Abstract
S-phase kinase-associated protein 2 (Skp2) belongs to the F-box protein family. It is a component of the SCF E3 ubiquitin ligase complex. Skp2 has been shown to regulate cellular proliferation by targeting several cell cycle-regulated proteins for ubiquitination and degradation, including cyclin-dependent kinase inhibitor p27. Skp2 has also been demonstrated to display an oncogenic function since its overexpression has been observed in many human cancers. This review discusses the recent discoveries on the novel roles of Skp2 in regulating cellular senescence, cancer progression, and metastasis, as well as the therapeutic potential of targeting Skp2 for human cancer treatment.
Keywords: Skp2, p53, RhoA, cellular senescence, metastasis, cancer therapy
Ubiquitin is a highly evolutionary conserved protein consisting of 76 amino acids. In eukaryotic cells, the ubiquitin proteasome system (UPS) mediates the majority of protein degradation, which plays important roles in the regulation of multiple responses to diverse signals during development and metabolism[1]. Posttranslational modification of proteins with ubiquitin is mediated by three enzymes: the E1 activating enzyme, the E2 conjugating enzyme, and the E3 ligase (Figure 1). Ubiquitination is a covalent reaction that attaches ubiquitin (s) to one or more lysine residues in a protein. In the human genome, 2 E1s, roughly 50 E2s, and 600 E3s have been identified[2]. The substrate specificity in the UPS is determined by the E3 ligase, which is subjected to regulation at multiple levels. Deregulation of E3 ligase has been implicated in human diseases such as cancer.
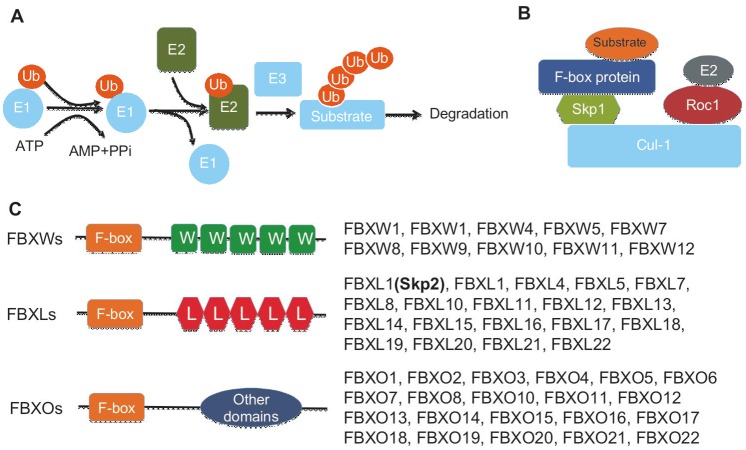
Figure 1. Skp2 belongs to the F-box protein family.
A, a simplified scheme for the ubiquitin proteasome system. Posttranslational modification of a protein with ubiquitin is mediated by three enzymes: the E1-activating enzyme, the E2-conjugating enzyme, and the E3 ligase. B, SCF E3 ligase complex is composed of Cul-1, Skp1, Roc1, and an F-box protein, such as Skp2. C, F-box proteins can be divided into three classes, WD40 repeat-containing F-box proteins (FBXWs), leucine-rich repeats (LRRs)-containing F-box proteins (FBXLs) including Skp2, and F-box proteins containing other diverse domains (FBXOs).
S-phase kinase-associated protein 2 (Skp2) is an F-box protein, which constitutes one of the four subunits of the Skp1-Cullin-1 (Cul-1)-F-Box (SCF) ubiquitin E3 ligase complex. Earlier studies have shown that Skp2 regulates cell cycle progression and proliferation by targeting ubiquitination and degradation of its substrates, such as cell cycle inhibitor p27[3],[4]. Subsequent studies with human cancer samples revealed that Skp2 is overexpressed in a variety of human cancers and is inversely correlated with p27 levels, suggesting that Skp2 overexpression may have essential functions in human cancer development[5],[6]. Other studies using xenograft mouse tumor models have supported the oncogenic role of Skp2 in cancer development[7]–[9]. Moreover, recent work using a Skp2-deficient mouse model has revealed that Skp2 is required for cancer development in multiple tumor-promoting conditions, including PTEN, ARF, and pRB inactivation[10],[11].
In this review, we will summarize recent findings on the novel roles of E3 ligase Skp2 in human cancers, with a particular emphasis on cellular senescence, cancer progression, and metastasis.
Skp2 Is a Member of the F-box Protein Family
The function of the SCF E3 ligase is to facilitate the transfer of ubiquitin from E2 ubiquitin conjugating enzymes to protein substrates. Currently, 68 F-box proteins have been identified in the human genome. Although structurally diverse, they all contain an F-box motif consisting of approximately 45–50 amino acids[12]. In addition to the F-box motif, all F-box proteins have a protein-protein interaction domain for substrate binding and recognition that falls into the following three classes (Figure 1) [13],[14]: (1) WD40 repeat-containing F-box proteins (FBXWs), such as β-TRCP and FBXW7; (2) leucine-rich repeats (LRRs)-containing F-box proteins (FBXLs), including Skp2; and (3) F-box proteins containing other diverse domains (FBXOs), which include proteins with other domain types in the C-terminal region. Both genetic and biochemical analyses have demonstrated that the SCF E3 ligase targets a variety of important proteins for ubiquitin-dependent proteasome degradation. Interestingly, phosphorylation of the substrates on either serine or threonine is required for SCF-mediated protein degradation, which is mediated through the WD40 and LRR repeats.
Among the large number of F-box proteins identified in the human genome, only a few, such as Skp2, β-TRCP, and FBXW7, have been extensively studied, including the identification of their substrates.
Skp2 Targets Proteins for Ubiquitination and Degradation
As mentioned above, Skp2 is a critical component of Skp2SCF ubiquitin E3 ligase, one of the best-characterized SCF complexes. Skp2 was first identified because of its overexpression in many cancer cell lines and its association with Skp1 and cyclin A/Cdk2/Cks1[15]. A variety of Skp2 substrates, which are involved in multiple cellular processes such as cell cycle and apoptosis, have been identified[13]. The best-known Skp2 substrate is cell cycle inhibitor p27, which is targeted by Skp2SCF for ubiquitination and degradation when threonine 187, located at the C-terminal end of p27, is phosphorylated[16]–[18]. The binding and recognition of p27 as a substrate by Skp2 requires an accessory protein, Cks1, as Cks1 deficiency prevents Skp2 from binding to p27, in turn leading to p27 up-regulation[19]–[21]. Strikingly, genetically engineered Skp2 knockout mice and Cks1-deficient mice share similar phenotypes. Mouse embryonic fibroblasts (MEFs) from Cks1−/− and Skp2−/−mice both display reduced cell proliferation, accompanied by enhanced p27 protein expression[3]. Thus, it appears that the function of Cks1 is to facilitate Skp2-mediated substrate polyubiquitination. Both in vitro and in vivo evidence suggests that p27 is a critical and relevant Skp2 substrate for Skp2 functions. Double deficiency of p27 and Skp2 rescues the cell proliferation defect observed in Skp2−/− MEFs as well as the reduced organ size and body weight observed in Skp2-deficient mice[4]. Importantly, there is an inverse correlation between Skp2 and p27 expression in human cancers. Notably, Skp2 is found to be overexpressed in various human cancer samples and associated with poor prognosis and is accompanied by p27 down-regulation[5],[6],[22]–[25].
Although p27 is a critical target of Skp2, many additional substrates of Skp2SCF have been identified, and the relevance of these proteins to Skp2 function remains to be determined. Many of these proteins, such as p21 [26],[27], p57[28], E2F-1[29], MEF[30], p130[31],[32], Tob1[33], cyclin D [27], cyclin E 1[34], Smad4 [35], Myc [36],[37], B-Myb [38], and RASSF1A[39], are cell cycle regulators. In addition, Skp2 also targets apoptosis regulators such as Myc[36],[37] and Foxo[36],[37] for protein degradation. Moreover, Skp2 targets many other proteins with diverse functions for degradation, including Orc1p[40] and Cdt1[41],[42], Rag-2[43], BRCA2 [44], Cdk9[45], MKP1[46], and UBP43[47]. These results suggest that Skp2 plays a role not only in cell cycle progression and apoptosis, but also in a wide range of other biological processes. To understand the relevance of these substrates to Skp2 function, genetic mouse models will be required for further study.
Overexpression of Skp2 is frequently observed in human cancers, and accumulating evidence suggests that Skp2 plays a proto-oncogenic role both in vitro and in vivo. Skp2 is able to cooperate with H-RasG12V to induce cellular transformation in soft agar assays and tumor formation assays in nude mice[48],[49]. Although overexpression of Skp2 in the T-cell compartment is not sufficient to induce T-cell lymphomas, the combined overexpression of Skp2 with N-ras leads to T-cell lymphomas with shorter latency and higher penetrance and results in a significant decrease in mouse survival compared to the N-ras transgenic mouse alone[49], suggesting a role for Skp2 in the progression of T-cell lymphoma. Moreover, overexpression of Skp2 in mouse prostate leads to prostate intraepithelial neoplasia (PIN)[50]. In line with these observations, our recent study showed that overexpression of Skp2 in prostate cancer cells significantly promotes prostate cancer cell growth and tumorigenesis in a xenograft tumor model[7], whereas overexpression of the Skp2 S72A mutant, which is unable to be phosphorylated by Akt, could not induce these same effects[7]. Therefore, our study underscores the critical role of Akt-mediated Skp2 phosphorylation at Ser 72 in the regulation of Skp2SCF activity and oncogenic transformation.
Skp2 Suppresses Cellular Senescence Induced by Oncogenic Stimuli Independent of ARF/p53
Cellular senescence, first described by Hayflick et al. in 1961[51],[52], is an irreversible form of cell cycle arrest that can be triggered by a variety of insults via two mechanisms: genetic reprogramming and in response to damaging conditions, which will lead to replicative senescence and premature senescence, respectively[53]–[55]. In addition to cultivation-induced proliferative exhaustion, several other stress stimuli, including telomere dysfunction, DNA damage accumulation, and genotoxic stress, have been shown to induce replicative senescence. Premature cellular senescence can be triggered by the overexpression of several oncogenes (also termed oncogene-induced senescence, OIS), such as HRasG12V and BrafE600E[55]–[57], as well as loss of tumor suppressor PTEN (also termed PTEN-loss-induced senescence, PICS) [55],[58],[59] or other tumor suppressors, such as neurofibromatosis type 1 (NF1) and Von Hippel-Lindau (VHL)[59]–[61]. Recent studies suggest that cellular senescence can serve as an important tumor-suppressive barrier to restrict tumor development in vivo[55],[59],[62]–[67].
Tumor suppressor p53 has been shown to be essential in inducing cell cycle arrest, apoptosis, and senescence in response to various stress signals[68]–[72]. Induction of p53 in response to these stress stimuli leads to cellular senescence both in vitro and in vivo[56],[63],[73]. On the contrary, silencing or inactivation of p53 by viral oncoproteins, such as SV40 large T antigen or HPV-16 E6 protein, suppresses senescence response[74]–[77]. Importantly, genetic inactivation of p53 in mice eliminates the senescence resulting from overexpression of oncogenic HRasG12V or BRafV600E, which ultimately leads to promotion of cancer progression in mouse models[56],[65],[78]–[80]. Thus, these findings indicate an important role of p53 in the regulation of cellular senescence.
Although cellular senescence critically depends on the ARF/p53 pathway, emerging evidence suggests that cellular senescence can also be triggered in an ARF/p53-independent manner. For example, acute inactivation of VHL, a tumor suppressor that is frequently mutated in many human cancers[81], triggers cellular senescence in vitro and in vivo. Surprisingly, the cellular senescence resulting from loss of VHL is dependent on pRB activation and p400 reduction[61]. Interestingly, Skp2 mRNA levels are significantly reduced when VHL is inactivated, thus leading to increased p27 expression[61]. This implies that Skp2 might also play a direct role in cellular senescence. In line with this notion, Skp2 down-regulation through the overexpression of the human T lymphotropic virus type 1 (HTLV-1) Tax protein also results in cellular senescence[82].
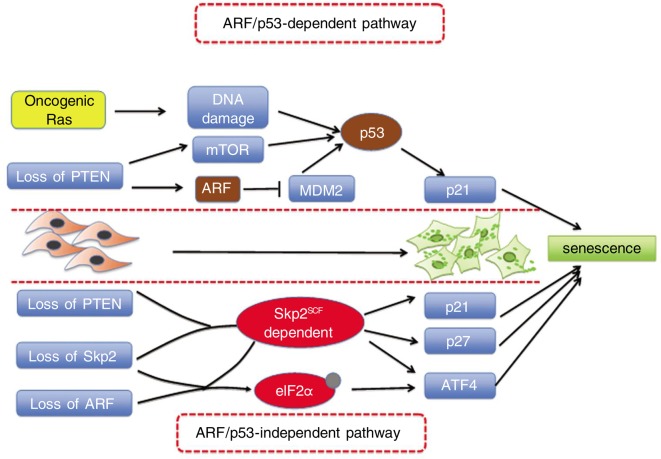
Our recent work provides strong evidence that Skp2 indeed plays a direct role in cellular senescence. Our study reveals that genetic Skp2 inactivation alone does not induce cellular senescence response. However, the combined inactivation of Skp2 and tumor suppressors PTEN or ARF results in cellular senescence both in vitro and in vivo[10]. Consistent with previous observations showing that Skp2 mediates p21 and p27 degradation[3],[4],[26], p21 and p27 protein levels are increased in Skp2−/− MEFs[10]. Interestingly, we found that Skp2 deficiency sensitizes cancer-prone cells expressing Ras and E1A oncoproteins or partially lacking tumor suppressor PTEN to senescence[10]. Such a senescence response triggered by inactivation of Skp2 in the presence of powerful oncogenic signals, even when the p19ARF/p53 response is evaded, suggests that Skp2 regulates a p53/ARF-independent senescence pathway. Furthermore, compound mutant mice deficient in both PTEN and Skp2 (PTEN+/−; Skp2−/−) are strongly protected from cancer[10]. Adrenal tumor formation and lymphoadenophathy are profoundly inhibited in these compound mutant mice as compared to the PTEN+/− mice. Similar results are observed in mice deficient for PTEN in the prostate and those deficient for tumor suppressor p19ARP[10]. In these two mouse models, Skp2 inactivation in combination with deficiency of PTEN or p19ARF renders mice resistant to cancer. Importantly, a significant increase in senescent cells and a reduction in proliferation rate are observed within the pre-tumoral tissues from the cancer-resistant organs, particularly the lymph nodes and prostate, as compared to those of mice expressing wild-type Skp2. Interestingly, the senescence response triggered by Skp2 inactivation in combination with PTEN inactivation or ARF loss does not result in p53 activation or DNA damage response (Figure 2). Our data suggest that combined deficiency for Skp2 and PTEN or ARF leads to induction of cell cycle inhibitors p27 and p21 and endoplasmic reticulum stress protein ATF4, which can synergistically contribute to senescence response[10]. Collectively, these findings suggest that Skp2 regulates a novel p19ARF/p53- independent senescence response to promote tumorigenesis.
Figure 2. ARF/p53-dependent and -independent pathways for cellular senescence.
Premature cellular senescence can be triggered by overexpression of several oncogenes, such as HRasG12V, as well as loss of tumor suppressors, such as PTEN, NF1, VHL, and ARF. Overexpression of Ras or loss of PTEN leads to ARF/p53 activation and cellular senescence. However, inactivation of Skp2 in combination with PTEN or ARF inactivation leads to an induction of p21, p27, and ATF4 in an ARF/p53-independent manner.
Skp2 Regulates Cell Migration and Metastasis by Affecting RhoA Transcription in Cooperation with Myc-Miz1-P300 and Independent of SCF-Skp2 E3 Ligase Activity
Metastasis is a complex process that accounts for a majority of death from cancer. Many key players involved in cell migration, invasion, and metastasis have been identified. RhoA, a member of the Rho family of GTPases, plays important roles in numerous biological processes such as migration and invasion and has been implicated in cancer metastasis[83]. Although no mutation of RhoA has been found in human cancers[84],[85], RhoA mRNA as well as protein levels are up-regulated in various human cancers[86]–[88]. However, how RhoA transcription is regulated remains largely unknown. Our recent work identified Skp2 as a key player in cell migration and invasion and uncovered transcriptional machinery, Skp2-Miz1-cMyc-p300, that is important for the regulation of RhoA transcription.
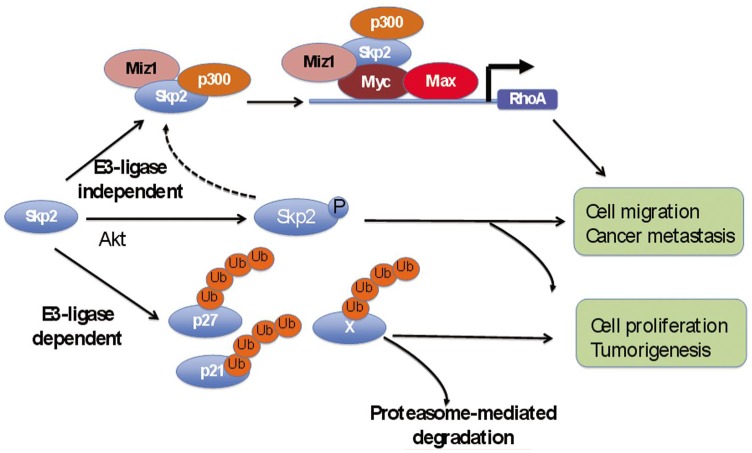
Skp2 overexpression has been reported to be associated with cancer progression and metastasis [7],[89]–[93]. Importantly, our recent work established a direct role for Skp2 in cell migration, invasion, and metastasis (Figure 3)[7],[94]. We found that Skp2-deficient cells (Skp2+/− MEFs or Skp2 knockdown cancer cells) were severely impaired in cell migration and invasion, whereas cells with Skp2 overexpression had significantly increased cell migration and invasion activities[7],[94]. Moreover, we have previously shown that Akt-mediated phosphorylation of Skp2 at serine 72 and subsequent cytosolic relocalization of phosphorylated Skp2 could promote cell migration[7]. Specifically, restoration of a cytosolic Skp2 mutant, Skp2-NES, could rescue the migration defects observed in the Skp2−/− MEFs, suggesting that cytosolic Skp2 may play an important role in cell migration and invasion (Figure 3). Recently, we identified a transcriptional complex of Skp2, including Myc, Miz1, and p300, that is important for the regulation of RhoA transcription. Skp2−/− MEFs or Skp2 knockdown cells showed a profound decrease in both RhoA mRNA and protein levels, whereas Skp2 overexpression induced RhoA protein expression, which further increased when Myc was co-expressed[94]. The restoration of RhoA expression partially rescued the migration and invasion defect in Skp2−/− MEFs and Skp2 knockdown cells, indicating that RhoA is an important downstream effector for Skp2 in cell migration[94]. Using a chromatin immunoprecipitation (ChIP) assay, we demonstrated that Skp2 is recruited to the RhoA promoter by Myc, as Myc knockdown prevents Skp2 from binding to the RhoA promoter, whereas Skp2 mutants defective in Myc binding fail to activate RhoA transcription[94]. These results suggest that the function of Skp2 in transcriptional regulation is critically dependent on Myc. Furthermore, we found that Skp2 recruits Miz1 and p300 to the Myc transcriptional complex to induce RhoA expression, and the recruitment of p300 and Miz1 to the RhoA promoter is impaired in Skp2 knockdown cells[94]. Importantly, using a well-established breast cancer lung metastasis model generated by tail vein injection of MDA-MB-231 breast cancer cells, we demonstrated that knockdown of Myc, Miz1, or Skp2 profoundly reduced metastasis to the lung whereas overexpression of Myc or Skp2 promoted lung metastasis, suggesting that the Myc-Skp2-Miz1 complex has essential functions in cell invasion and metastasis. Importantly, RhoA, Myc, Miz1, and Skp2 are all significantly correlated with human prostate cancer metastasis. Moreover, RhoA expression is strongly correlated with Myc, Skp2, and Miz1 expression in prostate cancer. Furthermore, we found there is also a positive correlation between Myc, Skp2, and Miz1. Taken together, these results demonstrate that Myc-Skp1-Miz1 has important functions in RhoA transcription and underscore the clinical relevance of the Myc-Skp2-Miz1 complex and RhoA expression in cancer metastasis (Figure 3). However, it is unclear whether Akt-mediated phosphorylation of Skp2 also contributes to Skp2-mediated recruitment of Miz1 and p300 and subsequent Myc-Skp2-Miz1 transcriptional complex formation.
Figure 3. E3-dependent and E3-independent functions of Skp2.
Skp2 E3 ligase can target various proteins, such as p27, for proteasome-mediated degradation. However, Skp2 can also function independently of its E3 ligase activity. Skp2 can be phosphorylated by Akt and promote cell migration and metastasis. In addition, Skp2 can recruit Miz1 and p300 and form a transcriptional complex of Myc-Skp2-Miz1-p300 to regulate RhoA expression. Such activities of Skp2 do not require its E3 ligase activity.
Interestingly, the ability of Skp2 to regulate RhoA transcription appears to be independent of its SCF-Skp2 E3 ligase activity. We found that both wild-type Skp2 and Skp2-LRR mutant, which is devoid of the amino terminus and F-box domain and defective in E3 ligase activity, similarly interacted with Myc, p300, and Miz1 and cooperated with them in the up-regulation of RhoA transcription[94]. In addition, the Skp2-LRR mutant was able to promote breast cancer cell metastasis to the lung as efficiently as wild-type Skp2 but was less efficient at promoting cell cycle progression, cell proliferation, and primary tumor formation compared to wild-type Skp2. Taken together, these data suggest that Skp2SCF E3 ligase activity is required for cell cycle progression and tumorigenesis but is dispensable for RhoA gene expression and cancer metastasis.
Targeting Skp2 for Cancer Therapy
Emerging evidence suggests that targeting Skp2 in human cancers may offer therapeutic benefits in various cancers, as Skp2 has been shown to be overexpressed in multiple types of human cancers. Recently, three groups reported that Skp2 is required for tumorigenesis in mouse models in the context of BCR-ABL overexpression, PTEN loss, or pRB inactivation[44],[62],[95], suggesting that targeting Skp2 may be very effective for treatment of many types of human cancers. In recent years, mounting evidence suggests that cellular senescence serves as a crucial barrier in restricting tumor progression. Thus, it has been proposed that approaches to enhance the pro-senescence response may be effective therapeutic strategies for human cancer[55]. However, as p53 is the most commonly mutated gene in human cancers, strategies targeting p53-dependent cellular senescence may not be applicable for tumors with p53 loss or inactivation. Therefore, identification and targeting of p53-independent cellular senescence pathways may be the key for the success of pro-senescence therapy. As Skp2 deficiency triggers p53-independent cellular senescence, targeting Skp2 may be an ideal approach for the treatment of advanced human cancers with p53 inactivation. Furthermore, targeting Skp2 may also be a potential strategy to treat metastatic cancers, as we demonstrated that overexpression of Skp2 promotes cell migration and metastasis in cooperation with Myc-Miz1 to regulate RhoA transcription, whereas inactivation of Skp2-Myc-Miz1 restricts cancer metastasis.
Given that Skp2 plays an important role in tumorigenesis, the development of specific Skp2 inhibitors will offer novel therapeutics for the treatment of human cancer. Recently, two small molecules targeting other components of the Skp2SCF complex were identified: a small molecule (compound A) targeting Skp2SCF E3 ligase activity towards p27 ubiquitination [95], and MLN4924, a small molecule inhibitor targeting Nedd8-activating enzyme, thereby affecting Cul-1 neddylation and Skp2SCF complex formation [96]. The first study demonstrated that administration of this inhibitor causes cell arrest, apoptosis, and autophagy in leukemia cells, whereas the second study demonstrated that MLN4924 reduced Cul-1 neddylation, accompanied by inducing p27 accumulation, cell arrest, apoptosis, and senescence[96],[97]. Furthermore, MLN4924 has potent effects on suppressing tumor growth in vivo using xenograft tumor models[96],[97]. Thus, these studies clearly demonstrated that developing specific inhibitors to target Skp2 could offer novel and effective therapeutics against human cancer. We are in urgent need of such inhibitors to combat human cancers.
Conclusions and Perspective
Overexpression of Skp2 has been observed in many types of human cancers. Recent studies demonstrate that Skp2 overexpression promotes cancer progression and metastasis, whereas loss of Skp2 function inhibits these processes. In addition, Skp2 deficiency in tumor cells can trigger p53-independent senescence response. Collectively, these studies suggest that targeting Skp2 may be an ideal therapeutic strategy for human cancer, therefore establishing a rationale for designing small molecule inhibitors of Skp2 for the treatment of human cancers. An alternative approach to reduce Skp2 expression and activity as a means of cancer treatment is to target the transcription, protein stability, and SCF complex formation of Skp2. Skp2 gene expression is regulated by the Notch, IKK/NF-κB, or Akt signaling pathways, thus inhibition of these oncogenic pathways is expected to shut down Skp2 gene expression. Importantly, small molecules targeting these pathways are currently in clinical trials. In addition, the stability of Skp2 protein is positively regulated by Akt and CDK2 activity. Thus, small molecule inhibitors targeting Akt and CDK2 will be able to trigger rapid Skp2 degradation, in turn inhibiting cancer development. Furthermore, the Skp2SCF E3 ligase can function to target protein degradation only when this complex is properly formed. Thus, targeting Cul-1 neddylation can efficiently disrupt the Skp2SCF complex formation and can lead to an impaired SCF E3 ligase activity. One approach to target Skp2SCF complex formation is to use MLN4924, a small molecule inhibitor known to target Cul-1 neddylation.
Although there is significant progress in the field of Skp2 research, future studies need to be conducted to address several important questions. First, although many substrates have been identified for Skp2SCF, it remains to be determined which substrate is specifically required for Skp2-mediated cell proliferation, apoptosis, and tumorigenesis. Second, CAND1 has been shown to inhibit Skp2SCF complex by binding to unneddylated Cul-1[98], but the exact roles of CAND1 in Skp2SCF complex formation and cancer progression remain unclear. It is conceivable that CAND1 may play a tumor suppressive role in human cancers. Third, the mechanism by which Akt-mediated Skp2 phosphorylation regulates cell migration needs further investigation. Fourth, it is unclear whether Skp2 is globally involved in cancer development in various human tissues. Lastly, there is no evidence for the requirement of Skp2 in cancer maintenance in various cancers. Future studies using multiple mouse models and in vitro approaches are required to address these challenging questions, which will ultimately lead to a more comprehensive understanding of how the Skp2SCF complex is regulated and its roles in cancer progression and metastasis.
Acknowledgments
We thank the members of Lin's laboratory, The University of Texas MD Anderson Cancer Center, for their valuable comments and suggestions. This work was supported by the MD Anderson Cancer Center Trust Scholar Award, NIH grants, CPRIT grant, and DOD prostate cancer New Investigator Award to H.K.L., as well as the MD Anderson Cancer Center Breast SPORE Career Development Award to C. H. C.
References
- 1.Welchman RL, Gordon C, Mayer RJ. Ubiquitin and ubiquitin-like proteins as multifunctional signals. Nat Rev Mol Cell Biol. 2005;6:599–609. doi: 10.1038/nrm1700. [DOI] [PubMed] [Google Scholar]
- 2.Bhoj VG, Chen ZJ. Ubiquitylation in innate and adaptive immunity. Nature. 2009;458:430–437. doi: 10.1038/nature07959. [DOI] [PubMed] [Google Scholar]
- 3.Nakayama K, Nagahama H, Minamishima YA, et al. Targeted disruption of Skp2 results in accumulation of cyclin E and p27 (Kip1), Polyploidy and centrosome overduplication. EMBO J. 2000;19:2069–2081. doi: 10.1093/emboj/19.9.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakayama K, Nagahama H, Minamishima YA, et al. Skp2-mediated degradation of p27 regulates progression into mitosis. Dev Cell. 2004;6:661–672. doi: 10.1016/s1534-5807(04)00131-5. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Izhak O, Lahav-Baratz S, Meretyk S, et al. Inverse relationship between Skp2 ubiquitin ligase and the cyclin dependent kinase inhibitor p27Kip1 in prostate cancer. J Urol. 2003;170:241–245. doi: 10.1097/01.ju.0000072113.34524.a7. [DOI] [PubMed] [Google Scholar]
- 6.Drobnjak M, Melamed J, Taneja S, et al. Altered expression of p27 and Skp2 proteins in prostate cancer of African-American patients. Clin Cancer Res. 2003;9:2613–2619. [PubMed] [Google Scholar]
- 7.Lin HK, Wang G, Chen Z, et al. Phosphorylation-dependent regulation of cytosolic localization and oncogenic function of Skp2 by Akt/Pkb. Nat Cell Biol. 2009;11:420–432. doi: 10.1038/ncb1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan CH, Lee SW, Li CF, et al. Deciphering the transcriptional complex critical for RhoA gene expression and cancer metastasis. Nat Cell Biol. 2010;12:457–467. doi: 10.1038/ncb2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan CH, Lee SW, Wang J, et al. Regulation of skp2 expression and activity and its role in cancer progression. Sci World J. 2010;10:1001–1015. doi: 10.1100/tsw.2010.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin HK, Chen Z, Wang G, et al. Skp2 targeting suppresses tumorigenesis by Arf-p53-independent cellular senescence. Nature. 2010;464:374–379. doi: 10.1038/nature08815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H, Bauzon F, Ji P, et al. Skp2 is required for survival of aberrantly proliferating Rb1-deficient cells and for tumorigenesis in Rb1+/− mice. Nat Genet. 2010;42:83–88. doi: 10.1038/ng.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bai C, Sen P, Hofmann K, et al. SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell. 1996;86:263–274. doi: 10.1016/s0092-8674(00)80098-7. [DOI] [PubMed] [Google Scholar]
- 13.Frescas D, Pagano M. Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the scales of cancer. Nat Rev Cancer. 2008;8:438–449. doi: 10.1038/nrc2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin J, Cardozo T, Lovering RC, et al. Systematic analysis and nomenclature of mammalian F-box proteins. Genes Dev. 2004;18:2573–2580. doi: 10.1101/gad.1255304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Kobayashi R, Galaktionov K, et al. P19Skp1 and p45Skp2 are essential elements of the cyclin A-CDK2 s phase kinase. Cell. 1995;82:915–925. doi: 10.1016/0092-8674(95)90271-6. [DOI] [PubMed] [Google Scholar]
- 16.Sheaff RJ, Groudine M, Gordon M, et al. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev. 1997;11:1464–1478. doi: 10.1101/gad.11.11.1464. [DOI] [PubMed] [Google Scholar]
- 17.Carrano AC, Eytan E, Hershko A, et al. Skp2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat Cell Biol. 1999;1:193–199. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- 18.Tsvetkov LM, Yeh KH, Lee SJ, et al. P27(Kip1) ubiquitination and degradation is regulated by the SCF(Skp2) complex through phosphorylated Thr187 in p27. Curr Biol. 1999;9:661–664. doi: 10.1016/s0960-9822(99)80290-5. [DOI] [PubMed] [Google Scholar]
- 19.Ganoth D, Bornstein G, Ko TK, et al. The cell-cycle regulatory protein Cks1 is required for SCF(Skp2)–mediated ubiquitinylation of p27. Nat Cell Biol. 2001;3:321–324. doi: 10.1038/35060126. [DOI] [PubMed] [Google Scholar]
- 20.Harper JW. Protein destruction: adapting roles for Cks proteins. Curr Biol. 2001;11:R431–R435. doi: 10.1016/s0960-9822(01)00253-6. [DOI] [PubMed] [Google Scholar]
- 21.Spruck C, Strohmaier H, Watson M, et al. A CDK-independent function of mammalian Cks1: targeting of SCF (Skp2) to the CDK inhibitor p27Kip1. Mol Cell. 2001;7:639–650. doi: 10.1016/s1097-2765(01)00210-6. [DOI] [PubMed] [Google Scholar]
- 22.Bloom J, Pagano M. Deregulated degradation of the cdk inhibitor p27 and malignant transformation. Semin Cancer Biol. 2003;13:41–47. doi: 10.1016/s1044-579x(02)00098-6. [DOI] [PubMed] [Google Scholar]
- 23.Fukuchi M, Masuda N, Nakajima M, et al. Inverse correlation between expression levels of p27 and the ubiquitin ligase subunit Skp2 in early esophageal squamous cell carcinoma. Anticancer Res. 2004;24:777–783. [PubMed] [Google Scholar]
- 24.Li Q, Murphy M, Ross J, et al. Skp2 and p27kip1 expression in melanocytic nevi and melanoma: an inverse relationship. J Cutan Pathol. 2004;31:633–642. doi: 10.1111/j.0303-6987.2004.00243.x. [DOI] [PubMed] [Google Scholar]
- 25.Lim MS, Adamson A, Lin Z, et al. Expression of Skp2, a p27 (Kip1) ubiquitin ligase, in malignant lymphoma: correlation with p27(Kip1) and proliferation index. Blood. 2002;100:2950–2956. doi: 10.1182/blood.V100.8.2950. [DOI] [PubMed] [Google Scholar]
- 26.Bornstein G, Bloom J, Sitry-Shevah D, et al. Role of the SCFSkp2 ubiquitin ligase in the degradation of p21Cip1 in s phase. J Biol Chem. 2003;278:25752–25757. doi: 10.1074/jbc.M301774200. [DOI] [PubMed] [Google Scholar]
- 27.Yu ZK, Gervais JL, Zhang H. Human CUL-1 associates with the SKP1/SKP2 complex and regulates p21 (CIP1/WAF1) and cyclin D proteins. proc Natl Acad Sci USA. 1998;95:11324–11329. doi: 10.1073/pnas.95.19.11324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamura T, Hara T, Kotoshiba S, et al. Degradation of p57Kip2 mediated by SCFSkp2-dependent ubiquitylation. Proc Natl Acad Sci USA. 2003;100:10231–10236. doi: 10.1073/pnas.1831009100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marti A, Wirbelauer C, Scheffner M, et al. Interaction between ubiquitin-protein ligase SCFSKP2 and E2F-1 underlies the regulation of E2F-1 degradation. Nat Cell Biol. 1999;1:14–19. doi: 10.1038/8984. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Hedvat CV, Mao S, et al. The ETS protein MEF is regulated by phosphorylation-dependent proteolysis via the protein-ubiquitin ligase SCFSkp2. Mol Cell Biol. 2006;26:3114–3123. doi: 10.1128/MCB.26.8.3114-3123.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhattacharya S, Garriga J, Calbo J, et al. Skp2 associates with p130 and accelerates p130 ubiquitylation and degradation in human cells. Oncogene. 2003;22:2443–2451. doi: 10.1038/sj.onc.1206339. [DOI] [PubMed] [Google Scholar]
- 32.Tedesco D, Lukas J, Reed SI. The pRb-related protein p130 is regulated by phosphorylation-dependent proteolysis via the protein-ubiquitin ligase SCF(Skp2) Genes Dev. 2002;16:2946–2957. doi: 10.1101/gad.1011202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hiramatsu Y, Kitagawa K, Suzuki T, et al. Degradation of Tob1 mediated by SCFSkp2-dependent ubiquitination. Cancer Res. 2006;66:8477–8483. doi: 10.1158/0008-5472.CAN-06-1603. [DOI] [PubMed] [Google Scholar]
- 34.Yeh KH, Kondo T, Zheng J, et al. The F-box protein SKP2 binds to the phosphorylated threonine 380 in cyclin E and regulates ubiquitin-dependent degradation of cyclin E. Biochem Biophys Res Commun. 2001;281:884–890. doi: 10.1006/bbrc.2001.4442. [DOI] [PubMed] [Google Scholar]
- 35.Liang M, Liang YY, Wrighton K, et al. Ubiquitination and proteolysis of cancer-derived Smad4 mutants by SCFSkp2. Mol Cell Biol. 2004;24:7524–7537. doi: 10.1128/MCB.24.17.7524-7537.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim SY, Herbst A, Tworkowski KA, et al. Skp2 regulates myc protein stability and activity. Mol Cell. 2003;11:1177–1188. doi: 10.1016/s1097-2765(03)00173-4. [DOI] [PubMed] [Google Scholar]
- 37.von der Lehr N, Johansson S, Wu S, et al. The F-box protein Skp2 participates in c-Myc proteosomal degradation and acts as a cofactor for c-Myc-regulated transcription. Mol Cell. 2003;11:1189–1200. doi: 10.1016/s1097-2765(03)00193-x. [DOI] [PubMed] [Google Scholar]
- 38.Charrasse S, Carena I, Brondani V, et al. Degradation of B-Myb by ubiquitin-mediated proteolysis: involvement of the Cdc34-SCF(p45Skp2) pathway. Oncogene. 2000;19:2986–2995. doi: 10.1038/sj.onc.1203618. [DOI] [PubMed] [Google Scholar]
- 39.Song MS, Song SJ, Kim SJ, et al. Skp2 regulates the antiproliferative function of the tumor suppressor RASSF1A via ubiquitin-mediated degradation at the G1–S transition. Oncogene. 2008;27:3176–3185. doi: 10.1038/sj.onc.1210971. [DOI] [PubMed] [Google Scholar]
- 40.Mendez J, Zou-Yang XH, Kim SY, et al. Human origin recognition complex large subunit is degraded by ubiquitin-mediated proteolysis after initiation of DNA replication. Mol Cell. 2002;9:481–491. doi: 10.1016/s1097-2765(02)00467-7. [DOI] [PubMed] [Google Scholar]
- 41.Kondo T, Kobayashi M, Tanaka J, et al. Rapid degradation of Cdt1 upon UV-induced DNA damage is mediated by SCFSkp2 complex. J Biol Chem. 2004;279:27315–27319. doi: 10.1074/jbc.M314023200. [DOI] [PubMed] [Google Scholar]
- 42.Li X, Zhao Q, Liao R, et al. The SCF (Skp2) ubiquitin ligase complex interacts with the human replication licensing factor Cdt1 and regulates Cdt1 degradation. J Biol Chem. 2003;278:30854–30858. doi: 10.1074/jbc.C300251200. [DOI] [PubMed] [Google Scholar]
- 43.Jiang H, Chang FC, Ross AE, et al. Ubiquitylation of RAG-2 by Skp2-SCF links destruction of the V(D)J recombinase to the cell cycle. Mol Cell. 2005;18:699–709. doi: 10.1016/j.molcel.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 44.Moro L, Arbini AA, Marra E, et al. Up-regulation of Skp2 after prostate cancer cell adhesion to basement membranes results in BRCA2 degradation and cell proliferation. J Biol Chem. 2006;281:22100–22107. doi: 10.1074/jbc.M604636200. [DOI] [PubMed] [Google Scholar]
- 45.Kiernan RE, Emiliani S, Nakayama K, et al. Interaction between cyclin T1 and SCF (SKP2) targets CDK9 for ubiquitination and degradation by the proteasome. Mol Cell Biol. 2001;21:7956–7970. doi: 10.1128/MCB.21.23.7956-7970.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin YW, Yang JL. Cooperation of ERK and SCFSkp2 for MKP-1 destruction provides a positive feedback regulation of proliferating signaling. J Biol Chem. 2006;281:915–926. doi: 10.1074/jbc.M508720200. [DOI] [PubMed] [Google Scholar]
- 47.Tokarz S, Berset C, La Rue J, et al. The ISG15 isopeptidase UBP43 is regulated by proteolysis via the SCFSkp2 ubiquitin ligase. J Biol Chem. 2004;279:46424–46430. doi: 10.1074/jbc.M403189200. [DOI] [PubMed] [Google Scholar]
- 48.Gstaiger M, Jordan R, Lim M, et al. Skp2 is oncogenic and overexpressed in human cancers. Proc Natl Acad Sci USA. 2001;98:5043–5048. doi: 10.1073/pnas.081474898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Latres E, Chiarle R, Schulman BA, et al. Role of the F-box protein Skp2 in lymphomagenesis. Proc Natl Acad Sci USA. 2001;98:2515–2520. doi: 10.1073/pnas.041475098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shim EH, Johnson L, Noh HL, et al. Expression of the F-box protein SKP2 induces hyperplasia, dysplasia, and low-grade carcinoma in the mouse prostate. Cancer Res. 2003;63:1583–1588. [PubMed] [Google Scholar]
- 51.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 52.Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 53.Gorospe M, Abdelmohsen K. MicroRegulators come of age in senescence. Trends Genet. 2011;27:233–241. doi: 10.1016/j.tig.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuilman T, Michaloglou C, Mooi WJ, et al. The essence of senescence. Genes Dev. 2010;24:2463–2479. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nardella C, Clohessy JG, Alimonti A, et al. Pro-senescence therapy for cancer treatment. Nat Rev Cancer. 2011;11:503–511. doi: 10.1038/nrc3057. [DOI] [PubMed] [Google Scholar]
- 56.Serrano M, Lin AW, McCurrach ME, et al. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 57.Ben-Porath I, Weinberg RA. The signals and pathways activating cellular senescence. Int J Biochem Cell Biol. 2005;37:961–976. doi: 10.1016/j.biocel.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 58.Alimonti A, Nardella C, Chen Z, et al. A novel type of cellular senescence that can be enhanced in mouse models and human tumor xenografts to suppress prostate tumorigenesis. J Clin Invest. 2010;120:681–693. doi: 10.1172/JCI40535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen Z, Trotman LC, Shaffer D, et al. Crucial role of p53-dependent cellular senescence in suppression of PTEN-deficient tumorigenesis. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Courtois-Cox S, Genther Williams SM, Reczek EE, et al. A negative feedback signaling network underlies oncogene-induced senescence. Cancer Cell. 2006;10:459–472. doi: 10.1016/j.ccr.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Young AP, Schlisio S, Minamishima YA, et al. VHL loss actuates a HIF-independent senescence programme mediated by Rb and p400. Nat Cell Biol. 2008;10:361–369. doi: 10.1038/ncb1699. [DOI] [PubMed] [Google Scholar]
- 62.Sharpless NE, DePinho RA. Cancer: crime and punishment. Nature. 2005;436:636–637. doi: 10.1038/436636a. [DOI] [PubMed] [Google Scholar]
- 63.Xue W, Zender L, Miething C, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ventura A, Kirsch DG, McLaughlin ME, et al. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445:661–665. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
- 65.Braig M, Lee S, Loddenkemper C, et al. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature. 2005;436:660–665. doi: 10.1038/nature03841. [DOI] [PubMed] [Google Scholar]
- 66.Michaloglou C, Vredeveld LC, Soengas MS, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- 67.Collado M, Gil J, Efeyan A, et al. Tumour biology: senescence in premalignant tumours. Nature. 2005;436:642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- 68.Brown CJ, Lain S, Verma CS, et al. Awakening guardian angels: drugging the p53 pathway. Nat Rev Cancer. 2009;9:862–873. doi: 10.1038/nrc2763. [DOI] [PubMed] [Google Scholar]
- 69.Junttila MR, Evan GI. P53—a jack of all trades but master of none. Nat Rev Cancer. 2009;9:821–829. doi: 10.1038/nrc2728. [DOI] [PubMed] [Google Scholar]
- 70.Donehower LA, Lozano G. 20 years studying p53 functions in genetically engineered mice. Nat Rev Cancer. 2009;9:831–841. doi: 10.1038/nrc2731. [DOI] [PubMed] [Google Scholar]
- 71.Lu WJ, Amatruda JF, Abrams JM. P53 ancestry: gazing through an evolutionary lens. Nat Rev Cancer. 2009;9:758–762. doi: 10.1038/nrc2732. [DOI] [PubMed] [Google Scholar]
- 72.Levine AJ, Oren M. The first 30 years of p53: growing ever more complex. Nat Rev Cancer. 2009;9:749–758. doi: 10.1038/nrc2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sugrue MM, Shin DY, Lee SW, et al. Wild-type p53 triggers a rapid senescence program in human tumor cells lacking functional p53. Proc Natl Acad Sci USA. 1997;94:9648–9653. doi: 10.1073/pnas.94.18.9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ide T, Tsuji Y, Ishibashi S, et al. Reinitiation of host DNA synthesis in senescent human diploid cells by infection with simian virus 40. Exp Cell Res. 1983;143:343–349. doi: 10.1016/0014-4827(83)90060-5. [DOI] [PubMed] [Google Scholar]
- 75.Gorman SD, Cristofalo VJ. Reinitiation of cellular DNA synthesis in BrdU-selected nondividing senescent WI-38 cells by simian virus 40 infection. J Cell Physiol. 1985;125:122–126. doi: 10.1002/jcp.1041250116. [DOI] [PubMed] [Google Scholar]
- 76.Hawley-Nelson P, Vousden KH, Hubbert NL, et al. HPV16 E6 and E7 proteins cooperate to immortalize human foreskin keratinocytes. EMBO J. 1989;8:3905–3910. doi: 10.1002/j.1460-2075.1989.tb08570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Munger K, Phelps WC, Bubb V, et al. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989;63:4417–4421. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dankort D, Filenova E, Collado M, et al. A new mouse model to explore the initiation, progression, and therapy of BRAFV600E-induced lung tumors. Genes Dev. 2007;21:379–384. doi: 10.1101/gad.1516407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dhomen N, Reis-Filho JS, da Rocha Dias S, et al. Oncogenic BRAF induces melanocyte senescence and melanoma in mice. Cancer Cell. 2009;15:294–303. doi: 10.1016/j.ccr.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 80.Sarkisian CJ, Keister BA, Stairs DB, et al. Dose-dependent oncogene-induced senescence in vivo and its evasion during mammary tumorigenesis. Nat Cell Biol. 2007;9:493–505. doi: 10.1038/ncb1567. [DOI] [PubMed] [Google Scholar]
- 81.Kaelin WG. Von hippel-lindau disease. Annu Rev Pathol. 2007;2:145–173. doi: 10.1146/annurev.pathol.2.010506.092049. [DOI] [PubMed] [Google Scholar]
- 82.Kuo YL, Giam CZ. Activation of the anaphase promoting complex by HTLV-1 tax leads to senescence. EMBO J. 2006;25:1741–1752. doi: 10.1038/sj.emboj.7601054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 84.Moscow JA, He R, Gnarra JR, et al. Examination of human tumors for RhoA mutations. Oncogene. 1994;9:189–194. [PubMed] [Google Scholar]
- 85.Rihet S, Vielh P, Camonis J, et al. Mutation status of genes encoding RhoA, Rac1, and Cdc42 GTPases in a panel of invasive human colorectal and breast tumors. J Cancer Res Clin Oncol. 2001;127:733–738. doi: 10.1007/s004320100272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bellizzi A, Mangia A, Chiriatti A, et al. RhoA protein expression in primary breast cancers and matched lymphocytes is associated with progression of the disease. Int J Mol Med. 2008;22:25–31. [PubMed] [Google Scholar]
- 87.Faried A, Faried LS, Usman N, et al. Clinical and prognostic significance of RhoA and RhoC gene expression in esophageal squamous cell carcinoma. Ann Surg Oncol. 2007;14:3593–3601. doi: 10.1245/s10434-007-9562-x. [DOI] [PubMed] [Google Scholar]
- 88.Horiuchi A, Imai T, Wang C, et al. Up-regulation of small GTPases, RhoA and RhoC, is associated with tumor progression in ovarian carcinoma. Lab Invest. 2003;83:861–870. doi: 10.1097/01.lab.0000073128.16098.31. [DOI] [PubMed] [Google Scholar]
- 89.Stanbrough M, Bubley GJ, Ross K, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 90.Yokoi S, Yasui K, Mori M, et al. Amplification and overexpression of SKP2 are associated with metastasis of non-small-cell lung cancers to lymph nodes. Am J Pathol. 2004;165:175–180. doi: 10.1016/S0002-9440(10)63286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li JQ, Wu F, Masaki T, et al. Correlation of SKP2 with Carcinogenesis, invasion, metastasis, and prognosis in colorectal tumors. Int J Oncol. 2004;25:87–95. [PubMed] [Google Scholar]
- 92.Salon C, Merdzhanova G, Brambilla C, et al. E2F-1, SKP2 and cyclin E oncoproteins are upregulated and directly correlated in high-grade neuroendocrine lung tumors. Oncogene. 2007;26:6927–6936. doi: 10.1038/sj.onc.1210499. [DOI] [PubMed] [Google Scholar]
- 93.Einama T, Kagata Y, Tsuda H, et al. High-level SKP2 expression in pancreatic ductal adenocarcinoma: correlation with the extent of lymph node metastasis, higher histological grade, and poorer patient outcome. Pancreas. 2006;32:376–381. doi: 10.1097/01.mpa.0000220862.78248.c4. [DOI] [PubMed] [Google Scholar]
- 94.Chan CH, Lee SW, Li CF, et al. Deciphering the transcriptional complex critical for RhoA gene expression and cancer metastasis. Nat Cell Biol. 2010;12:457–467. doi: 10.1038/ncb2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen Q, Xie W, Kuhn DJ, et al. Targeting the p27 E3 ligase SCF(Skp2) results in p27- and Skp2-mediated cell-cycle arrest and activation of autophagy. Blood. 2008;111:4690–4699. doi: 10.1182/blood-2007-09-112904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Soucy TA, Smith PG, Milhollen MA, et al. An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature. 2009;458:732–736. doi: 10.1038/nature07884. [DOI] [PubMed] [Google Scholar]
- 97.Lin HK, Chen Z, Wang G, et al. Skp2 targeting suppresses tumorigenesis by Arf-p53-independent cellular senescence. Nature. 2010;464:374–379. doi: 10.1038/nature08815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zheng J, Yang X, Harrell JM, et al. CAND1 binds to unneddylated CUL1 and regulates the formation of SCF ubiquitin E3 ligase complex. Mol Cell. 2002;10:1519–1526. doi: 10.1016/s1097-2765(02)00784-0. [DOI] [PubMed] [Google Scholar]