Abstract
Niclosamide, an oral antihelminthic drug, has been used to treat tapeworm infection for about 50 years. Niclosamide is also used as a molluscicide for water treatment in schistosomiasis control programs. Recently, several groups have independently discovered that niclosamide is also active against cancer cells, but its precise mechanism of antitumor action is not fully understood. Evidence supports that niclosamide targets multiple signaling pathways (NF-κB, Wnt/β-catenin, Notch, ROS, mTORC1, and Stat3), most of which are closely involved with cancer stem cells. The exciting advances in elucidating the antitumor activity and the molecular targets of this drug will be discussed. A method for synthesizing a phosphate pro-drug of niclosamide is provided. Given its potential antitumor activity, clinical trials for niclosamide and its derivatives are warranted for cancer treatment.
Keywords: Niclosamide, cancer, cancer stem cells, signal transduction
Niclosamide, whose systematic name is (5-chloro-N-2-chloro-4-nitrophenyl)-2-hydroxybenzamide, is an oral antihelminthic drug used for approximately 50 years for treating most tapeworm infections. It is also used as a molluscicide for water treatment in schistosomiasis control programs. The activity of niclosamide against these parasites is believed to be mediated by inhibition of mitochondrial oxidative phosphorylation and anaerobic ATP production[1]. Recently, several groups have independently discovered that niclosamide is active against cancer cells[2]–[4], though its precise mechanism of antitumor action is not fully understood. Accumulating evidence suggests that niclosamide targets multiple signaling pathways such as nuclear factor-kappaB (NF-kB), Wnt/β-catenin, and Notch, most of which are closely involved with cancer stem cell proliferation. This mini-review focuses on the exciting progress in elucidating the antitumor activity and the molecular targets of this drug.
Niclosamide Inhibits Tumor Cell Growth
Niclosamide inhibits the proliferation of tumor cells with minimal effect on normal cells
Niclosamide has shown antiproliferative activity in a broad spectrum of cancer cells including hematologic cancer cells (e.g., acute myeloid leukemia, AML) and solid tumor cells (e.g., colon cancer, breast cancer, and prostate cancer)[2]-[5]. The 50% inhibition concentration (IC50) values ranged from 0.18 to 1 µmol/L for AML cells (Table 1). Furthermore, niclosamide showed impressive in vivo antitumor activity in xenograft nude mouse models[2],[3].
Table 1. The half maximal inhibition concentration (IC50) values of niclosamide in some tumor cell lines and normal cells.
| Tumor type | Cell line | IC50 (µmol/L) | Reference |
| Leukemia | |||
| Acute myeloid leukemia | U937 | 0.41 | [2] |
| OCI-AML3 | 0.79 | [2] | |
| HL-60 | 0.48 | [2] | |
| Molm13 | 0.72 | [2] | |
| MV4-11 | 1.0 | [2] | |
| Solid tumor | |||
| Prostrate cancer | Du145 | 0.7 | [5] |
| PC3 | 11.7±2.3 | [5] | |
| Colorectal cancer | HT29 | 7.2±1.2 | [5] |
| HCT116 | 2.2 | [4] | |
| Cervical carcinoma | HeLa | 0.25±0.07 | [5] |
| Lung adenocarcinoma | A549 | 3.0±0.2 | [5] |
| Epithelial carcinoma | A431 | 8.8±0.9 | [5] |
| Normal cell | |||
| MEF | 4.54 | [2] | |
| NHFB | 5.78 | [2] | |
| Bone marrow | 9.00±4.87 | [2] |
Drug resistance and relapse are challenges for chemotherapy, but combining agents that target different pathways may help prevent these challenges. We have reported that niclosamide may be useful not only as a monotherapy but also in combination therapy. Indeed, we found that niclosamide showed synergistic effects with frontline chemotherapeutic agents for AML (e.g., cytarabine, etoposide, and daunorubicin)[2]. Similarly, the additive anti-proliferative activity of niclosamide when combined with the cytotoxic agent oxaliplatin was noted in colorectal cancer cell lines HCT116, HT29, and CaCO2[3].
Although niclosamide has a potent activity against tumor cells, it has minimal effects on the viability of normal cells. We reported that the IC50 of niclosamide in mouse embryonic fibroblast (MEF) cells and normal human fibroblast (NHFB) cells was 4.54 and 5.78 µmol/L, respectively, based on MTS assay after drug treatment for 72 h [2]. In addition, the IC50 value for bone marrow cells from four healthy donors was (9.00 ± 4.87) µmol/L[2]. Osada et al.[3] also reported that niclosamide did not have significant toxicity against nonmalignant cancer cells such as mammary epithelial cells (MCF-10A) and peripheral blood mononuclear cells from healthy individuals. These findings from us and others suggest that niclosamide possesses a therapeutic window for its antitumor effects.
Niclosamide inhibits cancer cell metastasis and migration
Sack et al.[4] evaluated the effect of niclosamide on the migration and invasion of cells overexpressing S100 calcium-binding protein A4 (S100A4), an 11-kDa protein originally identified as metastasin 1 (MTS1) that is essential for metastasis in colon cancer[6]. Exposure of colon cancer HCT116 cells to 1 µmol/L niclosamide for 24 h reduced cell migration to less than 50% of solvent-treated control cells as measured by Boyden chamber and scratch wound healing assays; however, ectopic expression of S100A4 counteracted the effect of niclosamide on cell migration[4]. In an assay conducted in Matrigel-covered Boyden chambers, niclosamide also showed potent activity against invasion of HCT116 cells (30% of control). Similarly, S100A4 overcame the niclosamide-mediated inhibition of cell motility, suggesting the involvement of S100A4 in this process.
By using noninvasive in vivo luminescence imaging, Sack et al.[4] monitored the effect of niclosamide on metastasis in a mouse colon cancer xenograft model. The results showed that niclosamide treatment inhibited S100A4-induced metastasis in vivo.
Niclosamide eradicates cancer stem cells
Human cancers are believed to originate from a small fraction of cancer stem cells capable of uncontrolled self-renewal and aberrant differentiation. The persistence of cancer stem cells is an important mechanism of chemotherapeutic resistance and relapse after conventional chemotherapy. Cancer stem cells share some key properties including signaling pathways (e.g., Wnt, Notch, and hedgehog) with normal stem cells[7]. Notably, several signaling pathways critical for self-renewal and proliferation of cancer stem cells (e.g., NF-κB, Notch, and Wnt/β-catenin) are targets of niclosamide[2],[4],[8]. Thus, we hypothesized that niclosamide is effective against cancer stem cells, and we indeed discovered that niclosamide at nanomolar cencentrations killed AML stem cells (CD34+CD38−) with minimal cytotoxicity against the progenitor cells in normal bone marrow cells from three healthy donors[2]. In this sense, niclosamide may be a promising chemotherapeutic agent to effectively eradicate leukemic stem cells while sparing normal hematopoietic tissue. This aspect of clinical efficacy remains to be investigated in clinical trials. In addition, future work should investigate whether niclosamide inhibits cancer stem cells in solid tumors and its effect on the “sternness” molecules.
Molecular Targets of Niclosamide
Niclosamide inhibits the NF-κB pathway
The transcription factor NF-κB has been demonstrated to promote cancer growth, angiogenesis, escape from apoptosis, and tumorigenesis. NF-κB is sequestered in the cytosol of resting cells through binding the inhibitory subunit IκBα. IκBα can be phosphorylated by the IκB kinase (IKK) complex (containing IKKα, IKKβ, and NEMO/IKKγ) subunits in response to diverse stimuli[9]. Phosphorylated IκBα is then degraded by the ubiquitin-proteosome pathway, allowing NF-κB translocation into the nucleus and transactivation of its target genes. IKK is regulated predominantly by TGF-β-activated kinase-1 (TAK1) in the canonical pathway of cytokine-induced NF-κB activation[10],[11]. Niclosamide blocked TNFα-induced IκBα phosphorylation, translocation of p65, and the expression of NF-κB-regulated genes (Figure 1). The inhibitory effects of niclosamide involved two steps: TAK1 →IKK and IKK →IκBα. Niclosamide also inhibited the DNA binding of NF-κB to the promoter of its target genes[2]. Inhibition of NF-κB pathway was a key molecular mechanism for the antileukemic action of niclosamide. Of note, leukemia stem cells but not normal hematopoietic stem cells display constitutive activation of NF-κB, and blocking the NF-κB pathway is believed to selectively kill leukemia stem cells.
Figure 1. Niclosamide targets nuclear factor-kappaB (NF-κB) and elevates reactive oxygen species (ROS) levels.
Niclosamide blocks the tumor necrosis factor-α (TNFα)-induced I B phosphorylation, translocation of p65, and the expression of NF-κB-regulated genes. On the other hand, niclosamide elevates the intracellular ROS content. TNFR, tumor necrosis factor receptor; TAK, TGF-β-activated kinase; IKK, IκB kinase.
Niclosamide elevates ROS levels
Reactive oxygen species (ROS) generation is an important mechanism by which certain chemotherapeutic agents (e.g., arsenic tetroxide, farnesyltransferase inhibitors, and phenethyl isothiocyanate) kill tumor cells[12]. Because niclosamide was reported to inhibit NADH → NADP+ transhydrogenation in the Hymenolepis diminuta submitochondrial particles assay, we tested whether niclosamide induces ROS generation and discovered that niclosamide treatment in AML cells led to a ∼20-fold elevation of ROS[2].
Quenching ROS by N-acetyl-cysteine (NAC) attenuated but did not completely abrogate niclosamide-mediated apoptosis[2]. In a separate approach, the mitochondrial respiration-deficient ρ-cells (C6F/HL-60 cells), which presumably generate less ROS, underwent the same extent of IκBα phosphorylation and p65 translocation as parental HL-60 cells. Therefore, niclosamide inhibited the TNFα-induced NF-κB activation in a ROS-independent manner. Conversely, TNFα treatment did not influence the niclosamide-induced ROS generation[2]. Taken together, our data support that niclosamide has two independent effects: NF-kB activation and ROS elevation (Figure 1).
Niclosamide inhibits the Wnt/β-catenin pathway
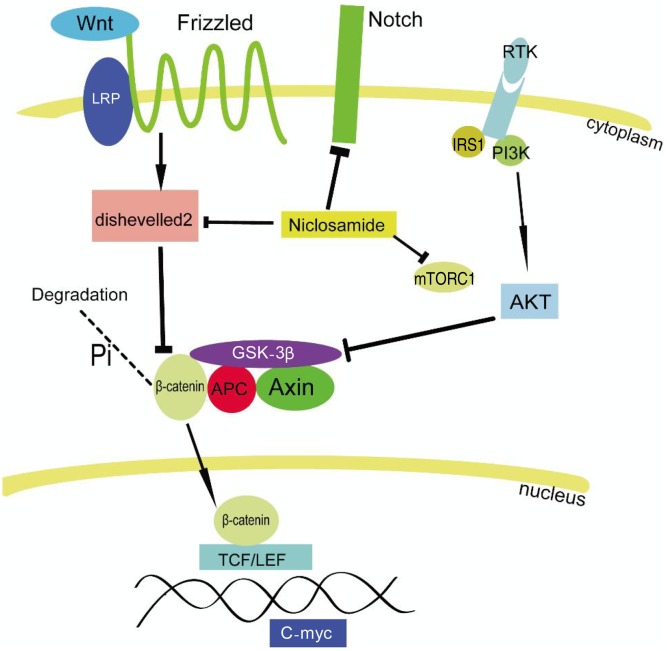
The Wnt signaling pathway plays fundamental roles in directing tissue patterning in embryonic development, in maintaining tissue homeostasis in differentiated tissue, and in tumorigenesis. In canonical Wnt signaling, Wnt binds and activates Frizzled receptor and low-density lipoprotein-related protein receptors 5 and 6 (LRP5 and LRP6) to regulate the stability of the downstream protein β-catenin by modulating its destruction complex containing axin, adenomatous polyposis kinase (APC), glycogen synthase kinase 3β (GSK3β), and casein kinase. Stabilized β-catenin then translocates to the nucleus to bind and initiate the transcriptional activity of TCF/LEF transcription factors.
In a recent study using a high-throughput screening approach, Chen et al.[13] validated that niclosamide is a potent inhibitor of the Wnt/β-catenin pathway (Figure 2). Niclosamide promoted Frizzled1 internalization, down-regulated the expression of Dishevelled-2 protein, and inhibited β-catenin stabilization[3]. Independently, Sack et al.[4] demonstrated that niclosamide inhibited β-catenin/TCF transcription-activating complex and diminished the expression of downstream target S100A4, which is a driving force of metastasis in colon cancer cells. Interestingly, a single treatment of 1 µmol/L niclosamide reduced S100A4 at levels of mRNA and protein in vitro, and discontinuous niclosamide treatment still had a major effect of prolonging overall survival in a xenograft mouse model[4].
Figure 2. Niclosamide targets the Wnt/β-catenin, Notch, and mTORC1 pathways.
Niclosamide down-regulates the expression of Dishevelled-2 and inhibits the stabilization of β-catenin. Niclosamide also inhibits Notch signaling pathway and mTORC1. LRP, low-density lipoprotein-related protein receptor; IRS1, insulin receptor substrate 1; RTK, receptor tyrosine kinase.
Niclosamide inhibits the Notch pathway
The Notch signaling pathway plays important roles in a variety of cellular processes such as proliferation, differentiation, apoptosis, cell fate decisions, and maintenance of stem cells. Upon activation, Notch receptor regulates downstream target genes through both C promoter-binding factor-1 (CBF-1)-dependent and CBF-1-independent pathways. Wang et al.[8] reported that niclosamide potently suppresses the luciferase activity of a CBF-1-dependent reporter gene in both a dose-dependent and a time-dependent manners in K562 leukemia cells (Figure 2). Taken together, these observations suggest that niclosamide has the potential to treat Notch signaling pathway-associated diseases.
Niclosamide inhibits the Stat3 pathway
Ren et al.[5] reported that niclosamide is a selective inhibitor of Stat3 but not the closely related Stat1 or Stat5. Namely, niclosamide treatment abrogated the epidermal growth factor (EGF)-stimulated dimerization and nuclear translocation and transcriptional activity of Stat3, and induced cell growth inhibition and apoptosis in several types of cancer cells (e.g. Du145, Hela, A549) that exhibit relatively higher levels of Stat3 constitutive activation[5].
Niclosamide inhibits the mTORC1 pathway and the proteasome
Using an automated cell-based screening assay, Balgi et al.[14] identified that niclosamide can rapidly increase autophagosome formation. Biochemical assays showed that niclosamide induced autophagy and inhibited mammalian target of rapamycin complex 1 (mTORC1) but not mTORC2 signaling in cells maintained in nutrient-rich conditions (Figure 2). Furthermore, niclosamide prevented the formation of large ubiquitin-containing aggregates caused by proteasome inhibition, suggesting that niclosamide may inhibit protein ubiquitination or promote the selective clearance of ubiquitinated proteins in the absence of proteasome activity.
Pharmacokinetics and Safety
Niclosamide is a Food and Drug Administration (FDA)-approved drug that is given orally for helminthosis. A single 5 mg/kg oral administration of niclosamide in rats could generate a maximal plasma concentration of 1.08 µmol/L[15], which was sufficient to kill AML cells[2]. However, poor bioavailability of niclosamide because of its limited water solubility and absorption is a challenge. Indeed, niclosamide is practically insoluble in water, but the solubility does increase sparingly over the pH range of 8-10. Preparation of solid dispersions with PEG-6000 and addition of polyamidoamine (PAMAM) dendrimers were reported to improve the solubility and in vitro dissolution properties of niclosamide[16].
To improve the water solubility of niclosamide, we modified this drug to a phosphate pro-drug[2]. In the addendum of this article, the procedure for synthesis of water-soluble p-niclosamide was made available, upon the request of many readers.
Niclosamide has low toxicity in mammals (oral median lethal dose in rats >5000 mg/kg)[17], which is obviously associated with poor absorption. The safety of niclosamide needs to be re-evaluated if it is translated to chemotherapy for cancer patients by systemic intravenous administration.
Conclusions
Niclosamide is active against cancer cells such as AML and colorectal cancer cells, not only as a monotherapy but also as part of combination therapy, in which it has been found to be synergistic with frontline chemotherapeutic agents (e.g., oxaliplatin, cytarabine, etoposide, and daunorubicin). Because niclosamide targets multiple signaling pathways (e.g., NF-κB, Wnt/β-catenin, and Notch), most of which are closely involved with cancer stem cells, it holds promise in eradicating cancer stem cells. These exciting findings warrant clinical trials of niclosamide in cancer patients.
Addendum
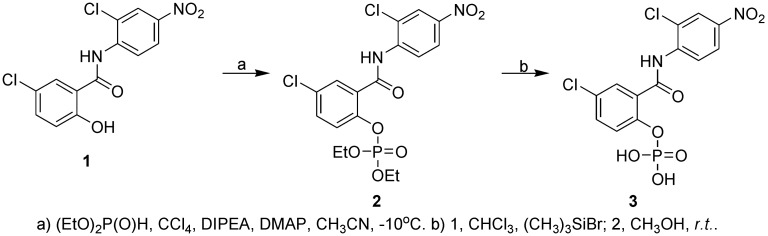
In order to improve the water solubility of niclosamide and hence to improve its bioavailability in vivo, we have synthesized the phosphate of niclosamide (Scheme 1).
Scheme 1. Synthesis of water-soluble p-niclosamide.
First, niclosamide (compound 1) was phosphorylated with diethyl phosphite to yield compound 2. The designed compound 3 was obtained by a simple deprotection of compound 2 with an excellent yield (Tetrahedron Letters, 1996, 37:771). To the stirred suspension of niclosamide (654 mg, 2 mmol) in anhydrous CH3CN (10 mL), CCI4 (1.54 g, 10 mmol), DIPEA (N,N-Diisopropylethylamine) (516 mg, 4 mmol), and DMAP (4-Dimethylaminopyridine) (24 mg, 0.2 mmol) were added at -10°C. After stirring for a further 10 min, diethyl phosphate (414 mg, 3 mmol) was added dropwise at the same temperature. The reaction was completed within approximately 1 h (as determined by thin layer chromatography). Aqueous KH2PO4 (0.5 mol/L) was then added to quench the reaction, and the mixture was allowed to warm to room temperature. The mixture was extracted three times with ethyl acetate. The organic phase was then combined and washed successively with water and brine, dried over Na2SO4, and concentrated in vacuo. The product was used in the next step without any further purification. The product obtained was dissolved in CHCI3 (20 mL), followed by addition of (CH3)3SiBr (3.06 g, 20 mmol) at room temperature. After stirring for 24 h, the mixture was concentrated under reduced pressure. CH3OH (20 mL) was then added, and the mixture was stirred for 30 min at room temperature. After the reaction was completed, the solvent was removed in vacuo and yielded a white solid (660 mg, 81% in two steps).
Acknowledgments
This study was supported in part by grants from National Natural Science Foundation of China for Distinguished Young Scholars (81025021), the National Basic Research Program of China (973 Program grant 2009CB825506), the Major Research Plan of the National Natural Science Foundation of China (90713036), the National High Technology Research and Development Program of China (863 Program grant 2008AA02Z420), and the Fundamental Research Foundation for the Central Universities to Jing-Xuan Pan.
References
- 1.Weinbach EC, Garbus J. Mechanism of action of reagents that uncouple oxidative phosphorylation. Nature. 1969;221:1016–1018. doi: 10.1038/2211016a0. [DOI] [PubMed] [Google Scholar]
- 2.Jin Y, Lu Z, Ding K, et al. Antineoplastic mechanisms of niclosamide in acute myelogenous leukemia stem cells: inactivation of the NF-kappa B pathway and generation of reactive oxygen species. Cancer Res. 2010;70:2516–2527. doi: 10.1158/0008-5472.CAN-09-3950. [DOI] [PubMed] [Google Scholar]
- 3.Osada T, Chen M, Yang XY, et al. Antihelminth compound niclosamide downregulates Wnt signaling and elicits antitumor responses in tumors with activating APC mutations. Cancer Res. 2011;71:4172–4182. doi: 10.1158/0008-5472.CAN-10-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sack U, Walther W, Scudiero D, et al. Novel effect of antihelminthic niclosamide on S100A4-mediated metastatic progression in colon cancer. J Natl Cancer Inst. 2011;103:1018–1036. doi: 10.1093/jnci/djr190. [DOI] [PubMed] [Google Scholar]
- 5.Ren XM, Duan L, He Q, et al. Identification of niclosamide as a new small-molecule inhibitor of the STAT3 signaling pathway. ACS Med Chem Lett. 2010;1:454–459. doi: 10.1021/ml100146z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grum-Schwensen B, Klingelhofer J, Berg CH, et al. Suppression of tumor development and metastasis formation in mice lacking the S100A4 (mts1) gene. Cancer Res. 2005;65:3772–3780. doi: 10.1158/0008-5472.CAN-04-4510. [DOI] [PubMed] [Google Scholar]
- 7.O'Brien CA, Kreso A, Jamieson CH. Cancer stem cells and self-renewal. Clin Cancer Res. 2010;16:3113–3120. doi: 10.1158/1078-0432.CCR-09-2824. [DOI] [PubMed] [Google Scholar]
- 8.Wang AM, Ku HH, Liang YC, et al. The autonomous notch signal pathway is activated by baicalin and baicalein but is suppressed by niclosamide in K562 cells. J Cell Biochem. 2009;106:682–692. doi: 10.1002/jcb.22065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen F, Castranova V. Nuclear factor-kappaB, an unappreciated tumor suppressor. Cancer Res. 2007;67:11093–11098. doi: 10.1158/0008-5472.CAN-07-1576. [DOI] [PubMed] [Google Scholar]
- 10.C Wang, Deng L, Hong M, et al. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 11.Sakurai H, Miyoshi H, Toriumi W, et al. Functional interactions of transforming growth factor beta-activated kinase 1 with IkappaB kinases to stimulate NF-kappaB activation. J Biol Chem. 1999;274:10641–10648. doi: 10.1074/jbc.274.15.10641. [DOI] [PubMed] [Google Scholar]
- 12.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 13.Chen M, Wang J, Lu J, et al. The anti-helminthic niclosamide inhibits Wnt/Frizzled1 signaling. Biochemistry. 2009;48:10267–10274. doi: 10.1021/bi9009677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balgi AD, Fonseca BD, Donohue E, et al. Screen for chemical modulators of autophagy reveals novel therapeutic inhibitors of mTORC1 signaling. PLoS One. 2009;4:e7124. doi: 10.1371/journal.pone.0007124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mercer-Haines N, Fioravanti CF. Hymenolepis diminuta: mitochondrial transhydrogenase as an additional site for anaerobic phosphorylation. Exp Parasitol. 2008;119:24–29. doi: 10.1016/j.exppara.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Devarakonda B, Hill RA, Liebenberg W, et al. Comparison of the aqueous solubilization of practically insoluble niclosamide by polyamidoamine (PAMAM) dendrimers and cyclodextrins. Int J Pharm. 2005;304:193–209. doi: 10.1016/j.ijpharm.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 17.Merschjohann K, Steverding D. In vitro trypanocidal activity of the anti-helminthic drug niclosamide. Exp Parasitol. 2008;118:637–640. doi: 10.1016/j.exppara.2007.12.001. [DOI] [PubMed] [Google Scholar]