Abstract
Biallelic inactivation of fumarate hydratase (FH) causes type 2 papillary renal cell carcinoma (PRCC2), uterine fibroids, and cutaneous leimyomas, a condition known as hereditary leiomyomatosis and renal cell cancer (HLRCC). The most direct effect of FH inactivation is intracellular fumarate accumulation. A majority of studies on FH inactivation over the past decade have focused on the theory that intracellular fumarate stabilizes hypoxia-inducible factor 1α (HIF1A) through competitive inhibition of HIF prolyl hydroxylases. Recently, a competing theory that intracellular fumarate activates nuclear factor (erythroid-derived 2)-like 2 (NRF2) through post-translational modification of its negative regulator. Kelch-like ECH-associated protein 1 (KEAP1) has emerged from a computational modeling study and mouse model studies. This review dissects the origin of these two governing theories and highlights the presence of chromatin-structure-regulated targets of transcription factors, which we refer to as “cryptic targets” of transcription factors. One such cryptic target is heme oxygenase I (HMOX1), the expression of which is known to be modulated by the gene product of SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily a, member 4 (SMARCA4, also known as BRG1).
Keywords: HLRCC, fumarate hydratase, HIF1A, NRF2, renal cancer
Hereditary leiomyomatosis and renal cell carcinoma (HLRCC) is a familial cancer syndrome that is characterized by increased frequency of skin leiomyoma, uterine fibroids, and renal cancer in affected families[1]. The associated renal cancer is commonly described as a very aggressive subtype known as type 2 papillary renal cell carcinoma (PRCC2)[2]. This type of renal cancer is usually metastatic at presentation and is refractory to all reported radiation therapies, chemotherapies, and molecularly targeted therapies. Consequently, PRCC2 is the major cause of mortality in HLRCC patients[2].
The gene associated with HLRCC is fumarate hydratase (FH)[3]. FH encodes a TCA cycle enzyme that catalyzes the hydration of fumarate to form malate (Figure 1). Patients with HLRCC carry a loss-of-function FH allele that is usually inherited from an afflicted parent[3]. The remaining functional copy of FH is always lost (or inactivated) in disease tissues, suggesting that complete FH inactivation is the cause of HLRCC-related complications.
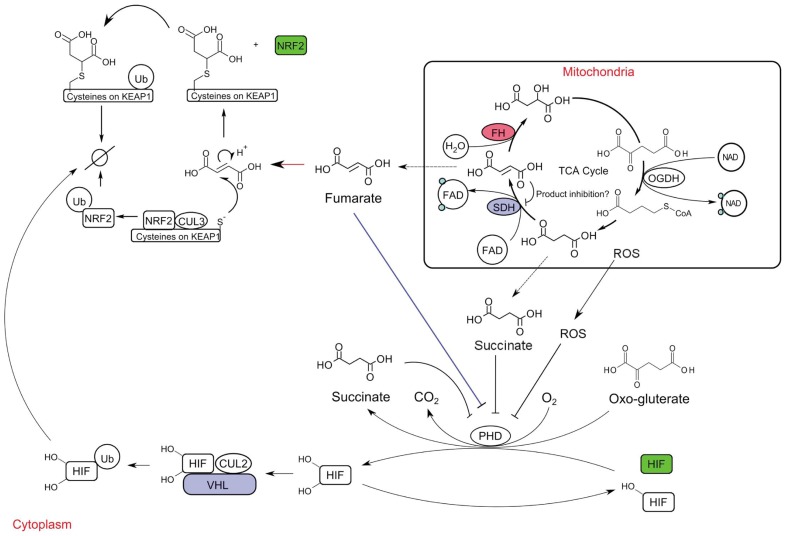
Figure 1. Consequences of FH inactivation.
The FH protein is a TCA cycle enzyme that catalyzes the hydration of fumarate to form malate. When FH function is impared (red circle), fumarate will accumulate and be pumped out of the mitochondria into the cytosol. The HIF1A activation theory states that cytosolic fumarate can inhibit HIF prolyl hydroxylase (blue line), resulting in HIF1A accumulation and activation (protein accumulation and activation are shown by green rectangle). The NRF2 activation theory states that cytosolic fumarate can undergo a Michael's addition reaction with the sulfhydryl group of cysteine residues on the surface of KEAP1 (red arrow). This prevents KEAP1 from binding to NRF2 and results in NRF2 accumulation and activation. Succinate also accumulates in FH-inactivated cells. The mechanism of succinate accumulation in FH-inactivated cells has not been studied, but the accumulated fumarate may inhibit SDH. The cytosolic succinate may inhibit HIF prolyl hydroxylase, giving rise to the observed phenotype of HIF accumulation that is the focus of the majority of HLRCC research. SDH and VHL mutations (violet circle and rectangle, respectively) give rise to the same spectrum of tumors (paraganglioma, pheochromocytoma, clear cell renal cell carcinoma). These tumors share HIF activation as the main biochemical phenotype.
The fundamental mechanism by which FH inactivation leads to renal tumor formation in HLRCC-afflicted individuals is not well established. The most studied theory posits that FH inactivation causes activation of the hypoxia-inducible factor (HIF) by indirectly inducing the expression of its a subunit (HIFα) (Figure 1)[4],[5]. More recently, a new theory explaining the fundamental mechanism of FH inactivation in tumor formation has arisen. This new theory proposes that the activation of nuclear factor (erythroid-derived 2)-like 2 (NRF2) is the main consequence of FH inactivation (Figure 1)[6],[7]. In this review, we describe the two theories behind the biochemical consequences of FH inactivation and propose a hypothesis on the tumorigenic mechanism of FH inactivation.
The HIF1A Activation Theory
FH catalyzes the hydration of fumarate to form malate in the TCA cycle. In cells devoid of FH function, fumarate can accumulate to approximately 200 times the normal level[4]. In addition, in FH-mutated cells, succinate can accumulate to approximately 15 times the normal level[4]. The reason for fumarate accumulation is intuitively apparent: FH is the enzyme that converts it into malate and ensures the continuation of the TCA cycle. The accumulation of succinate, however, has yet to be investigated. One possible explanation is product inhibition, which happen when the activity of succinate dehydrogenase (SDH) is inhibited by fumarate.
Accumulation of TCA metabolites has measurable and direct consequences in cells and tissues[8]. Accumulation of succinate in cells harboring SDH mutations results in the activation of HIFα[9]. Succinate is purportedly a competitive inhibitor of HIF prolyl hydroxylases, which use oxoglutarate to hydroxylate HIFα, marking HIFα proteins for ubiquitination by the Von Hippel-Lindau (VHL) complex[9]. Excessive succinate prevents the hydroxylation reaction, causing HIFα to accumulate. Likewise, loss of VHL function prevents proper formation of the ubiquitination complex, resulting in HIFα accumulation[10]. Consequently, SDH and VHL mutations give rise to the same spectrum of tumors, and the unifying feature of those tumors is the activation of HIFα (Figure 1)[11].
Activation of HIFα has also been described as the main consequence of FH inactivation[4],[5]. Similar to succinate, fumarate is also reported to be a competitive inhibitor of HIF prolyl hydroxylases[5]. This theory arises from experimental observations that were similar to HIFα activation by succinate[5]. Because oxoglutarate is a reducing agent in the HIFα hydroxylation reaction, the inhibition of prolyl hydrolyase enzymes by succinate can be partially reversed by exposing cells to oxoglutarate. Similarly, exposing cells to oxoglutarate can also abrogate HIFα accumulation in FH-inactivated cells[5]. Much research into the consequences of FH inactivation has focused on the effects of HIFα activation. In addition, reactive oxygen species (ROS) accumulate in cells devoid of FH activity. ROS accumulation also leads to HIFα accumulation. This alternative ROS-mediated mechanism of HIFα stabilization arises from the experimental observation that the HIFα activation phenotype could be neutralized by antioxidants such as N-acetyl-cystene or pyruvate[12]. In either case, the precise mechanism of that leads to HIFα activation in FH-inactivated cells remains unclear. Controlled experiments to investigate this cellular signaling have been confounded because succinate is elevated in FH-inactivated cells and is itself capable of activating HIFα (Figure 1).
There are two species of the α subunit of HIF, HIF1α (HIF1A) and HIF2α (HIF2A, also known as EPAS1), that are encoded by two different genes. HIF1A and HIF2A have been shown to recognize the same cis-acting hypoxia response element in target gene promoters, but their transcription targets do not entirely overlap[13]. Thus, HIF1A and HIF2A have apparent phenotypic differences. New evidence suggests that HIF1A has some tumor suppressive activity or at least is not associated with aggressive renal tumor development. In contrast, HIF2A is believed to have oncogenic activity and is associated with more aggressive tumors[14],[15]. In concordance with this, loss-of-function mutation of HIF1A is regularly found in aggressive clear cell renal cell carcinoma[16]. In these cancers, HIF1A mutations result in specific constitutive activation of HIF2A and drive the aggressive phenotype. In an in vitro study, reconstitution of functional VHL into the VHL-mutant clear cell renal cell carcinoma cell line 786-0 resulted in daughter cell lines that were not tumorigenic. Notably, tumorigenecity was restored by knock-in of constitutively active HIF2A but not HIF1A, providing supporting evidence that HIF2A is associated with tumorigenecity[17]. In contrast to aggressive clear cell renal cell carcinoma, hereditary PRCC2 exhibits activation of HIF1A but not HIF2A, with HIF2A expression being suppressed at translational level[18].
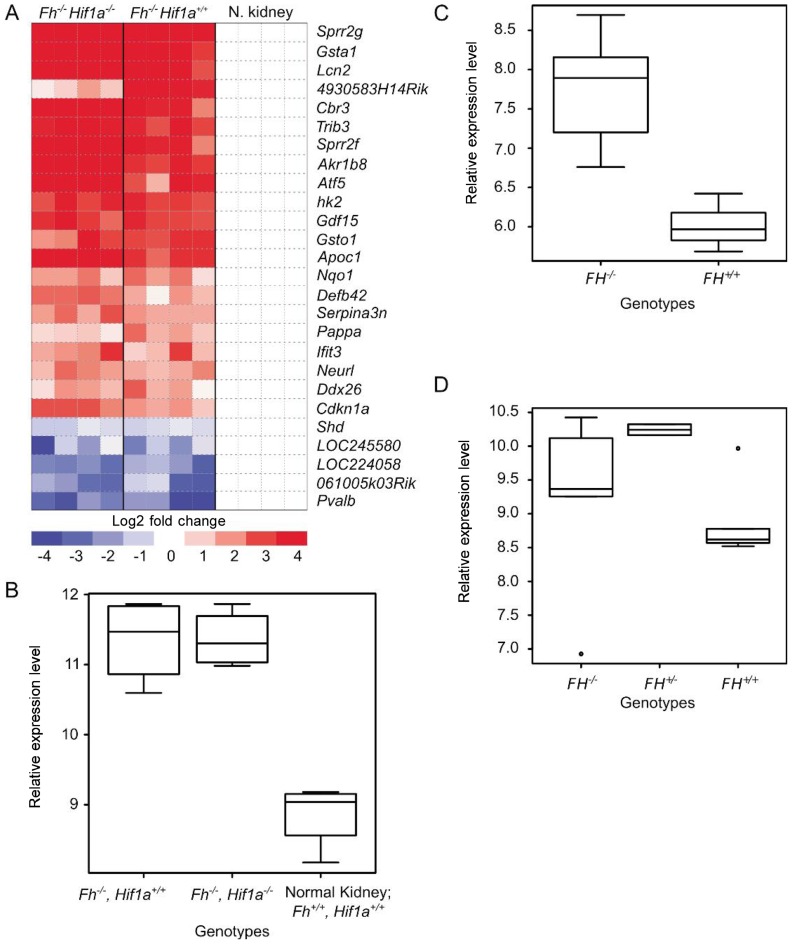
Besides the paradoxical differential activation of HIF1A vs. HIF2A in hereditary PRCC2, FH mutation also causes an entirely different spectrum of tumors from those associated with VHL and SDH mutations[4]. Furthermore, the robust response seen in HIFα-driven tumors towards the antiangiogenic agent sunitinib is not observed in HLRCC, suggesting that HIF1A activation may be a secondary effect[19]. Through gene expression profile analysis, we recently found that the angiogenic subset of HIF1A transcription targets was not overexpressed in hereditary PRCC2 clinical samples[6]. Another study examining renal cyst formation in conditional Fh knockout mice compared to conditional Fh/Hif1a and Fh/Hif2a double knockout mice showed that Hif1a but not Hif2a knockout in the background of Fh knockout exacerbates the Fh knockout associated renal cyst formation[7]. We examined the gene expression data from the study and only managed to find minimal differences between renal cysts in Fh knockout mice and Fh/Hif1a double knockout mice (Figure 2A). These observations further substantiate the possibility that HIFα activation observed in cells devoid of FH activity is likely to be a secondary effect rather than the primary consequence of FH inactivation.
Figure 2. Gene expression profiles of tissues with FH inactivation.
A, differential expression of genes in renal tissues from normal vs. single or double knockout mice. Gene expression data previously published by Adam and co-workers; GSE29988[7]. B, expression of Hmox1 in the Fh knockout, Fh/Hif1a double knockout, and control mice. Gene expression data previously published by Adam and co-workers; GSE29988[7]. C, expression of HMOX1 in FH−/− and FH+/+ uterine fibroids. Gene expression data previously published by Vanharanta and co-workers; GSE2152[41]. D, expression of HMOX1 in hereditary type 2 papillary renal cell carcinoma (FH−/−), normal renal tissues from HLRCC patients (FH+/−), and normal renal tissues from non-HLRCC patients (FH+/+). Gene expression data previously published by Ooi and co-workers; GSE26574[6].
The NRF2 Activation Theory
It is clear that one of the main consequences of FH inactivation is fumarate accumulation[4]. Fumarate is a thiol-reactive compound that is capable of reacting with the sulphydryl moiety of cysteine residues to produce 2-succinyl-cysteine in physiologic conditions[20]. As such, the reaction is appropriately named a succination to differentiate it from protein succinylation. The availability of an antibody specific to 2-succinyl-cysteine has made detection of the succination reaction in cells with high fumarate possible. This antibody, which stains FH-mutant tumor samples with high specificity, can be used as a highly sensitive and specific marker for HLRCC, which is characterized by FH inactivation and high levels of fumarate[21].
Succination is known to modify the activity of a large number of cysteine-containing proteins[22]–[24]. Kelch-like ECH-associated protein 1 (KEAP1), which has many exposed cysteine residues, is a prime example of a protein regulated by succination. KEAP1 is an adaptor protein that positions its binding partners in close proximity to CULLIN3 ubiquitin ligase for ubiquitination (Figure 1). Exposed sulfhydryl groups in the cysteine residues on KEAP1's surface are readily modified by thiol-reactive compounds. Upon covalent modification of its surface cysteine residues, KEAP1 undergoes a conformational change that blocks its interaction with its binding partners, I eading to binding partner accumulation[25]; as such KEAP1 acts as an electrophile sensor. The NRF2 transcription factor is one of the best studied binding partners of KEAP1. NRF2 modulates the expression of a large number of genes that carry out diverse cellular functions. NRF2 recognizes the cis-acting antioxidant response element (ARE) in target gene promoters and, upon binding, typically leads to increased expression of these genes. Classical NRF2 targets includes enzymes involved in phase I xenobiotic metabolism, such as cytochrome p450-dependent mixed-function oxidases and NADPH quinone reductase 1, and phase II xenobiotic metabolism, such as UDP-glucosyl transferases and glutathione-s-transferases. NRF2 also modulates the synthesis of antioxidant molecules such as glutathione, and thioredoxin as well as enzymes involved in pentose phosphate pathway, such as glucose 6-phosphate dehydrogenase and transketolases[26].
Transcription targets of NRF2 are coordinately overexpressed in FH-inactivated tissues[6],[7]. Our group and others have recently shown that KEAP1 is succinated in FH-inactivated cells, causing accumulation and activation of NRF2[6],[7]. In addition, re-expression of functional FH in FH-null cells reduces NRF2 levels. The effect of FH inactivation on NRF2 activity became apparent in a gene expression profile analysis, which showed that the gene expression signature for FH inactivation significantly overlapped with that of KEAP1 knockdown and that NRF2 targets were specifically overexpressed in FH-inactivated tissues[6]. While there is a highly significant overlap in NRF2 target genes between cells in which FH is inactive or cells in which KEAP1 is knocked down, cell lineage-specific differences are also apparent. One of the genes that displays this type of differential expression pattern across different FH-mutated tissues is HMOX1. This gene is a focus of recent HLRCC research because it was shown to be a possible therapeutic target for FH-inactive cells[27]. However, expression profile analysis on clinical samples has shown that HMOX1 is overexpressed in uterine fibroids associated with FH mutation in relative to uterine fibroids that arise sporadically (4-fold increase) but not in hereditary PRCC2 (1.5-fold increase relative to normal renal tissues) (Figures 2B-D). One possible explanation for this difference is that the availability of some transcription targets is dependent upon chromatin structure, and chromatin structure is affected by cell lineage. In an alternative model, the modulation of HMOX1 expression by NRF2 has been shown to require an intact SWItch/Sucrose NonFermentable (SWI/SNF) complex, where mutation of SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily a, member 4 (SMARCA4, also known as BRG1) was shown to decouple NRF2 and HMOX1 expression[28]. Recently, components of SWI/SNF complex were shown to be a common feature of the somatic mutation landscape of renal cancers[29]. The differential expression of HMOX1 suggests possible SWI/SNF complex disruption in hereditary PRCC2 and cautions against inferring phenomena in human genetics by extrapolating animal model data.
It should be noted that there is no direct evidence to indicate that NRF2 activation is a dominant oncogenic pathway resulting from FH inactivation. Nevertheless, NRF2 activation is a feature of many cancers, including lung cancer, breast cancer, colorectal cancer, and both the familial and sporadic forms of PRCC2[6],[30]–[32]. While FH mutation has yet to be identified in sporadic tumors, NRF2 activation has been reported as a consequence of either direct NRF2 gain-of-function mutation, KEAP1 loss-of-function mutation, or gain-of-function mutation of oncogenes such as MYC, BRAF, and KRAS in disparate cancer types[32]–[34]. Furthermore, expression of constitu-tively active NRF2 in immortalized embryonic renal cells (HEK293) has also been shown to induce transformation, providing compelling evidence that NRF2 activation could be oncogenic[35].
Glycolytic Shift in FH-inactivated Tumors
Because of the role of FH in the TCA cycle, inactivation of this enzyme is associated with inefficient oxidative phosphorylation and a shift to glycolysis under normoxic conditions (also known as Warburg's effect). Warburg's effect is a prominent phenotype of HLRCC tumors[36]. The precise mechanism underlying the shift of ATP synthesis from oxidative phosphorylation in normal cells to glycolysis during the course of tumorigenesis remains unknown. Some studies suggest that HIFα activation alone can account for most of the transcriptional reprogramming required to cause the observed glycolytic shift[18],[36]. HIFα modulates the expression of many genes that encode enzymes involved in glycolysis[37]. Active HIFα also diverts pyruvate away from the mitochondria and reduces the number of mitochondria[38].
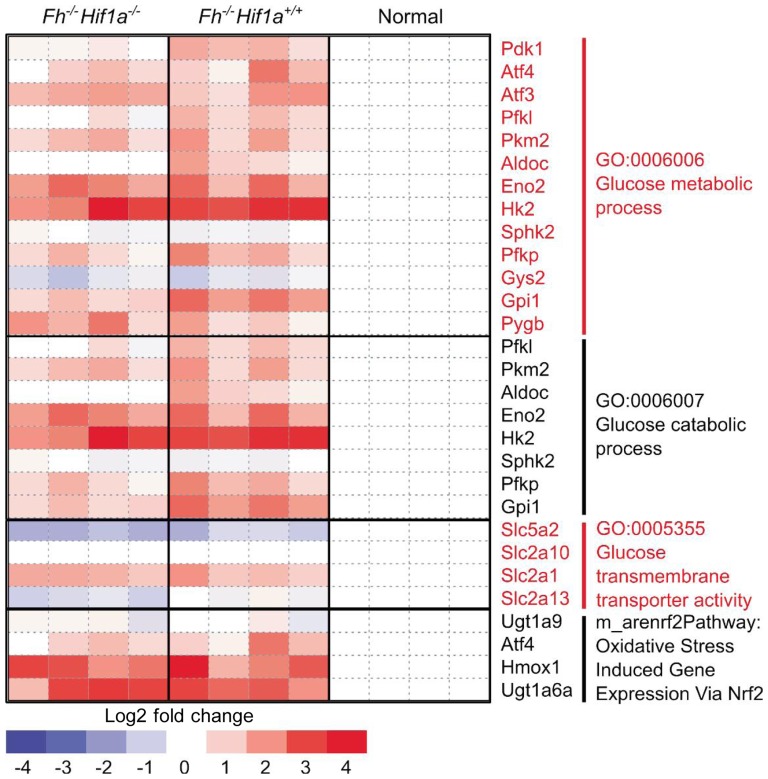
Glycolytic pathway genes regulated by HIFα, particularly glucose transporter 1 (GLUT1, also known as SLC2A1), hexokinase 2 (HK2), and pyruvate kinase (PK)[37],[39], are indeed overexpressed in FH-inactivated tissues when compared to corresponding normal tissues. This suggests that the HIFα activation theory does offer a potential mechanism for the observed metabolic shift in FH-inactivated tissues. However, this explanation of the glycolytic shift is not supported by experiments in animal models. Analysis of the data from the knockout mouse model study[7] in dicate that the expression level of GLUT1, HK2, and PK does not seem to change with the addition of Hif1a knockout on the Fh knockout background, suggesting that the observed overexpression of these genes were the result of activation of transcription factors other than HIF (Figure 3). Exploration into how these pro-glycolytic genes become up-regulated in the Fh/Hif1a double knockout mouse may provide additional understanding of this aggressive tumor subtype.
Figure 3. Grouping of differentially expressed genes according to gene ontology.
Expression of glucose metabolism genes including classical HIF1A target, Glut1(Slc2a1), is not dependent on Hif1a status, suggesting that other transcription factors may be responsible for the glycolytic shift observed in the Fh−/− tissues. Gene expression data previously published by Adam and coworkers; GSE29988[7].
Conclusions
In this article, we have presented evidence for the role of HIF1A and NRF2 in the development of renal tumors. However, it is important to note that the activation of HIF1A or NRF2 could be incidental to tumor development. The activation of these two transcription factors can happen in normal cells without causing cancer. Therefore, the true question is how these seemingly normal cellular signaling components give rise to fatal consequences. One answer is that activity of these transcription factors, particularly HIF1A and NRF2, are prolonged in the tumor. Therefore, sustained activation, rather than transient activation, is associated with tumor growth. A second answer could be the accessibility of cis-acting elements recognized by these transcription factors. Access to these sites is strongly influenced by chromatin structure, which allows transcription factors to differentially control the expression of their target genes in a chromatin-structure-specific manner[40]. Effects of cell lineage or somatic mutations in genes that control chromatin structure could allow aberrant regulation or availability of the targets of NRF2 or HIF1A. Transcription factor targets regulated by both the level of transcription factors and chromatin structure may hold clues to understand why activation of seemingly normal cellular signaling pathways can lead to malignancy in a tissue-specific manner. We refer to these transcription targets as “cryptic targets”, since, due to specific chromatin structure, their regulation by transcription factors is not universally present in all cell types.
Cryptic targets may be ideal candidates for therapeutic intervention since these genes are truly unique to tumor cells. As such, understanding whether the sustained activation and/or the presence of “cryptic targets” most strongly influence tumor development should be the next milestone in furthering our understanding of the fundamental causes of PRCC2 and other cancers.
References
- 1.Launonen V, Vierimaa O, Kiuru M, et al. et al. Inherited susceptibility to uterine leiomyomas and renal cell cancer. Proc Natl Acad Sci U S A. 2001;98:3387–3392. doi: 10.1073/pnas.051633798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lehtonen HJ. Hereditary leiomyomatosis and renal cell cancer: update on clinical and molecular characteristics. Fam Cancer. 2011;10:397–411. doi: 10.1007/s10689-011-9428-z. [DOI] [PubMed] [Google Scholar]
- 3.Tomlinson IP, Alam NA, Rowan AJ, et al. et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet. 2002;30:406–410. doi: 10.1038/ng849. [DOI] [PubMed] [Google Scholar]
- 4.Pollard PJ, Briere JJ, Alam NA, et al. et al. Accumulation of Krebs cycle intermediates and over-expression of HIF1alpha in tumours which result from germline FH and SDH mutations. Hum Mol Genet. 2005;14:2231–2239. doi: 10.1093/hmg/ddi227. [DOI] [PubMed] [Google Scholar]
- 5.Isaacs JS, Jung YJ, Mole DR, et al. et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell. 2005;8:143–153. doi: 10.1016/j.ccr.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 6.Ooi A, Wong JC, Petillo D, et al. et al. An antioxidant response phenotype shared between hereditary and sporadic type 2 papillary renal cell carcinoma. Cancer Cell. 2011;20:511–523. doi: 10.1016/j.ccr.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 7.Adam J, Hatipoglu E, O'Flaherty L, et al. et al. Renal cyst formation in Fh1-deficient mice is independent of the Hif/Phd pathway: roles for fumarate in KEAP1 succination and Nrf2 signaling. Cancer Cell. 2011;20:524–537. doi: 10.1016/j.ccr.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deberardinis RJ, Thompson CB. Cellular metabolism and disease: what do metabolic outliers teach us? Cell. 2012;148:1132–1144. doi: 10.1016/j.cell.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selak MA, Armour SM, MacKenzie ED, et al. et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 10.Maxwell PH, Wiesener MS, Chang GW, et al. et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 11.Linehan WM, Srinivasan R, Schmidt LS. The genetic basis of kidney cancer: a metabolic disease. Nat Rev Urol. 2010;7:277–285. doi: 10.1038/nrurol.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sudarshan S, Sourbier C, Kong HS, et al. et al. Fumarate hydratase deficiency in renal cancer induces glycolytic addiction and hypoxia-inducible transcription factor 1alpha stabilization by glucose-dependent generation of reactive oxygen species. Mol Cell Biol. 2009;29:4080–4090. doi: 10.1128/MCB.00483-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loboda A, Jozkowicz A, Dulak J. HIF-1 and HIF-2 transcription factors—similar but not identical. Mol Cells. 2010;29:435–442. doi: 10.1007/s10059-010-0067-2. [DOI] [PubMed] [Google Scholar]
- 14.Keith B, Johnson RS, Simon MC. HIF1alpha and HIF2alpha: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2012;12:9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordan JD, Lai P, Dondeti VR, et al. et al. HIF-alpha effects on c-Myc distinguish two subtypes of sporadic VHL-deficient clear cell renal carcinoma. Cancer Cell. 2008;14:435–446. doi: 10.1016/j.ccr.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mandriota SJ, Turner KJ, Davies DR, et al. et al. HIF activation identifies early lesions in VHL kidneys: evidence for site-specific tumor suppressor function in the nephron. Cancer Cell. 2002;1:459–468. doi: 10.1016/s1535-6108(02)00071-5. [DOI] [PubMed] [Google Scholar]
- 17.Kondo K, Kim WY, Lechpammer M, et al. et al. Inhibition of HIF2alpha is sufficient to suppress pVHL-defective tumor growth. PLoS Biol. 2003;1:E83. doi: 10.1371/journal.pbio.0000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tong WH, Sourbier C, Kovtunovych G, et al. et al. The glycolytic shift in fumarate-hydratase-deficient kidney cancer lowers AMPK levels, increases anabolic propensities and lowers cellular iron levels. Cancer Cell. 2011;20:315–327. doi: 10.1016/j.ccr.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molina AM, Feldman DR, Ginsberg MS, et al. et al. Phase II trial of sunitinib in patients with metastatic non-clear cell renal cell carcinoma. Invest New Drugs. 2012;30:335–340. doi: 10.1007/s10637-010-9491-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alderson NL, Wang Y, Blatnik M, et al. et al. S-(2-Succinyl)cysteine: a novel chemical modification of tissue proteins by a Krebs cycle intermediate. Arch Biochem Biophys. 2006;450:1–8. doi: 10.1016/j.abb.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Bardella C, El-Bahrawy M, Frizzell N, et al. et al. Aberrant succination of proteins in fumarate hydratase-deficient mice and HLRCC patients is a robust biomarker of mutation status. J Pathol. 2011;225:4–11. doi: 10.1002/path.2932. [DOI] [PubMed] [Google Scholar]
- 22.Frizzell N, Lima M, Baynes JW. Succination of proteins in diabetes. Free Radic Res. 2011;45:101–109. doi: 10.3109/10715762.2010.524643. [DOI] [PubMed] [Google Scholar]
- 23.Blatnik M, Thorpe SR, Baynes JW. Succination of proteins by fumarate: mechanism of inactivation of glyceraldehyde-3-phosphate dehydrogenase in diabetes. Ann N Y Acad Sci. 2008;1126:272–275. doi: 10.1196/annals.1433.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blatnik M, Frizzell N, Thorpe SR, et al. et al. Inactivation of glyceraldehyde-3-phosphate dehydrogenase by fumarate in diabetes: formation of S-(2-succinyl)cysteine, a novel chemical modification of protein and possible biomarker of mitochondrial stress. Diabetes. 2008;57:41–49. doi: 10.2337/db07-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang DD, Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol. 2003;23:8137–8151. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malhotra D, Portales-Casamar E, Singh A, et al. et al. Global mapping of binding sites for Nrf2 identifies novel targets in cell survival response through CHIP-Seq profiling and network analysis. Nucleic Acids Res. 2010;38:5718–5734. doi: 10.1093/nar/gkq212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frezza C, Zheng L, Folger O, et al. et al. Haem oxygenase is synthetically lethal with the tumour suppressor fumarate hydratase. Nature. 2011;477:225–228. doi: 10.1038/nature10363. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, Ohta T, Maruyama A, et al. et al. BRG1 interacts with Nrf2 to selectively mediate HO-1 induction in response to oxidative stress. Mol Cell Biol. 2006;26:7942–7952. doi: 10.1128/MCB.00700-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varela I, Tarpey P, Raine K, et al. et al. Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature. 2011;469:539–542. doi: 10.1038/nature09639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoo NJ, Kim HR, Kim YR, et al. et al. Somatic mutations of the KEAP1 gene in common solid cancers. Histopathology. 2012;60:943–952. doi: 10.1111/j.1365-2559.2012.04178.x. [DOI] [PubMed] [Google Scholar]
- 31.Li QK, Singh A, Biswal S, et al. et al. KEAP1 gene mutations and NRF2 activation are common in pulmonary papillary adenocarcinoma. J Hum Genet. 2011;56:230–234. doi: 10.1038/jhg.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh A, Misra V, Thimmulappa RK, et al. et al. Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLoS Med. 2006;3:e420. doi: 10.1371/journal.pmed.0030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeNicola GM, Karreth FA, Humpton TJ, et al. et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim YR, Oh JE, Kim MS, et al. et al. Oncogenic NRF2 mutations in squamous cell carcinomas of oesophagus and skin. J Pathol. 2010;220:446–451. doi: 10.1002/path.2653. [DOI] [PubMed] [Google Scholar]
- 35.Shibata T, Saito S, Kokubu A, et al. et al. Global downstream pathway analysis reveals a dependence of oncogenic NF-E2-related factor 2 mutation on the mTOR growth signaling pathway. Cancer Res. 2010;70:9095–9105. doi: 10.1158/0008-5472.CAN-10-0384. [DOI] [PubMed] [Google Scholar]
- 36.Yang Y, Valera VA, Padilla-Nash HM, et al. et al. UOK 262 cell line, fumarate hydratase deficient (FH−/FH−) hereditary leiomyomatosis renal cell carcinoma: in vitro and in vivo model of an aberrant energy metabolic pathway in human cancer. Cancer Genet Cytogenet. 2010;196:45–55. doi: 10.1016/j.cancergencyto.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer. 2008;8:705–713. doi: 10.1038/nrc2468. [DOI] [PubMed] [Google Scholar]
- 38.Semenza GL. Hypoxia-inducible factor 1: regulator of mitochondrial metabolism and mediator of ischemic preconditioning. Biochim Biophys Acta. 2011;1813:1263–1268. doi: 10.1016/j.bbamcr.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolf A, Agnihotri S, Micallef J, et al. et al. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J Exp Med. 2011;208:313–326. doi: 10.1084/jem.20101470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baylin SB, Ohm JE. Epigenetic gene silencing in cancer—a mechanism for early oncogenic pathway addiction? Nat Rev Cancer. 2006;6:107–116. doi: 10.1038/nrc1799. [DOI] [PubMed] [Google Scholar]
- 41.Vanharanta S, Pollard PJ, Lehtonen HJ, et al. et al. Distinct expression profile in fumarate-hydratase-deficient uterine fibroids. Hum Mol Genet. 2006;15:97–103. doi: 10.1093/hmg/ddi431. [DOI] [PubMed] [Google Scholar]