Abstract
Mantle cell lymphoma (MCL), a special type of non-Hodgkin's lymphoma, is incurable through conventional treatment. This study aimed to analyze the clinical features, therapeutic responses, and prognosis of patients with MCL. Clinical data of 30 patients with MCL treated in our hospital between April 2006 and July 2011 were analyzed. Eighteen patients were treated with CHOP plus rituximab (R-CHOP) regimen, 12 underwent conventional chemotherapy. The median age of the 30 patients was 58 years, 23 were men, all patients had Cyclin D1 overexpression, 29 (96.7%) had advanced disease, 11 (36.7%) had bone marrow involvement, 9 (30.0%) had gastrointestinal involvement, and 15 (50.0%) had splenomegaly. The complete response (CR) rate and overall response rate (ORR) were significantly higher in patients undergoing R-CHOP immunochemotherapy than in those undergoing conventional chemotherapy (38.9% vs. 16.7%, P = 0.187; 72.2% vs. 41.4%, P = 0.098). The difference of 2-year overall survival rate between the two groups was not significant (P = 0.807) due to the short follow-up time. The 2-year progression-free survival (PFS) rate was higher in R-CHOP group than in conventional chemotherapy group (53% vs. 25%, P = 0.083), and was higher in patients with a lower mantle cell lymphoma international prognostic index (MIPI) (51% for MIPI 0-3, 33% for MIPI 4-5, and 0% for MIPI 6-11, P = 0.059). Most patients with MCL were elderly; in an advanced stage; showed a male predominance; and usually had bone marrow involvement, gastrointestinal involvement, or splenomegaly. R-CHOP regimen could improve the CR rate and ORR of MCL patients. MIPI can be a new prognostic index for predicting the prognosis of advanced MCL.
Keywords: Mantle cell lymphoma, clinical features, therapeutic efficacy, prognosis
Mantle cell lymphoma (MCL) is a class of B-cell-originated non-Hodgkin's lymphoma (NHL) accounting for 5%–7% of total NHL. MCL is common in older men and is usually diagnosed at a late stage with extranodal involvement[1]. MCL has characteristics of both indolent and aggressive lymphomas. The first-line therapy of MCL is highly effective. The commonly used regimen, rituximab in combination with chemotherapy, shows a total efficiency of 75%–96%[1]. However, this regimen often results in chemoresistance or tumor recurrence[1]. A few patients can be cured by allogeneic hematopoietic stem cell transplantation, whereas most patients have poor prognosis, with a median overall survival (OS) of 4–6 years[2]. This study retrospectively analyzed the clinical features, treatment responses, and prognosis of patients with newly diagnosed MCL treated in our hospital.
Patients and Methods
Patient information
Clinical data of patients with untreated MCL diagnosed at Beijing Cancer Hospital between April 2006 and July 2011 according to the 2001 WHO Classification of Tumors of Hematopoietic and Lymphoid Tissue were collected. Before treatment, all patients underwent routine blood test, erythrocyte sedimentation rate (ESR) detection, serum lactate dehydrogenase (LDH) detection, serum β2-microglobulin (BMG) detection, ultrasound, bone marrow puncture, and chest, abdominal and pelvic CT examination. The patients who had failed to complete scheduled treatment were excluded from the analysis. Informed consent was signed by all patients to allow the use of their clinical data for research. This study was approved by the Institutional Review Board of Beijing Cancer Hospital.
Treatment
The patients underwent immunochemotherapy with R-CHOP regimen (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone), or conventional chemotherapy with CHOP regimen (cyclophosphamide, doxorubicin, vincristine, and prednisone), CHOPE regimen (cyclophosphamide, doxorubicin, vincristine, prednisone, and etoposide), or COP regimen (cyclophosphamide, vincristine, and prednisone) according to their disease status. Autologous stem cell transplantation was performed when appropriate.
Clinical response evaluation
Malignant lymphoma response evaluation criteria[3] were used to evaluate the short-term treatment response, which was classified as complete remission (CR), partial remission (PR), stable disease (SD), and progressive disease (PD). The overall response rate (ORR) was calculated as the percentage of CR plus PR.
Survival evaluation
OS was assessed from the date of diagnosis to the date of death for any reason or the last follow-up visit. Progression-free survival (PFS) was assessed from the date of diagnosis to the date of tumor progression discovery, death, or the last follow-up visit.
Statistical analysis
SPSS 16.0 software was used for statistical analysis. The Chi-square test was used to analyze the relationships between clinical factors and short-term responses. The Kaplan-Meier method and life table method were used to determine survival rate, and the log-rank test was used to compare survival rates. The difference was considered significant when P < 0.05.
Results
General information
Between April 2006 and July 2011, 32 patients were diagnosed with MCL. Among them, 1 refused treatment; 1 completed only one cycle of chemotherapy and was lost thereafter. Clinical data of 30 patients were included for the below analysis.
The age at tumor onset was 30–82 years, with a median of 58 years. Of the 30 patients, 18 (12 men and 6 women, 45–82 years old with a median of 58 years) underwent immunochemotherapy with R-CHOP regimen (median, 4.5 cycles); 12 underwent conventional chemotherapy, including 9 (8 men and 1 woman, 30–78 years old with a median of 55 years) with CHOP regimen (median, 2 cycles), 1 (man, 66 years old) with CHOPE regimen (2 cycles), and 2 (80 and 55 years old) with COP regimen (6 cycles) due to the old age or poor physical condition. Five patients underwent autologous stem cell transplantation: 3 in the R-CHOP group and 2 in the conventional chemotherapy group.
The patients were treated with 1–10 cycles of first-line chemotherapy, with a median of 4.5 cycles. Among them, 13 underwent 6–8 cycles of chemotherapy; 10 turned to second-line chemotherapy due to tumor progression or poor response to first-line chemotherapy; 7 completed 1–5 cycles of chemotherapy and achieved remission, but they either refused or were unable to continue further treatment due to adverse events. Clinical features of the 30 patients are summarized in Table 1.
Table 1. Relationships between clinical characteristics and treatment responses as well as 2-year PFS and OS rates of 30 patients with previously untreated mantle cell lymphoma.
| Variate | Total (cases) | Treatment response (cases) |
2-year survival rate (%) |
|||||||||
| CR | PR | SD | PD | ORR (%) | P | OS | P | PFS | P | |||
| Gender | 0.125 | 0.690 | ||||||||||
| Male | 23 | 6 | 6 | 4 | 7 | 52.2 | 80 | 40 | ||||
| Female | 7 | 3 | 3 | 0 | 1 | 85.7 | 16 | 0.140 | 49 | |||
| Age (years) | 0.410 | 0.149 | ||||||||||
| < 60 | 17 | 4 | 7 | 2 | 4 | 64.7 | 85 | 50 | ||||
| ≥ 60 | 13 | 5 | 2 | 2 | 4 | 53.8 | 51 | 0.049 | 30 | |||
| ECOG | 0.054 | 0.049 | ||||||||||
| < 2 | 27 | 9 | 9 | 3 | 6 | 66.7 | 76 | 46 | ||||
| ≥ 2 | 3 | 0 | 0 | 1 | 3 | 0 | 0 | <0.001 | 0 | |||
| Ann Arbor stage | 0.600 | 0.424 | ||||||||||
| I-II | 1 | 0 | 1 | 0 | 0 | 100 | 0 | 0 | ||||
| III-IV | 29 | 9 | 8 | 4 | 8 | 58.6 | 72 | 0.278 | 42 | |||
| B symptoms | 0.296 | 0.126 | ||||||||||
| Yes | 12 | 3 | 3 | 0 | 6 | 50 | 29 | 32 | ||||
| No | 18 | 6 | 6 | 4 | 2 | 66.7 | 87 | 0.015 | 49 | |||
| Bulky disease | 0.197 | 0.499 | ||||||||||
| Yes | 11 | 2 | 3 | 1 | 5 | 45.5 | 66 | 42 | ||||
| No | 19 | 7 | 6 | 3 | 3 | 68.4 | 72 | 0.054 | 42 | |||
| Extranodal sites | 0.588 | 0.698 | ||||||||||
| < 2 | 4 | 3 | 3 | 2 | 58.3 | 54 | 45 | |||||
| ≥ 2 | 5 | 6 | 1 | 6 | 61.1 | 80 | 0.209 | 39 | ||||
| Splenomegaly | 0.355 | 0.347 | ||||||||||
| Yes | 15 | 4 | 6 | 2 | 3 | 66.7 | 70 | 37 | ||||
| No | 15 | 5 | 3 | 2 | 5 | 53.3 | 64 | 0.634 | 46 | |||
| Bone marrow involvement | 0.245 | 0.762 | ||||||||||
| Yes | 4 | 4 | 2 | 1 | 72.7 | 61 | 42 | |||||
| No | 5 | 5 | 2 | 7 | 52.6 | 71 | 0.924 | 40 | ||||
| Gastrointestinal involvement | 0.528 | 0.611 | ||||||||||
| Yes | 9 | 3 | 2 | 0 | 4 | 55.6 | 87 | 42 | ||||
| No | 21 | 6 | 7 | 4 | 4 | 61.9 | 61 | 0.162 | 40 | |||
| WBC (×109/L) | 0.469 | 0753 | ||||||||||
| < 10 | 7 | 8 | 4 | 7 | 57.7 | 68 | 39 | |||||
| ≥ 10 | 2 | 1 | 0 | 1 | 75.0 | 67 | 0.726 | 50 | ||||
| LYM (×109/L) | 0.112 | 0.170 | ||||||||||
| < 5 | 26 | 6 | 8 | 4 | 8 | 53.8 | 68 | 34 | ||||
| ≥ 5 | 4 | 3 | 1 | 0 | 0 | 100 | 60 | 0.818 | 75 | |||
| HB (g/L) | 0.645 | 0.428 | ||||||||||
| < 120 | 4 | 5 | 1 | 5 | 60 | 58 | 28 | |||||
| ≥ 120 | 5 | 4 | 3 | 3 | 60 | 74 | 0.126 | 51 | ||||
| ALB (g/L) | 0.098 | 0.002 | ||||||||||
| < 40 | 12 | 2 | 3 | 1 | 6 | 41.7 | 48 | 0 | ||||
| ≥ 40 | 18 | 7 | 6 | 3 | 2 | 72.2 | 82 | 0.008 | 61 | |||
| LDH (IU/L) | 0.153 | 0.065 | ||||||||||
| < 240 | 24 | 7 | 9 | 2 | 6 | 66.7 | 86 | 49 | ||||
| ≥ 240 | 6 | 2 | 0 | 2 | 2 | 33.3 | 0 | <0.001 | 0 | |||
| BMG | 0.038 | 0.087 | ||||||||||
| Normal | 12 | 4 | 6 | 1 | 1 | 83.3 | 67 | 55 | ||||
| Elevated | 18 | 5 | 3 | 3 | 7 | 44.4 | 66 | 0.603 | 32 | |||
| ESR (mm/h) | 0.508 | 0.447 | ||||||||||
| < 20 | 11 | 3 | 4 | 1 | 3 | 63.6 | 100 | 51 | ||||
| ≥ 20 | 16 | 4 | 5 | 3 | 4 | 56.2 | 43 | 0.030 | 26 | |||
| NA | 3 | |||||||||||
| Ki-67 (%) | 0.383 | 0.511 | ||||||||||
| < 30 | 20 | 5 | 6 | 3 | 6 | 55.0 | 68 | 50 | ||||
| ≥ 30 | 7 | 2 | 3 | 1 | 1 | 71.4 | 70 | 0.698 | 24 | |||
| NA | 3 | |||||||||||
| Treatment regimen | 0.098 | 0.083 | ||||||||||
| R-CHOP | 18 | 7 | 6 | 2 | 3 | 72.7 | 59 | 53 | ||||
| Conventional chemotherapy | 12 | 2 | 3 | 2 | 5 | 41.7 | 72 | 0.807 | 25 | |||
| IPI score | 0.466 | 0.137 | ||||||||||
| < 3 | 19 | 5 | 7 | 2 | 5 | 63.2 | 88 | 50 | ||||
| ≥ 3 | 11 | 4 | 2 | 2 | 3 | 54.5 | 41 | 0.047 | 26 | |||
| MIPI score | 0.053 | 0.059 | ||||||||||
| 0-3 | 21 | 6 | 9 | 2 | 4 | 71.4 | 89 | 49 | ||||
| 4-5 | 6 | 3 | 0 | 1 | 2 | 50 | 48 | 33 | ||||
| 6-11 | 3 | 0 | 0 | 1 | 2 | 0 | 0 | <0.001 | 0 | |||
CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; ECOG, Eastern Cooperative Oncology Group; WBC, white blood cell; LYM, lymphocyte; HB, hemoglobin; ALB, albumin; LDH, lactate dehydrogenase; BMG, β2-microglobulin; ESR, erythrocyte sedimentation rate; NA, not available; IPI, international prognostic index; MIPI, mantle cell lymphoma international prognostic index.
Short-term treatment response
The treatment responses in the 30 patients were as follows: CR in 9 patients (30%), PR in 9 patients (30%), SD in 6 patients (20%), and PD in 6 patients (20%); the ORR was 60%. The CR rate and ORR were higher in the R-CHOP group than in the conventional chemotherapy group, but the difference was not significant (38.9% vs. 16.7%, P = 0.187; 72.2% vs. 41.7%, P = 0.098). Among various clinical factors, only the BMG level was associated with the short-term treatment response (P = 0.038) (Table 1).
Survival
All follow-ups ended in October 2011, with a median follow-up time of 23.2 months (2.8–66.7 months). Nine patients died: 4 in the R-CHOP group, and 5 in the conventional chemotherapy group.
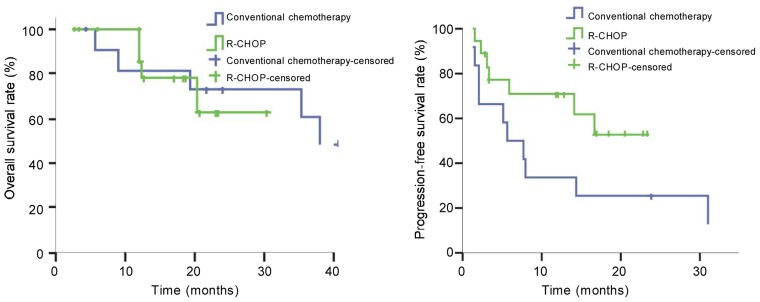
Of the 30 patients, the 1-, 2-, and 3-year OS rates were 89%, 67%, and 57%, respectively, with a median OS not reached. The 1- and 2-year OS rates were 93% and 59% in the R-CHOP group and were 82% and 72% in the conventional chemotherapy group. The difference was not significant (P = 0.807) (Figure 1A). The median OS was not reached in the R-CHOP group, but was 38.0 months in the conventional chemotherapy group.
Figure 1. Kaplan-Meier survival curves of patients with mantle cell lymphoma treated with R-CHOP regimen (n = 18) or conventional chemotherapy (n = 12). Both the overall survival (A) and the progression-free survival (B) are better in the R-CHOP group than in the conventional chemotherapy group, but the differences are not significant.
The 1- and 2-year PFS rates of the 30 patients were 55% and 41%, with a median PFS of 14.0 months. The R-CHOP group had a median follow-up of 18.3 months (range, 2.8–54.3 months), with 1- and 2-year PFS rates of 71% and 53%. The conventional chemotherapy group had a median follow-up of 41.9 months (range, 4.5-66.7 months), with 1- and 2-year PFS rates of 33% and 25%. The median PFS was not reached in the R-CHOP group, but was 5.8 months in the conventional chemotherapy group. No significant difference in PFS was observed between the two groups (P = 0.083) (Figure 1B).
Prognostic analysis
Univariate analysis showed that age (P = 0.049), ECOG performance status (P < 0.001), B symptoms (P = 0.015), serum albumin level (P = 0.008), serum LDH level (P < 0.001), and ESR (P = 0.030) were associated with 2-year OS rate and that ECOG performance status (P = 0.049) and serum albumin level (P = 0.002) were associated with 2-year PFS rate (Table 1).
Discussion
In the early 1990s, Raffeld et al.[4] and Banks et al.[5] adopted the term “mantle cell lymphoma” to describe a special subtype of small lymphocytic lymphoma (SLL) with t(11;14)(q13;32) mutation, which can induce Cyclin D1 overexpression. Up-regulated Cyclin D1 induces cells into a proliferating state from the resting phase. However, some cases of Cyclin D1-negative MCL without t (11; 14) (q13;32) mutation have been reported recently[6]. Identifying Cyclin D1-negative MCL is challenging in pathologic study. Ek et al.[7] found that the expression of SOX11 (a neuron transcription factor) was elevated in patients with MCL, including those with Cyclin D1-negative MCL, but was negative in normal lymphocytes and most other lymphomas. Therefore, they concluded that SOX11 could be used as a specific marker of MCL. In the present study, all 30 patients were Cyclin D1-positive.
MCL, which is more common in older men, is usually diagnosed at a late stage and often invades the bone marrow, gastrointestinal tract, spleen, and peripheral blood[1]. In this study, the clinical characteristics of our patients were consistent with available reports (Table 1). To date, there is no standard first-line therapy for MCL. A CHOP-like or more intense regimen tends to be selected as first-line treatment. The standard CHOP-based chemotherapy has an RR of 20%–50% and a low CR rate[8],[9]. In the present study, the RR of CHOP-like chemotherapy was 41.4% and the CR rate was 16.7%, both of which were consistent with previously reported results.
In Germany, a study comparing the efficacy of CHOP and R-CHOP regimens on previously untreated MCL found that rituximab increased the ORR from 75% to 94% and the CR rate from 7% to 34%, and prolonged the median PFS from 14 months to 28 months[10],[11]. In the present study, the CR rate and ORR were higher in the R-CHOP group than in the conventional chemotherapy group, but the difference was not significant (both P > 0.05). The 2-year PFS rate showed a similar pattern (P > 0.05). This discrepancy may be due to the nature of a retrospective study, the relatively small sample size, and short follow-up time.
Although rituximab can improve the short-term treatment effect and PFS in MCL patients, its effect on OS is still controversial. A retrospective study has found that rituximab combined with chemotherapy could significantly improve OS in MCL patients[12], whereas another study concluded the opposite[13]. In the present study, due to the short follow-up time, we did not find significant difference in the 2-year OS rate between the two groups (P = 0.807). After analyzing the clinical and pathologic information of 455 patients with late-stage MCL from three randomized clinical trials in Europe, Hoster et al.[14] developed a new MCL international prognostic index (MIPI), which included patient age, ECOG performance status, LDH level, and white blood cell count. Based on these parameters, late-stage MCL patients were divided into a low-risk group (44%, the median OS was not reached), an intermediate-risk group (35%, the median OS was 51 months), and a high-risk group (21%, the median OS was 29 months)[14]. In the present study, the 2-year PFS rate was associated with MIPI scores (P = 0.063). However, due to the small sample size, the low-risk group had much more patients than the high-risk group (21 vs. 3). Therefore, more patients and extended follow-up are needed to verify this conclusion.
In addition, our univariate analysis showed that the BMG level was associated with short-term treatment effect (P = 0.038), and ECOG performance status and serum albumin level were associated with the 2-year PFS rate (P = 0.049, P = 0.002). Age, ECOG performance status, B symptoms, serum albumin level, serum LDH level, and ESR were associated with the 2-year OS rate (all P > 0.05). However, these findings need to be verified by prospective trials with more patients.
Although rituximab in combination with CHOP chemotherapy can improve RR and prolong PFS, a complete cure cannot be obtained. New treatment regimens, especially regimens containing cytarabine, such as Hyper CVAD and Nordic regimens, are now under study. The regimens containing cytarabine can improve not only the short-term treatment effect but also PFS and OS. In combination with rituximab, these regimens have become a first-line therapy recommended by the National Comprehensive Cancer Network (NCCN). In addition, new drugs, such as bortezomib, bendamustine and mTOR inhibitors, are highly expected. The value of autologous hematopoietic stem cell transplantation in MCL treatment has gradually being recognized. However, the present study included only a few patients who underwent autologous stem cell transplantation and could not analyze its impact on the survival of MCL patients. MIPI is becoming increasingly important in predicting prognosis and guiding treatment of patients with late-stage MCL. Patients with different prognoses can choose regimens with different intensities. This study provides the basis to carry out more individualized treatment.
References
- 1.Goy A, Kahl B. Mantle cell lymphoma: the promise of new treatment options. Crit Rev Oncol Hematol. 2011;80:69–86. doi: 10.1016/j.critrevonc.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Chandran R, Gardiner SK, Simon M, et al. et al. Survival trends in mantle cell lymphoma in the United States over 16 years, 1992–2007. Leuk Lymphoma. 2012 Feb 13; doi: 10.3109/10428194.2012.656628. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Cheson BD, Pfistner B, Juweid ME, et al. et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 4.Raffeld M, Jaffe ES. bcl-1, t(11;14), and mantle cell-derived lymphomas. Blood. 1991;78:259–263. [PubMed] [Google Scholar]
- 5.Banks PM, Chan J, Cleary ML, et al. et al. Mantle cell lymphoma. A proposal for unification of morphologic, immunologic, and molecular data. Am J Surg Pathol. 1992;16:637–640. doi: 10.1097/00000478-199207000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Fu K, Weisenberger DD, Greiner TC, et al. et al. Cyclin D1-negative mantle cell lymphoma: a clinicopathologic study based on gene expression profiling. Blood. 2005;106:4315–21. doi: 10.1182/blood-2005-04-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ek S, Dictor M, Jerkeman M, et al. et al. Nuclear expression of the non B-cell lineage Sox11 transcription factor identifies mantle cell lymphoma. Blood. 2008;111:800–805. doi: 10.1182/blood-2007-06-093401. [DOI] [PubMed] [Google Scholar]
- 8.Bosch F, López-Guillermo A, Campo E, et al. et al. Mantle cell lymphoma: presenting features, response to therapy, and prognostic factors. Cancer. 1998;82:567–575. doi: 10.1002/(sici)1097-0142(19980201)82:3<567::aid-cncr20>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 9.Nickenig C, Dreyling M, Hoster E, et al. et al. Combined cyclophosphamide, vincristine, doxorubicin, and prednisone (CHOP) improves response rates but not survival and has lower hematologic toxicity compared with combined mitoxantrone, chlorambucil, and prednisone (MCP) in follicular and mantle cell lymphomas: results of a prospective randomized trial of the German Low-Grade Lymphoma Study Group. Cancer. 2006;107:1014–22. doi: 10.1002/cncr.22093. [DOI] [PubMed] [Google Scholar]
- 10.Lenz G, Dreyling M, Hoster E, et al. et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG) J Clin Oncol. 2005;23:1984–1992. doi: 10.1200/JCO.2005.08.133. [DOI] [PubMed] [Google Scholar]
- 11.Hoster E, Unterhalt M, Wormmann B, et al. et al. The addition of rituximab to first-line chemotherapy (RCHOP) results in superior response rates, time to treatment failure and response duration in patients with advanced stage mantle cell lymphoma: long term results of a randomized GLSG trial [abstract] Blood. 2008;112:16. [Google Scholar]
- 12.Dreyling MH, Griffiths R, Gleeson M, et al. et al. Improved overall survival after the addition of rituximab to chemotherapy alone as first-line therapy for mantle cell lymphoma (MCL): evidence from SEER-Medicare. J Clin Oncol. 2011;29:abstract e18500. doi: 10.1182/blood-2011-04-348367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balta A, Panitsas F, Skertsou M, et al. et al. First line treatment with anthracycline-based regimen with or without rituximab in patients with mantle cell lymphoma. Ann Oncol. 2011;22:iv220. [Google Scholar]
- 14.Hoster E, Dreyling M, Klapper W, et al. et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111:558–565. doi: 10.1182/blood-2007-06-095331. [DOI] [PubMed] [Google Scholar]