.Bakos E, Hegedus T, Hollo Z, et al. Membrane topology and glycosylation of the human multidrug resistance–associated protein. J Biol Chem. 1996;271:12322–12326. doi: 10.1074/jbc.271.21.12322. [DOI] [PubMed] [Google Scholar]
- 24.Hipfner DR, Almquist KC, Leslie EM, et al. Membrane topology of the multidrug resistance protein (MRP). A study of glycosylation-site mutants reveals an extracytosolic NH2 terminus. J Biol Chem. 1997;272:23623–23630. doi: 10.1074/jbc.272.38.23623. [DOI] [PubMed] [Google Scholar]
- 25.Cole SP, Sparks KE, Fraser K, et al. Pharmacological characterization of multidrug resistant MRP-transfected human tumor cells. Cancer Res. 1994;54:5902–5910. [PubMed] [Google Scholar]
- 26.Zaman GJ, Flens MJ, van Leusden MR, et al. The human multidrug resistance–associated protein MRP is a plasma membrane drug-efflux pump. Proc Natl Acad Sci U S A. 1994;91:8822–8826. doi: 10.1073/pnas.91.19.8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Breuninger LM, Paul S, Gaughan K, et al. Expression of multidrug resistance–associated protein in NIH/3T3 cells confers multidrug resistance associated with increased drug efflux and altered intracellular drug distribution. Cancer Res. 1995;55:5342–5347. [PubMed] [Google Scholar]
- 28.Williams GC, Liu A, Knipp G, et al. Direct evidence that saquinavir is transported by multidrug resistance-associated protein (MRP1) and canalicular multispecific organic anion transporter (MRP2) Antimicrob Agents Chemother. 2002;46:3456–3462. doi: 10.1128/AAC.46.11.3456-3462.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen ZS, Furukawa T, Sumizawa T, et al. ATP-dependent efflux of CPT-11 and SN-38 by the multidrug resistance protein (MRP) and its inhibition by PAK-104P. Mol Pharmacol. 1999;55:921–928. [PubMed] [Google Scholar]
- 30.Morrow CS, Peklak-Scott C, Bishwokarma B, et al. Multidrug resistance protein 1 (MRP1, ABCC1) mediates resistance to mitoxantrone via glutathione-dependent drug efflux. Mol Pharmacol. 2006;69:1499–1505. doi: 10.1124/mol.105.017988. [DOI] [PubMed] [Google Scholar]
- 31.Allen JD, Brinkhuis RF, van Deemter L, et al. Extensive contribution of the multidrug transporters P-glycoprotein and Mrp1 to basal drug resistance. Cancer Res. 2000;60:5761–5766. [PubMed] [Google Scholar]
- 32.Lin ZP, Johnson DR, Finch RA, et al. Comparative study of the importance of multidrug resistance-associated protein 1 and P-glycoprotein to drug sensitivity in immortalized mouse embryonic fibroblasts. Mol Cancer Ther. 2002;1:1105–1114. [PubMed] [Google Scholar]
- 33.Czyzewski K, Styczynski J. Imatinib is a substrate for various multidrug resistance proteins. Neoplasma. 2009;56:202–207. doi: 10.4149/neo_2009_03_202. [DOI] [PubMed] [Google Scholar]
- 34.Jedlitschky G, Leier I, Buchholz U, et al. ATP-dependent transport of glutathione S-conjugates by the multidrug resistance–associated protein. Cancer Res. 1994;54:4833–4836. [PubMed] [Google Scholar]
- 35.Leier I, Jedlitschky G, Buchholz U, et al. The MRP gene encodes an ATP-dependent export pump for leukotriene C4 and structurally related conjugates. J Biol Chem. 1994;269:27807–27810. [PubMed] [Google Scholar]
- 36.Jedlitschky G, Leier I, Buchholz U, et al. Transport of glutathione, glucuronate, and sulfate conjugates by the MRP gene-encoded conjugate export pump. Cancer Res. 1996;56:988–994. [PubMed] [Google Scholar]
- 37.Loe DW, Almquist KC, Cole SP, et al. ATP-dependent 17 beta- estradiol 17-(beta-D-glucuronide) transport by multidrug resistance protein (MRP). Inhibition by cholestatic steroids. J Biol Chem. 1996;271:9683–9689. doi: 10.1074/jbc.271.16.9683. [DOI] [PubMed] [Google Scholar]
- 38.Wijnholds J, Evers R, van Leusden MR, et al. Increased sensitivity to anticancer drugs and decreased inflammatory response in mice lacking the multidrug resistance–associated protein. Nat Med. 1997;3:1275–1279. doi: 10.1038/nm1197-1275. [DOI] [PubMed] [Google Scholar]
- 39.Evers R, Zaman GJ, van Deemter L, et al. Basolateral localization and export activity of the human multidrug resistance–associated protein in polarized pig kidney cells. J Clin Invest. 1996;97:1211–1218. doi: 10.1172/JCI118535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishikawa T. The ATP-dependent glutathione S-conjugate export pump. Trends Biochem Sci. 1992;17:463–468. doi: 10.1016/0968-0004(92)90489-v. [DOI] [PubMed] [Google Scholar]
- 41.Kruh GD, Gaughan KT, Godwin A, et al. Expression pattern of MRP in human tissues and adult solid tumor cell lines. J Natl Cancer Inst. 1995;87:1256–1258. doi: 10.1093/jnci/87.16.1256. [DOI] [PubMed] [Google Scholar]
- 42.Zaman GJ, Lankelma J, van Tellingen O, et al. Role of glutathione in the export of compounds from cells by the multidrug resistance–associated protein. Proc Natl Acad Sci U S A. 1995;92:7690–7694. doi: 10.1073/pnas.92.17.7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Versantvoort CH, Broxterman HJ, Bagrij T, et al. Regulation by glutathione of drug transport in multidrug resistant human lung tumour cell lines overexpressing multidrug resistance–associated protein. Br J Cancer. 1995;72:82–89. doi: 10.1038/bjc.1995.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Renes J, de Vries EG, Nienhuis EF, et al. ATP- and glutathione-dependent transport of chemotherapeutic drugs by the multidrug resistance protein MRP1. Br J Pharmacol. 1999;126:681–688. doi: 10.1038/sj.bjp.0702360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rappa G, Lorico A, Flavell RA, et al. Evidence that the multidrug resistance protein (MRP) functions as a co-transporter of glutathione and natural product toxins. Cancer Res. 1997;57:5232–5237. [PubMed] [Google Scholar]
- 46.Loe DW, Almquist KC, Deeley RG, et al. Multidrug resistance protein (MRP)–mediated transport of leukotriene C4 and chemotherapeutic agents in membrane vesicles. Demonstration of glutathione-dependent vincristine transport. J Biol Chem. 1996;271:9675–9682. doi: 10.1074/jbc.271.16.9675. [DOI] [PubMed] [Google Scholar]
- 47.Filipits M, Suchomel RW, Dekan G, et al. MRP and MDR1 gene expression in primary breast carcinomas. Clin Cancer Res. 1996;2:1231–1237. [PubMed] [Google Scholar]
- 48.Nooter K, de la Riviere GB, Klijn J, et al. Multidrug resistance protein in recurrent breast cancer. Lancet. 1997;349:1885–1886. doi: 10.1016/s0140-6736(05)63876-7. [DOI] [PubMed] [Google Scholar]
- 49.Tiwari AK, Sodani K, Dai CL, et al. Revisiting the ABCs of multidrug resistance in cancer chemotherapy. Curr Pharm Biotechnol. 2011;12:570–594. doi: 10.2174/138920111795164048. [DOI] [PubMed] [Google Scholar]
- 50.Nagengast WB, Oude Munnink TH, Dijkers EC, et al. Multidrug resistance in oncology and beyond: from imaging of drug efflux pumps to cellular drug targets. Methods Mol Biol. 2010;596:15–31. doi: 10.1007/978-1-60761-416-6_2. [DOI] [PubMed] [Google Scholar]
- 51.Buchler M, Konig J, Brom M, et al. cDNA cloning of the hepatocyte canalicular isoform of the multidrug resistance protein, cMrp, reveals a novel conjugate export pump deficient in hyperbilirubinemic mutant rats. J Biol Chem. 1996;271:15091–15098. doi: 10.1074/jbc.271.25.15091. [DOI] [PubMed] [Google Scholar]
- 52.Paulusma CC, Kool M, Bosma PJ, et al. A mutation in the human canahcular multispecific organic anion transporter gene causes the dubin-johnson syndrome. Hepatology. 1997;25:1539–1542. doi: 10.1002/hep.510250635. [DOI] [PubMed] [Google Scholar]
- 53.Mottino AD, Hoffman T, Jennes L, et al. Expression of multidrug resistance–associated protein 2 in small intestine from pregnant and postpartum rats. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1261–G1273. doi: 10.1152/ajpgi.2001.280.6.G1261. [DOI] [PubMed] [Google Scholar]
- 54.Schaub TP, Kartenbeck J, Konig J, et al. Expression of the conjugate export pump encoded by the MRP2 gene in the apical membrane of kidney proximal tubules. J Am Soc Nephrol. 1997;8:1213–1221. doi: 10.1681/ASN.V881213. [DOI] [PubMed] [Google Scholar]
- 55.Kool M, de Haas M, Scheffer GL, et al. Analysis of expression of cMOAT (MRP2), MRP3, MRP4, and MRP5, homologues of the multidrug resistance–associated protein gene (MRP1), in human cancer cell lines. Cancer Res. 1997;57:3537–3547. [PubMed] [Google Scholar]
- 56.Rost D, Konig J, Weiss G, et al. Expression and localization of the multidrug resistance proteins MRP2 and MRP3 in human gallbladder epithelia. Gastroenterology. 2001;121:1203–1208. doi: 10.1053/gast.2001.28648. [DOI] [PubMed] [Google Scholar]
- 57.Koike K, Kawabe T, Tanaka T, et al. A canalicular multispecific organic anion transporter (cMOAT) antisense cdna enhances drug sensitivity in human hepatic cancer cells. Cancer Res. 1997;57:5475–5479. [PubMed] [Google Scholar]
- 58.Evers R, Kool M, van Deemter L, et al. Drug export activity of the human canalicular multispecific organic anion transporter in polarized kidney mdck cells expressing cMOAT (MRP2) cDNA. J Clin Invest. 1998;101:1310–1319. doi: 10.1172/JCI119886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hooijberg JH, Broxterman HJ, Kool M, et al. Antifolate resistance mediated by the multidrug resistance proteins MRP1 and MRP2. Cancer Res. 1999;59:2532–2535. [PubMed] [Google Scholar]
- 60.Cui Y, Konig J, Buchholz JK, et al. Drug resistance and ATP-dependent conjugate transport mediated by the apical multidrug resistance protein, MRP2, permanently expressed in human and canine cells. Mol Pharmacol. 1999;55:929–937. [PubMed] [Google Scholar]
- 61.Ishikawa T, Ali-Osman F. Glutathione-associated cis-diamminedichloroplatinum (II) metabolism and ATP-dependent efflux from leukemia cells. Molecular characterization of glutathione-platinum complex and its biological significance. J Biol Chem. 1993;268:20116–20125. [PubMed] [Google Scholar]
- 62.Evers R, de Haas M, Sparidans R, et al. Vinblastine and sulfinpyrazone export by the multidrug resistance protein MRP2 is associated with glutathione export. Br J Cancer. 2000;83:375–383. doi: 10.1054/bjoc.2000.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nies AT, Keppler D. The apical conjugate efflux pump ABCC2 (MRP2) Pflugers Arch. 2007;453:643–659. doi: 10.1007/s00424-006-0109-y. [DOI] [PubMed] [Google Scholar]
- 64.Konig J, Nies AT, Cui Y, et al. Conjugate export pumps of the multidrug resistance protein (MRP) family: localization, substrate specificity, and MRP2-mediated drug resistance. Biochim Biophys Acta. 1999;1461:377–394. doi: 10.1016/s0005-2736(99)00169-8. [DOI] [PubMed] [Google Scholar]
- 65.Kawabe T, Chen ZS, Wada M, et al. Enhanced transport of anticancer agents and leukotriene C4 by the human canalicular multispecific organic anion transporter (cMOAT/MRP2) FEBS Lett. 1999;456:327–331. doi: 10.1016/s0014-5793(99)00979-5. [DOI] [PubMed] [Google Scholar]
- 66.Kartenbeck J, Leuschner U, Mayer R, et al. Absence of the canalicular isoform of the MRP gene–encoded conjugate export pump from the hepatocytes in dubin-johnson syndrome. Hepatology. 1996;23:1061–1066. doi: 10.1053/jhep.1996.v23.pm0008621134. [DOI] [PubMed] [Google Scholar]
- 67.Jansen PL, Peters WH, Lamers WH. Hereditary chronic conjugated hyperbilirubinemia in mutant rats caused by defective hepatic anion transport. Hepatology. 1985;5:573–579. doi: 10.1002/hep.1840050408. [DOI] [PubMed] [Google Scholar]
- 68.Hosokawa S, Tagaya O, Mikami T, et al. A new rat mutant with chronic conjugated hyperbilirubinemia and renal glomerular lesions. Lab Anim Sci. 1992;42:27–34. [PubMed] [Google Scholar]
- 69.Hirohashi T, Suzuki H, Ito K, et al. Hepatic expression of multidrug resistance–associated protein-like proteins maintained in eisai hyperbilirubinemic rats. Mol Pharmacol. 1998;53:1068–1075. [PubMed] [Google Scholar]
- 70.Narasaki F, Oka M, Nakano R, et al. Human canalicular multispecific organic anion transporter (cMOAT) is expressed in human lung, gastric, and colorectal cancer cells. Biochem Biophys Res Commun. 1997;240:606–611. doi: 10.1006/bbrc.1997.7703. [DOI] [PubMed] [Google Scholar]
- 71.Taniguchi K, Wada M, Kohno K, et al. A human canalicular multispecific organic anion transporter (cMOAT) gene is overexpressed in cisplatin-resistant human cancer cell lines with decreased drug accumulation. Cancer Res. 1996;56:4124–4129. [PubMed] [Google Scholar]
- 72.Korita PV, Wakai T, Shirai Y, et al. Multidrug resistance–associated protein 2 determines the efficacy of cisplatin in patients with hepatocellular carcinoma. Oncol Rep. 2010;23:965–972. doi: 10.3892/or_00000721. [DOI] [PubMed] [Google Scholar]
- 73.Konig J, Rost D, Cui Y, et al. Characterization of the human multidrug resistance protein isoform MRP3 localized to the basolateral hepatocyte membrane. Hepatology. 1999;29:1156–1163. doi: 10.1002/hep.510290404. [DOI] [PubMed] [Google Scholar]
- 74.Kool M, van der Linden M, de Haas M, et al. MRP3, an organic anion transporter able to transport anti-cancer drugs. Proc Natl Acad Sci U S A. 1999;96:6914–6919. doi: 10.1073/pnas.96.12.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ortiz DF, Li S, Iyer R, et al. MRP3, a new ATP-binding cassette protein localized to the canalicular domain of the hepatocyte. Am J Physiol. 1999;276:G1493–G1500. doi: 10.1152/ajpgi.1999.276.6.G1493. [DOI] [PubMed] [Google Scholar]
- 76.Borst P, Zelcer N, van de Wetering K. MRP2 and 3 in health and disease. Cancer Lett. 2006;234:51–61. doi: 10.1016/j.canlet.2005.05.051. [DOI] [PubMed] [Google Scholar]
- 77.Scheffer GL, Kool M, de Haas M, et al. Tissue distribution and induction of human multidrug resistant protein 3. Lab Invest. 2002;82:193–201. doi: 10.1038/labinvest.3780411. [DOI] [PubMed] [Google Scholar]
- 78.Zeng H, Bain LJ, Belinsky MG, et al. Expression of multidrug resistance protein-3 (multispecific organic anion transporter-D) in human embryonic kidney 293 cells confers resistance to anticancer agents. Cancer Res. 1999;59:5964–5967. [PubMed] [Google Scholar]
- 79.Zelcer N, Saeki T, Reid G, et al. Characterization of drug transport by the human multidrug resistance protein 3 (ABCC3) J Biol Chem. 2001;276:46400–46407. doi: 10.1074/jbc.M107041200. [DOI] [PubMed] [Google Scholar]
- 80.Lagas JS, Fan L, Wagenaar E, et al. P-glycoprotein (P-gp/ Abcb1), Abcc2, and Abcc3 determine the pharmacokinetics of etoposide. Clin Cancer Res. 2010;16:130–140. doi: 10.1158/1078-0432.CCR-09-1321. [DOI] [PubMed] [Google Scholar]
- 81.Kubo K, Sekine S, Saito M. Compensatory expression of MRP3 in the livers of MRP2-deficient EHBRs is promoted by DHA intake. Biosci Biotechnol Biochem. 2009;73:2432–2438. doi: 10.1271/bbb.90387. [DOI] [PubMed] [Google Scholar]
- 82.Belinsky MG, Dawson PA, Shchaveleva I, et al. Analysis of the in vivo functions of MRP3. Mol Pharmacol. 2005;68:160–168. doi: 10.1124/mol.104.010587. [DOI] [PubMed] [Google Scholar]
- 83.Zeng H, Liu G, Rea PA, et al. Transport of amphipathic anions by human multidrug resistance protein 3. Cancer Res. 2000;60:4779–4784. [PubMed] [Google Scholar]
- 84.Hirohashi T, Suzuki H, Sugiyama Y. Characterization of the transport properties of cloned rat multidrug resistance–associated protein 3 (Mrp3) J Biol Chem. 1999;274:15181–15185. doi: 10.1074/jbc.274.21.15181. [DOI] [PubMed] [Google Scholar]
- 85.Nies AT, Konig J, Pfannschmidt M, et al. Expression of the multidrug resistance proteins MRP2 and MRP3 in human hepatocellular carcinoma. Int J Cancer. 2001;94:492–499. doi: 10.1002/ijc.1498. [DOI] [PubMed] [Google Scholar]
- 86.Ohishi Y, Oda Y, Uchiumi T, et al. ATP-binding cassette superfamily transporter gene expression in human primary ovarian carcinoma. Clin Cancer Res. 2002;8:3767–3775. [PubMed] [Google Scholar]
- 87.Plasschaert SL, de Bont ES, Boezen M, et al. Expression of multidrug resistance–associated proteins predicts prognosis in childhood and adult acute lymphoblastic leukemia. Clin Cancer Res. 2005;11:8661–8668. doi: 10.1158/1078-0432.CCR-05-1096. [DOI] [PubMed] [Google Scholar]
- 88.Russel FG, Koenderink JB, Masereeuw R. Multidrug resistance protein 4 (MRP4/ABCC4): a versatile efflux transporter for drugs and signalling molecules. Trends Pharmacol Sci. 2008;29:200–207. doi: 10.1016/j.tips.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 89.Schuetz JD, Connelly MC, Sun D, et al. MRP4: a previously unidentified factor in resistance to nucleoside-based antiviral drugs. Nat Med. 1999;5:1048–1051. doi: 10.1038/12487. [DOI] [PubMed] [Google Scholar]
- 90.Allikmets R, Gerrard B, Hutchinson A, et al. Characterization of the human ABC superfamily: isolation and mapping of 21 new genes using the expressed sequence tags database. Hum Mol Genet. 1996;5:1649–1655. doi: 10.1093/hmg/5.10.1649. [DOI] [PubMed] [Google Scholar]
- 91.Lee K, Belinsky MG, Bell DW, et al. Isolation of MOAT-b, a widely expressed multidrug resistance–associated protein/ canalicular multispecific organic anion transporter-related transporter. Cancer Res. 1998;58:2741–2747. [PubMed] [Google Scholar]
- 92.Borst P, Evers R, Kool M, et al. A family of drug transporters: the multidrug resistance–associated proteins. J Natl Cancer Inst. 2000;92:1295–1302. doi: 10.1093/jnci/92.16.1295. [DOI] [PubMed] [Google Scholar]
- 93.Borst P, de Wolf C, van de Wetering K. Multidrug resistance–associated proteins 3, 4, and 5. Pflugers Arch. 2007;453:661–673. doi: 10.1007/s00424-006-0054-9. [DOI] [PubMed] [Google Scholar]
- 94.Sauna ZE, Nandigama K, Ambudkar SV. Multidrug resistance protein 4 (ABCC4)–mediated ATP hydrolysis: effect of transport substrates and characterization of the post-hydrolysis transition state. J Biol Chem. 2004;279:48855–48864. doi: 10.1074/jbc.M408849200. [DOI] [PubMed] [Google Scholar]
- 95.Li C, Krishnamurthy PC, Penmatsa H, et al. Spatiotemporal coupling of cAMP transporter to CFTR chloride channel function in the gut epithelia. Cell. 2007;131:940–951. doi: 10.1016/j.cell.2007.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zelcer N, Reid G, Wielinga P, et al. Steroid and bile acid conjugates are substrates of human multidrug–resistance protein (MRP) 4 (ATP-binding cassette C4) Biochem J. 2003;371:361–367. doi: 10.1042/BJ20021886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hopper-Borge E, Chen ZS, Shchaveleva I, et al. Analysis of the drug resistance profile of multidrug resistance protein 7 (ABCC10): resistance to docetaxel. Cancer Res. 2004;64:4927–4930. doi: 10.1158/0008-5472.CAN-03-3111. [DOI] [PubMed] [Google Scholar]
- 98.Rius M, Nies AT, Hummel-Eisenbeiss J, et al. Cotransport of reduced glutathione with bile salts by MRP4 (ABCC4) localized to the basolateral hepatocyte membrane. Hepatology. 2003;38:374–384. doi: 10.1053/jhep.2003.50331. [DOI] [PubMed] [Google Scholar]
- 99.Adachi M, Sampath J, Lan LB, et al. Expression of MRP4 confers resistance to ganciclovir and compromises bystander cell killing. J Biol Chem. 2002;277:38998–39004. doi: 10.1074/jbc.M203262200. [DOI] [PubMed] [Google Scholar]
- 100.Chen ZS, Lee K, Walther S, et al. Analysis of methotrexate and folate transport by multidrug resistance protein 4 (ABCC4): MRP4 is a component of the methotrexate efflux system. Cancer Res. 2002;62:3144–3150. [PubMed] [Google Scholar]
- 101.Chen ZS, Lee K, Kruh GD. Transport of cyclic nucleotides and estradiol 17-beta-D-glucuronide by multidrug resistance protein 4. Resistance to 6-mercaptopurine and 6-thioguanine. J Biol Chem. 2001;276:33747–33754. doi: 10.1074/jbc.M104833200. [DOI] [PubMed] [Google Scholar]
- 102.Lai L, Tan TM. Role of glutathione in the multidrug resistance protein 4 (MRP4/ABCC4)–mediated efflux of cAMP and resistance to purine analogues. Biochem J. 2002;361:497–503. doi: 10.1042/0264-6021:3610497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reid G, Wielinga P, Zelcer N, et al. Characterization of the transport of nucleoside analog drugs by the human multidrug resistance proteins MRP4 and MRP5. Mol Pharmacol. 2003;63:1094–1103. doi: 10.1124/mol.63.5.1094. [DOI] [PubMed] [Google Scholar]
- 104.Wielinga PR, Reid G, Challa EE, et al. Thiopurine metabolism and identification of the thiopurine metabolites transported by MRP4 and MRP5 overexpressed in human embryonic kidney cells. Mol Pharmacol. 2002;62:1321–1331. doi: 10.1124/mol.62.6.1321. [DOI] [PubMed] [Google Scholar]
- 105.Wu CP, Calcagno AM, Hladky SB, et al. Modulatory effects of plant phenols on human multidrug-resistance proteins 1, 4 and 5 (ABCC1, 4 and 5) FEBS J. 2005;272:4725–4740. doi: 10.1111/j.1742-4658.2005.04888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wielinga PR, van der Heijden I, Reid G, et al. Characterization of the MRP4- and MRP5-mediated transport of cyclic nucleotides from intact cells. J Biol Chem. 2003;278:17664–17671. doi: 10.1074/jbc.M212723200. [DOI] [PubMed] [Google Scholar]
- 107.Hofer AM, Lefkimmiatis K. Extracellular calcium and cAMP: second messengers as “third messengers”? Physiology (Bethesda) 2007;22:320–327. doi: 10.1152/physiol.00019.2007. [DOI] [PubMed] [Google Scholar]
- 108.Chen C, Slitt AL, Dieter MZ, et al. Up-regulation of Mrp4 expression in kidney of MRP2-deficient TR- rats. Biochem Pharmacol. 2005;70:1088–1095. doi: 10.1016/j.bcp.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 109.Denk GU, Soroka CJ, Takeyama Y, et al. Multidrug resistance-associated protein 4 is up-regulated in liver but down-regulated in kidney in obstructive cholestasis in the rat. J Hepatol. 2004;40:585–591. doi: 10.1016/j.jhep.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 110.Klokouzas A, Wu CP, van Veen HW, et al. cGMP and glutathione-conjugate transport in human erythrocytes. Eur J Biochem. 2003;270:3696–3708. doi: 10.1046/j.1432-1033.2003.03753.x. [DOI] [PubMed] [Google Scholar]
- 111.Sager G, Orbo A, Pettersen RH, et al. Export of guanosine 3′, 5′-cyclic monophosphate (cGMP) from human erythrocytes characterized by inside-out membrane vesicles. Scand J Clin Lab Invest. 1996;56:289–293. doi: 10.3109/00365519609090579. [DOI] [PubMed] [Google Scholar]
- 112.Schultz C, Vaskinn S, Kildalsen H, et al. Cyclic AMP stimulates the cyclic GMP egression pump in human erythrocytes: effects of probenecid, verapamil, progesterone, theophylline, ibmx, forskolin, and cyclic AMP on cyclic GMP uptake and association to inside-out vesicles. Biochemistry. 1998;37:1161–1166. doi: 10.1021/bi9713409. [DOI] [PubMed] [Google Scholar]
- 113.Sundkvist E, Jaeger R, Sager G. Pharmacological characterization of the ATP-dependent low K(m) guanosine 3′, 5′-cyclic monophosphate (cGMP) transporter in human erythrocytes. Biochem Pharmacol. 2002;63:945–949. doi: 10.1016/s0006-2952(01)00940-6. [DOI] [PubMed] [Google Scholar]
- 114.Wu CP, Woodcock H, Hladky SB, et al. CGMP (guanosine 3′, 5′-cyclic monophosphate) transport across human erythrocyte membranes. Biochem Pharmacol. 2005;69:1257–1262. doi: 10.1016/j.bcp.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 115.Lin ZP, Zhu YL, Johnson DR, et al. Disruption of cAMP and prostaglandin E2 transport by multidrug resistance protein 4 deficiency alters cAMP-mediated signaling and nociceptive response. Mol Pharmacol. 2008;73:243–251. doi: 10.1124/mol.107.039594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rius M, Hummel-Eisenbeiss J, Hofmann AF, et al. Substrate specificity of human ABCC4 (MRP4)–mediated cotransport of bile acids and reduced glutathione. Am J Physiol Gastrointest Liver Physiol. 2006;290:G640–G649. doi: 10.1152/ajpgi.00354.2005. [DOI] [PubMed] [Google Scholar]
- 117.Van Aubel RA, Smeets PH, van den Heuvel JJ, et al. Human organic anion transporter MRP4 (ABCC4) is an efflux pump for the purine end metabolite urate with multiple allosteric substrate binding sites. Am J Physiol Renal Physiol. 2005;288:F327–F333. doi: 10.1152/ajprenal.00133.2004. [DOI] [PubMed] [Google Scholar]
- 118.Schuetz EG, Strom S, Yasuda K, et al. Disrupted bile acid homeostasis reveals an unexpected interaction among nuclear hormone receptors, transporters, and cytochrome P450. J Biol Chem. 2001;276:39411–39418. doi: 10.1074/jbc.M106340200. [DOI] [PubMed] [Google Scholar]
- 119.Aleksunes LM, Slitt AM, Cherrington NJ, et al. Differential expression of mouse hepatic transporter genes in response to acetaminophen and carbon tetrachloride. Toxicol Sci. 2005;83:44–52. doi: 10.1093/toxsci/kfi013. [DOI] [PubMed] [Google Scholar]
- 120.Aleksunes LM, Scheffer GL, Jakowski AB, et al. Coordinated expression of multidrug resistance–associated proteins (MRPs) in mouse liver during toxicant-induced injury. Toxicol Sci. 2006;89:370–379. doi: 10.1093/toxsci/kfi332. [DOI] [PubMed] [Google Scholar]
- 121.Adachi M, Reid G, Schuetz JD. Therapeutic and biological importance of getting nucleotides out of cells: a case for the ABC transporters, MRP4 and 5. Adv Drug Deliv Rev. 2002;54:1333–1342. doi: 10.1016/s0169-409x(02)00166-7. [DOI] [PubMed] [Google Scholar]
- 122.Hitzl M, Klein K, Zanger UM, et al. Influence of omeprazole on multidrug resistance protein 3 expression in human liver. J Pharmacol Exp Ther. 2003;304:524–530. doi: 10.1124/jpet.102.043547. [DOI] [PubMed] [Google Scholar]
- 123.Haimeur A, Conseil G, Deeley RG, et al. The MRP-related and BCRP/ABCG2 multidrug resistance proteins: biology, substrate specificity and regulation. Curr Drug Metab. 2004;5:21–53. doi: 10.2174/1389200043489199. [DOI] [PubMed] [Google Scholar]
- 124.Belinsky MG, Bain LJ, Balsara BB, et al. Characterization of MOAT-C and MOAT-D, new members of the MRP/cMOAT subfamily of transporter proteins. J Natl Cancer Inst. 1998;90:1735–1741. doi: 10.1093/jnci/90.22.1735. [DOI] [PubMed] [Google Scholar]
- 125.McAleer MA, Breen MA, White NL, et al. Pabc11 (also known as MOAT-C and MRP5), a member of the ABC family of proteins, has anion transporter activity but does not confer multidrug resistance when overexpressed in human embryonic kidney 293 cells. J Biol Chem. 1999;274:23541–23548. doi: 10.1074/jbc.274.33.23541. [DOI] [PubMed] [Google Scholar]
- 126.Wijnholds J, Mol CA, van Deemter L, et al. Multidrug-resistance protein 5 is a multispecific organic anion transporter able to transport nucleotide analogs. Proc Natl Acad Sci U S A. 2000;97:7476–7481. doi: 10.1073/pnas.120159197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Carvajal JA, Germain AM, Huidobro-Toro JP, et al. Molecular mechanism of cGMP-mediated smooth muscle relaxation. J Cell Physiol. 2000;184:409–420. doi: 10.1002/1097-4652(200009)184:3<409::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 128.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 129.Dazert P, Meissner K, Vogelgesang S, et al. Expression and localization of the multidrug resistance protein 5 (MRP5/ ABCC5), a cellular export pump for cyclic nucleotides, in human heart. Am J Pathol. 2003;163:1567–1577. doi: 10.1016/S0002-9440(10)63513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Flesch M, Kilter H, Cremers B, et al. Acute effects of nitric oxide and cyclic GMP on human myocardial contractility. J Pharmacol Exp Ther. 1997;281:1340–1349. [PubMed] [Google Scholar]
- 131.Touyz RM, Picard S, Schiffrin EL, et al. Cyclic GMP inhibits a pharmacologically distinct Na+/H+ exchanger variant in cultured rat astrocytes via an extracellular site of action. J Neurochem. 1997;68:1451–1461. doi: 10.1046/j.1471-4159.1997.68041451.x. [DOI] [PubMed] [Google Scholar]
- 132.Vogelgesang S, Kunert-Keil C, Cascorbi I, et al. Expression of multidrug transporters in dysembryoplastic neuroepithelial tumors causing intractable epilepsy. Clin Neuropathol. 2004;23:223–231. [PubMed] [Google Scholar]
- 133.Nies AT, Jedlitschky G, Konig J, et al. Expression and immunolocalization of the multidrug resistance proteins, MRP1–MRP6 (ABCC1–ABCC6), in human brain. Neuroscience. 2004;129:349–360. doi: 10.1016/j.neuroscience.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 134.Nies AT, Konig J, Cui Y, et al. Structural requirements for the apical sorting of human multidrug resistance protein 2 (ABCC2) Eur J Biochem. 2002;269:1866–1876. doi: 10.1046/j.1432-1033.2002.02832.x. [DOI] [PubMed] [Google Scholar]
- 135.Nies AT, Spring H, Thon WF, et al. Immunolocalization of multidrug resistance protein 5 in the human genitourinary system. J Urol. 2002;167:2271–2275. [PubMed] [Google Scholar]
- 136.Robbins BL, Connelly MC, Marshall DR, et al. A human T lymphoid cell variant resistant to the acyclic nucleoside phosphonate 9-(2-phosphonylmethoxyethyl)adenine shows a unique combination of a phosphorylation defect and increased efflux of the agent. Mol Pharmacol. 1995;47:391–397. [PubMed] [Google Scholar]
- 137.Pratt S, Shepard RL, Kandasamy RA, et al. The multidrug resistance protein 5 (ABCC5) confers resistance to 5-fluorouracil and transports its monophosphorylated metabolites. Mol Cancer Ther. 2005;4:855–863. doi: 10.1158/1535-7163.MCT-04-0291. [DOI] [PubMed] [Google Scholar]
- 138.Jedlitschky G, Burchell B, Keppler D. The multidrug resistance protein 5 functions as an ATP-dependent export pump for cyclic nucleotides. J Biol Chem. 2000;275:30069–30074. doi: 10.1074/jbc.M005463200. [DOI] [PubMed] [Google Scholar]
- 139.Sundkvist E, Jaeger R, Sager G. Leukotriene C(4) (LTC(4)) does not share a cellular efflux mechanism with cGMP: characterisation of cGMP transport by uptake to inside-out vesicles from human erythrocytes. Biochim Biophys Acta. 2000;1463:121–130. doi: 10.1016/s0005-2736(99)00184-4. [DOI] [PubMed] [Google Scholar]
- 140.Garthwaite J. Neural nitric oxide signalling. Trends Neurosci. 1995;18:51–52. [PubMed] [Google Scholar]
- 141.Kool M, van der Linden M, de Haas M, et al. Expression of human MRP6, a homologue of the multidrug resistance protein gene MRP1, in tissues and cancer cells. Cancer Res. 1999;59:175–182. [PubMed] [Google Scholar]
- 142.Cai J, Daoud R, Alqawi O, et al. Nucleotide binding and nucleotide hydrolysis properties of the ABC transporter MRP6 (ABCC6) Biochemistry. 2002;41:8058–8067. doi: 10.1021/bi012082p. [DOI] [PubMed] [Google Scholar]
- 143.Belinsky MG, Kruh GD. MOAT-E (ARA) is a full-length MRP/ cMOAT subfamily transporter expressed in kidney and liver. Br J Cancer. 1999;80:1342–1349. doi: 10.1038/sj.bjc.6690527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Madon J, Hagenbuch B, Landmann L, et al. Transport function and hepatocellular localization of Mrp6 in rat liver. Mol Pharmacol. 2000;57:634–641. doi: 10.1124/mol.57.3.634. [DOI] [PubMed] [Google Scholar]
- 145.Beck K, Hayashi K, Nishiguchi B, et al. The distribution of ABCC6 in normal mouse tissues suggests multiple functions for this ABC transporter. J Histochem Cytochem. 2003;51:887–902. doi: 10.1177/002215540305100704. [DOI] [PubMed] [Google Scholar]
- 146.Bergen AA, Plomp AS, Schuurman EJ, et al. Mutations in ABCC6 cause pseudoxanthoma elasticum. Nat Genet. 2000;25:228–231. doi: 10.1038/76109. [DOI] [PubMed] [Google Scholar]
- 147.Ringpfeil F, Lebwohl MG, Christiano AM, et al. Pseudoxanthoma elasticum: mutations in the MRP6 gene encoding a transmembrane ATP-binding cassette (ABC) transporter. Proc Natl Acad Sci U S A. 2000;97:6001–6006. doi: 10.1073/pnas.100041297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Struk B, Cai L, Zach S, et al. Mutations of the gene encoding the transmembrane transporter protein ABC-C6 cause pseudoxanthoma elasticum. J Mol Med (Berl) 2000;78:282–286. doi: 10.1007/s001090000114. [DOI] [PubMed] [Google Scholar]
- 149.Gheduzzi D, Guidetti R, Anzivino C, et al. ABCC6 mutations in italian families affected by pseudoxanthoma elasticum (PXE) Hum Mutat. 2004;24:438–439. doi: 10.1002/humu.9284. [DOI] [PubMed] [Google Scholar]
- 150.Cai L, Lumsden A, Guenther UP, et al. A novel Q378X mutation exists in the transmembrane transporter protein ABCC6 and its pseudogene: implications for mutation analysis in pseudoxanthoma elasticum. J Mol Med (Berl) 2001;79:536–546. doi: 10.1007/s001090100275. [DOI] [PubMed] [Google Scholar]
- 151.Meloni I, Rubegni P, De Aloe G, et al. Pseudoxanthoma elasticum: Point mutations in the ABCC6 gene and a large deletion including also ABCC1 and MYH11. Hum Mutat. 2001;18:85. doi: 10.1002/humu.1157. [DOI] [PubMed] [Google Scholar]
- 152.Ringpfeil F, Nakano A, Uitto J, et al. Compound heterozygosity for a recurrent 16.5-kb Alu-mediated deletion mutation and single-base-pair substitutions in the ABCC6 gene results in pseudoxanthoma elasticum. Am J Hum Genet. 2001;68:642–652. doi: 10.1086/318807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hu X, Plomp A, Wijnholds J, et al. ABCC6/MRP6 mutations: further insight into the molecular pathology of pseudoxanthoma elasticum. Eur J Hum Genet. 2003;11:215–224. doi: 10.1038/sj.ejhg.5200953. [DOI] [PubMed] [Google Scholar]
- 154.Maher JM, Slitt AL, Cherrington NJ, et al. Tissue distribution and hepatic and renal ontogeny of the multidrug resistance- associated protein (MRP) family in mice. Drug Metab Dispos. 2005;33:947–955. doi: 10.1124/dmd.105.003780. [DOI] [PubMed] [Google Scholar]
- 155.Maher JM, Cherrington NJ, Slitt AL, et al. Tissue distribution and induction of the rat multidrug resistance-associated proteins 5 and 6. Life Sci. 2006;78:2219–2225. doi: 10.1016/j.lfs.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 156.Scheffer GL, Hu X, Pijnenborg AC, et al. MRP6 (ABCC6) detection in normal human tissues and tumors. Lab Invest. 2002;82:515–518. doi: 10.1038/labinvest.3780444. [DOI] [PubMed] [Google Scholar]
- 157.Sinko E, Ilias A, Ujhelly O, et al. Subcellular localization and N- glycosylation of human ABCC6, expressed in MDCKII cells. Biochem Biophys Res Commun. 2003;308:263–269. doi: 10.1016/s0006-291x(03)01349-4. [DOI] [PubMed] [Google Scholar]
- 158.Uitto J, Pulkkinen L, Ringpfeil F. Molecular genetics of pseudoxanthoma elasticum: a metabolic disorder at the environment-genome interface? Trends Mol Med. 2001;7:13–17. doi: 10.1016/s1471-4914(00)01869-4. [DOI] [PubMed] [Google Scholar]
- 159.Belinsky MG, Chen ZS, Shchaveleva I, et al. Characterization of the drug resistance and transport properties of multidrug resistance protein 6 (MRP6, ABCC6) Cancer Res. 2002;62:6172–6177. [PubMed] [Google Scholar]
- 160.Ilias A, Urban Z, Seidl TL, et al. Loss of ATP-dependent transport activity in pseudoxanthoma elasticum-associated mutants of human ABCC6 (MRP6) J Biol Chem. 2002;277:16860–16867. doi: 10.1074/jbc.M110918200. [DOI] [PubMed] [Google Scholar]
- 161.Hopper E, Belinsky MG, Zeng H, et al. Analysis of the structure and expression pattern of MRP7 (ABCC10), a new member of the MRP subfamily. Cancer Lett. 2001;162:181–191. doi: 10.1016/s0304-3835(00)00646-7. [DOI] [PubMed] [Google Scholar]
- 162.Takayanagi S, Kataoka T, Ohara O, et al. Human ATP-binding cassette transporter ABCC10: expression profile and p53- dependent upregulation. J Exp Ther Oncol. 2004;4:239–246. [PubMed] [Google Scholar]
- 163.Kao HH, Chang MS, Cheng JF, et al. Genomic structure, gene expression, and promoter analysis of human multidrug resistance–associated protein 7. J Biomed Sci. 2003;10:98–110. doi: 10.1007/BF02256002. [DOI] [PubMed] [Google Scholar]
- 164.Wooden SL, Kalb SR, Cotter RJ, et al. Cutting edge: HLA-E binds a peptide derived from the ATP-binding cassette transporter multidrug resistance–associated protein 7 and inhibits NK cell–mediated lysis. J Immunol. 2005;175:1383–1387. doi: 10.4049/jimmunol.175.3.1383. [DOI] [PubMed] [Google Scholar]
- 165.Bessho Y, Oguri T, Ozasa H, et al. ABCC10/MRP7 is associated with vinorelbine resistance in non–small cell lung cancer. Oncol Rep. 2009;21:263–268. [PubMed] [Google Scholar]
- 166.Huisman MT, Chhatta AA, van Tellingen O, et al. MRP2 (ABCC2) transports taxanes and confers paclitaxel resistance and both processes are stimulated by probenecid. Int J Cancer. 2005;116:824–829. doi: 10.1002/ijc.21013. [DOI] [PubMed] [Google Scholar]
- 167.Hopper-Borge E, Xu X, Shen T, et al. Human multidrug resistance protein 7 (ABCC10) is a resistance factor for nucleoside analogues and epothilone B. Cancer Res. 2009;69:178–184. doi: 10.1158/0008-5472.CAN-08-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hopper-Borge EA, Churchill T, Paulose C, et al. Contribution of ABCC10 (MRP7) to in vivo paclitaxel resistance as assessed in ABCC10(-/-) mice. Cancer Res. 2011;71:3649–3657. doi: 10.1158/0008-5472.CAN-10-3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Risinger AL, Jackson EM, Polin LA, et al. The taccalonolides: microtubule stabilizers that circumvent clinically relevant taxane resistance mechanisms. Cancer Res. 2008;68:8881–8888. doi: 10.1158/0008-5472.CAN-08-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chen ZS, Hopper-Borge E, Belinsky MG, et al. Characterization of the transport properties of human multidrug resistance protein 7 (MRP7, ABCC10) Mol Pharmacol. 2003;63:351–358. doi: 10.1124/mol.63.2.351. [DOI] [PubMed] [Google Scholar]
- 171.Naramoto H, Uematsu T, Uchihashi T, et al. Multidrug resistance–associated protein 7 expression is involved in cross-resistance to docetaxel in salivary gland adenocarcinoma cell lines. Int J Oncol. 2007;30:393–401. [PubMed] [Google Scholar]
- 172.Oguri T, Ozasa H, Uemura T, et al. MRP7/ABCC10 expression is a predictive biomarker for the resistance to paclitaxel in non–small cell lung cancer. Mol Cancer Ther. 2008;7:1150–1155. doi: 10.1158/1535-7163.MCT-07-2088. [DOI] [PubMed] [Google Scholar]
- 173.Dabrowska M, Sirotnak FM. Regulation of transcription of the human MRP7 gene. Characteristics of the basal promoter and identification of tumor-derived transcripts encoding additional 5′ end heterogeneity. Gene. 2004;341:129–139. doi: 10.1016/j.gene.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 174.Bera TK, Lee S, Salvatore G, et al. MRP8, a new member of ABC transporter superfamily, identified by est database mining and gene prediction program, is highly expressed in breast cancer. Mol Med. 2001;7:509–516. [PMC free article] [PubMed] [Google Scholar]
- 175.Yabuuchi H, Shimizu H, Takayanagi S, et al. Multiple splicing variants of two new human ATP-binding cassette transporters, ABCC11 and ABCC12. Biochem Biophys Res Commun. 2001;288:933–939. doi: 10.1006/bbrc.2001.5865. [DOI] [PubMed] [Google Scholar]
- 176.Tammur J, Prades C, Arnould I, et al. Two new genes from the human ATP-binding cassette transporter superfamily, ABCC11 and ABCC12, tandemly duplicated on chromosome 16q12. Gene. 2001;273:89–96. doi: 10.1016/s0378-1119(01)00572-8. [DOI] [PubMed] [Google Scholar]
- 177.Bortfeld M, Rius M, Konig J, et al. Human multidrug resistance protein 8 (MRP8/ABCC11), an apical efflux pump for steroid sulfates, is an axonal protein of the CNS and peripheral nervous system. Neuroscience. 2006;137:1247–1257. doi: 10.1016/j.neuroscience.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 178.Reid G, Wielinga P, Zelcer N, et al. The human multidrug resistance protein MRP4 functions as a prostaglandin efflux transporter and is inhibited by nonsteroidal antiinflammatory drugs. Proc Natl Acad Sci U S A. 2003;100:9244–9249. doi: 10.1073/pnas.1033060100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chen ZS, Guo Y, Belinsky MG, et al. Transport of bile acids, sulfated steroids, estradiol 17-beta-D-glucuronide, and leukotriene C4 by human multidrug resistance protein 8 (ABCC11) Mol Pharmacol. 2005;67:545–557. doi: 10.1124/mol.104.007138. [DOI] [PubMed] [Google Scholar]
- 180.Guo Y, Kotova E, Chen ZS, et al. MRP8, ATP-binding cassette c11 (ABCC11), is a cyclic nucleotide efflux pump and a resistance factor for fluoropyrimidines 2′,3′-dideoxycytidine and 9′-(2′-phosphonylmethoxyethyl)adenine. J Biol Chem. 2003;278:29509–29514. doi: 10.1074/jbc.M304059200. [DOI] [PubMed] [Google Scholar]
- 181.Guo Y, Kock K, Ritter CA, et al. Expression of ABCC-type nucleotide exporters in blasts of adult acute myeloid leukemia: relation to long-term survival. Clin Cancer Res. 2009;15:1762–1769. doi: 10.1158/1078-0432.CCR-08-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yabuuchi H, Takayanagi S, Yoshinaga K, et al. ABCC13, an unusual truncated ABC transporter, is highly expressed in fetal human liver. Biochem Biophys Res Commun. 2002;299:410–417. doi: 10.1016/s0006-291x(02)02658-x. [DOI] [PubMed] [Google Scholar]
- 183.Annilo T, Dean M. Degeneration of an ATP-binding cassette transporter gene, ABCC13, in different mammalian lineages. Genomics. 2004;84:34–46. doi: 10.1016/j.ygeno.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 184.Tomita H, Nagamitsu S, Wakui K, et al. Paroxysmal kinesigenic choreoathetosis locus maps to chromosome 16p11.2–q12.1. Am J Hum Genet. 1999;65:1688–1697. doi: 10.1086/302682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bera TK, lavarone C, Kumar V, et al. MRP9, an unusual truncated member of the ABC transporter superfamily, is highly expressed in breast cancer. Proc Natl Acad Sci U S A. 2002;99:6997–7002. doi: 10.1073/pnas.102187299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ono N, Van der Heijden I, Scheffer GL, et al. Multidrug resistance–associated protein 9 (ABCC12) is present in mouse and boar sperm. Biochem J. 2007;406:31–40. doi: 10.1042/BJ20070292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zelcer N, Saeki T, Bot I, et al. Transport of bile acids in multidrug-resistance-protein 3-overexpressing cells cotransfected with the ileal Na+-dependent bile-acid transporter. Biochem J. 2003;369:23–30. doi: 10.1042/BJ20021081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gillet JP, Efferth T, Remacle J. Chemotherapy-induced resistance by ATP-binding cassette transporter genes. Biochim Biophys Acta. 2007;1775:237–262. doi: 10.1016/j.bbcan.2007.05.002. [DOI] [PubMed] [Google Scholar]