Abstract
Establishing Epstein-Barr virus (EBV)-specific cytolytic T lymphocytes (EBV-CTLs) from peripheral blood mononuclear cells (PBMCs) for adoptive immunotherapy has been reported in EBV-associated malignancies including Hodgkin's lymphoma and nasopharyngeal carcinoma (NPC). In the current study, we performed ex vivo expansion of tumor-infiltrating lymphocytes (TILs) obtained from NPC biopsy specimens with a rapid expansion protocol using anti-CD3 monoclonal antibody (OKT3), recombinant human interleukin (IL)-2, and irradiated PBMCs from healthy donors to initiate the growth of TILs. Young TIL cultures comprised of more than 90% of CD3+ T cells, a variable percentage of CD3+CD8+ and CD3+ CD4+ T cells, and less than 10% of CD3−CD16+ natural killer cells, a similar phenotype of EBV-CTL cultures from PBMCs. Interestingly, TIL cultures secreted high levels of the Th1 cytokines, interferon gamma (IFNγ) and tumor necrosis factor-alpha (TNF-α), and low levels of the Th2 cytokines, IL-4 and IL-10. Moreover, young TILs could recognize autologous EBV-transformed B lymphoblast cell lines, but not autologous EBV-negative blast cells or allogeneic EBV-negative tumor cells. Taken together, these data suggest that ex vivo expansion of TILs from NPC biopsy tissue is an appealing alternative method to establish T cell-based immunotherapy for NPC.
Keywords: Tumor-infiltrating lymphocytes, immunotherapy, nasopharyngeal carcinoma
Nasopharyngeal carcinoma (NPC) is an Epstein-Barr virus (EBV)-related malignancy with high incidence in South China and Southeast Asia[1]. Conventional chemotherapy and radiotherapy only have limited efficacy in NPC patients with late-stage disease. With the advantage of safety and minimal side effects, T-cell-based immunotherapy has become an alternative choice for patients with late-stage cancer[2]–[5]. The latent type II EBV antigens, including EBV nuclear antigen 1 (EBNA1), latent membrane protein 1 and 2 (LMP1 and LMP2), and BARF0, are often presented by tumor cells in EBV-associated malignancies of lymphoid or epithelial origin, including Hodgkin's lymphoma (HL) and NPC[6]–[9]. In contrast, the latent type III EBV antigens, including EBNA1-6, LMP1 and LMP2, and BARFO, are presented by EBV-infected cells in post-transplantation lymphopro-liferative disorder (PTLD)[10],[11]. Several groups have treated patients with PTLD or EBV-positive HL and NPC with adoptive immunotherapy using EBV-specific cytolytic T lymphocytes (EBV-CTLs) derived from the peripheral blood of patients and have obtained significant clinical response, including control of serum EB viral load and even tumor regression[12]–[16]. However, the generation of EBV-CTLs or EBV peptide-specific CTLs from peripheral blood is a complex, time-consuming, and laborious process, which includes setting up EBV-positive transformed B lymphoblast cell lines (LCLs), limited dilution cloning, culture establishment by in vitro restimulations, and genetic engineering.
In the present study, we report a rapid and simple method to expand young TILs from NPC biopsy specimens ex vivo using anti-CD3 monoclonal antibody (OKT3), recombinant human interleukin (IL)-2, and irradiated allogenic PBMCs to initiate rapid lymphocyte growth. In addition, we evaluated the expansion fold, viability, phenotype, and specific lysis of the expanded young TIL cultures from NPC patients when compared to the EBV-CTL cultures derived from peripheral blood of NPC patients. Our data suggest that this method is a new strategy for T-cell-based immunotherapy for patients with NPC with the advantages of simple, rapid expansion in vitro, and stable EBV-antigen specific lysis activity.
Materials and Methods
Generation of young TIL cultures
Bulk TILs were procured and expanded ex vivo by the methods previously described[17]. Briefly, bulk TILs were isolated from NPC biopsy specimens by mincing the tissues up and digesting them with 0.1 µg/mL collagenase type IV (sigma-Aldrich, St. Louis, MO, USA) for 2 h, followed by culture in 24-well plates in X-VIVO-15 medium (Lonza, Walkersville, MD, USA) that contained 5% human AB serum, and 150 IU/mL recombinant human IL-2 to generate enough T cells for rapid expansion in 15 to 20 days.
A minimally rapid expansion protocol (REP) of “young TILs” was performed as others reported in melanoma[18]. On the day of initiation (day 0), 1 × 106 TILs were suspended in 20 mL of X-VIVO-15 medium containing 5% human AB serum mixed with 30 ng/mL anti-CD3 antibody (OKT3, R&D Systems, Minneapolis, MN), 1000 IU/mL IL-2, as well as irradiated (40 Gy) allogenic feeder cells attained from 3 different donors (at a 200:1 feeder to TIL ratio) and irradiated (40 Gy) allogenic LCLs from 2 different donors (at a 50:1 LCL to TIL ratio). The mixture was placed in vertically positioned T25 flasks. Half of the medium was exchanged on days 5 and 8, and cells were split as needed thereafter. Cell phenotype and activity were assessed on day 14 of the rapid expansion.
The generation of EBV-CTLs derived from PBMCs was performed as described before[19]. Briefly, the cryopreserved PBMCs were thawed and aliquots of 3 × 106 cells were stimulated in 24-well plates with irradiated (40 Gy) LCLs at a 40:1 responder:stimulator (R:S) ratio. After 7 days, viable cells were re-stimulated with the irradiated LCLs (40:1 R:S ratio) and, 3 days later, the cells were expanded in complete medium containing 10 IU/mL IL-2 (R&D Systems, Inc). The cultures were re-stimulated weekly with irradiated LCLs in the presence of IL-2.
EBV-transformed LCLs and phytohemagglutinin (PHA)-stimulated blast cells were set up from the PBMCs of NPC patients as described before[19]. The blast cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum and IL-2 (100 IU/mL), whereas LCLs, NPC tumor cell line C666, and leukemia cell line K562 were maintained in complete RPMI-1640 medium supplemented with 10% fetal bovine serum.
All human tissues used in this study were collected with written informed consent provided by the patients, and this protocol was approved by the Research Ethics Committee of the Sun Yat-sen University Cancer Center.
Immunologic monitoring assays
TIL phenotype was determined by fluorescence-activated cell sorting (FACS) analysis using antibodies against CD3, CD4, CD8, and CD16 conjugated with different fluorescent dyes (purchased from eBioscience or BD Biosciences, San Diego, CA, USA). Intracellular staining for interferon gamma (IFNγ), IL-4, IL-10, and other cytokines was performed on T cells stimulated by phorbol 12-myristate 13-acetate (PMA) and inomycin or autologous LCLs (auto-LCLs), autologous blast (auto-blast) cells at a 3:1 R:S ratio for 4 h in the presence of brefeldin A (10 µg/mL, Sigma- Aldrich). Intracellular cytokines were detected using a fixation and permeabilization protocol and buffers purchased from eBioscience. For each sample, 1 × 105 cells were analyzed using an FC500 flow cytometer and CXP analysis software (Beckman Coulter, Inc., Fullerton, CA, USA).
Cytotoxic activity assay
Cytotoxic activity was evaluated by co-culture of target cells and TILs at different ratios for 4 h in 96-well plates. The panel of target cells included auto-LCLs, auto-blasts, NPC tumor cell line C666, and leukemia cell line K562. The target cells were labeled with 5- or 6-(N-Succinimidyloxycarbonyl)-3′, 6′-0,0′'- diacetylfluorescein (CFSE; Molecular Probes™, Invitrogen, Paisley, UK), co-cultured with TILs for 5 h, and then harvested and stained with 7-amino-actinomycin D (7-AAD) (eBioScience) to detect dead cells. The percentage of specific lysis was evaluated using flow cytometry and CXP software.
Statistical analysis
Numerical data are expressed as mean ± standard error in this study. Statistical differences between the means for different groups were evaluated with SPSS 13.0 software, using one-way analysis of variance or Student's t test. P values less than 0.05 were considered significant.
Results
Generation of young TIL cultures
Young TIL cultures were established using fresh tumor tissues from 15 NPC patients following the young TIL production process (Figure 1). At the initiation stage, TIL cultures were grown in IL-2 (150 IU/mL) medium in 24-well plates and reached a number of (5-50) × 106 cells in 18 to 21 days. Next, young TILs were expanded using a 14-day minimal REP as described in the Methods section. After 14 days, young TIL cultures achieved numbers of (1.2-20) × 109 cells, and the average expansion fold was 1000 (Table 1). These data indicated that the expanded TIL number was sufficient to establish clinical therapy.
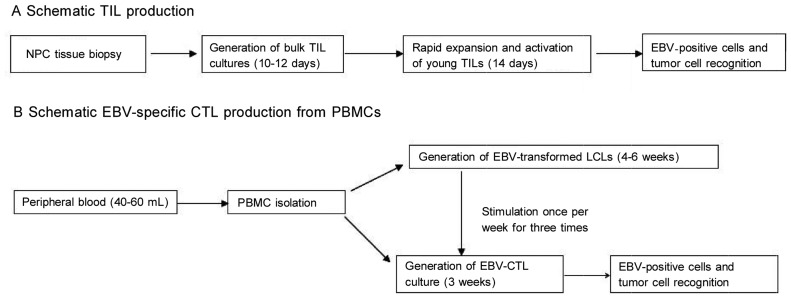
Figure 1. The procedure of generating tumor-infiltrating lymphocytes (TILs) and Epstein-Barr virus (EBV)-specific cytolytic T lymphocytes (CTLs) from nasopharyngeal carcinoma (NPC) tissues.
A, TIL production from NPC biopsy specimens. B, EBV-CTL production from the peripheral blood mononuclear cells (PBMCs) of NPC patients.
Table 1. Rapid expansion and phenotype of young tumor-infiltrating lymphocytes (TILs) from patients with nasopharyngeal carcinoma (NPC).
| TIL No. | Expansion fold | Cell number |
Culture phenotype (% of final cells) |
||||
| Initial | Final | CD3 | CD8 | CD4 | CD16 | ||
| 1 | 2000 | 6.0E+06 | 1.2E+09 | 98.1 | 38.6 | 54.2 | 1.0 |
| 2 | 1724 | 1.45E+07 | 2.5E+10 | 97.4 | 87.6 | 9.5 | 3.8 |
| 3 | 264 | 1.36E+07 | 3.6E+09 | 98.1 | 66.3 | 32.8 | 2.7 |
| 4 | 669 | 1.42E+07 | 9.51E+09 | 94.7 | 76.9 | 8.2 | 2.7 |
| 5 | 1777 | 4.5E+06 | 8.0E+09 | 94.0 | 11.4 | 82.1 | 0.9 |
| 6 | 950 | 6.5E+06 | 6.2E+09 | 91.2 | 16.7 | 74.1 | 1.0 |
| 7 | 157 | 9.5E+06 | 1.5E+09 | 89.4 | 76.0 | 9.7 | 1.2 |
| 8 | 159 | 8.16E+06 | 1.3E+09 | 93.2 | 82.0 | 4.1 | 1.0 |
| 9 | 1382 | 1.28E+07 | 1.7E+09 | 92.3 | 13.6 | 79.6 | 1.1 |
| 10 | 2000 | 9.5E+06 | 2.0E+10 | 90.5 | 19.6 | 72.3 | 1.9 |
| 11 | 1884 | 5.2E+06 | 9.8E+09 | 91.5 | 20.9 | 73.3 | 1.5 |
| 12 | 554 | 1.28E+07 | 7.1E+09 | 88.6 | 55.7 | 28.4 | 0.2 |
| 13 | 1260 | 6.0E+06 | 7.6E+09 | 93.3 | 43.7 | 41.9 | 1.2 |
| 14 | 292 | 1.3E+07 | 3.8E+09 | 96.4 | 66.9 | 23.1 | 2.5 |
| 15 | 1578 | 7.2E+06 | 1.2E+10 | 89.3 | 52.0 | 36.6 | 3.2 |
| Average | 1110 | 9.56E+06 | 7.89E+09 | 93.2 | 48.5 | 41.9 | 1.7 |
| Standard error | 709.4 | 3.6E+06 | 6.91E+09 | 3.2 | 27.1 | 28.5 | 1.0 |
Phenotype and cytokine profiles in young TIL cultures
Analysis of lymphocyte subsets in the TIL cultures from 15 NPC patients demonstrated that young TILs had a high percentage of CD3+ T cells [(93.2 ± 3.2)%] and a low percentage of CD3−CD16+ natural killer (NK) cells [(1.7 ± 1.0)%]. The percentages of CD3+CD8+ T cells [(48.5 ±27.1)%] and CD3+CD4+ T cells [(41.9 ± 28.5)%] were variable in individual TIL cultures (Table 1).
To determine the immune response ability of young TILs after 14-day rapid expansion, we evaluated the cytokine profiles, including type 1 cytokines IFNγ and TNFα and type 2 cytokines IL-4 and IL-10, in TIL cultures from 15 NPC patients treated with PMA/inomycin (Table 2). The TIL cultures had a high percentage of CD4+ and CD8+ IFNγ-producing T cells [(18.8 ± 5.9)% and (50.5 ± 25.3)%] and CD4+ and CD8+ TNFα-producing T cells [(16.9 ± 6.1)% and (45.3 ± 23.3)%], and had a low percentage of CD4+ and CD8+ IL-4-producing T cells [(2.5 ± 2.1)% and (1.8 ± 2.4)%] and CD4+ and CD8+ IL-10-producing T cells [(0.1 ± 0.1)% and (0.04 ± 0.07)%]. These results suggest that the young TIL cultures from NPC patients have high levels of immune reactivity and are prioritized to secret type 1 cytokines after T-cell receptor stimulation.
Table 2. Cytokine profiles of the expanded young TIL cultures.
| TIL No. | % of IFNγ-producing cells |
% of TNFα-producing cells |
% of IL-4-producing cells |
% of IL-10-producing cells |
||||
| CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | CD4 | CD8 | |
| 1 | 15.5 | 70.9 | 12.1 | 72.3 | 2.1 | 1.1 | 0.1 | 0.1 |
| 2 | 14.1 | 48.0 | 8.8 | 37.2 | 2.4 | 1.0 | 0.3 | 0 |
| 3 | 23.4 | 52.1 | 19.7 | 44.1 | 1.5 | 0.7 | 0 | 0 |
| 4 | 26.9 | 44.8 | 20.1 | 51.3 | 1.6 | 0.4 | 0 | 0.2 |
| 5 | 18.3 | 11.5 | 17.3 | 5.1 | 7.8 | 8.9 | 0.3 | 0 |
| 6 | 18.7 | 10.1 | 25.3 | 5.3 | 6.1 | 6.5 | 0.1 | 0 |
| 7 | 29.8 | 43.0 | 24.0 | 37.9 | 2.4 | 1.2 | 0 | 0.1 |
| 8 | 26.8 | 71.4 | 19.1 | 65.1 | 1.3 | 0.1 | 0 | 0 |
| 9 | 15.6 | 89.4 | 19.3 | 83.1 | 2.1 | 1.2 | 0 | 0 |
| 10 | 16.0 | 87.7 | 22.0 | 81.2 | 1.2 | 0.4 | 0.2 | 0 |
| 11 | 16.7 | 35.7 | 15.8 | 42.2 | 1.3 | 1.8 | 0.1 | 0 |
| 12 | 8.6 | 44.9 | 7.3 | 34.3 | 0.9 | 1.2 | 0 | 0.1 |
| 13 | 21.3 | 35.6 | 24.0 | 36.1 | 1.3 | 0.8 | 0.1 | 0 |
| 14 | 11.3 | 29.3 | 12.7 | 32.6 | 5.5 | 2.1 | 0 | 0 |
| 15 | 19.4 | 83.5 | 7.1 | 52.6 | 1.3 | 0.7 | 0.3 | 0.2 |
| Average | 18.8 | 50.5 | 16.9 | 45.3 | 2.5 | 1.8 | 0.1 | 0.04 |
| Standard error | 5.9 | 25.3 | 6.1 | 23.3 | 2.1 | 2.4 | 0.1 | 0.07 |
TIL cultures were stimulated with phorbol 12-myristate 13-acetate(PMA)/inomycin for 4 h before fluorescence-activated cell sorting (FACS) analysis. IFN-γ, interferon gamma; TNFα, tumor necrosis factor-alpha; IL-4, interleukin-4.
EBV-specific reactivity of young TILs
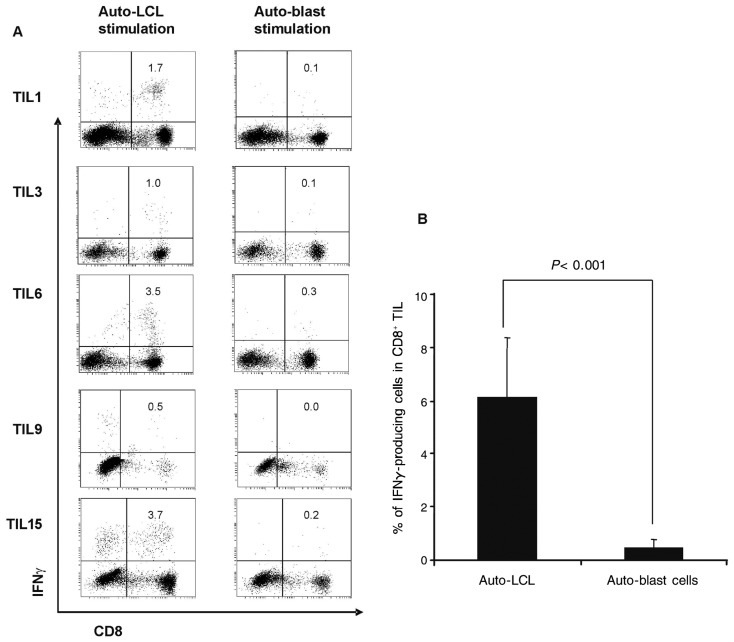
Next, we further examined the ability of young TILs to specifically recognize autologous EBV-positive target cells. IFNγ production after EBV antigen-specific stimulation and cytotoxic activity assay were performed to determine the recognition of auto-LCLs (EBV+) or auto-blast cells (EBV−) by TILs. The percentage of CD8+ IFNγ-producing TILs was evaluated by FACS analysis after auto-LCL or auto-blast cell stimulation (Figure 2A). The percentage of CD8+ IFNγ-producing TILs after auto-LCL antigenic stimulation was significantly higher than that after auto-blast cell non-antigenic stimulation (P < 0.001, Figure 2B).
Figure 2. TIL cultures expanded ex vivo produce high levels of Interferon gamma (IFN-γ) after EBV-positive lymphoblast cell line (LCL) stimulation compared to EBV-negative blast stimulation.
A, flow cytometric analysis of IFN7 release from TILs after 14 days of culture ex vivo and stimulation with autologous-LCLs or autologous-blast cells (effector:target = 3:1). B, average percentage of IFNγ-producing cells in expanded TIL culture (n = 15) stimulated with autologous-LCLs or autologous-blast cells.
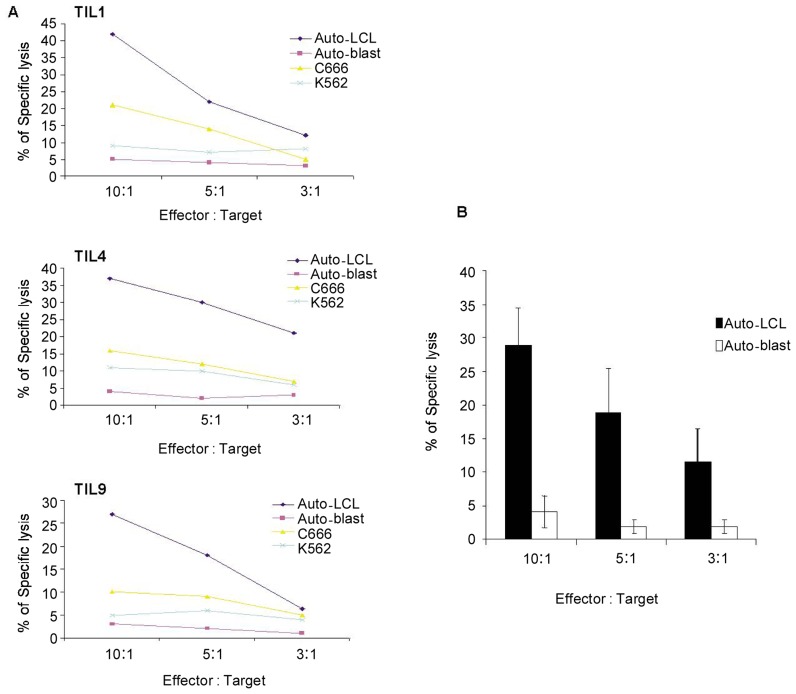
Furthermore, the presence of EBV-specific cytotoxic activity was investigated in 15 young TIL cultures. In concordance with the production of IFNγ stimulated by auto-LCLs, the level of specific lysis of TILs to auto-LCLs was approximately 30% at 10:1 (effector:target ratio), whereas the level of specific lysis of TILs to auto-blast cells was below 10% at any effector:target ratio (Figure 3). Interestingly, the level of specific lysis of TILs (TIL1, TIL4, and TIL9) to NPC cell line C666 (EBV+, not completely HLA-matched) was lower than that of auto-LCLs (EBV+, completely HLA matched) but higher than that of auto-blast cells (EBV−) and leukemia cell line K562 (EBV−), which was only recognized by NK cells for lack of major histocompatibility complex (Figure 3A). Collectively, our results indicated that TIL cultures from NPC patients have a stable EBV-specific cytotoxicity with major histocompatibility complex restriction and do not recognize EBV-negative cells.
Figure 3. EBV-specific cytotoxic activity of TIL cultures.
A, representative experiments illustrate the EBV-specific cytotoxic activity of TILs from three different NPC patients to target a panel of cells, including auto-LCL (EBV+), auto-blast cells (EBV−), NPC tumor cell line C666 (EBV+), and leukemia cell line K562 (EBV−). The effector cell and target cell panels were co-cultured at different ratios for 5 h, and target cells were labeled by 5- or 6-(N-Succinimidyloxycarbonyl)-3′,6′-O,O′-diacetylfluorescein (CFSE). The percentage of specific lysis of target cells was determined by 7-amino-actinomycin D (7-AAD) staining and fluorescence-activated cell sorting (FACS) detection. One of three experiments is shown in the figure. B, the average percentage of specific lysis of TILs to target cells, including auto-LCLs and auto-blast cells (n = 15), is shown in the figure. Error bars represent standard deviation.
Discussion
In this study, we established a new method to expand a large amount of young TILs from small NPC biopsy specimens ex vivo. These young TIL cultures had a high percentage of CD3+CD8+ and CD3+CD4+ T cells and low percentage of CD3−CD16+ T cells, The classical phenotype of EBV-CTL culture includes a high percentage of CD3+ T cells (>90%) and CD3+CD8+ T cells (>80%), as well as a low percentage of CD3+CD4+ T cells (<20%) and CD3−CD16+ NK cells (<10%)[20]–[22]. Our data showed that the lymphocyte composition of young TIL cultures in NPC was similar to EBV-CTL culture derived from PBMCs, with a high percentage of CD3+ T cells and low percentage of CD16+ NK cells. However, unlike EBV-CTL cultures from PBMCs, the percentage of CD4+ and CD8+ varied in TIL cultures. Interestingly, we observed in a recent study that a portion of EBV-CTL cultures from the PBMCs of NPC patients had a high percentage of CD3−CD16+ NK cells (20%-60%) and a low percentage of CD3+CD8+ T cells (<60% ), and these EBV-CTLs had a non-specific activity[23]. The young TIL cultures generated in the present study had a higher percentage of CD3+ T cells and a lower percentage of CD3−CD16+ NK cells, which may help to maintain the specific activity of TILs. Furthermore, these young TILs had stable EBV-specific activity and were easier to generate and expand in vitro compared to EBV-CTLs from PBMCs.
After performing LMP1 and LMP2 tetramer staining, we observed that TIL cultures also contained some LMP1 and LMP2 epitope antigen-specific CTLs[19]. Indeed, accumulation of EBV-specific T cells in tumor tissues has been demonstrated previously[13],[19],[23]. In the present study, the specific lysis of TILs to LCLs was approximately 30% at a ratio of 10:1. These results indicated that the young TIL cultures maintained a stable EBV-specific reactivity after expansion and reactivation ex vivo using a REP method. These data support that the rapid expansion of young TIL cultures ex vivo has not only expanded the T cell number but also reactivated the EBV-specific effector cells. On the other hand, our previous results showed that EBV-CTLs from the peripheral blood of NPC patients has a different pattern of cytotoxic activity, including pattern I, EBV-specific cytotoxicity, characterized by efficient lysis (≥ 25% at 10:1 R:S ratio) of the autologous and allogenic HLA class I-matched LCLs and no lysis of autologous PHA-stimulated blast cells or HLA class I-mismatched targets; pattern II, lymphokine-activated killer cell-type cytotoxicity, characterized by equal lysis of HLA class I-matched and -mismatched LCL; and pattern III, no cytotoxicity, where the specific lysis against all targets was ≤ 10% at 10:1 R:S ratio[19]. Moreover, 66% of EBV-CTL cultures from the PBMCs of NPC patients had a pattern I lysis, 20% had a pattern II lysis, and 13% had a pattern III lysis[23]. Compared to EBV-CTL cultures from peripheral blood, young TIL cultures all had a pattern I specific lysis in this study. Furthermore, we have also previously observed that the EBV-CTLs from PBMCs and TIL cultures in NPC have different EBV epitope antigen choice[19]. EBV-CTL cultures stimulated by auto-LCLs often contained a higher population of EBNA3A, EBNA3B, and EBNA3C antigen-specific T cells than LMP1 and LMP2 antigen-specific T cells[24],[25]. In contrast, EBV antigen-specific T cells in TILs homed to tumor tissues via their attraction to the EBV antigens, including EBNA1, LMP1, LMP2, and BARFO, which were presented by NPC cells in vivo. Therefore, TILs should contain a high concentration of NPC-recognized EBV antigen-specific T cells. As demonstrated in our study, young TIL cultures in NPC may have a stronger EBV-specific activity to auto-tumor cells when compared to EBV-CTL cultures from the PBMCs of NPC patients. The specific lysis of TILs to auto-tumor cells was not examined in this study because establishing individual primary tumor cell lines from NPC patients was difficult. The antigen-specific immune response of TIL cultures and the safety and efficacy of autologous immunotherapy with young TILs have been identified in phases I and II clinical trials in melanoma and other solid tumors[18],[26]–[31]. The findings presented here support further phase I/II clinical trials of immunotherapy with young TILs in patients with NPC in the near future. Young TIL-based immunotherapy can be reserved for the treatment of NPC patients from whom tumor biopsy tissues can be obtained, whereas EBV-specific CTL-based immunotherapy is a better choice for NPC patients from whom tumor biopsy tissues can not be obtained.
Establishing young TIL-based immunotherapy in NPC may have several potential advantages. First, the experimental method for establishing young TILs is simple and rapid, and TIL cultures can be successfully established for most NPC patients with tumor biopsy tissues. Second, the young TIL cultures have a low non-specific activity to HLA-mismatched cells because these cultures contain a high percentage of CD3+ T cells and a low percentage of CD3−CD16+ NK cells. Finally, young TIL cultures have a stable EBV-specific activity and contain a higher percentage of tumor-recognized EBV antigen-specific T cells compared to EBV-CTLs stimulated by auto-LCLs from peripheral blood. Collectively, our findings indicate that we have developed an appealing alternative method to establish EBV-CTL immunotherapy for NPC.
Acknowledgments
We thank members of Dr. Yi-Xin Zeng's team, Sun Yat-sen University Cancer Center, for helpful discussions. This work was supported by grants from the National Natural Science Foundation of China (No. 224-30872981) and Guangdong Province Natural Science Foundation (No. 10151008901000156).
References
- 1.Spano JP, Busson P, Atlan D, et al. et al. Nasopharyngeal carcinomas: an update. Eur J Cancer. 2003;39:2121–2135. doi: 10.1016/s0959-8049(03)00367-8. [DOI] [PubMed] [Google Scholar]
- 2.Chan B, Lee W, Hu CX, et al. et al. Adoptive cellular immunotherapy for non -small cell lung cancer: a pilot study. Cytotherapy. 2003;5:46–54. doi: 10.1080/14653240310000074. [DOI] [PubMed] [Google Scholar]
- 3.Dalgleish A, Whelan M. Novel immunotherapeutic approaches to prostate cancer. Curr Opin Mol Ther. 2005;7:30–34. [PubMed] [Google Scholar]
- 4.June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest. 2007;117:1466–1476. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santin AD, Bellone S, Roman JJ, et al. et al. Therapeutic vaccines for cervical cancer: dendritic cell-based immunotherapy. Curr Pharm Des. 2005;11:3485–3500. doi: 10.2174/138161205774414565. [DOI] [PubMed] [Google Scholar]
- 6.Griffin BE. Epstein-Barr virus (EBV) and human disease: facts, opinions and problems. Mutat Res. 2000;462:395–405. doi: 10.1016/s1383-5742(00)00028-4. [DOI] [PubMed] [Google Scholar]
- 7.Gross TG, Hilden JM, Manivel JC, et al. et al. Concurrent Epstein-Barr virus associated non-Hodgkin lymphoma and recurrent Hodgkin disease. J Pediatr Hematol Oncol. 1996;18:182–186. doi: 10.1097/00043426-199605000-00018. [DOI] [PubMed] [Google Scholar]
- 8.Jevon GP, Elavathil LJ, Harnish DG, et al. et al. Epstein-Barr virus in non-Hodgkin's lymphomas and lymphoid tissue in children. Pediatr Pathol Lab Med. 1995;15:283–290. doi: 10.3109/15513819509026963. [DOI] [PubMed] [Google Scholar]
- 9.Murray PG, Young LS. Epstein-Barr virus infection: basis of malignancy and potential for therapy. Expert Rev Mol Med. 2001;3:1–20. doi: 10.1017/S1462399401003842. [DOI] [PubMed] [Google Scholar]
- 10.Gottschalk S, Rooney CM, Heslop HE. Post-transplant lymphoproliferative disorders. Annu Rev Med. 2005;56:29–44. doi: 10.1146/annurev.med.56.082103.104727. [DOI] [PubMed] [Google Scholar]
- 11.Hsieh WS, Lemas MV, Ambinder RF. The biology of Epstein-Barr virus in post-transplant lymphoproliferative disease. Transpl Infect Dis. 1999;1:204–212. doi: 10.1034/j.1399-3062.1999.010308.x. [DOI] [PubMed] [Google Scholar]
- 12.Louis CU, Straathof K, Bollard CM, et al. et al. Enhancing the in vivo expansion of adoptively transferred EBV-specific CTL with lymphodepleting CD45 monoclonal antibodies in NPC patients. Blood. 2009;113:2442–2450. doi: 10.1182/blood-2008-05-157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merlo A, Turrini R, Dolcetti R, et al. et al. Adoptive cell therapy against EBV-related malignancies: a survey of clinical results. Expert Opin Biol Ther. 2008;8:1265–1294. doi: 10.1517/14712598.8.9.1265. [DOI] [PubMed] [Google Scholar]
- 14.Gottschalk S, Heslop HE, Rooney CM. Adoptive immunotherapy for EBV-associated malignancies. Leuk Lymphoma. 2005;46:1–10. doi: 10.1080/10428190400002202. [DOI] [PubMed] [Google Scholar]
- 15.Comoli P, De Palma R, Siena S, et al. et al. Adoptive transfer of allogeneic Epstein-Barr virus (EBV)-specific cytotoxic T cells with in vitro antitumor activity boosts LMP2-specific immune response in a patient with EBV-related nasopharyngeal carcinoma. Ann Oncol. 2004;15:113–117. doi: 10.1093/annonc/mdh027. [DOI] [PubMed] [Google Scholar]
- 16.Lutzky VP, Davis JE, Crooks P, et al. et al. Optimization of LMP-specific CTL expansion for potential adoptive immunotherapy in NPC patients. Immunol Cell Biol. 2009;87:481–488. doi: 10.1038/icb.2009.25. [DOI] [PubMed] [Google Scholar]
- 17.Tran KQ, Zhou J, Durflinger KH, et al. et al. Minimally cultured tumor-infiltrating lymphocytes display optimal characteristics for adoptive cell therapy. J Immunother. 2008;31:742–751. doi: 10.1097/CJI.0b013e31818403d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Itzhaki O, Hovav E, Ziporen Y, et al. et al. Establishment and large-scale expansion of minimally cultured “young” tumor infiltrating lymphocytes for adoptive transfer therapy. J Immunother. 2011;34:212–220. doi: 10.1097/CJI.0b013e318209c94c. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Zeng XH, Mo HY, et al. et al. Functional inactivation of EBV-specific T-lymphocytes in nasopharyngeal carcinoma: implications for tumor immunotherapy. PLoS One. 2007;2:e1122. doi: 10.1371/journal.pone.0001122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffmann T, Russell C, Vindelov L. Generation of EBV-specific CTLs suitable for adoptive immunotherapy of EBV-associated lymphoproliferative disease following allogeneic transplantation. APMIS. 2002;110:148–157. doi: 10.1034/j.1600-0463.2002.100205.x. [DOI] [PubMed] [Google Scholar]
- 21.Savoldo B, Goss J, Liu Z, et al. et al. Generation of autologous Epstein-Barr virus-specific cytotoxic T cells for adoptive immunotherapy in solid organ transplant recipients. Transplantation. 2001;72:1078–1086. doi: 10.1097/00007890-200109270-00017. [DOI] [PubMed] [Google Scholar]
- 22.Wilkie GM, Taylor C, Jones MM, et al. et al. Establishment and characterization of a bank of cytotoxic T lymphocytes for immunotherapy of Epstein-Barr virus-associated diseases. J Immunother. 2004;27:309–316. doi: 10.1097/00002371-200407000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Chen QY, Mo H, et al. et al. Immunophenotyping at the time of diagnosis distinguishes two groups of nasopharyngeal carcinoma patients: implications for adoptive immunotherapy. Int J Biol Sci. 2011;7:607–617. doi: 10.7150/ijbs.7.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khanna R, Busson P, Burrows SR, et al. et al. Molecular characterization of antigen-processing function in nasopharyngeal carcinoma (NPC): evidence for efficient presentation of Epstein-Barr virus cytotoxic T-cell epitopes by NPC cells. Cancer Res. 1998;58:310–314. [PubMed] [Google Scholar]
- 25.Taylor GS. T cell-based therapies for EBV-associated malignancies. Expert Opin Biol Ther. 2004;4:11–21. doi: 10.1517/14712598.4.1.11. [DOI] [PubMed] [Google Scholar]
- 26.Besser MJ, Shapira-Frommer R, Treves AJ, et al. et al. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2010;16:2646–2655. doi: 10.1158/1078-0432.CCR-10-0041. [DOI] [PubMed] [Google Scholar]
- 27.Dillman R, Schiltz P, DePriest C, et al. et al. Tumor-infiltrating lymphocytes and interleukin-2: dose and schedules of administration in the treatment of metastatic cancer. Cancer Biother Radiopharm. 2004;19:730–737. doi: 10.1089/cbr.2004.19.730. [DOI] [PubMed] [Google Scholar]
- 28.Huang J, Khong HT, Dudley ME, et al. et al. Survival, persistence, and progressive differentiation of adoptively transferred tumor-reactive T cells associated with tumor regression. J Immunother. 2005;28:258–267. doi: 10.1097/01.cji.0000158855.92792.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen LT, Yen PH, Nie J, et al. et al. Expansion and characterization of human melanoma tumor-infiltrating lymphocytes (TILs) PLoS One. 2010;5:e13940. doi: 10.1371/journal.pone.0013940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weber J, Atkins M, Hwu P, et al. et al. White paper on adoptive cell therapy for cancer with tumor-infiltrating lymphocytes: a report of the CTEP subcommittee on adoptive cell therapy. Clin Cancer Res. 2011;17:1664–1673. doi: 10.1158/1078-0432.CCR-10-2272. [DOI] [PubMed] [Google Scholar]
- 31.Zuliani T, David J, Bercegeay S, et al. et al. Value of large scale expansion of tumor infiltrating lymphocytes in a compartmentalised gas-permeable bag: interests for adoptive immunotherapy. J Transl Med. 2011;9:63. doi: 10.1186/1479-5876-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]