Abstract
Breast cancer is a major health problem that affects more than 24% of women in Bangladesh. Furthermore, among low-income countries including Bangladesh, individuals have a high risk for developing breast cancer. This study aimed to identify candidate mitochondrial DNA (mtDNA) biomarkers for breast cancer diagnosis in Bangladeshi women to be used as a preventive approach. We screened the blood samples from 24 breast cancer patients and 20 healthy controls to detect polymorphisms in the D-loop and the ND3- and ND4-coding regions of mtDNA by direct sequencing. Among 14 distinct mutations, 10 polymorphisms were found in the D-loop, 3 were found in the ND3-coding region, and 1 was found in the ND4-coding region. The frequency of two novel polymorphisms in the D-loop, one at position 16290 (T-ins) and the other at position 16293 (A-del), was higher in breast cancer patients than in control subjects (position 16290: odds ratio = 6.011, 95% confidence interval = 1.2482 to 28.8411, P = 0.002; position 16293: odds ratio = 5.6028, 95% confidence interval = 1.4357 to 21.8925, P = 0.010). We also observed one novel mutation in the ND3-coding region at position 10316 (A > G) in 69% of breast cancer patients but not in control subjects. The study suggests that two novel polymorphisms in the D-loop may be candidate biomarkers for breast cancer diagnosis in Bangladeshi women.
Keywords: Breast cancer, diagnosis, low-income country, biomarker, mtDNA
In Bangladesh, breast cancer burden is increasing due to lack of awareness and lack of population-based biomarkers for screening the disease. Approximately 24% of women suffer from breast cancer[1], which is the second leading cause of cancer mortality. Worldwide, it is estimated that more than a million women are diagnosed with breast cancer[2]. Cancer poses serious health problems both in developed and developing countries. The prevention and control of breast cancer in developing countries deserve urgent attention because the incidence of the disease is expected to double in these countries in the next 20 to 25 years[2]. The problem of breast cancer in Bangladesh is particularly acute because of poverty and lack of consciousness about health matter.
Cancer control means primary prevention, early detection (i.e. secondary prevention), treatment, follow-up, treatment of recurrence, and palliative care, including relief of pain for advanced and incurable cases. In other words, the real object of breast cancer control is to minimize the total impact of cancer by reducing mortality from breast cancer. Based on the World Health Statistics, new cancer cases in Bangladesh have been estimated at 167 per 100 000 populations[3]. In low-income countries like Bangladesh, breast cancer diagnosis is poor due to lack of advanced diagnostic tools, genetic markers, and inadequate resources; hence, women with breast cancer may not receive proper treatment and care. Other causes are poor health awareness and education, lack of early screening programs, lack of governmental support, and social barriers[4] Countries with the most developed breast cancer registries have documented an increasing trend: rates in Japan, Singapore and Korea have doubled in the last 35 years, and rates in China have increased >30% in the past decade[5]. The most common reasons for the global increase in breast cancer are social factors, including smoking, alcohol, poor diet, and obesity. In addition, many hormone-related risk factors like reduced breast feeding are responsible for breast cancer in low-income countries[6].
There are not sufficient data on breast cancer epidemiology in Bangladesh, and treatment is delayed due to lack of appropriate biomarker. Therefore, development of cost effective and rapid biomarker can be useful for early diagnosis of disease. In contrast, cancer mortality in Western countries decreases due to patient awareness and early disease diagnosis[7]. Recently, genetic markers, particularly mitochondrial DNA (mtDNA)-based markers, have drawn much attention for early diagnosis of primary breast cancer[8],[9]. Identification of such markers for breast cancer in Bangladeshi populations would facilitate early detection, and then reduce disease burden and increase survival rate.
Proper screening is also a necessary part of breast cancer prevention and early detection. However, for low-income countries, the cost of techniques such as mammography, ultrasonography, magnetic resonance imaging, and the like make them inaccessible, thereby rendering screening unfeasible. Thus, novel inexpensive alternatives are needed to allow implementation of screening in low-income countries. To address this need, we sought to determine if markers based on the mtDNA D-loop would be useful for inexpensive and easy screening of breast cancer.
mtDNA is a 16569 base pair, circular, maternally-inherited, double-stranded DNA encoding 13 genes for respiratory chain subunits. It represents <1.0% of total cellular DNA and contains no introns or histones[10]. The number of mitochondria and, therefore, content of mtDNA varies in different tissues. The mtDNA content can either increase or decrease in various cancers in comparison to control[11],[12]. Lack of histone proteins and the presence of reactive oxygen species in the mitochondrial matrix makes mtDNA prone to mutations[13]; the mutation frequency is 20 times greater than that of nuclear DNA[14]. In this study, we report the screening of breast cancer samples for polymorphisms in the mtDNA D-loop, a mutational “hot spot” in human breast cancer wherein there are two hypervariable sites, HVR-I (16024-16383) and HVR-II (57-372)[15]. This region regulates transcription and replication of mtDNA, and thus, instability in this region may be involved in carcinogenesis[16]. In recent years, mtDNA polymorphisms have drawn attention as diagnostic markers for early breast cancer detection[17]. With modern advancements in the sequencing technique, we have taken the opportunity to screen polymorphisms of mtDNA in samples from breast cancer patients and controls.
Materials and Methods
Sample collection
This study was approved by the local ethical committee of Bangladesh Medical Research Council (BMRC) and University of Dhaka. All patients were females and ranged in age from 33 to 65 years (median, 42 years). Twenty age-matched (range, 28–58 years; median, 44 years) healthy women from the mainstream population was also included. No data on the tumor size or lymph nodes were obtained. Both groups have the same ethnicity and nationality and reside in Bangladesh. Approximately 5 mL of blood sample was collected in EDTA-coated tubes from each patient who visited the hospital for treatment and 5 mL of blood was collected from each control. The samples were kept at -20°C until analyzed.
Reagents and instrument
All reagents for DNA isolation were purchased from Sigma Aldrich. Primers were designed and purchased from Applied Biosystems (USA). PCR components, Taq polymerase, MgCl2, and dNTPs were purchased from Eppendorf India Ltd. Sequencing reagents, POP7, 10 × buffer, and BDT v3.1 were obtained from Life Technologies (USA).
DNA isolation
Total DNA were isolated from the blood samples by standard proteinase K treatment followed by phenol/ chloroform/isoamyl alcohol extraction. DNA was precipitated with 0.3 mol/L sodium acetate in 70% ethanol at -20°C overnight and resuspended in Tris-EDTA (TE) buffer (pH 8.0). DNA quantification was performed with measurements at an absorbance of 260 nm[14]. Extracted DNA was tested using 0.8% agarose gel electrophoresis.
Primer sequences
The following overlapping primer sequences were used for amplification of mtDNA D-loop: 5′-TCATTGGACAAGTAGCATCC-3′ for 23F and 5′-GAGTGGTTAATAGGGTGATAG-3′ for 23R; 5′-CACCATTCTCCGTGAAATCA-3′ for 24F and 5′-AGGCTAAGCGTTTTGAGCTG-3′ for 24R. Two other primers used for ND3 and ND4 amplification were not shown.
PCR
Partial mitochondrial genome was amplified using four sets of primers, and the resultant amplicons were tested using 2% agarose gel electrophoresis. Primers were designed to amplify the D-loop, ND3, and ND4 regions. After PCR, gel electrophoresis was done along with 1 kb DNA ladder to check the amplification of distinct PCR products. PCR reactions (20 µL of total volume) contained 10-20 ng of DNA and 0.5 µmol/L primers, 0.2 mmol/L each deoxynucleotide triphosphate (dNTP), 1 U of TaqMan™ DNA polymerase, and 2.5 mmol/L MgCl2. PCR amplification of specific regions of mtDNA was performed with the following cycling conditions: initial denaturation at 95°C for 5 min, followed by 94°C for 30 s, annealing at 58°C for 30 s, and 72°C for 2 min for 35 cycles and final extension at 72°C for 7 min. The ABI-prism Big Dye Terminator V.1.1 containing ampliTaq polymerase, dye terminators (fluorescent label), dNTP, and MgCl2 was used for direct sequencing of PCR products for specific primers (forward/reverse primers). The sequencing PCR (cycle sequencing) was performed with following cycling conditions: initial denaturation at 95°C for 1 min, followed by 94°C for 10 s, annealing at 55°C for 30 s, and extension at 60°C for 4 min for 35 cycles.
Purification of amplicons
We used commercially available products for PCR product purification. A protocol for one such product, Centricon®-100 columns (P/N N930-2119), was given here, but in general, the manufacturer's protocol was followed for each commercial product.
Centricon ®-100 columns contain an ultrafiltration membrane that separates primers and dNTPs from larger PCR products. However, they may not work as well for short PCR products (<125 bases). To purify PCR fragments by ultrafiltration, the Centricon-100 column should be assembled as directed. Then 2 mL of deionized water is loaded onto the column, followed by the entire sample. The column is then centrifuged at 3000 × g in a fixed-angle centrifuge for 10 min. After removing the waste receptacle and attaching the collection vial, the column is then inverted and centrifuged at 270 × g for 2 min to collect the sample. This process should yield 40–60 µL of sample. Deionized water was then added to bring the purified PCR fragments to the original volume.
mtDNA sequence analysis
The purified sequencing PCR products were analyzed by electrophoresis in the ABI-Prism 3130 Genetic Analyzer (Applied Biosystems, USA). The sequence patterns were observed and edited using Auto Assembler V 3.0 and BioEdit Sequence Alignment Editor V 7.0.9.0 (http://vww.mbio.ncsu.edu/bioedit/bioedit.html). The sequences were aligned by using the bl2seq tool of NCBI (http://blast.ncbi.nlm.nih.gov/Blast.cgi) and compared with the revised Cambridge Reference Sequence, rCRS (NCBI reference sequence: NC_012920.1). mtDNA polymorphisms were compared with the mitochondrial genome database of world population by using Mitomap (www.mitomap.org). The haplogroup of cancer patients was identified by using mtDNA manager (www.mtmanager.yonsei.ac.kr).
Statistical analyses
Two-tailed, non-directional Fisher's exact tests were used to verify the mutation probability difference in populations with or without cancer (healthy controls)[18]. To confirm the results of these common tests, particularly in light of the expected low number in the healthy population, Yates's chi- and uncorrected chi-squared test (“N-1” chi-squared test) have been used because they are expected to have relatively low type I error. The analysis was performed as previously described[19].
To further understand the significance of 16290T-ins and 16293A-del polymorphisms, related risk factors and effectiveness of diagnostic criteria were assessed. The parameters and the confidence intervals for the estimated components were computed by using standard methods[20].
Results
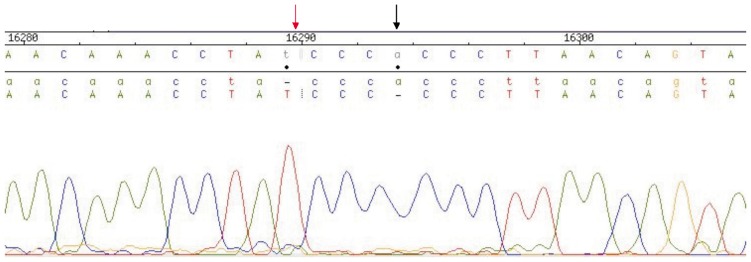
We sequenced the partial mitochondrial genome D-loop and two coding regions, ND3 and ND4, using blood samples collected from 24 breast cancer patients and 20 age-matched control individuals and identified more than one mutation in each patient. Among 14 distinct mutations, 6 had been reported in other studies and 8 had not been reported. Overall, 7 novel mutations in the D-loop (Table 1) and 1 in the ND3 region (Table 2) were identified in breast cancer patients. The most frequent mutations were found at nucleotide position (np) 16290 (T-ins) and np 16293 (A-del) (Figure 1), which respectively occurred in 95% and 75% of breast cancer patients, but only in 1 (5% ) control (Table 3). The 16290T-ins and 16293A-del SNPs have high sensitivity (0.75; 95% CI, 0.617 to 0.826) and specificity (0.7692; 95% CI, 0.649 to 0.769 ), with a subsequent diagnostic OR of 5.571 (95% CI, 1.549 to 20.042) and number needed to diagnose of 2.500 (95% CI, 1.592 to 9.333)[20],[21]. Mutation in the ND3 region at np 10316 (A>G) occurred in 69% of breast cancer patients but was absent in control subjects. Approximately 56% of mutations were homoplasmic, and 44% were heteroplasmic.
Table 1. Polymorphisms in the mtDNA D-loop Identified In breast cancer patients of Bangladesh.
| Position | Mutations | Codon | AA changes | Mitomap |
| 182 | C-ins | HVR-II | Non-coding | Novel |
| 319 | T-C | HVR-II | Non-coding | Reported in Ref. [28] |
| 315 | C-CC-ins(n) | HVR-II | Non-coding | Reported in Ref. [28] |
| 590 | A-ins | MT-TF | Non-coding | Novel |
| 600 | T-ins | MT-TF | Non-coding | Novel |
| 16093 | T>C | HVR-I | Non-coding | Reported in Ref. [22] |
| 16290 | T-ins | HVR-I | Non-coding | Novel |
| 16293 | A-del | HVR-I | Non-coding | Novel |
| 16260 | G-ins | HVR-I | Non-coding | Novel |
| 16268 | 2G-ins | HVR-I | Non-coding | Novel |
Table 2. Polymorphisms in the mtDNA coding regions in breast cancer patients of Banglades.
Figure 1. Two major polymorphisms in breast cancer patients of Bangladesh.
Red arrow shows 16290T-ins and black arrow shows 16293A-del.
Table 3. Statistical detail of D-loop polymorphisms In this case-control study.
| Allele | Cases (n = 24) | Controls (n = 20) | Odds ratio | 95% Confidence interval | P |
| 16290T-ins | 95% | 5% | 6.001 | 1.248-28.841 | 0.002 |
| 16293A-del | 75% | 5% | 5.603 | 1.436-21.892 | 0.010 |
Discussion
Over the past decade, a variety of approaches have been developed to improve the results of conventional cancer screening by detecting molecular marker in clinical samples. In this study, we present comprehensive data of 14 mtDNA mutations in the D-loop and in two coding regions in breast cancer patients of Bangladesh. six mutations were previously reported, and the remaining 8 are reported for the first time here. In the control region, the two most frequent polymorphisms in breast cancer patients occurred at np 16290T-ins and 16293A-del in 95% and 75% of cases, respectively, but only in <5% of control individuals. Our results oppose the previous findings that mtDNA mutations in breast cancer could not be detected in serum samples[22]. Recent survey reports revealed that most studies on mtDNA mutations in breast cancer focused on the D-loop and regions containing microsa-tellites[23],[24], which is consistent with the result in our study.
One novel mutation in ND3 region at np 10316 (A>G) was observed in 69% patients but absent in control subjects. The pathogenic role of this mutation in the ND3 subunit is unknown, but it may have adverse effects in ATP synthesis process. However, more extensive biochemical and molecular studies will be essential to determine the effect of this single novel mutation on energy metabolism. The D-loop alteration may interfere with the DNA sequence in the promoter region and thereby affect the rate of transcription or replication of mtDNA. Thus, mutations in this region may contribute to mtDNA depletion in cancer.
This study shows that 56% of the detected mutations were homoplasmic. However, previous studies have reported that most pathogenic mutations are heteroplasmic, whereas homoplasmic mutations are often benign polymorphisms[24]. Long-term accumulation of homoplasmic polymorphisms may eventually result in changes in the oxidative phosphoralytion (OXPOS) system. Homoplasmic mutations have also been confirmed to occur in cancerous lesions, indicating that mutant mtDNA becomes dominant in tumor cells[25],[26]. Thus, in late onset of a disease like breast cancer, mtDNA polymorphisms can potentially play a role in modifying the risk of cancer[26].
Taken together, these observations provide evidence that mitochondrial dysfunction is a factor in cancer etiology, an insight that may suggest new approaches for diagnosis and treatment. Our results revealed that polymorphisms were more frequent in the D-loop than in coding regions of mtDNA in breast cancer patients. Thus, these D-loop polymorphisms can be considered candidate biomarkers for breast cancer diagnosis in Bangladeshi patients. The present study was unable to sufficiently substantiate detecting and tracking breast cancer by screening mtDNA mutations via analysis of body fluid; screening via analysis of tumor tissue and benign tissue would be more practical.
The results of this study therefore suggest continued sequencing of various types of early-stage breast tumor and normal tissues to investigate mtDNA mutations for potential diagnostic marker development. Further, complete mtDNA genome sequencing studies can be used to determine the haplogroups of patients, which would be useful for diagnosis of high-risk individuals.
Acknowledgments
This work was supported by grants from the Centre for Advanced Research in Sciences (CARS), University of Dhaka. The authors also thank Drs. Zakaria Shamim and Bacchu (Enam Medical College Hospital) for collecting blood samples. We specially thank patients for their kind cooperation and donation of blood samples.
References
- 1.El Saghir NS, Khalil MK, Eid T, et al. et al. Trends in epidemiology and management of breast cancer in developing Arab countries: a literature and registry analysis. Int J Surg. 2007;5:225–233. doi: 10.1016/j.ijsu.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 2.Welcsh PL, King MC. BRCA1 and BRCA2 and the genetics of breast and ovarian cancer. Hum Mol Genet. 2001;10:705–713. doi: 10.1093/hmg/10.7.705. [DOI] [PubMed] [Google Scholar]
- 3.Curado PM, Edwards B, Shin HR. Lyon, France: International Agency for Research on Cancer; 2007. Cancer incidence in five continents; pp. 223–243. [Google Scholar]
- 4.The Lancet Breast cancer in developing countries. Lancet. 2009;374:1567. doi: 10.1016/S0140-6736(09)61930-9. [DOI] [PubMed] [Google Scholar]
- 5.Colditz GA, Rosner BA, Chen WY, et al. et al. Risk factors for breast cancer according to estrogen and progesterone receptor status. J Natl Cancer Inst. 2004;96:218–228. doi: 10.1093/jnci/djh025. [DOI] [PubMed] [Google Scholar]
- 6.Ezzat AA, Ibrahim EM, Raja MA, et al. et al. Locally advanced breast cancer in Saudi Arabia: high frequency of stage III in young population. Med Oncol. 1999;16:95–103. doi: 10.1007/BF02785842. [DOI] [PubMed] [Google Scholar]
- 7.Foulkes WD, Narod SA. Hereditary breast and ovarian cancer: epidemiology, genetics, screening and predictive testing. Clin Invest Med. 1995;18:473–483. [PubMed] [Google Scholar]
- 8.Nomoto S, Yamashita K, Koshikawa K, et al. et al. Mitochondrial D-loop mutations as clonal markers in multicentric hepatocellular carcinoma and plasma. Clin Cancer Res. 2002;8:481–487. [PubMed] [Google Scholar]
- 9.Porter P. “Westernizing” women's risk? Breast cancer in lower-income countries. New Eng J Med. 2008;358:213–216. doi: 10.1056/NEJMp0708307. [DOI] [PubMed] [Google Scholar]
- 10.Anderson S, Bankier AT, Barrell BG, et al. et al. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 11.Lee YS, Kennedy WD, Yin YW. Structural insight into processive human mitochondrial DNA synthesis and disease-related polymerase mutations. Cell. 2009;139:312–324. doi: 10.1016/j.cell.2009.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brandon M, Baldi P, Wallace DC. Mitochondrial mutations in cancer. Oncology. 2006;25:4647–4662. doi: 10.1038/sj.onc.1209607. [DOI] [PubMed] [Google Scholar]
- 13.Vives-Bauza C, Gonzalo R, Manfredi G, et al. et al. Enhanced ROS production and antioxidant defenses in cybrids harbouring mutations in mtDNA. Neurosci Lett. 2006;391:136–141. doi: 10.1016/j.neulet.2005.08.049. [DOI] [PubMed] [Google Scholar]
- 14.Ishikawa K, Takenaga K, Akimoto M, et al. et al. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science. 2008;320:661–664. doi: 10.1126/science.1156906. [DOI] [PubMed] [Google Scholar]
- 15.Stoneking M, Hedgecock D, Higuchi RG, et al. et al. Population variation of human mitochondrial DNA control region sequences detected by enzymatic amplification and sequence-specific oligonucleotide probes. Am J Hum Genet. 1991;48:370–382. [PMC free article] [PubMed] [Google Scholar]
- 16.Mims MP, Hayes TG, Zheng S, et al. et al. Mitochondrial DNA G10398A polymorphism and invasive breast cancer in African-American women. Cancer Res. 2006;66:1880–1881. doi: 10.1158/0008-5472.CAN-05-3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sidransky D. Emerging molecular markers of cancer. Nat Rev Cancer. 2002;2:210–219. doi: 10.1038/nrc755. [DOI] [PubMed] [Google Scholar]
- 18.Fleiss JL, Bruce L, Cho Paik M, et al. et al. San Francisco, USA: Wiley-Interscience Inc; 2003. Statistical methods for rates and proportions; pp. 17–49. [Google Scholar]
- 19.Sultana GNN, Rahman A, Karim MM, et al. et al. Breast cancer risk associated mitochondrial NADH-dehydrogenase subunit-3 (ND3) polymorphisms (G10398A and T10400C) in Bangladeshi women. J Med Genet. 2011;3:131–135. [Google Scholar]
- 20.Campbell I. Chi-squared and Fisher-Irwin tests of two-by-two tables with small sample recommendations. Stat Med. 2007;26:3661–3675. doi: 10.1002/sim.2832. [DOI] [PubMed] [Google Scholar]
- 21.Sheskin DJ. Boca Raton: Chapman & Hall; 2007. Handbook of parametric and non parametric statistical procedures; p. 229. [Google Scholar]
- 22.Cai FF, Kohler C, Zhang B, et al. et al. Mutations of mitochondrial DNA as potential biomarkers in breast cancer. Anticancer Res. 2011;31:4267–4271. [PubMed] [Google Scholar]
- 23.Canter JA, Kallianpur AR, Pari FF, et al. et al. Mitochondrial DNA G10398A polymorphism and invasive breast cancer in African-American women. Cancer Res. 2005;65:8028–8033. doi: 10.1158/0008-5472.CAN-05-1428. [DOI] [PubMed] [Google Scholar]
- 24.Yeh JJ, Lunetta KL, van Orsouw NJ, et al. et al. Somatic mitochondrial DNA (mtDNA) mutations in papillary thyroid carcinomas and differential mtDNA sequence variants in cases with thyroid tumors. Oncogene. 2000;19:2060–2066. doi: 10.1038/sj.onc.1203537. [DOI] [PubMed] [Google Scholar]
- 25.Osama O, Kenji H, Takanori U, et al. et al. Detection of mitochondrial DNA alteration in the serum of hepatocellular carcinoma patients. Clin Cancer Res. 2002;8:2875–2878. [PubMed] [Google Scholar]
- 26.Yin PH, Lee HC, Chau GY. Alteration of the copy number and deletion of mitochondrial DNA in human hepatocellular carcinoma. Br J Cancer. 2004;90:2390–2396. doi: 10.1038/sj.bjc.6601838. [DOI] [PMC free article] [PubMed] [Google Scholar]