Abstract
A recently identified protein, FAN1 (FANCD2-associated nuclease 1, previously known as KIAA1018), is a novel nuclease associated with monoubiquitinated FANCD2 that is required for cellular resistance against DNA interstrand crosslinking (ICL) agents. The mechanisms of FAN1 regulation have not yet been explored. Here, we provide evidence that FAN1 is degraded during mitotic exit, suggesting that FAN1 may be a mitotic substrate of the anaphase-promoting cyclosome complex (APC/C). Indeed, Cdh1, but not Cdc20, was capable of regulating the protein level of FAN1 through the KEN box and the D-box. Moreover, the up- and down-regulation of FAN1 affected the progression to mitotic exit. Collectively, these data suggest that FAN1 may be a new mitotic substrate of APC/CCdh1 that plays a key role during mitotic exit.
Keywords: KIAA1018/FAN1, APC/C, Cdh1, cell cycle
DNA interstrand crosslinks (ICLs) are formed when bifunctional agents covalently link the two strands in a double helix. ICLs are toxic lesions that prevent strand separation, which is necessary for transcription and DNA replication. Forks that stall at ICLs recruit signaling complexes, including the Fanconi Anemia (FA) proteins and FA-associated proteins. FA is an inherited recessive condition characterized by developmental defects, skeletal abnormalities, bone marrow failure, and a predisposition to cancer[1],[2]. The FA pathway, which includes at least 13 known FA-associated genes, plays a role in repairing ICL and damaged DNA. Eight FA proteins comprise a multisubunit ubiquitin E3 ligase complex (referred to as the FA core complex). After DNA damage has occurred, the FA core complex adds a single molecule of ubiquitin to FANCD2, which is a key step in the activation of the pathway. FANCD2 then associates with other downstream proteins to promote the repair of damaged DNA[3],[4].
FANCD2-associated nuclease 1 (FAN1), previously known as KIAA1018, is a newly identified nuclease that contains an N-terminal ubiquitin zinc finger (UBZ) domain and a C-terminal nuclease domain[5]–[8]. Although FAN1 was discovered independently using different approaches, different groups collectively found that FAN1 possesses nuclease activity that is required for cellular resistance against ICL agents. FAN1 is recruited to the damaged DNA via the UBZ domain through associating specifically with monoubiquitinated FANCD2.
To date, most studies of FAN1 have focused on its role in regulating DNA ICLs during S phase. The FA pathway was thought to be largely inactivated during mitosis due to the absence of monoubiquitinated FANCD2, as determined by western blotting[9]. Mitotic phosphorylation and degradation of FANCM (Fanconi anemia, complementation group M), which leads to the release of the FA core complex, may be one mechanism for inhibiting FANCD2 monoubiquitination during mitosis[10],[11]. Because FAN1 associates rather specifically with monoubiquitinated FANCD2, which is absent during mitosis, the regulation and the role of FAN1 during mitosis have not yet been explored.
The anaphase-promoting cyclosome complex (APC/C), an E3 ubquitin ligase complex, plays crucial roles in mitosis and during G1 phase by degrading cell cycle-related proteins, such as Securin, Cyclins, Plk1, and Skp2[12]. The temporal cell cycle specificity of APC/C is achieved by the activation of two closely related proteins, Cdc20 and Cdh1, which facilitate the recruitment of substrates and ubiquitination by the core complex[13]–[15]. In early mitosis, APC/C binding to Cdc20 leads to the initiation of anaphase. In late mitosis, the association of APC/C with Cdh1 maintains APC/C activity throughout the subsequent G1 phase[13]–[20]. The switch from Cdc20 binding to Cdh1 binding by APC/C is induced by the inactivation of Cdk1, which also leads to destruction of Cdc20 mediated by Cdh1[21],[22]. The Cdc20-and Cdh1-bound forms of APC/C represent different substrate specificities. Cdc20 recognizes D-box-containing proteins[23], whereas Cdh1 recognizes proteins containing either D-box or KEN-box sequences[24]. Interestingly, both a KEN-box and a D-box motif present at the N-terminus of FAN1. The presence of these sequences in FAN1 prompted us to test whether FAN1 is regulated by APC/C and whether it plays a role in mitotic exit. As described below, FAN1 may be a new mitotic substrate of APC/CCdh1.
Materials and Methods
Cell lines
Human embryonic kidney (HEK) 293T and U20S cells were cultured in Dulbecco's modified Eagle's medium (DMEM, Invitrogen) supplemented with 10% fetal bovine serum (FBS, Hyclone), 1 mmol/L glutamine, and 100 U/mL each of penicillin and streptomycin. Cells were maintained at 37°C in a humidified atmosphere containing 5% CO2.
Plasmids
Wild-type SFB (S protein tag, Flag epitope tag, and Streptavidin-binding peptide tag)-FAN1 was a generous gift from Dr. Junjie Chen (University of Texas M.D. Anderson Cancer Center, USA). Human Myc-Cdh1 and Myc-Cdc20 have been previously described[25],[26]. Mutations were introduced using the MutanBEST site-directed mutagenesis kit (Takara) and were verified by DNA sequencing.
Antibodies
Human anti-Cdh1 antibody was obtained from Abeam. Human anti-phospho-histone H3 (Ser 10) antibody was obtained from Millipore (Cat. #06-570). Other primary antibodies used for western blotting, which included anti-Cdc20 (SC-13162), anti-Cyclin B1 (SC-245), anti-Skp2 (SC-2652), and anti-GAPDH antibodies (SC-166574), were obtained from Santa Cruz Biotechnology. Anti-FAN1 antibody was a gift from Dr. Jun Huang (School of Basic Medical Science, Zhejiang University, Hangzhou, China). Anti-Myc antibody (A-14 or M10E, Santa Cruz Biotechnology) and anti-Flag antibody (Sigma, F7425) were used to detect tagged proteins. Bound primary antibodies were detected with either horseradish peroxidase-conjugated anti-mouse IgG HRP or horseradish peroxidase-conjugated anti-rabbit HRP (Promega), and proteins were visualized by chemiluminescence.
Transfection experiments
Transfections were performed as previously described[25],[27]. Briefly, asynchronously growing cells were seeded at 2.5 × 105 cells per well in a 6-well tissue culture plate or at 1 × 106 cells per 100 mm in a tissue culture dish and were transfected with 2 µg or 12 µg plasmid DNA, respectively, with Lipofectamine™ 2000 (Invitrogen) according to the manufacturer's protocol.
RNA interference
Knockdown of FAN1 expression was accomplished using Smartpool siRNA (Dharmacon). Cdh1 and Cdc20 siRNAs were designed according to previously validated oligonucleotides and synthesized by Invitrogen[25],[28]. Approximately 2 × 105 U20S cells per well were seeded in a 6-well tissue culture dish the day before transfection. Transfections were performed according to the manufacturer's instructions using the Lipofectamine™ RNAiMAX transfection reagent (Invitrogen) and 100 nmol/L siRNA. At 48 h after transfection, cells were harvested in MCL buffer [50 mmol/L Tris-HCl pH 8.0, 2 mmol/L DTT, 5 mmol/L EDTA, 0.5% Nonidet P-40, 100 mmol/L NaCl, 1 mmol/L microcystin, 1 mmol/L sodium orthovanadate, 2 mmol/L phenylmethanesulfonyl fluoride (PMSF), 1 × protease (Sigma Chemical Co.) supplemented with a phosphatase inhibitor cocktail (Calbiochem)].
Protein stability experiments
To determine the half-life of FAN1, 20 µg/mL cycloheximide (CHX) (Sigma) was added to the cell culture to inhibit protein synthesis, and cells were harvested in MCLB at the indicated time intervals. To determine the effects of proteasome inhibitors on FAN1 protein stability, cells were incubated with 20 µmol/L MG132 (benzyloxy-carbonyl-Leu-Leu-Leu-aldehyde) 8 h before harvest.
Western blotting and immunoprecipitations
These procedures were performed as previously described. Briefly, cells were lysed in MCLB, and the purified lysates were resolved by SDS-PAGE and transferred to nitrocellulose membranes for western blotting. Proteins were detected with ECL detection reagents (Beyotime Co.). For immunoprecipitations, the purified supernatants were first incubated with anti-Myc antibody conjugated to agarose beads (Santa Cruz, SC-40AC) or anti-Flag antibody conjugated to agarose beads (Sigma Chemical Co. A4596) for 1 to 2 h at 4°C. Precipitates were washed 4 times with MCLB. To investigate the interaction between FAN1 and Cdh1 at the endogenous level, the purified supernatants were first incubated with anti-Cdh1 or negative control IgG antibodies for 2 h at 4°C. Then, protein A/G agarose beads were added overnight. Precipitates were washed 4 times with MCLB and analyzed by western blotting.
Synchronization of U2OS cells
This method was described previously[25],[28]. Briefly, U2OS cells were cultured in the presence of 100 ng/mL nocodazole for 16 h, and mitotic cells were isolated by shake off. Cells were washed twice with PBS and cultured for indicated time. Cells were collected and analyzed by flow cytometry, reverse transcription-polymerase chain reaction (RT-PCR), and western blotting.
Flow cytometry
This procedure was detailed previously[25],[28]. Briefly, cells were harvested by trypsinization and collected by centrifugation. Cells were washed once with PBS and fixed in 5 mL of 70% ethanol at 4°C. Cells were washed once with PBS/1% bovine serum albumin (BSA), and then incubated with 1 mL of PBS/1% BSA containing 30 µg/mL propidium iodide (PI) and 0.25 mg/mL RNase A for 1 h at room temperature. Cells were analyzed for DNA content by flow cytometry with a Cytomics FC 500 flow cytometer (Beckman). The data were analyzed using Multicycle AV for Windows (Beckman).
RNA extraction and RT-PCR
These procedures were performed as previously described[25],[27],[29]. Cells were collected at each indicated time point after nocodazole-block release. RNAs were extracted using Trizol (Invitrogen) according to the manufacturer's instructions. The primers used for amplifying FAN1 and GAPDH were as follows: FAN1-F, 5′-CAATGGGAACTGGGAAGAAG-3′; FAN1-R, 5′-GTGAAACACCGCAGGAAGAG-3′; GAPDH-F, 5′-ACAGTCAGCCGCATCTTCTT-3′; GAPDH-R, 5′-GACAAGCTTCCCGTTCTCAG-3′.
Results
FAN1 was regulated by Cdh1
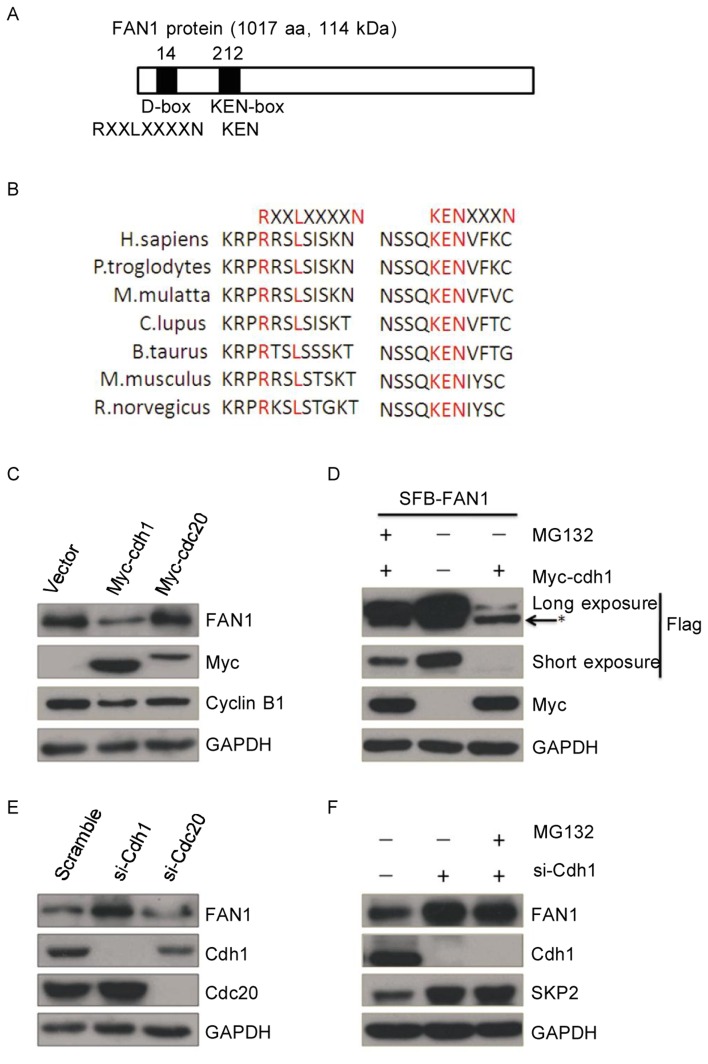
Cdc20- and Cdh1-activated APC/C plays key roles in mediating the ubiquitination-dependent destruction of many KEN-box- and D-box-containing proteins during mitotic exit. Interestingly, there is a D-box (aa 14-17) and a KEN-box (aa 212-214) in the FAN1 protein sequence (Figure 1A), and these two motifs are evolutionarily conserved in different species (Figure 1B). This promoted us to test whether FAN1 is a substrate of Cdh1 or Cdc20. As shown in Figure 1C, endogenous protein levels of FAN1 were significantly decreased in cells transiently transfected with Cdh1, but not with Cdc20. As expected[30],[31], Cyclin B1 protein expression was decreased in cells transiently transfected with either Cdh1 or Cdc20.
Figure 1. FAN1 is regulated by Cdh1.
A, a schematic presentation of FAN1 (a 1017-aa, 114-kDa protein) denoting the two putative degradation signals, including one D-box (RxxLxxxxN) and one KEN-box. The numbers along the top of this diagram denote the amino acid number at which the consensus sequences start in relation to the start codon. Conserved amino acids are indicated below the bar in bold print, and nonconserved residues are denoted by X. B, a schematic presentation of the KEN-box and D-box of KIAA1018/MTMR15/FAN1 orthologs from different species. The relevant protein identification codes are as follows: Homo sapiens NP_055782.3; Pan Troglodytes XP_510266.2; Macaca mulatta Xp_001109813.1; Canis lupus familiaris XP_856650.1; Bos taurus NP_001158142.1; Mus musculus NP_808561.2; and Rattus norvegicus XP_219706.4. C, U20S cells were transiently transfected with 2 µg of the indicated plasmids for 24 h and then subjected to western blotting. D, U20S cells transiently transfected with 2 µg of the indicated plasmids for 24 h were treated with or without MG132 for 8 h and then were subjected to western blotting. An asterisk (*) indicates a non-specific band. E, U2OS cells were transiently transfected with Cdh1 siRNA, Cdc20 siRNA, or scrambled siRNA for 48 h and then were subjec-ted to western blotting. F, U2OS cells were transiently transfected with Cdh1 siRNA or scrambled siRNA for 48 h, treated with or without MG132 for 8 h, and then subjected to western blotting.
To test whether the degradation of FAN1 is mediated by an APC/C-catalyzed ubiquitin-dependent proteasome pathway, cells were treated with MG132, a selective inhibitor of the proteasome, and FAN1 protein levels were analyzed. As shown in Figure 1D, MG132 treatment partially rescued the protein levels of exogenous FAN1, which were significantly decreased in the cells transiently transfected with Cdh1.
The endogenous protein levels of FAN1 were increased when cells were transfected with siRNA targeting Cdh1, but not Cdc20 (Figure. 1E). Levels of Cdh1 were not increased by the treatment of MG132 (Figure 1F). Skp2, a well known substrate of Cdh1, was used as the positive control. Taken together, Cdh1 was capable of down-regulating FAN1 protein through the proteasome pathway.
FAN1 interacted with Cdh1
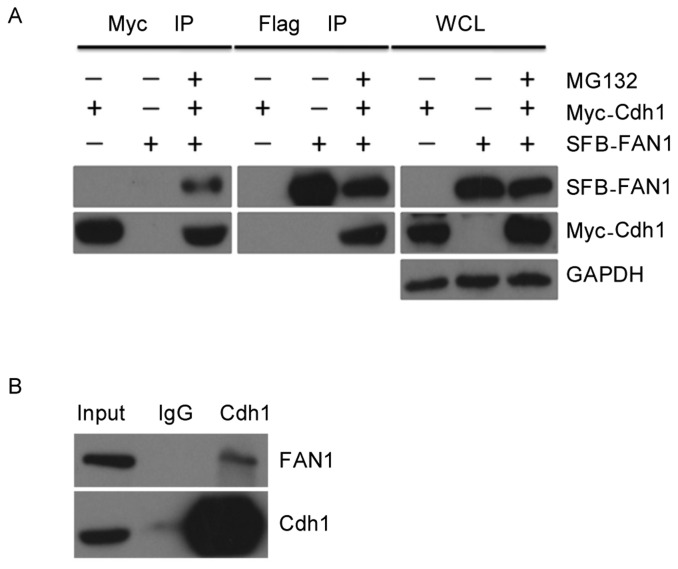
Next, we tested whether there was an interaction between FAN1 and Cdh1. Myc-tagged Cdh1 was co-transfected with SFB-tagged FAN1 into HEK-293T cells. Reciprocal co-immunoprecipitation (co-IP) using anti-Myc and anti-Flag conjugated agarose beads was performed. Because endogenous FAN1 protein levels were decreased when cells were co-transfected with Myc-tagged Cdh1, the cells were incubated with MG132 for 8 h prior to the cell harvest. As shown in Figure 2A, an interaction between these two proteins was clearly detected in cell lysates. This interaction was further confirmed at the endogenous level, as shown in Figure 2B. Endogenous FAN1 was clearly detected in immunoprecipitation reactions with an anti-Cdh1 antibody. These results indicate that FAN1 physically interacts with Cdh1 in cells.
Figure 2. FAN1 interacts with Cdh1.
A, Human embryonic kidney (HEK) 293T cells transfected with the indicated plasmids for 24 h were lysed with RIPA buffer, and underwent immunoprecipitation (IP) using anti-Myc or anti-Flag conjugated agarose beads. Analysis by western blotting was conducted with the indicated antibodies. WCL: whole cell lysate. B, U2OS cells were lysed with RIPA buffer, and lysates were subjected to IP using anti-Cdh1 or anti-IgG conjugated agarose beads followed by western blotting.
Both the KEN-box and the D-box were required for the degradation of FAN1
Considering that Cdh1 interacts with and down-regulates the expression of FAN1, we hypothesized that both the KEN-box and the D-box within FAN1 are responsible for its degradation. To test this hypothesis, we mutated conserved residues within the KEN-box and the D-box (Figure 3A). The half-life of mutated FAN1 was much longer than that of wild-type FAN1 (Figure 3B and 3C), indicating that Cdh1 down-regulates FAN1 through recognizing these two motifs.
Figure 3. Stability of wild-type FAN1 versus mutant FAN1.
A, summary of mutations in FAN1. B, U2OS cells transfected with wild-type FAN1 or mutant FAN1 for 24 h were treated with 20 µg/mL cycloheximide and collected at the indicated time points for western blotting. C, the densities of the FAN1 protein bands at each time point were normalized to GAPDH and were calculated into percentages using 1.0 as the value of the zero time point.
FAN1 was degraded during mitotic exit
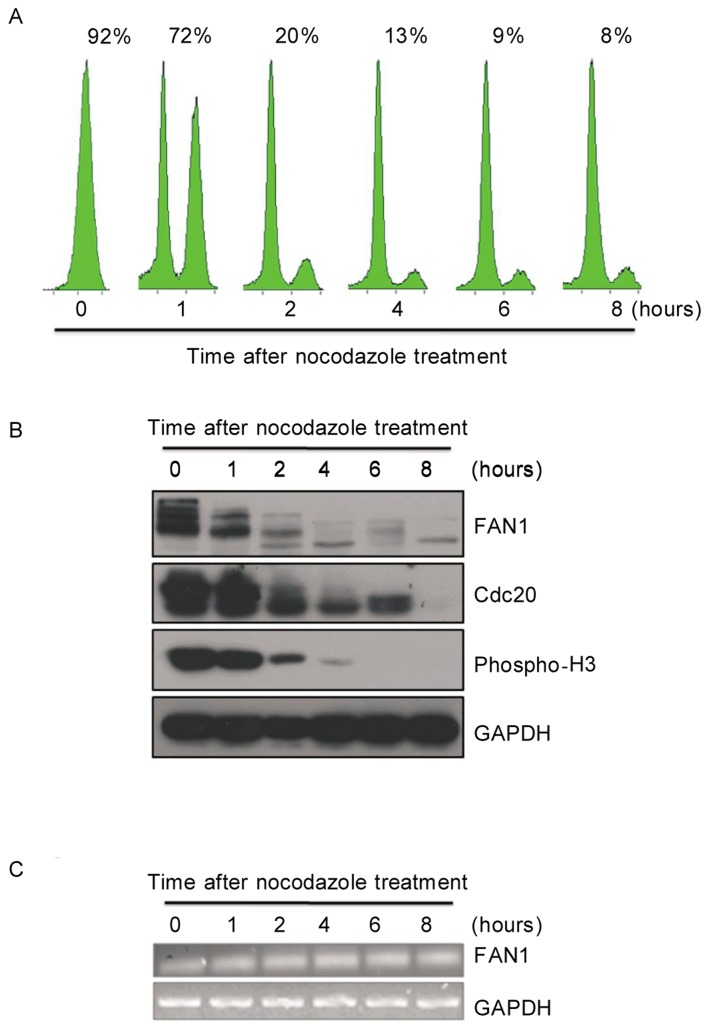
As APC/CCdh1 plays a key role in mitotic exit and G1 phase, we monitored FAN1 protein levels during mitotic exit. U2OS cells were synchronized at mitotic pro-metaphase by nocodazole, a microtubule polymerization inhibitor, and released at indicated time points. The cell cycle profile was monitored by flow cytometry (Figure 4A). The levels of FAN1 protein were analyzed, and it appeared that FAN1 was gradually degraded during the progression through mitotic exit (Figure 4B). This degradation of FAN1 during mitotic exit was similar to that of Cdc20, a well-known mitotic substrate of APC/CCdh1. As expected, the mRNA levels of FAN1 remained constant during mitotic exit (Figure 4C). Notably, multiple bands at different molecular weights were observed by western blotting for FAN1 in M phase, but not in G1 phase, indicating that FAN1 may be phosphorylated in M phase, which is common for mitotic substrates of APC/CCdh1[27]. These observations indicate that FAN1 may be a mitotic substrate of APC/CCdh1 during mitotic exit and G1 phase.
Figure 4. FAN1 is degraded during mitotic exit.
U2OS cells were synchronized in M phase by incubation with 100 ng/mL nocodazole for 16 h. M phase cells selected by shake-off were released at indicated time points. Some cells were collected for analysis by flow cytometry (A). The percentages of G2/M cells are provided. The rest cells were analyzed by western blotting (B) or RT-PCR (C) at indicated time points.
FAN1 might play a crucial role during mitotic exit
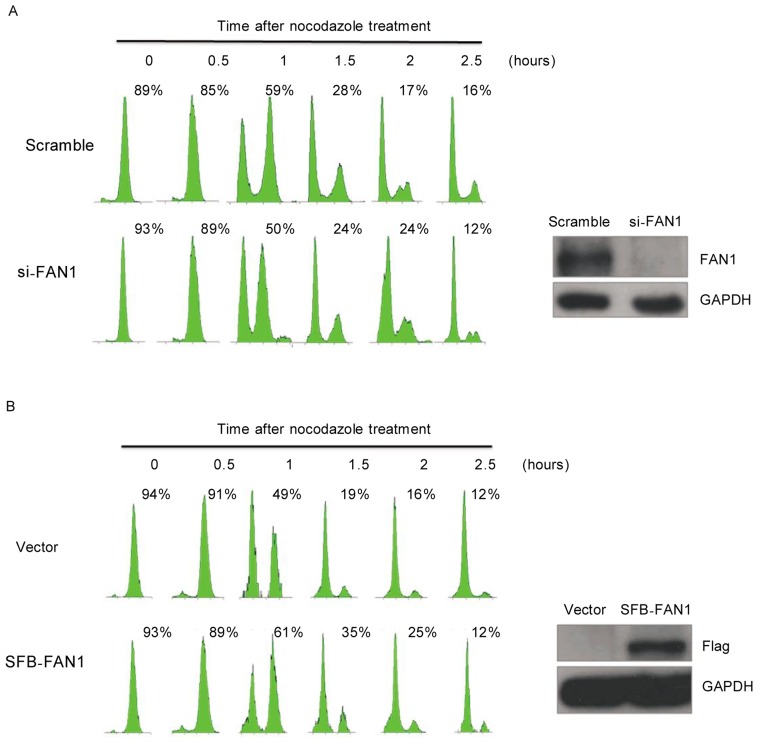
Because FAN1 is regulated by APC/CCdh1 and is degraded during the mitotic exit, we sought to determine whether FAN1 plays a role in the mitotic progression. U2OS cells treated with siRNAs targeting FAN1 or scrambled siRNA were synchronized in M phase using nocodazole and then released. As shown in Figure 5A, the mitotic exit was promoted in cells depleted of FAN1. Consistent with this observation, ectopic expression of FAN1 delayed mitotic exit in the cells (Figure 5B). Collectively, these data indicate that FAN1 may play a critical role during mitotic exit.
Figure 5. FAN1 may play a crucial role during mitotic exit.
U2OS cells were transfected with FAN1-siRNA or scrambled siRNA for 48 h (A) or with the indicated plasmids for 24 h (B). Then, the cells were synchronized in M phase by incubation with 100 ng/mL nocodazole for 16 h. M phase cells selected by shake-off were released at indicated time points. Cells were collected for analysis by flow cytometry (left) and western blotting (right). Note: The percentages of G2/M phase cells are indicated.
Discussion
In this study, we demonstrated that human FAN1 was degraded during mitotic exit and that APC/CCdh1 potentially mediated this degradation through interactions with FAN1. Thus, FAN1 may be a new mitotic substrate of APC/CCdh1 and play an important role in mitotic exit.
It has been shown that regulators of early mitosis are typically targeted for degradation by association with Cdc20 via a D-box motif, whereas Cdh1 targets regulators of late mitosis for degradation via D-box and KEN-box motifs. Interestingly, although there are both D-box and KEN-box sequences in FAN1, we demonstrated that only Cdh1 targets FAN1 for degradation. In agreement with these observations, Cdh1 was able to interact with FAN1 in vivo, and modulating FAN1 protein levels in cells altered the progression of mitotic exit.
Several studies have indicated that the FA pathway plays important roles in mitosis. FA-deficient cells exhibit increased chromosomal breaks at fragile sites, and the high incidence of these breaks may be linked to high cancer rates in FA patients[32],[33]. Here, we found that overexpression or depletion of FAN1, an FA-related protein, affected the progression of mitotic exit in U2OS cells. Therefore, it will be very interesting to investigate whether FAN1 has functional interactions with the FA pathway during mitosis to prevent chromosomal instability. As FAN1 specifically interacts with monoubi-quitinated FANCD2, which is absent in mitosis[9], it is possible that FAN1 may have additional functions during mitotic exit that are not related to the FA pathway. SNM1A, another nuclease, is also involved both in the DNA damage response and in an early mitotic prophase checkpoint in response to spindle stress[34],[35]. The exact function of FAN1 in mitotic exit remains to be further explored, and the identification of the physiologic substrate (s) of FAN1 during ICL will be the key to understanding the role of this nuclease.
The FAN1 protein contains 1017 amino acids and has a theoretical molecular weight of 114 kDa. However, we noticed that there were multiple bands observed by western blotting with higher molecular weights of approximately 130 kDa when U2OS cells were synchronized at mitotic phase, and there were fewer bands with lower molecular weights during progression through mitotic exit. This indicates that FAN1 may become phosphorylated in M phase, which is common for mitotic substrates of APC/CCdh1 [27]. In fact, sequence analysis using the NetPhos 2.0 computer program revealed several putative serine/threonine residues that are potentially phosphorylated by Cdks during M phase. Further research needs to be performed to elucidate the mechanisms of posttranslational regulation of FAN1 activity to shed light on the role of FAN1 in mitosis.
Acknowledgments
We thank Dr. Junjie Chen (University of Texas M.D. Anderson Cancer Center, USA) for providing SFB-FAN1 and Dr. Jun Huang (School of Basic Medical Science, Zhejiang University) for providing Anti-FAN1 antibody. This work was supported by grants from Natural Science Foundation of Guangdong Province (No. 10251008901000000 to T. K.), Ph.D. Program Foundation of Ministry of Education of China (No. 20100171110079 to T. K.), and China Postdoctoral Science Foundation (No. 20110490966).
References
- 1.Grompe M, D'Andrea A. Fanconi anemia and DNA repair. Hum Mol Genet. 2001;10:2253–2259. doi: 10.1093/hmg/10.20.2253. [DOI] [PubMed] [Google Scholar]
- 2.Joenje H, Patel KJ. The emerging genetic and molecular basis of fanconi anaemia. Nat Rev Genet. 2001;2:446–457. doi: 10.1038/35076590. [DOI] [PubMed] [Google Scholar]
- 3.D'Andrea AD, Grompe M. The fanconi anaemia/BRCA pathway. Nat Rev Cancer. 2003;3:23–34. doi: 10.1038/nrc970. [DOI] [PubMed] [Google Scholar]
- 4.Wang W. Emergence of a DNA-damage response network consisting of fanconi anaemia and BRCA proteins. Nat Rev Genet. 2007;8:735–748. doi: 10.1038/nrg2159. [DOI] [PubMed] [Google Scholar]
- 5.MacKay C, Délais AC, Lundin C, et al. et al. Identification of KIAA1018/FAN1, a DNA repair nuclease recruited to DNA damage by monoubiquitinated FANCD2. Cell. 2010;142:65–76. doi: 10.1016/j.cell.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kratz K, Schöpf B, Kaden S, et al. et al. Deficiency of FANCD2-associated nuclease KIAA1018/FAN1 sensitizes cells to interstrand crosslinking agents. Cell. 2010;142:77–88. doi: 10.1016/j.cell.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 7.Liu T, Ghosal G, Yuan J, et al. et al. FAN1 acts with FANCI-FANCD2 to promote DNA interstrand cross-link repair. Science. 2010;329:693–696. doi: 10.1126/science.1192656. [DOI] [PubMed] [Google Scholar]
- 8.Smogorzewska A, Desetty R, Saito TT, et al. et al. A genetic screen identifies FAN1, a fanconi anemia-associated nuclease necessary for DNA interstrand crosslink repair. Mol Cell. 2010;39:36–47. doi: 10.1016/j.molcel.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taniguchi T, Garcia-Higuera I, Andreassen PR, et al. et al. S-phase-specific interaction of the fanconi anemia protein, FANCD2, with BRCA1 and RAD51. Blood. 2002;100:2414–2420. doi: 10.1182/blood-2002-01-0278. [DOI] [PubMed] [Google Scholar]
- 10.Kim JM, Kee Y, Gurtan A, et al. et al. Cell cycle-dependent chromatin loading of the fanconi anemia core complex by FANCM/FAAP 24. Blood. 2008;111:5215–5222. doi: 10.1182/blood-2007-09-113092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kee Y, Kim JM, D'Andrea A. Regulated degradation of FANCM in the Fanconi anemia pathway during mitosis. Genes Dev. 2009;23:555–560. doi: 10.1101/gad.1761309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Acquaviva C, Pines J. The anaphase-promoting complex/cyclosome: APC/C. J Cell Sci. 2006;119:2401–2404. doi: 10.1242/jcs.02937. [DOI] [PubMed] [Google Scholar]
- 13.Schwab M, Lutum AS, Seufert W. Yeast Hct1 is a regulator of Clb2 cyclin proteolysis. Cell. 1997;90:683–693. doi: 10.1016/s0092-8674(00)80529-2. [DOI] [PubMed] [Google Scholar]
- 14.Sigrist SJ, Lehner CF. Drosophila fizzy-related down-regulates mitotic cyclins and is required for cell proliferation arrest and entry into endocycles. Cell. 1997;90:671–681. doi: 10.1016/s0092-8674(00)80528-0. [DOI] [PubMed] [Google Scholar]
- 15.Visintin R, Prinz S, Amon A. Cdc20 and Cdh1: a family of substrate-specific activators of APC-dependent proteolysis. Science. 1997;278:460–463. doi: 10.1126/science.278.5337.460. [DOI] [PubMed] [Google Scholar]
- 16.Kitamura K, Maekawa H, Shimoda C. Fission yeast Ste9, a homolog of Hct1/Cdh1 and Fizzy-related, is a novel negative regulator of cell cycle progression during G1-phase. Mol Biol Cell. 1998;9:1065–1080. doi: 10.1091/mbc.9.5.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blanco MA, Sanchez-Diaz A, de Prada JM, et al. et al. APCste9/srw1 promotes degradation of mitotic cyclins in G1 and is inhibited by cdc2 phosphorylation. EMBO J. 2000;19:3945–3955. doi: 10.1093/emboj/19.15.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bashir T, Dorrello NV, Amador V, et al. et al. Control of the SCFskp2−cks1ubiquitin ligase by the APC/Ccdh1 ubiquitin ligase. Nature. 2004;428:190–193. doi: 10.1038/nature02330. [DOI] [PubMed] [Google Scholar]
- 19.Wei W, Ayad NG, Wan Y, et al. et al. Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature. 2004;428:194–198. doi: 10.1038/nature02381. [DOI] [PubMed] [Google Scholar]
- 20.Buschhorn BA, Peters JM. How APC/C orders destruction. Nat Cell Biol. 2006;8:209–211. doi: 10.1038/ncb0306-209. [DOI] [PubMed] [Google Scholar]
- 21.Hagting A, den Elzen N, Vodermaier HC, et al. et al. Human securing proteolysis is controlled by the spindle checkpoint and reveals when the APC/C switches from activation by Cdc20 to Cdh1. J Cell Biol. 2002;157:1125–1137. doi: 10.1083/jcb.200111001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raff JW, Jeffers K, Huang JY. The roles of Fzy/Cdc20 and Fzr/Cdh1 in regulating the destruction of cyclin B in space and time. J Cell Biol. 2002;157:1139–1149. doi: 10.1083/jcb.200203035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang G, Yu H, Kirschner MW. Direct binding of CDC20 protein family members activates the anaphase-promoting complex in mitosis and G1. Mol Cell. 1998;2:163–171. doi: 10.1016/s1097-2765(00)80126-4. [DOI] [PubMed] [Google Scholar]
- 24.Pfleger CM, Kirschner MW. The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev. 2000;14:655–665. [PMC free article] [PubMed] [Google Scholar]
- 25.Lv XB, Xie F, Hu K, et al. et al. Damaged DNA-binding protein 1 (DDB1) interacts with Cdh1 and modulates the function of APC/Ccdh1. J Biol Chem. 2010;285:18234–18240. doi: 10.1074/jbc.M109.094144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu H, Cheng EHY, Hsieh JJD. Bimodal degradation of MLL by SCFskp2 and APCcdc20 assures cell cycle execution: a critical regulatory circuit lost in leukemogenic MLL fusions. Genes Dev. 2007;21:2385–2398. doi: 10.1101/gad.1574507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang T, Wei Y, Honaker Y, et al. et al. GSK-3β targets Cdc25A for ubiquitin-mediated proteolysis and GSK-3β inactivation correlates with Cdc25A overproduction in human cancers. Cancer Cell. 2008;13:36–47. doi: 10.1016/j.ccr.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Westhorpe FG, Tighe A, Lara-Gonzalez P, et al. et al. P31comet-mediated extraction of Mad2 from the MCC promotes efficient mitotic exit. J Cell Sci. 2011;124:3905–3916. doi: 10.1242/jcs.093286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu S, Feng Z, Zhang M, et al. et al. hSSB1 binds and protects p21 from ubiquitin-mediated degradation and positively correlates with p21 in human hepatocellular carcinomas. Oncogene. 2011;30:2219–2229. doi: 10.1038/onc.2010.596. [DOI] [PubMed] [Google Scholar]
- 30.Zhou Y, Ching YP, Ng RWM, et al. et al. Differential expression, localization and activity of two alternatively spliced isoforms of human APC regulator CDH1. Biochem J. 2003;374:349–358. doi: 10.1042/BJ20030600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geley S, Kramer E, Gieffers C, et al. et al. Anaphase-promoting complex/cyclosome-dependent proteolysis of human cyclin A starts at the beginning of mitosis and is not subject to the spindle assembly checkpoint. J Cell Biol. 2001;153:137–148. doi: 10.1083/jcb.153.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chan KL, Hickson ID. On the origins of ultra-fine anaphase bridges. Cell Cycle. 2009;8:3065–3066. doi: 10.4161/cc.8.19.9513. [DOI] [PubMed] [Google Scholar]
- 33.Howlett NG, Taniquchi T, Durkin SG, et al. et al. The Fanconi anemia pathway is required for the DNA replication stress response and for the regulation of common fragile site stability. Hum Mol Genet. 2005;14:693–701. doi: 10.1093/hmg/ddi065. [DOI] [PubMed] [Google Scholar]
- 34.Akhter S, Richie CT, Deng JM, et al. et al. Deficiency in SNM1 abolishes an early mitotic checkpoint induced by spindle stress. Mol Cell Biol. 2004;24:10448–10455. doi: 10.1128/MCB.24.23.10448-10455.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan Y, Akhter S, Zhang X, et al. et al. The multifunctional SNM1 gene family: not just nucleases. Future Oncol. 2010;6:1015–1029. doi: 10.2217/fon.10.47. [DOI] [PMC free article] [PubMed] [Google Scholar]