Abstract
Serum enzymes that play potential roles in tumor growth have recently been reported to have prognostic relevance in a diverse array of tumors. However, prognosis-related serum enzymes are rarely reported for nasopharyngeal carcinoma (NPC). To clarify whether the level of serum enzymes is linked to the prognosis of NPC, we reviewed the pretreatment data of lactate dehydrogenase (LDH), alkaline phosphatase (ALP), and glutamyl transferase (GGT) in 533 newly diagnosed NPC patients who underwent radical radiotherapy between May 2002 and October 2003 at Sun Yat-sen University Cancer Center. Patients were grouped according to the upper limit of normal values of LDH, ALP, and GGT. The Kaplan-Meier method and log-rank test were used for selecting prognostic factors from clinical characteristics and serum enzymes, and the Chi-square test was applied to analyze the relationships of clinical characteristics and serum enzymes. Finally, a Cox proportional hazards model was used to identify the independent prognostic factors. We found that increased levels of LDH had poor effects on both overall survival and distant metastasis-free survival (P = 0.009 and 0.035, respectively), and increased pretreatment level of serum ALP had poor effects on both overall survival and local recurrence-free survival (P = 0.037 and 0.039, respectively). In multivariate analysis, increased LDH level was identified as an independent prognostic factor for overall survival. Therefore, we conclude that increased pretreatment serum LDH and ALP levels are poor prognostic factors for NPC.
Keywords: Nasopharyngeal carcinoma, LDH, ALP, GGT, prognosis
Nasopharyngeal carcinoma (NPC) is a malignancy with a high incidence in South China, particularly in Guangdong district. With the development of radiotherapy technology and improvement of chemotherapy regimens, the 5-year survival rate of patients with NPC has reached approximately 60% to 70%[1],[2]. However, locoregional recurrence and distant metastasis after radiotherapy are still the main patterns of failure affecting the survival rate of patients with NPC. To improve the long-term survival rate and quality of life for patients, implementing individualized therapy is a critical step[3]. This strategy calls for the study of clinical and biological characteristics of NPC and the search for markers indicating prognosis of NPC patients[4]. The transformation of normal cells into malignant cells often leads to abnormal serum enzyme synthesis, even before changes in tumor morphology[5]. Therefore, enzyme studies have recently received widespread attention. For example, Fantin et al.[6] reported that lactate dehydrogenase (LDH) was closely related to glycolysis and mitochondrial deficiency that is required for tumor maintenance. Alkaline phosphatase (ALP) has been suggested as a tumor marker by Wei et al. [7] and Stinghen et al.[8]. Corti et al.[9] also showed that glutamyl transferase (GGT), which is associated with tumor progression, might be an indicator for tumor prediction and therapy. As for NPC, increased metastatic potential of NPC in patients with high serum LDH levels has been reported, but only 42 patients were enrolled in the study, thereby limiting the strength of the conclusions because of the small sample size [10]. Furthermore, multivariate analysis has shown that LDH > 410 U/L might independently predict poor prognosis in NPC patients; however, that study stressed the prognostic impact of anatomical factors and did not define the cutoff value for LDH[11],[12]. In addition to LDH, the influences of other serum enzymes in NPC have not yet been reported. Therefore, to explore the relationship between serum enzymes and NPC patient prognosis, we evaluated the levels of serum enzymes LDH, ALP, and GGT using clinical data collected from 533 NPC patients treated in our hospital.
Patients and Methods
Population
From 1582 newly diagnosed NPC patients treated in Sun Yat-sen University Cancer Center between May 1, 2002 and October 31, 2003, a total of 550 patients were randomly selected on the computer using SAS software. The patients meeting the following criteria were enrolled: (1) had pathologically proven NPC; (2) were examined to exclude distant metastasis; (3) underwent computed tomography (CT) or magnetic resonance imaging (MRI) of the nasopharynx and neck before radiotherapy; (4) underwent radical radiotherapy; and (5) had complete biochemical records documenting pretreatment fasting serum levels (including data on LDH, GGT, and ALP). A total of 533 patients met the above criteria.
Treatment
All patients underwent radical radiotherapy for NPC. For stages I–II disease, the treatment was radiotherapy alone, whereas for stages III–IV disease, chemotherapy was added to radiotherapy.
For radiotherapy, most patients underwent conventional fractionation radiotherapy with high energy 6–8 MV X-ray by a linear accelerator. Four radiotherapy technologies were used: (1) conventional two-dimensional radiotherapy (2D-RT); (2) CT simulation treatment planning radiotherapy; (3) three-dimensional conformal radiotherapy (3D-CRT); and (4) intensity-modulated radiotherapy (IMRT). The accumulated dose to the gross primary tumor and involved neck lymph nodes was 66 to 74 Gy, and the uninvolved areas received 50 to 60 Gy.
Chemotherapy consisted of cisplatin (DDP) and 5-fluorouracil (5-FU). A bolus injection of 70–100 mg/m2 DDP was delivered on day 1, and 500–1000 mg/m2 5-FU was administered by 96-hour continuous infusion daily on days 2 through 5. A total of 304 (570%) patients underwent chemotherapy: 112 patients with concurrent chemotherapy, 81 patients with induction plus concurrent chemotherapy, 6 patients with concurrent chemotherapy plus adjuvant chemotherapy, and 105 patients with induction chemotherapy or adjuvant chemotherapy.
Serum enzyme detection
A pretreatment fasting serum sample (5 mL) was obtained from each patient by venipuncture of the antecubital vein using a sterile needle and syringe between 8 and 10 o'clock in the morning. The blood samples were then transferred into clean, sterile centrifuge tubes and allowed to clot. Each clotted sample was centrifuged for 10 min to obtain the serum. Enzyme assays were carried out within 24 h of serum collection. Serum enzyme activity was assayed according to the specification described by Stroeve et al.[13]. The LDH activity assay involved incubation of the serum sample with nicotinamide adenine dinuceotide (3 mg/mL) and DL-lactic acid (0.45 mol/L) with sodium pyrophosphate buffer (pH 8.8). The ALP activity assay involved aminomenthyl propanol, magnesium, and nitrophenyl phosphate disodium. The GGT activity assay involved Tris buffer, glycylglycine, and carboxy substrate (Glupa). Enzyme activity was reported in units (U).
The cutoff values for the target serum enzymes were defined by the upper limit of normal (ULN) values set by the Hitachi 7600 automatic biochemical detector, the machine used in our hospital for biochemical analysis. The cutoff values were 240 U/L, 110 U/L, and 50 U/L for LDH, ALP, and GGT, respectively. A serum enzyme level above the cutoff value was defined as an increased serum enzyme level.
Observation index
The below indices were observed:(1) the pretreatment levels of LDH, ALP, and GGT; (2) overall survival (OS), the survival time between the beginning of radiotherapy and either death or the time of the last follow-up; (3) local recurrence-free survival (LRFS), the survival time without primary lesions in the nasopharynx or regional lymph node recurrence; and (4) distant metastasis-free survival (DMFS), the survival time without distant metastasis.
Follow-up
After completion of radiotherapy, patients were followed up every 3 months for the first 3 years, and the intervals gradually increased from 6 months to 1 year after 3 years.
Statistical analysis
SPSS version 13.0 (SPSS, Chicago, IL) was used for data processing. Univariate analysis was performed using Kaplan-Meier methods and the log-rank test, and chi-square tests were conducted to determine the correlations between prognostic factors of clinical characteristics and prognostic factors of serum enzymes. Multivariate prognosis analysis was performed using the Cox proportional hazards model (variable excluded when P> 0.10). P < 0.05 was considered significant.
Results
Clinical characteristics and their prognostic values
Clinical characteristics and 5-year OS rates of the 533 patients are listed in Table 1. The male-to-female ratio was 2.7:1 (396/137). Age ranged from 10 to 78 years, with a median age of 46 years. A total of 465 (87.2%) patients had tumors exhibiting World Health Organization (WHO) type III histology. All patients were staged according to American Joint Committee on Cancer (AJCC) 2009 Edition, with 28 patients at stage I, 201 patients at stage II, 200 patients at stage III, and 104 patients at stage IV. A majority of the patients (76.3%) underwent 2D-RT.
Table 1. Clinical characteristics of 533 patients with nasopharyngeal carcinoma and univariate analysis related to overall survival.
| Clinical characteristic | Number of patients (%) | Five-year overall survival rate (%) | Log-rank P value |
| Gender | 0.125 | ||
| Male | 396 (74.3) | 71 | |
| Female | 137 (25.7) | 79 | |
| Age (years) | 0.002 | ||
| <46 | 259 (48.6) | 78 | |
| ≥46 | 274 (51.4) | 68 | |
| Histologya | 0.364 | ||
| WHO type I | 2 (0.4) | 50 | |
| WHO type II | 66 (12.4) | 76 | |
| WHO type III | 465 (87.2) | 73 | |
| AJCC T category (2009) | <0.001 | ||
| T1–2 | 314 (58.9) | 78 | |
| T3–4 | 219 (41.1) | 66 | |
| AJCC N category (2009) | 0.013 | ||
| N0–1 | 370 (68.4) | 76 | |
| N2–3 | 163 (30.6) | 66 | |
| Radiotherapy typeb | 0.316 | ||
| Type I | 407 (76.3) | 71 | |
| Type II | 97 (18.2) | 77 | |
| Type III | 17 (3.2) | 76 | |
| Type IV | 12 (2.3) | 92 |
aWHO type I, keratinizing squamous cell carcinoma; WHO type II, differentiated non-keratinizing carcinoma; WHO type III, undifferentiated non-keratinizing carcinoma (according to World Health Organization histological classification). btype I, conventional two-dimensional radiotherapy; type II, computed tomography simulation treatment planning radiotherapy; type III, intensity-modulated radiotherapy; type IV, three-dimensional conformal radiotherapy.
The median time of follow-up was 84 months (range, 3–98 months). The 3- and 5-year follow-up rates were 96.4% and 92.5%, respectively. The 3-, 5-, and 7-year OS rates were 79%, 73%, and 64%; LRFS rates were 91%, 89%, and 89%; and DMFS rates were 87%, 85%, and 84% for all patients. The clinical variables related to OS according to the log-rank test included age at diagnosis, T classification, and N classification (P = 0.002, P < 0.001, and P = 0.013, respectively). These variables were included in the final multivariate analysis.
Levels of serum LDH, ALP, GGT and their prognostic values
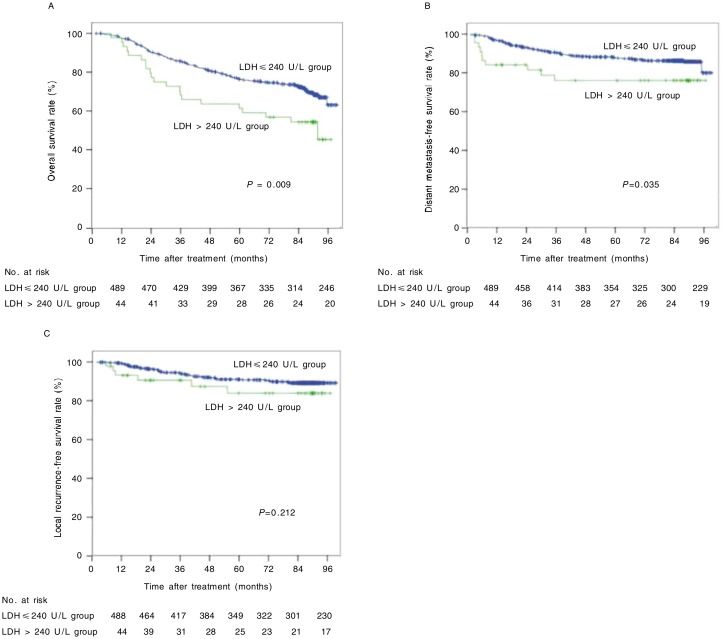
Pretreatment LDH levels of the 533 patients ranged from 22 to 751 U/L, with a median value of 165 U/L. Among all patients, 44 (8.3%) had increased LDH levels (LDH > 240 U/L). The OS, LRFS, and DMFS curves for the normal LDH level group and the increased LDH level group are shown in Figure 1. Patients in the increased LDH level group had a poorer OS and a poorer DMFS than those in the normal LDH level group (χ2 = 6.908, P = 0.009; χ2 =4.450, P = 0.035); there were no statistical differences in LRFS curves between the two groups (P = 0.212).
Figure 1. Overall survival (OS), distant metastasis-free survival (DMFS), and local recurrence-free survival (LRFS) curves of 533 nasopharyngeal carcinoma (NPC) patients with different pretreatment levels of lactate dehydrogenase (LDH).
A, the 5-year OS rates were 57% in the increased LDH level group and 75% in the normal LDH level group (χ2 = 6.908, P = 0.009). B, the 5-year DMFS rates were 76% in the increased LDH level group and 86% in the normal LDH level group (χ2 = 4.450, P = 0.035). C, the 5-year LRFS rates were 84% in the increased LDH level group and 90% in the normal LDH level group (χ2 = 1.558, P = 0.212).
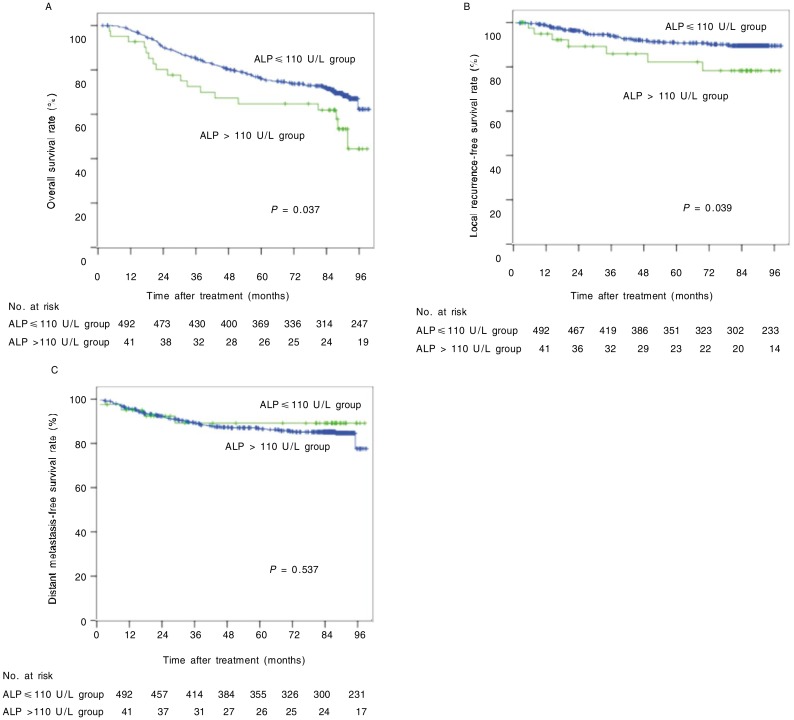
Pretreatment ALP levels ranged from 16 to 262 U/L, with a median value of 72 U/L. There were 41 (7.7%) patients with increased ALP levels (ALP > 110 U/L). Patients in the increased ALP level group had a poorer OS and a poorer LRFS than those in the normal ALP level group (χ2 = 4.329, P = 0.037; χ2 = 4.274, P = 0.039), but there were no significant differences in DMFS between the two groups (P = 0.537) (Figure 2).
Figure 2. OS, LRFS, and DMFS curves of 533 NPC patients with different pretreatment levels of alkaline phosphatase (ALP).
A, the 5-year OS rates were 65% in the increased ALP level group and 74% in the normal ALP level group (χ2 = 4.329, P = 0.037). B, the 5-year LRFS rates were 79% in the increased ALP level group and 90% in the normal ALP level group (χ2 = 4.274, P = 0.039). C, the 5-year DMFS rates were 89% in the increased ALP level group and 85% in the normal ALP level group (χ2 = 0.382, P = 0.537).
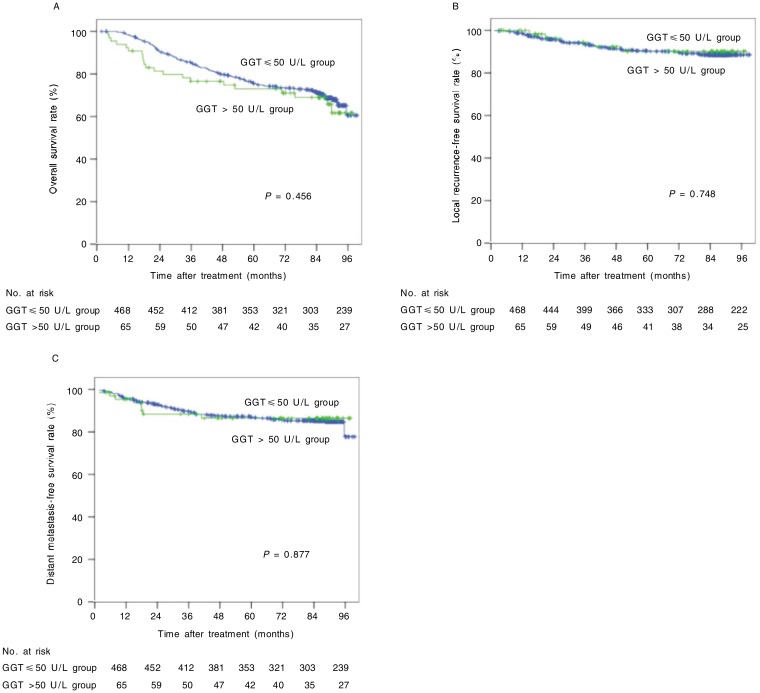
Pretreatment GGT levels ranged from 4 to 331 U/L, with a median value of 24 U/L. There were 65 (12.2%) patients with increased GGT levels (GGT > 50 U/L). However, we did not find distinct differences in OS, LRFS, or DMFS between the normal GGT level group and the increased GGT level group (Figure 3).
Figure 3. OS, LRFS, and DMFS curves of 533 NPC patients with different pretreatment levels of glutamyl transferase (GGT).
A, the 5-year OS rates were 71% in the increased GGT level group and 73% in the normal GGT level group (χ2 = 0.555, P = 0.456). B, the 5-year LRFS rates were 90% in the increased GGT level group and 89% in the normal GGT level group (χ2 = 0.103, P = 0.748). C, the 5-year DMFS rates were 86% in the increased GGT level group and 85% in the normal GGT level group (χ2 = 0.024, P = 0.877).
Based on these findings, we considered LDH and ALP serum enzymes associated with NPC prognosis and included them in the final multivariate analysis.
Further stratified analysis
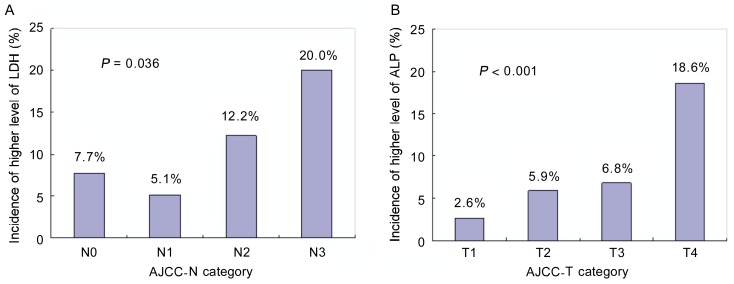
The potential relationships between prognostic factors of clinical characteristics and prognostic factors of serum enzymes are shown in Table 2. We found that patients with N2–3 tumors were more likely to have elevated pretreatment LDH levels than patients with N0–1 tumors (P = 0.01). Elevated ALP levels were more likely to appear in the older group (age > 46 years) and T3–4 group than in the counterpart groups (P = 0.024 and P = 0.007). The incidence of higher LDH levels (> 240 U/L) was increased with N stage progression (P = 0.036) (Figure 4A), whereas the incidence of higher ALP levels (> 110 U/L) was increased with T stage progression (P< 0.001) (Figure 4B).
Table 2. The relationships between prognostic factors of serum enzymes and prognostic factors of clinical characteristics.
| Characteristic | LDH |
P | ALP |
P | ||
| ≤240 U/L | >240 U/L | ≤110 U/L | >110 U/L | |||
| Age (years) | 0.905 | 0.024 | ||||
| <46 | 238 (91.9) | 21 (8.1) | 246 (95.0) | 13 (5.0) | ||
| ≥46 | 251 (91.6) | 23 (8.4) | 246 (89.8) | 28 (10.2) | ||
| AJCC T category (2009) | 0.506 | 0.007 | ||||
| T1–2 | 286 (91.1) | 28 (8.9) | 298 (94.9) | 16 (5.1) | ||
| T3–4 | 203 (92.7) | 16 (7.3) | 194 (88.6) | 25 (11.4) | ||
| AJCC N category (2009) | 0.010 | 0.385 | ||||
| N0–1 | 347 (93.8) | 23 (6.2) | 344 (93.0) | 26 (7.0) | ||
| N2–3 | 142 (87.1) | 21 (12.9) | 148 (90.8) | 15 (9.2) | ||
Values are presented as number of cases, with percentages in parentheses.
Figure 4. The relationships between the incidence of higher level of LDH and AJCC N category and between the incidence of higher level of ALP and AJCC T category.
A, the incidence of higher LDH levels (> 240 U/L) was increased in patients with N stage pregression (P = 0.036); B, the incidence of higher ALP levels (> 110 U/L) was increased in patients with T stage pregression(P < 0.001).
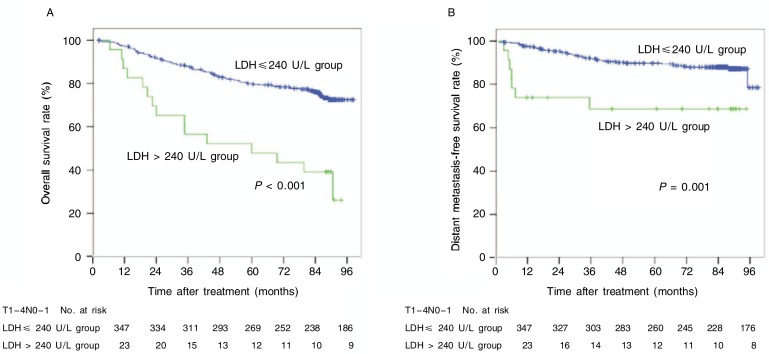
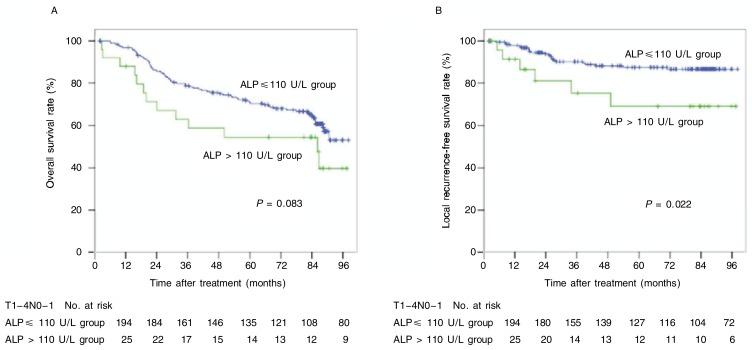
Stratified analysis showed that increased level of LDH associated with poor survival remarkably, both in the OS and DMFS curves (P < 0.001 and P = 0.001), among patients with N0–1 tumors (Figure 5) and that increased level of ALP nearly associated with poor prognosis, both in the OS and LRFS curves (P = 0.083 and P = 0.022), among patients with T3–4 tumors (Figure 6).
Figure 5. OS and DMFS curves of N0–1 NPC patients with different pretreatment levels of LDH.
A, 5-year OS rates were 43% in the increased LDH level group and 78% in the normal LDH level group (χ2 = 21.615, P < 0.001). B, 5-year DMFS rates were 68% in the increased LDH level group and 88% in the normal LDH level group (χ2 = 11.023, P = 0.001).
Figure 6. OS and LRFS curves of T3–4 NPC patients with different pretreatment levels of ALP.
A, 5-year OS rates were 69% in the increased ALP level group and 87% in the normal ALP level group (χ2 = 3.003, P = 0.083). B, 5-year LRFS rates were 55% in the increased ALP level group and 68% in the normal ALP level group (χ2 = 5.261, P = 0.022).
Multivariate analysis of overall survival rate
Multivariate analysis, including both clinical characteristic and serum enzyme prognostic factors, revealed that the independent poor prognostic factors associated with OS were pretreatment serum LDH level > 240 U/L, age > 46 years, T3–4 classification, and N2–3 classification (Table 3).
Table 3. Multivariate analysis of overall survival using the Cox proportional hazards regression model.
| Step | Factor | HR | 95% CI | P |
| One | AJCC T category | 1.638 | 1.196–2.244 | 0.002 |
| AJCC N category | 1.379 | 0.993–1.913 | 0.055 | |
| Age | 1.589 | 1.152–2.192 | 0.005 | |
| LDH levels | 1.592 | 0.991–2.557 | 0.055 | |
| ALP levels | 1.310 | 0.791–2.169 | 0.294 | |
| Two | AJCC T category | 1.657 | 1.210–2.268 | 0.002 |
| AJCC N category | 1.381 | 0.995–1.916 | 0.053 | |
| Age | 1.614 | 1.172–2.222 | 0.003 | |
| LDH levels | 1.660 | 1.042–2.644 | 0.033 |
Discussion
Tumors are composed of cell clones capable of rapid proliferation. Tumor proliferation has its own unique metabolic characteristics involving changes in many molecular indicators in the serum, including enzymes, proteins, and hormones. Exploring these objective indicators is extremely important for clinical practice[14]–[16]. To produce energy, cancer cells preferentially use the anaerobic pathway of glycolysis despite the presence of oxygen, a phenomenon historically known as the Warburg effect[17]. Anaerobic glycolysis results in transformation of pyruvate to lactate. LDH, a key enzyme of glycolysis, has five component isoenzymes because the LDH subunits (M and H), which are encoded by two distinct genes, produce five different combinations. The higher the number of H subunits an LDH isoenzyme contains, the lower the ability of the enzyme to catalyze the reaction of anaerobic glycolysis. Many diseases induce increases in isoenzyme levels. For example, liver disease causes an increase in LDH-4 and -5, myocardial infarction leads to a high level of LDH-1, and pulmonary embolism impacts the level of LDH-3. The isoenzyme most closely related to cancer is LDH-5, which is composed of four M subunits and catalyzes the pyruvate to lactate transformation most efficiently among the LDH isoenzymes. Several independent studies have shown that increased serum LDH levels or LDH-5 expression (as detected by immunohistochemistry) predicted poor prognosis and high metastasis risk in a spectrum of neoplastic diseases, including breast cancer, colorectal cancer, non–small cell lung cancer, endometrial cancer, and gastric cancer [18]–[23]. Thus, LDH was recently recommended as an objective indicator of tumor prognosis[24],[25]. Fantin et al.[6] and Xie et al.[26] reported that knockdown of LDH-A, which encodes LDH-5, significantly diminished tumor growth in mouse models, indicating that targeting LDH-A may be a novel therapeutic strategy for cancer treatment. In our study, increased pretreatment serum LDH, as expected, was related to poor OS and DMFS among NPC patients. By analyzing the relationship between LDH and clinical characteristic prognostic factors, we unexpectedly found that patients with tumors in higher N categories were more likely to have increased levels of LDH. Moreover, patients with N0-1 tumors who had elevated LDH levels had poorer prognosis and a higher risk of distant metastasis than those without elevated LDH levels. These observations could be attributed to several mechanisms. First, the growth and metastasis of an aggressive tumor requires more intense anaerobic glycolysis to produce energy, which may result in an elevated level of LDH, a key enzyme in the anaerobic glycolysis pathway[17]. In addition, increased LDH levels result in low extracellular pH due to lactic acid production. This, combined with up-regulated activity of the hypoxia-inducible factor (HIF) pathway, which regulates gene expression and tumor angiogenesis, may trigger activation of pathways that control tumor growth and aggressiveness[27]–[31].
ALP, a phosphate monoester hydrolase that is related to bone metabolism in the human body, catalyzes the hydrolysis and transfer of phosphate groups in alkaline conditions[32]. Kojima et al.[33] reported elevation of ALP activity in mice bearing Ehrlich ascites tumors and thought the change was primarily caused by the tumor itself, not by hormonal imbalance provoked secondarily. Moreover, many studies suggest that ALP may be a tumor-associated antigen and a monitor for drug efficacy in serum [34]–[36]. However, because it is ubiquitously presented in body tissues, ALP is easy to be up-regulated due to the metabolic disorders of bone disease and hepatic impairment, which may explain why the link between ALP and tumor prognosis reported in literature remains unclear. The 533 untreated NPC patients in our study had few serious complications caused by non-tumor factors, hence the increase of their serum ALP levels could be attributed to their tumors. In this study, we found that elevated ALP levels occurred more frequently in T3–4 tumors and that increased ALP levels affected the OS and LRFS of NPC patients. These findings suggest that increased ALP levels may be associated with a more locally invasive tumor phenotype. This association is possibly caused by increased ALP secretion by osteoblasts due to severe invasion of the base bone of the skull in patients with T3–4 NPC. Indeed, the skull base has become the most common site of recurrence because of attenuation of the reached radiation dose[12]. Therefore, we speculated that severe skull base invasion was the major cause of the increased levels of ALP. In further stratified analysis, we confirmed that increased ALP levels were present only in patients with T3–4 NPC, the only categories in which skull base invasion occurred. Furthermore, we observed that increased ALP levels occurred more frequently in the older group (age >46 years). One possible reason is that older patients had a higher incidence of T3–4 disease (122/274, 44.5%) than younger patients (97/259, 37.4%).
GGT, a key enzyme involved in glutathione metabolism, is often highly expressed in human malignancies. Beltran-Martinez et al.[37] reported that GGT was useful as an indicator of metastatic disease in patients with renal cell carcinoma. Fentiman et al.[38] suggested that premenopausal women with elevated levels of GGT had increased risk of breast cancer. Corti et al.[9] reviewed the recent investigation of the role of GGT in tumors and recommended GGT as a diagnostic/prognostic marker and a target for anticancer treatments. However, in our study, increased GGT level (>50 U/L) had no significant impact on survival. Nevertheless, after using a receiver operating characteristic curve to determine the optimal diagnostic cutoff value for which GGT was predictive of mortality and dividing patients into low and high GGT groups according to that cutoff value (28.5 U/L), we found that the 202 patients in the high GGT group (> 28.5 U/L) had a worse OS compared to the 331 patients in the low GGT group (≤ 28.5 U/L) (P = 0.024) (data not shown). The practical significance of this phenomenon requires further study.
We continued to collect dynamic serum enzyme level data during therapy (ΔLDH, ΔALP, and ΔGGT) for the 533 NPC patients, of which 175 were eligible. We found that the majority of patients had decreased levels of these serum enzymes during therapy. The rates of decrease were 74.9%, 74.3%, and 60%, and the mean values of these decreases were 38, 10, and 2 U/L for LDH, ALP, and GGT, respectively. There was no difference in survival between the patients with decreased serum enzyme levels and those without decreased levels (data not shown).
In this exploration of prognosis-related serum enzymes for NPC, we concluded that increased pretreatment levels of LDH and ALP can be use as prognostic factors for NPC. These findings prompt us to adjust treatment strategies ahead of time, considering not only TNM stage but also these prognosis-related serum enzymes. Only in this way can we achieve better individualized therapy for patients with NPC. Nevertheless, to better understand the true status of serum enzymes in tumor tissues and their correlation with tumor behavior, further research is needed to investigate their immunhistochemical expression pattern and to identify their molecular mechanisms.
Acknowledgments
This work was supported by grant from Hi-Tech Research and Development Program of China (No. 2006AA02Z4B4).
References
- 1.Li ZQ, Xia YF, Liu Q, et al. Radiotherapy-related typing in 842 patients in canton with nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2006;66:1011–1016. doi: 10.1016/j.ijrobp.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 2.Cho WC. Most common cancers in Asia-Pacific region: nasopharyngeal carcinoma. In: Cancer report of Asian-Pacific region 2010. Asian Pacific Organization for Cancer Prevention. 2010:284–289. [Google Scholar]
- 3.Leung TW, Tung SY, Sze WK, et al. Treatment results of 1070 patients with nasopharyngeal carcinoma: an analysis of survival and failure patterns. Head Neck. 2005;27:555–565. doi: 10.1002/hed.20189. [DOI] [PubMed] [Google Scholar]
- 4.Xia YF, Qian JY, Zhang EP. Beijing: Peking University Medical Publishing House; 2003. Practical radiotherapy of nasopharyngeal carcinoma. [Google Scholar]
- 5.Cascante M, Centelles JJ, Veech RL, et al. Role of thiamin (vitamin B-1) and transketolase in tumor cell proliferation. Nutr Cancer. 2000;36:150–154. doi: 10.1207/S15327914NC3602_2. [DOI] [PubMed] [Google Scholar]
- 6.Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425–434. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 7.Wei JS, Chung NC, Wei LL, et al. High-molecular-mass alkaline phosphatase as a tumor marker for colorectal cancer: comparison of two test methods. Clin Chem. 1993;39:540–543. [PubMed] [Google Scholar]
- 8.Stinghen ST, Moura JF, Zancanella P, et al. Specific immunoassays for placental alkaline phosphatase as a tumor marker. J Biomed Biotechnol. 2006;2006:56087. doi: 10.1155/JBB/2006/56087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corti A, Franzini M, Paolicchi A, et al. Gamma-glutamyltransferase of cancer cells at the crossroads of tumor progression, drug resistance and drug targeting. Anticancer Res. 2010;30:1169–1181. [PubMed] [Google Scholar]
- 10.Lu XG, Zhu YQ, Feng Y, et al. The relationship between serum LDH and distant metastasis of nasopharyngeal carcinoma. Acta Academiae Medicinae Suzhou. 2000;20:243–253. [in Chinese] [Google Scholar]
- 11.Cheng SH, Jian JJ, Tsai SY, et al. Prognostic features and treatment outcome in locoregionally advanced nasopharyngeal carcinoma following concurrent chemotherapy and radiotherapy. Int J Radiat Oncol Biol Phys. 1998;41:755–762. doi: 10.1016/s0360-3016(98)00092-3. [DOI] [PubMed] [Google Scholar]
- 12.Cheng SH, Tsai SY, Horng CF, et al. A prognostic scoring system for locoregional control in nasopharyngeal carcinoma following conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66:992–1003. doi: 10.1016/j.ijrobp.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Stroeve EA, Makarova VG. Moscow: MIR Publishers; 1989. Laboratory Manual in Biochemistry. [Google Scholar]
- 14.Bidart JM, Thuillier F, Augereau C, et al. Kinetics of serum tumor marker concentrations and usefulness in clinical monitoring. Clin Chem. 1999;45:1695–1707. [PubMed] [Google Scholar]
- 15.Nicolaides C, Fountzilas G, Zoumbos N, et al. Diffuse large cell lymphomas: identification of prognostic factors and validation of the International Non-Hodgkin's Lymphoma Prognostic Index. A Hellenic Cooperative Oncology Group Study. Oncology. 1998;55:405–415. doi: 10.1159/000011886. [DOI] [PubMed] [Google Scholar]
- 16.Patel PS, Rawal GN, Balar DB. Combined use of serum enzyme levels as tumor markers in cervical carcinoma patients. Tumour Biol. 1994;15:45–51. doi: 10.1159/000217872. [DOI] [PubMed] [Google Scholar]
- 17.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 18.Koukourakis MI, Giatromanolaki A, Sivridis E, et al. Lactate dehydrogenase-5 (LDH-5) overexpression in non-small-cell lung cancer tissues is linked to tumor hypoxia, angiogenic factor production and poor prognosis. Br J Cancer. 2003;89:877–885. doi: 10.1038/sj.bjc.6601205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stokkel MP, van Eck-Smit BL, Zwiderman AH, et al. Pretreatment serum lactate dehydrogenase as additional staging parameter in patients with small-cell lung carcinoma. J Cancer Res Clin Oncol. 1998;124:215–219. doi: 10.1007/s004320050157. [DOI] [PubMed] [Google Scholar]
- 20.Zhao YH, Zhou M, Liu H, et al. Upregulation of lactate dehydrogenase A by ErbB2 through heat shock factor 1 promotes breast cancer cell glycolysis and growth. Oncogene. 2009;28:3689–3701. doi: 10.1038/onc.2009.229. [DOI] [PubMed] [Google Scholar]
- 21.Koukourakis MI, Giatromanolaki A, Simopoulos C, et al. Lactate dehydrogenase 5 (LDH5) relates to up-regulated hypoxia inducible factor pathway and metastasis in colorectal cancer. Clin Exp Metastasis. 2005;22:25–30. doi: 10.1007/s10585-005-2343-7. [DOI] [PubMed] [Google Scholar]
- 22.Giatromanolaki A, Sivridis E, Gatter KC, et al. Lactate dehydrogenase 5 (LDH-5) expression in endometrial cancer relates to the activated VEGF/VEGFR2 (KDR) pathway and prognosis. Gynecol Oncol. 2006;103:912–918. doi: 10.1016/j.ygyno.2006.05.043. [DOI] [PubMed] [Google Scholar]
- 23.Kolev Y, Uetake H, Takagi Y, et al. Lactate dehydrogenase-5 (LDH-5) expression in human gastric cancer: association with hypoxia-inducible factor (HIF-1alpha) pathway, angiogenic factors production and poor prognosis. Ann Surg Oncol. 2008;15:2336–2344. doi: 10.1245/s10434-008-9955-5. [DOI] [PubMed] [Google Scholar]
- 24.Arkenau HT, Barriuso J, Olmos D, et al. Prospective validation of a prognostic score to improve patient selection for oncology phase I trials. J Clin Oncol. 2009;27:2692–2696. doi: 10.1200/JCO.2008.19.5081. [DOI] [PubMed] [Google Scholar]
- 25.Fussenich LM, Desar IM, Peters ME, et al. A new, simple and objective prognostic score for phase I cancer patients. Eur J Cancer. 2011;47:1152–1160. doi: 10.1016/j.ejca.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 26.Xie H, Valera VA, Merino MJ, et al. LDH-A inhibition, a therapeutic strategy for treatment of hereditary leiomyomatosis and renal cell cancer. Mol Cancer Ther. 2009;8:626–635. doi: 10.1158/1535-7163.MCT-08-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stubbs M, McSheehy PM, Griffiths JR, et al. Causes and consequences of tumour acidity and implications for treatment. Mol Med Today. 2000;6:15–19. doi: 10.1016/s1357-4310(99)01615-9. [DOI] [PubMed] [Google Scholar]
- 28.Colgan SM, Mukherjee S, Major P. Hypoxia-induced lactate dehydrogenase expression and tumor angiogenesis. Clin Colorectal Cancer. 2007;6:442–446. doi: 10.3816/CCC.2007.n.014. [DOI] [PubMed] [Google Scholar]
- 29.Harris AL. Hypoxia: a key regulatory factor in tumor growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 30.Rofstad EK. Microenvironment-induced cancer metastasis. Int J Radiat Biol. 2000;76:589–605. doi: 10.1080/095530000138259. [DOI] [PubMed] [Google Scholar]
- 31.Maxwell PH. The HIF pathway in cancer. Semin Cell Dev Biol. 2005;16:523–530. doi: 10.1016/j.semcdb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Harris H. The human alkaline phosphatases: what we know and what we don't know. Clin Chim Acta. 1990;186:133–150. doi: 10.1016/0009-8981(90)90031-m. [DOI] [PubMed] [Google Scholar]
- 33.Kojima Y, Sakurada T. Increase in alkaline phosphatase activity in the liver of mice bearing Ehrlich ascites tumor. Cancer Res. 1976;36:23–27. [PubMed] [Google Scholar]
- 34.Nilsson EE, Westfall SD, McDonald C, et al. An in vivo mouse reporter gene (human secreted alkaline phosphatase) model to monitor ovarian tumor growth and response to therapeutics. Cancer Chemother Pharmacol. 2002;49:93–100. doi: 10.1007/s00280-001-0396-0. [DOI] [PubMed] [Google Scholar]
- 35.Rassam MB, Al-Bashir NN, Al-Salihi AR, et al. Heat-stable alkaline phosphatase. A putative tumor marker of head and neck squamous cell carcinoma. Acta Oncol. 1995;34:49–52. doi: 10.3109/02841869509093638. [DOI] [PubMed] [Google Scholar]
- 36.Bao R, Selvakumaran M, Hamilton TC. Use of a surrogate marker (human secreted alkaline phosphatase) to monitor in vivo tumor growth and anticancer drug efficacy in ovarian cancer xenografts. Gynecol Oncol. 2000;78:373–379. doi: 10.1006/gyno.2000.5925. [DOI] [PubMed] [Google Scholar]
- 37.Beltran-Martinez RJ, Correa-Chacon AJ. Diagnostic value of gamma-glutamyltransferase in the metastatic disease of patients with renal cell carcinoma. Gac Med Mex. 2003;139:123–125. [PubMed] [Google Scholar]
- 38.Fentiman IS, Allen DS. Gamma-glutamyl transferase and breast cancer risk. Br J Cancer. 2010;103:90–93. doi: 10.1038/sj.bjc.6605719. [DOI] [PMC free article] [PubMed] [Google Scholar]