Abstract
Response criteria remain controversial in therapeutic evaluation for locally advanced esophageal carcinoma treated with neoadjuvant chemotherapy. We aimed to identify the predictive value of tumor regression grading (TRG) in tumor response and prognosis. Fifty-two patients who underwent neoadjuvant chemotherapy followed by esophagectomy and radical 2-field lymphadenectomy between June 2007 and June 2011 were included in this study. All tissue specimens were reassessed according to the TRG scale. Potential prognostic factors, including clinicopathologic factors, were evaluated. Survival curves were generated by using the Kaplan-Meier method and compared with the log-rank test. Prognostic factors were determined with multivariate analysis by using the Cox regression model. Our results showed that of 52 cases, 43 (83%) were squamous cell carcinoma and 9 (17%) were adenocarcinoma. TRG was correlated with pathologic T (P = 0.006) and N (P < 0.001) categories. Median overall survival for the entire cohort was 33 months. The 1- and 2-year overall survival rates were 71% and 44%, respectively. Univariate survival analysis results showed that favorable prognostic factors were histological subtype (P = 0.003), pathologic T category (P = 0.026), pathologic N category (P < 0.001), and TRG G0 (P = 0.041). Multivariate analyses identified pathologic N category (P < 0.001) as a significant independent prognostic parameter. Our results indicate that histomorphologic TRG can be considered as an alternative option to predict the therapeutic efficacy and prognostic factor for patients with locally advanced esophageal carcinoma treated by neoadjuvant chemotherapy.
Keywords: Tumor regression grading, esophageal cancer, neoadjuvant chemotherapy, efficacy assessment
Esophageal carcinoma is a common malignant tumor. Surgical resection and reconstruction is a major treatment for this type of cancer. Because of the application of comprehensive therapy and advancements in surgical techniques, the clinical efficacy on esophageal cancer has been greatly improved. The 5-year survival rate remains between 15% and 39%[1]. A recent study indicated that the 5-year survival rate of patients with stage I esophageal cancer was approximately 60%–80%; however, for patients with locally advanced esophageal carcinoma (stage III), this rate dropped to less than 25%[2]. Hence, preoperative neoadjuvant chemotherapy or chemoradiotherapy combined with surgery have gained more attention in the treatment of locally advanced esophageal cancer[3],[4].
Nevertheless, accurate evaluation of the efficacy of neoadjuvant therapy is a challenge in esophageal carcinoma treatment. The esophagus is a cavitary organ. The morphology of esophageal tumors is irregular, and uneven tumor regression has been noted after treatment. In addition, pathologic T category of esophageal cancer is determined by the depth of tumor infiltration rather than tumor length[5]. Thus, although Response Evaluation Criteria in Solid Tumors (RECIST) is currently used to determine solid tumor response to chemotherapy[6],[7], these criteria are not ideal for assessing the response of esophageal carcinoma to neoadjuvant chemotherapy.
Here, the percentage of residual cancer cells, that is, tumor regression grading (TRG), was used to evaluate the efficacy of neoadjuvant therapy on esophageal carcinoma. TRG was first proposed in the National Comprehensive Cancer Network (NCCN) guidelines (second edition) for gastroesophageal junction tumors in 2011. Standards for TRG are hotly debated. For example, some scholars categorize TRG into three grades[8],[9], whereas others indicate that there are four[9]. Likewise, some studies suggest that the extent of proliferation in the fibrous tissue of lesions should be regarded as an evaluation criterion[10], whereas others do not[8],[9],[11]. Study samples in current investigations are limited to cases treated with neoadjuvant chemoradiotherapy, in which the fibrosis degree after neoadjuvant chemoradiotherapy was more severe than that after neoadjuvant chemotherapy. Thus, the value of histological TRG in evaluating the efficacy of neoadjuvant chemotherapy remains to be elucidated. This retrospective study was designed to determine the clinical significance and prognostic value of histological TRG and to screen prognostic factors for locally advanced esophageal cancer treated with neoadjuvant chemotherapy.
Materials and Methods
Study subject
Clinical data of patients with locally advanced esophageal carcinoma who underwent neoadjuvant chemotherapy at Sun Yat-sen Cancer Center between June 2007 and June 2011 were retrospectively analyzed. The screening criteria were as follows: (1) patients were clinically diagnosed with esophageal cancer or gastroesophageal junction tumors by gastroscopic biopsy; (2) patients presented with tumor infiltration into the esophageal adventitia layer and invasion into the mediastinum (≥ T3), or suspected local lymph mode metastasis and unresectable tumors as evaluated by imaging methods; (3) patients underwent 2 to 3 cycles of neoadjuvant chemotherapy combined with surgery; (4) all patients had appreciable postoperative pathologic specimen before and after chemotherapy. Neoadjuvant chemotherapy consisted of docetaxel (75 mg/m2) and nedaplatin (80 mg/m2) repeated every 21 days. Treatment efficacy and performance status were evaluated 3 to 4 weeks after chemotherapy. Patients with progression-free tumors and a performance status score ≤ 2 underwent radical surgical resection. Exclusion criteria were as follows: 1) patients had progressive esophageal carcinoma after chemotherapy; 2) patients had a performance status score >2.
Efficacy evaluation
Resected tumor foci were evaluated by using the TRG criteria reported by Wu et al.[8] and recommended by NCCN guidelines for esophageal carcinoma (2011 edition)[12]: G0, no residual cancer cells; G1, 1% to 50% residual cancer cells; G2, >50% residual cancer cells.
Statistical analysis
SPSS 16.0 software was used for statistical analysis. Chi-square test and Fisher's exact test were used to determine the correlation between clinicopathologic factors and TRG. Grading data were analyzed by Spearman rank correlation. Survival, which was considered the time from diagnosis to death or final follow-up, was calculated by using the Kaplan-Meier method, and survival curves were compared with the log-rank test. Multivariate analysis with Cox regression was used to determine the prognostic factors. P < 0.05 was considered significant.
Results
Patient information
Among 122 patients with locally advanced esophageal carcinoma who underwent neoadjuvant chemotherapy between June 2007 and June 2011, 52 met the inclusion criteria, including 43(82.7%) men and 9(17.3%) women ranging in age from 39 to 73 years (median, 55 years). There were 43 cases (82.7%) of esophageal squamous cell cancer and 9 cases (17.3%) of esophageal adenocarcinoma. All patients completed 2 cycles of chemotherapy. All specimens were pathologically proved negative at the incision edge (R0 resection).
Efficacy evaluation
Spearman analysis showed that TRG was significantly correlated with postoperative pathologic T category (P = 0.006) and N category (P < 0.001), but was not correlated with sex, age, histological subtype, or tumor site (Table 1).
Table 1. Correlation between tumor regression grade (TRG) and clinicopathologic characteristics of 52 patients with locally advanced esophageal cancer.
| Characteristic | Total [cases (%)] | TRG |
r | P | ||
| G0 (n = 9) | G1 (n = 20) | G2 (n = 23) | ||||
| Gender | 0.066 | 0.893 | ||||
| Male | 43 (82.7) | 7 | 17 | 19 | ||
| Famale | 9 (17.3) | 2 | 3 | 4 | ||
| Age (years) | 0.109 | 0.442 | ||||
| < 55 | 29 (55.8) | 5 | 13 | 11 | ||
| ≥55 | 23 (44.2) | 4 | 7 | 12 | ||
| Tumor type | 0.241 | 0.202 | ||||
| Squamous cell carcinoma | 43 (82.7) | 9 | 17 | 17 | ||
| Adenocarcinoma | 9 (17.3) | 0 | 3 | 6 | ||
| Tumor location | 0.169 | 0.681 | ||||
| Upper esophagus | 6 (11.5) | 1 | 2 | 3 | ||
| Middle esophagus | 30 (57.7) | 7 | 12 | 11 | ||
| Lower esophagus | 9 (17.3) | 1 | 4 | 4 | ||
| Gastroesophageal junction | 7 (13.5) | 0 | 2 | 5 | ||
| YpT | 0.374 | 0.006 | ||||
| T0 | 6 (11.5) | 5 | 1 | 0 | ||
| T1 | 5 (9.6) | 1 | 2 | 2 | ||
| T2 | 11 (21.2) | 2 | 3 | 6 | ||
| T3 | 30 (57.7) | 1 | 14 | 15 | ||
| YpN | 0.518 | <0.001 | ||||
| N0 | 21 (40.4) | 9 | 7 | 5 | ||
| N1 | 15 (28.8) | 0 | 9 | 6 | ||
| N2 | 8 (15.4) | 0 | 2 | 6 | ||
| N3 | 8 (15.4) | 0 | 2 | 6 | ||
YpT, pathologic T stage after neoadjuvant chemotherapy; YpN, pathologic N stage after neoadjuvant chemotherapy.
Survival analysis
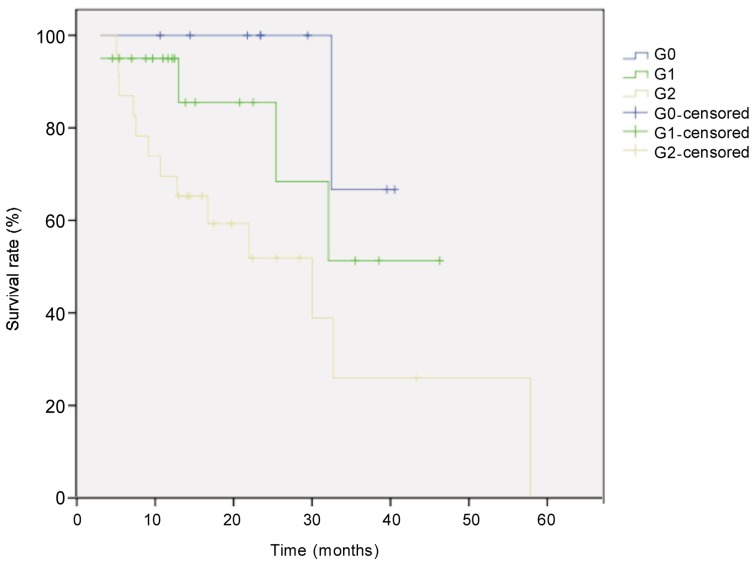
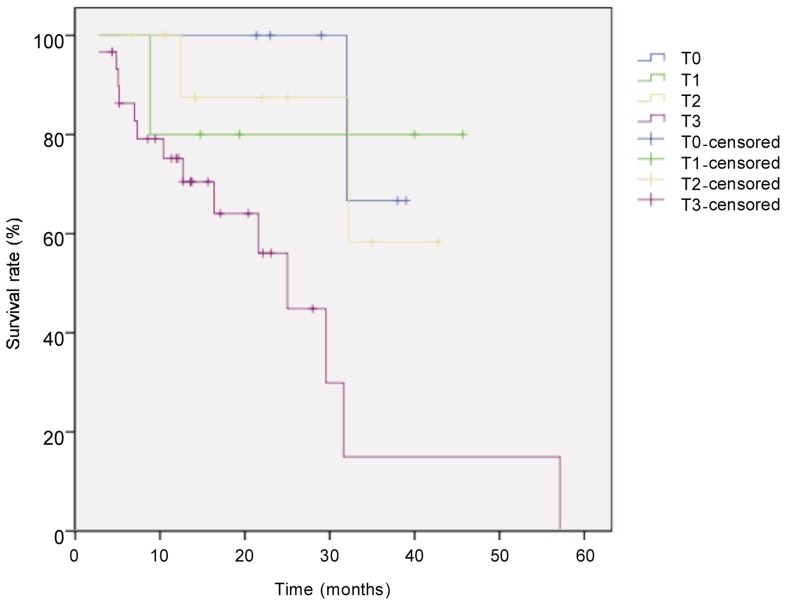
The median follow-up for all 52 patients with locally advanced esophageal cancer was 21.6 months, and the median survival time was 33 months. Eighteen patients died. The survival rate was 71% in the first year and 44% in the second year. Survival was better in patients with low TRG than in those with high TRG (χ2 = 6.405, P = 0.041, log-rank test) (Figure 1). Univariate analysis revealed that histological subtype (P = 0.003), tumor site (P = 0.044), postoperative pathologic T category (P = 0.026), pathologic N category (P < 0.001), and TRG (P = 0.041) were prognostic factors (Figures 2–5). Multivariate analysis with Cox regression suggested that pathologic N category was an independent prognostic parameter (Table 2).
Figure 1. Kaplan-Meier overall survival curves for patients with locally advanced esophageal carcinoma of different tumor regression grades (TRG).
The difference between groups (G0, n = 9; G1, n = 20; G3, n = 23) was compared with the log-rank test. The survival rate of the was significantly higher in G0 group than in other groups (P = 0.041).
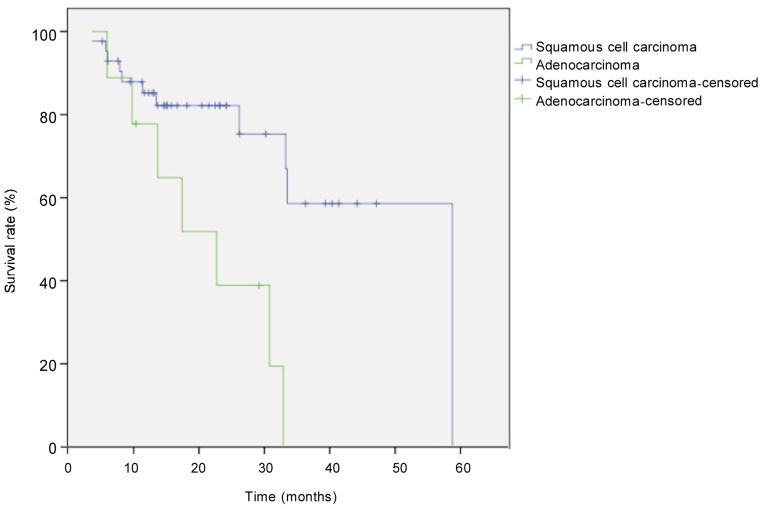
Figure 2. Kaplan-Meier overall survival curves for patients with locally advanced esophageal carcinoma of different types.
The difference between tumor types (squamous carcinoma, n = 43; adenocarcinoma, n = 9) was compared with the log-rank test. The survival rate was significantly higher in patients with squamous cell carcinoma than in those with adenocarcinoma (P = 0.003).
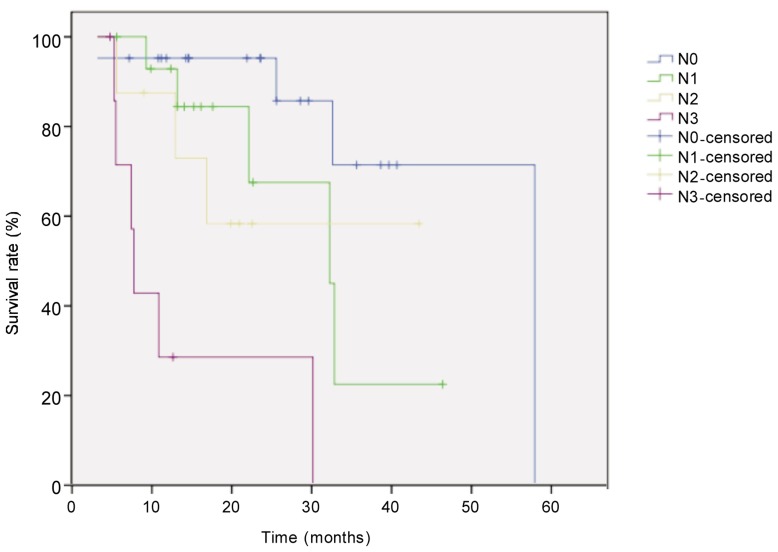
Figure 5. Kaplan-Meier overall survival curves for patients with locally advanced esophageal carcinoma of different pathologic N stage after neoadjuvant chemotherapy.
The difference between groups (N0, n = 21; N1, n = 11; N2, n = 8; N3, n = 8) was compared with the log-rank test. The survival rate was significantly higher in N0 group than in other groups (P < 0.001).
Table 2. Univariate and multivariate survival analyses on 52 patients with locally advanced esophageal cancer.
| Variate | Total number | Number of deaths | Univariate analysis (P) | Multivariate analysis (P) |
| Gender | 0.879 | 0.897 | ||
| Male | 43 | 16 | ||
| Female | 9 | 2 | ||
| Age (years) | 0.422 | 0.682 | ||
| <55 | 29 | 10 | ||
| ≥55 | 23 | 8 | ||
| Tumor type | 0.003 | 0.104 | ||
| Squamous cell carcinoma | 43 | 11 | ||
| Adenocarcinoma | 9 | 7 | ||
| Tumor location | 0.044 | 0.099 | ||
| Upper Esophagus | 6 | 2 | ||
| Middle Esophagus | 30 | 8 | ||
| Lower Esophagus | 9 | 3 | ||
| Gastroesophageal junction | 7 | 5 | ||
| YpT | 0.026 | 0.107 | ||
| T0 | 6 | 1 | ||
| T1 | 5 | 1 | ||
| T2 | 11 | 2 | ||
| T3 | 30 | 14 | ||
| YpN | <0.001 | <0.001 | ||
| N0 | 21 | 4 | ||
| N1 | 15 | 5 | ||
| N2 | 8 | 3 | ||
| N3 | 8 | 6 | ||
| TRG | 0.041 | 0.464 | ||
| G0 | 9 | 1 | ||
| G1 | 20 | 4 | ||
| G2 | 23 | 13 |
Footnotes as in Table 1.
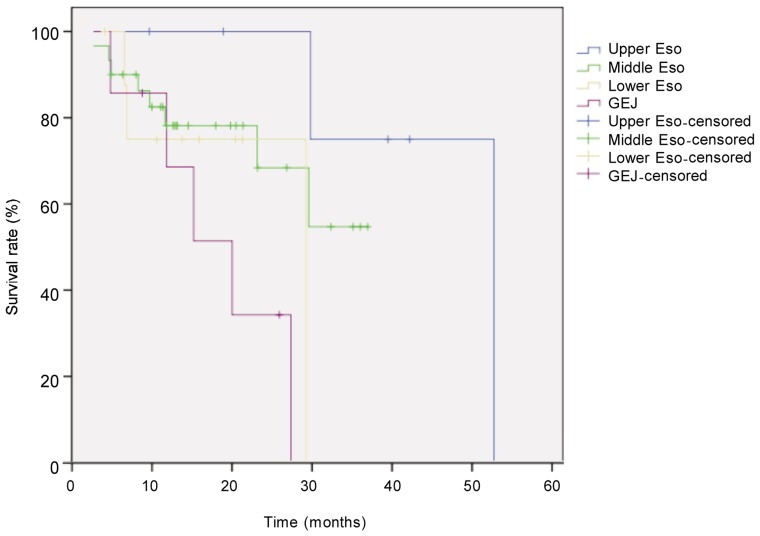
Figure 3. Kaplan-Meier overall survival curves for patients with locally advanced esophageal carcinoma at different locations.
The difference between tumor locations (upper esophagus, n = 6; middle esophagus, n = 30; lower esophagus, n = 9; gastroesophageal junction, n = 7) was compared with the log-rank test. The survival rate was significantly higher in upper esophagus group than in other groups (P = 0.044).
Figure 4. Kaplan-Meier overall survival curves for patients with locally advanced esophageal carcinoma of different pathologic T stage after neoadjuvant chemotherapy.
The difference between groups (T0, n = 6; T1, n = 5; T2, n = 11; T3, n = 30) was compared with the log-rank test. The survival rate was significantly higher in T0 group than in other groups (P = 0.026).
Discussion
There has been no standard treatment for locally advanced esophageal carcinoma. Preoperative neoadjuvant chemotherapy and chemoradiotherapy are being explored as treatment strategies for this type of cancer. However, the value of neoadjuvant chemotherapy or chemoradiotherapy in treating esophageal cancer remains elusive[13]. Some investigations indicated that neoadjuvant concurrent chemoradiotherapy yielded higher response rate than neoadjuvant chemotherapy. However, concurrent chemoradiotherapy produced more adverse events, elevated surgical risk, prolonged treatment duration, and increased financial burden compared with chemotherapy alone[14],[15]. The value of neoadjuvant chemotherapy in treating esophageal carcinoma is under debate. Different and even opposing results have been obtained due to different treatment schemes and chemotherapy cycles[16]. However, neoadjuvant treatment has generally satisfactory outcomes. After neoadjuvant chemotherapy, potentially resectable cases of locally advanced esophageal cancer evolved into completely resectable cases. Furthermore, the R0 resection rate of significantly increased and postoperative quality of life was enhanced[17]. Previously used chemotherapy for esophageal cancer was primarily 5-fluorouracil (5-FU) combined with cisplatin continuous infusion. However, chemotherapy agents, such as taxol, docetaxel, oxaliplatin, nedaplatin, have been widely used in clinical settings. Thus, whether neoadjuvant chemotherapy and chemoradiotherapy can bring survival benefits remains unanswered[18]–[20]. Previous phase III CROSS[21] and GALGB9781[22] trials suggested that neoadjuvant chemoradiotherapy increased overall survival compared to surgery alone. Phase III FFCD9901[23] and FFCD9102[24] clinical trials failed to confirm that neoadjuvant chemoradiotherapy improved overall survival. In contrast, results from the MRCOEO2[25] and FNLCCACCORD07-FFCD9703[26] clinical trials and MAGIC experiment[27] showed that neoadjuvant chemotherapy plus surgery enhanced survival rate compared to surgery alone. The RTOG8911 clinical trial[28] showed that the survival between neoadjuvant chemotherapy with surgery group and surgery alone group did not significantly differ. Sjoquist et al.[29] conducted a meta-analysis of esophageal cancer with neoadjuvant therapy incorporating 17 previous meta-analysis studies and 7 new investigations. Twelve studies compared neoadjuvant chemoradiotherapy and surgery alone (n = 1854); 9 trials compared neoadjuvant chemotherapy and surgery alone (n = 1981); 2 compared neoadjuvant chemoradiotherapy and neoadjuvant chemotherapy (n = 181); and 1 performed pairwise comparison, including both neoadjuvant chemoradiotherapy and chemotherapy (n = 81). The results validated that neoadjuvant chemoradiotherapy and chemotherapy yielded greater survival benefits compared to surgery alone. However, these studies failed to confirm that neoadjuvant chemoradiotherapy afforded advantages over neoadjuvant chemotherapy. The divergent outcomes are ascribed to multiple factors, such as histological subtypes, chemotherapy schemes, chemotherapy cycles, tumor grade, and so on. The evaluation criterion of the efficacy of neoadjuvant therapy may be a possible factor.
Objective and accurate evaluation of clinical efficacy can provide guidance for treatment and is valuable for predicting prognosis and survival. Postoperative TRG is the golden standard for determining the clinical efficacy of neoadjuvant chemotherapy and chemoradiotherapy. At present, TRG is frequently adopted to assess the efficacy of neoadjuvant therapy on esophageal carcinoma[30]. Mandard et al.[10] classified the percentage of residual cancer cells and the degree of lesion fibrosis into five grades. Chirieac et al.[9] divided the percentage of residual cancer cells into four grades (G0, 0%; G1, 1%–10%; G2, 11%–50%; and G3, >50%). Survival analysis revealed that patients in the G0 group had significantly longer survival compared with their counterparts in the G3 group, whereas the survival between the G1 and G2 groups did not differ. Wu et al.[8] categorized TRG into three grades based upon previous investigations (G0, 0%, G1, 1%–50%, and G2, >50%), which reflected prognosis and survival in a more object manner. Although current TRG evaluation criteria have not been applied, the 3-grade classification used by Wu et al.[8] has been widely recognized by pathologists. The 2011 NCCN guidelines for esophageal cancer recommended the TRG criterion for evaluating the efficacy of neoadjuvant therapy on esophageal cancer.
A recent report indicated that an R0 resection rate of 89% was achieved for esophageal cancer after neoadjuvant chemotherapy. Survival analysis revealed that positive lesion incision edge was an indicator of poor prognosis[31]. Another multi-center clinical trial (SAKK75/02) found that the R0 resection rate of the patients with locally advanced esophageal carcinoma was 93% following neoadjuvant chemoradiotherapy[32]. In our study, we retrospectively analyzed 52 cases of stage III esophageal cancer treated with neoadjuvant TP scheme (docetaxel and nedaplatin) preoperatively. The R0 resection rate was 100%. Collectively, our results and results from other studies suggest that both neoadjuvant chemotherapy and chemoradiotherapy can enhance the rate of radical operation, increase R0 resection rate, and lower the incidence of recurrent esophageal tumors.
This study showed that 9 (17.3%) patients had complete histopathologic response. Correlation analysis revealed that histopathologic response was highly correlated with pathologic T category (P = 0.006) and N category (P < 0.001), which were in line with previous studies. Langer et al.[33] analyzed TRG in 92 cases of esophageal cancer treated with neoadjuvant chemotherapy. The results indicated that TRG was significantly correlated with incision edge (P = 0.016), postoperative pathologic T category (P < 0.001), and N category (P = 0.001). Some studies showed that complete pathologic response was achieved in >20% of cases after neoadjuvant chemoradiotherapy[34],[35], which was higher than in the current study. This is possibly because concurrent chemoradiotherapy increased TRG.
Survival analysis in our study indicated that the survival among the G0, G1, and G2 groups significantly differed (P = 0.041), suggesting that TRG has a good value for predicting prognosis of esophageal cancer patients after neoadjuvant chemotherapy. Indeed, the patients who showed complete pathologic response (G0) had significantly prolonged survival. In univariate analysis, histological subtype, tumor site, pathologic T category, and N category were prognostic parameters. Histological subtype has been widely recognized as a prognostic factor for esophageal carcinoma. Previous studies noted that the prognosis of squamous cell cancer and adenocarcinoma significantly differed[36]. In the 7th edition of the American Joint Committee on Cancer (AJCC) manual, separate grading systems were applied for these two different histological subtypes. In addition, Gebski et al.[37] performed a meta-analysis study and found that adenocarcinoma patients were more likely to gain survival benefits from neoadjuvant therapy compared to squamous cell cancer subjects. Our results were similar. Previous investigations found that the effect of tumor site on survival was limited to early-stage squamous cell cancer patients (stage IB–IIB). Therefore, tumor site should be considered for this population. In our retrospective study, we observed that tumor site is a prognostic parameter, probably due to the influence of gastroesophageal cancer. However, histological subtype exerted a higher effect on survival compared to tumor site.
Multivariate analysis with Cox regression revealed that postoperative pathologic N category was an independent prognostic parameter. Based on previous studies, participants were classified into two groups according to the percentage of residual cancer cells (<10% versus ≥10%). Survival curves indicated that the group with fewer residual cancer cells (<10% ) have better survival (P < 0.004). Cox regression model analysis revealed that postoperative residual cancer cells (P < 0.028) and postoperative lymph node (YpN) status (P < 0.036) were prognostic factors but pathologic T category was not (P = 0.900)[38], suggesting that TRG better reflects the changes in tumor lesions following neoadjuvant treatment compared to pathologic T category. In our study, multivariate analysis indicated that TRG was not an independent prognostic factor for esophageal cancer patients, possibly due to limited sample size of patients with G0 in this cohort analysis. Thus, the survival status of this population was not objectively illustrated. The prognostic significance of pathologic T category upon survival indicate that neoadjuvant chemotherapy controlled primary lesions well. Local lymph node status became a more vital influencing factor for survival. Hence, the patients diagnosed with lymph node metastasis were required to undergo adjuvant chemotherapy postoperatively to reduce distant metastasis.
Conclusions
After neoadjuvant chemotherapy, TRG is highly correlated with pathologic T category and N category. Furthermore, TRG is a prognostic factor. Similarly, pathologic N category is also an independent prognostic factor for esophageal cancer patients treated with neoadjuvant chemotherapy. TRG criteria can be used to predict long-term survival of esophageal cancer patients. Hence, the TRG evaluation system has significant potential for assessing the clinical efficacy of neoadjuvant chemotherapy on esophageal carcinoma.
Acknowledgments
This study was supported by a grant from the National Key Technology Research and Development Program of China (No. 2006AA02A403).
References
- 1.Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC cancer staging manual: esophagus and esophagogastric junction. Ann Surg Oncol. 2010;17:1721–1724. doi: 10.1245/s10434-010-1024-1. [DOI] [PubMed] [Google Scholar]
- 2.Refaely Y, Krasna MJ. Multimodality therapy for esophageal cancer. Surg Clin North Am. 2002;82:729–746. doi: 10.1016/s0039-6109(02)00029-4. [DOI] [PubMed] [Google Scholar]
- 3.Hyngstrom JR, Posner MC. Neoadjuvant strategies for the treatment of locally advanced esophageal cancer. J Surg Oncol. 2010;101:299–304. doi: 10.1002/jso.21479. [DOI] [PubMed] [Google Scholar]
- 4.Gao XS. Considerations of treatment standardization from the procession of NCCN guideline of esophageal cancer. Chin J Cancer. 2010;29:860–864. doi: 10.5732/cjc.010.10251. [DOI] [PubMed] [Google Scholar]
- 5.Kim TJ, Kim HY, Lee KW, et al. et al. Multimodality assessment of esophageal cancer: preoperative staging and monitoring of response to therapy. Radiographics. 2009;29:403–421. doi: 10.1148/rg.292085106. [DOI] [PubMed] [Google Scholar]
- 6.Eisenhauer EA, Therasse P, Bogaerts J, et al. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 7.Therasse P, Arbuck SG, Eisenhauer EA, et al. et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 8.Wu TT, Chirieac LR, Abraham SC, et al. et al. Excellent interobserver agreement on grading the extent of residual carcinoma after preoperative chemoradiation in esophageal and esophagogastric junction carcinoma: a reliable predictor for patient outcome. Am J Surg Pathol. 2007;31:58–64. doi: 10.1097/01.pas.0000213312.36306.cc. [DOI] [PubMed] [Google Scholar]
- 9.Chirieac LR, Swisher SG, Ajani JA, et al. et al. Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer. 2005;103:1347–1355. doi: 10.1002/cncr.20916. [DOI] [PubMed] [Google Scholar]
- 10.Mandard AM, Dalibard F, Mandard JC, et al. et al. Pathologic assessment of tumor regression after preoperative chemo-radiotherapy of esophageal carcinoma. Clinicopathologic correlations Cancer. 1994;73:2680–2686. doi: 10.1002/1097-0142(19940601)73:11<2680::aid-cncr2820731105>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 11.Swisher SG, Hofstetter W, Wu TT, et al. et al. Proposed revision of the esophageal cancer staging system to accommodate pathologic response (PP) following preoperative chemoradiation (CRT) Ann Surg. 2005;241:810–820. doi: 10.1097/01.sla.0000161983.82345.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ajani JA, Barthel JS, Bentrem DJ, et al. et al. Esophageal and esophagogastric junction cancers. J Natl Compr Canc Netw. 2011;9:830–887. doi: 10.6004/jnccn.2011.0072. [DOI] [PubMed] [Google Scholar]
- 13.Iyer R, Wilkinson N, Demmy T, et al. et al. Controversies in the multimodality management of locally advanced esophageal cancer: evidence-based review of surgery alone and combined-modality therapy. Ann Surg Oncol. 2004;11:665–673. doi: 10.1245/ASO.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Imdahl A, Schoffel U, Ruf G. Impact of neoadjuvant therapy of perioperative morbidity in patients with esophageal cancer. Am J Surg. 2004;187:64–68. doi: 10.1016/j.amjsurg.2002.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Doty JR, Salazar JD, Forastiere AA, et al. et al. Postesophagectomy morbidity, mortality, and length of hospital stay after preoperative chemoradiation therapy. Ann Thorac Surg. 2002;74:227–231. doi: 10.1016/s0003-4975(02)03655-x. [DOI] [PubMed] [Google Scholar]
- 16.Hingorani M, Crosby T, Maraveyas A, et al. et al. Neoadjuvant chemoradiotherapy for resectable oesophageal and gastro-oesophageal junction cancer—do we need another randomised trial? Clin Oncol (R Coll Radiol) 2011;23:696–705. doi: 10.1016/j.clon.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 17.van Meerten E, van der Gaast A, Looman CW, et al. et al. Quality of life during neoadjuvant treatment and after surgery for resectable esophageal carcinoma. Int J Radiat Oncol Biol Phys. 2008;71:160–166. doi: 10.1016/j.ijrobp.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 18.Burmeister BH, Smithers BM, Gebski V, et al. et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol. 2005;6:659–668. doi: 10.1016/S1470-2045(05)70288-6. [DOI] [PubMed] [Google Scholar]
- 19.Kaklamanos IG, Walker GR, Ferry K, et al. et al. Neoadjuvant treatment for resectable cancer of the esophagus and the gastroesophageal junction: a meta-analysis of randomized clinical trials. Ann Surg Oncol. 2003;10:754–761. doi: 10.1245/aso.2003.03.078. [DOI] [PubMed] [Google Scholar]
- 20.Lv J, Cao XF, Zhu B, et al. et al. Effect of neoadjuvant chemoradiotherapy on prognosis and surgery for esophageal carcinoma. World J Gastroenterol. 2009;15:4962–4968. doi: 10.3748/wjg.15.4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaast AV, van Hagen P, Hulshof M, et al. et al. Effect of preoperative concurrent chemoradiotherapy on survival of patients with resectable esophageal or esophagogastric junction cancer: results from a multicenter randomized phase III study. ASCO Meeting Abstracts. 2010;28:4004. [Google Scholar]
- 22.Tepper J, Krasna MJ, Niedzwiecki D, et al. et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26:1086–1092. doi: 10.1200/JCO.2007.12.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mariette C, Seitz JF, Maillard E, et al. et al. Surgery alone versus chemoradiotherapy followed by surgery for localized esophageal cancer: analysis of a randomized controlled phase III trial FFCD 9901. ASCO Meeting Abstracts. 2010;28:4005. [Google Scholar]
- 24.Bedenne L, Michel P, Bouche O, et al. et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25:1160–1168. doi: 10.1200/JCO.2005.04.7118. [DOI] [PubMed] [Google Scholar]
- 25.Medical Research Council Oesophageal Cancer Working Group Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet. 2002;359:1727–1733. doi: 10.1016/S0140-6736(02)08651-8. [DOI] [PubMed] [Google Scholar]
- 26.Boige V, Pignon J, Saint-Aubert B, et al. et al. Final results of a randomized trial comparing preoperative 5-fluorouracil (F)/ cisplatin (P) to surgery alone in adenocarcinoma of stomach and lower esophagus (ASLE): FNLCC ACCORD07-FFCD 9703 trial. ASCO Meeting Abstracts. 2007;25:4510. [Google Scholar]
- 27.Cunningham D, Allum WH, Stenning SP, et al. et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 28.Kelsen DP, Ginsberg R, Pajak TF, et al. et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med. 1998;339:1979–1984. doi: 10.1056/NEJM199812313392704. [DOI] [PubMed] [Google Scholar]
- 29.Sjoquist KM, Burmeister BH, Smithers BM, et al. et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12:681–692. doi: 10.1016/S1470-2045(11)70142-5. [DOI] [PubMed] [Google Scholar]
- 30.Chang F, Deere H, Mahadeva U, et al. et al. Histopathologic examination and reporting of esophageal carcinomas following preoperative neoadjuvant therapy: practical guidelines and current issues. Am J Clin Pathol. 2008;129:252–262. doi: 10.1309/CCR3QN4874YJDJJ7. [DOI] [PubMed] [Google Scholar]
- 31.Pennathur A, Luketich JD, Landreneau RJ, et al. et al. Long-term results of a phase II trial of neoadjuvant chemotherapy followed by esophagectomy for locally advanced esophageal neoplasm. Ann Thorac Surg. 2008;85:1930–1937. doi: 10.1016/j.athoracsur.2008.01.097. [DOI] [PubMed] [Google Scholar]
- 32.Ruhstaller T, Widmer L, Schuller JC, et al. et al. Multicenter phase II trial of preoperative induction chemotherapy followed by chemoradiation with docetaxel and cisplatin for locally advanced esophageal carcinoma (SAKK 75/02) Ann Oncol. 2009;20:1522–1528. doi: 10.1093/annonc/mdp045. [DOI] [PubMed] [Google Scholar]
- 33.Langer R, Ott K, Feith M, et al. et al. Prognostic significance of histopathological tumor regression after neoadjuvant chemotherapy in esophageal adenocarcinomas. Mod Pathol. 2009;22:1555–1563. doi: 10.1038/modpathol.2009.123. [DOI] [PubMed] [Google Scholar]
- 34.Yano M, Yasuda T, Miyata H, et al. et al. Correlation between histological effects on the main tumors and nodal status after chemoradiotherapy for squamous cell carcinoma of the esophagus. J Surg Oncol. 2005;89:244–250. doi: 10.1002/jso.20209. [DOI] [PubMed] [Google Scholar]
- 35.Prenzel KL, Konig A, Schneider PM, et al. et al. Reduced incidence of nodal micrometastasis after major response to neoadjuvant chemoradiation in locally advanced esophageal cancer. Ann Surg Oncol. 2007;14:954–959. doi: 10.1245/s10434-006-9141-6. [DOI] [PubMed] [Google Scholar]
- 36.Siewert JR, Stein HJ, Feith M, et al. et al. Histologic tumor type is an independent prognostic parameter in esophageal cancer: lessons from more than 1,000 consecutive resections at a single center in the western world. Ann Surg. 2001;234:360–369. doi: 10.1097/00000658-200109000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gebski V, Burmeister B, Smithers BM, et al. et al. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol. 2007;8:226–234. doi: 10.1016/S1470-2045(07)70039-6. [DOI] [PubMed] [Google Scholar]
- 38.Schneider PM, Baldus SE, Metzger R, et al. et al. Histomorphologic tumor regression and lymph node metastases determine prognosis following neoadjuvant radiochemotherapy for esophageal cancer: implications for response classification. Ann Surg. 2005;242:684–692. doi: 10.1097/01.sla.0000186170.38348.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]