Abstract
Sapacitabine is an orally bioavailable prodrug of the nucleoside analog 2′-C-cyano-2′-deoxy-1-β-D-arabino-pentofuranosylcytosine (CNDAC). Both the prodrug and active metabolite are in clinical trials for hematologic malignancies and/or solid tumors. CNDAC has a unique mechanism of action: after incorporation into DNA, it induces single-strand breaks (SSBs) that are converted into double-strand breaks (DSBs) when cells go through a second S phase. In our previous studies, we demonstrated that CNDAC-induced SSBs can be repaired by the transcription-coupled nucleotide excision repair pathway, whereas lethal DSBs are mainly repaired through homologous recombination. In the current work, we used clonogenic assays to compare the DNA damage repair mechanism of CNDAC with two other deoxycytidine analogs: cytarabine, which is used in hematologic malignacies, and gemcitabine, which shows activity in solid tumors. Deficiency in two Rad51 paralogs, Rad51D and XRCC3, greatly sensitized cells to CNDAC, but not to cytarabine or gemcitabine, indicating that homologous recombination is not a major mechanism for repairing damage caused by the latter two analogs. This study further suggests clinical activity and application of sapacitabine that is distinct from that of cytarabine or gemcitabine.
Keywords: Sapacitabine, CNDAC, cytarabine, gemcitabine, homologous recombination
Nucleoside analogs are more prevalent than other classes of structurally related therapeutic agents in the treatment of cancer and viral diseases. However, enzymes governing DNA synthesis and metabolism presumably have differential and largely unpredictable affinities for nucleoside analog substrates due to their structural differences, which could be very small in some cases. It is remarkable that nucleosides with similar structures vary so broadly with regard to cellular metabolic characteristics, mechanisms of action, and disease targets. Most impressively, nucleoside analogs that possess closely related structures, share metabolic pathways, and inhibit similar target enzymes, still exhibit a diverse spectrum of anticancer activities in the clinic[1]. In this study we compared the relative importance of the homologous recombination (HR) repair mechanism for the biological activities of three cytosine nucleoside analogs that are structurally and metabolically closely related.
Cytarabine (cytosine arabinoside), is the paradigm of cytosine nucleoside analogs in cancer chemotherapy. Since its introduction to clinical use over 40 years ago, it has become the major drug in acute myeloid leukemia (AML) treatment and can cure a fraction of newly-diagnosed adults when administered alone at a pharmacologically indicated dose[2]. Despite this performance, the spectrum of activity of cytarabine is focused on hematologic malignancies, with little activity alone or in combination with other drugs or modalities in solid tumors. Because of this, considerable effort has been directed at the synthesis and evaluation of other cytosine nucleoside analogs in the hope of identifying a potential drug with activity in other tumors. Although a number of compounds from these groups have reached clinical trials, only the 2′,2′-difluoro-derivative, gemcitabine[3], has shown convincing clinical activity[4]. However, this provides substantial evidence that the nucleoside analog can be delivered to solid tumors and implies that a wide spectrum of malignancies (pancreatic, breast, ovarian, small cell lung, non-small cell lung, bladder, and germ cell cancers) has the enzymes required to anabolize cytosine nucleoside analogs such as gemcitabine to active nucleotides.
In recent years, the promising activities of CNDAC in preclinical models has provided rationale for clinical trials of this novel deoxycytidine analog and its prodrug[1]. Most nucleoside analogs are administered intravenously in practice. N-(1-(2-cyano-)2-deoxy-β-D-arabino-pentofuranosyl)-N4-palmitoylcytosine (sapacitabine) is a nucleoside analog prodrug that has the advantage of oral administration. The fatty acid side chain (palmitoyl group) on the N4 group of the cytosine moiety improves oral bioavailability and reduces inactivation by deamination[5]. The same prodrug has been designated chronologically as CS-682, CYC-682, and sapacitabine by pharmaceutical companies that have sponsored its clinical development[1]. It is currently in clinical trials for hematologic malignancies and solid tumors, showing encouraging clinical activities. Table 1 summarizes clinical trials of sapacitabine at various phases in a variety of cancers[6]–[16]. Two multicenter phase I trials with different CS-682 schedules in patients with advanced solid tumors were reported in 2006[14],[15]. Cyclacel Pharmaceuticals (Berkeley Heights, NJ, USA) is currently sponsoring a phase I trial of sapacitabine in combination with the Cyclin-dependent kinase (CDK) inhibitor seliciclib in advanced solid tumors and a phase II trial of sapacitabine for non-small cell lung cancer.
Table 1. Clinical trials of sapacitabine in solid tumors and hematologic malignancies.
| Disease | Stage of clinical trial | Number of Patients | Status | Reference (s) |
| Advanced solid tumors | Phase I | 48 | Completed | [15] |
| Advanced solid tumors | Phase I | 40 | Completed | [14] |
| Relapsed acute leukemia & MDS | Phase I | 47 | Completed | [12],[16] |
| Advanced solid tumors (in combination with CDK inhibitor seliciclib) | Phase I | NA | Ongoing | [9] |
| Cutaneous T-cell lymphoma | Phase II | NA | Ongoing | [13] |
| Elderly AML & MDS | Phase II | NA | Ongoing | [10] |
| Elderly AML (alternating with decitabine) | Phase I/II | NA | Ongoing | [8] |
| Elderly AML (SEAMLESS pivotal trial) | Phase III | NA | Ongoing | [7] |
| CLL (in patients with del11q22-23) | Phase II | NA | Ongoing | [6] |
| Non-small cell lung cancer | Phase II | NA | Ongoing | [1] |
MDS, myelodysplastic syndrome; CDK, Cyclin-dependent kinase; AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; NA, not available.
By the action of amidases, sapacitabine is metabolized into its active form, 2′-C-cyano-2′-deoxy-1-β-D-arabino-pentofuranosylcytosine (CNDAC). Conceptualized by Matsuda et al.[17], CNDAC exhibited a unique mechanism of causing single-strand breaks (SSBs) through a β-elimination rearrangement after incorporation into DNA during the first replication. CNDAC-induced SSBs, which could be repaired by the transcription-coupled nucleotide excision repair (TC-NER) mechanism[18], are converted into one-ended double-strand breaks (DSBs) when cells enter a second Sphase[19]. Such DSBs are predominantly repaired through the HR pathway, demonstrated by significantly increased sensitivity to CNDAC[19] and compromised level of sister chromatid exchanges (Liu et al., unpublished data) in cells lacking HR components.
To better understand the diversity in mechanisms of action of nucleoside analogs, in particular deoxycytidine analogs, we compared sapacitabine/CNDAC with cytarabine and gemcitabine in respect to the role of HR in DNA damage repair.
Materials and Methods
Cell culture
The Chinese hamster ovary (CHO) cell line AA8 (wild type) was purchased from the American Type Culture Collection. irslSF, an AA8-derived line mutant in XRCC3[20], was a kind gift from Dr. R. Legerski (The University of Texas M. D. Anderson Cancer Center, Houston, TX, USA). Another AA8-derived Rad51D knockout line, 51D1, and Rad51D-complemented line, 51D1.3[21], were generous gifts from Dr. L. Thompson (Lawrence Livermore National Laboratory, Livermore, CA, USA). Cells were maintained in exponential growth phase in α-MEM (free of ribonucleosides and deoxyribonucleosides) supplemented with 10% heat-inactivated fetal bovine serum at 37°C in a humidified atmosphere with 5% CO2. The population doubling time of the CHO lines was approximately 15 h. All cells were free of mycoplasma, as determined by Characterized Cell Line Core Facility at M. D. Anderson using the MycoAlert kit (Lonza, Basel, Switzerland).
Chemicals
The nucleoside analog CNDAC was synthesized as previously described[17]. Cytarabine and gemcitabine were from Sigma-Aldrich (St. Louis, MO, USA) and Eli Lilly Co. (Indianapolis, IN, USA), respectively. A stock solution of CNDAC (15-25 mmol/L) was prepared in phosphate-buffered saline (PBS) (pH 6.5), sterilized by filtration, stored at -20°C, and diluted in sterile PBS (pH 6.5) just before use. Solutions of cytarabine and gemcitabine (1 mmol/L each) were made by the same procedures, except in PBS (pH 7.4). All other chemicals were reagent-grade.
Clonogenic survival assay
Exponentially growing cells were seeded in 6-well plates overnight to allow attachment before cells were exposed to a range of concentrations of drugs for 24 h. After washing into drug-free medium, cells were incubated for 4 to 6 days, and colonies in triplicate wells were stained with crystal violet in 100% ethanol. GelCount (Oxford Optronix Ltd., Oxford, UK) was used to scan the plates and electronically count colony numbers.
Statistical analysis
IC50, values were calculated by using GraphPad Prism5 software (GraphPad Software, Inc.) based on survival curves generated in this program. Intergroup comparisons of clonogenic survival in response to drugs were performed using two-tailed distribution, two-sample unequal variance t test.
Results
Sapacitabine/CNDAC acts by a mechanism of action distinct from cytarabine and gemcitabine
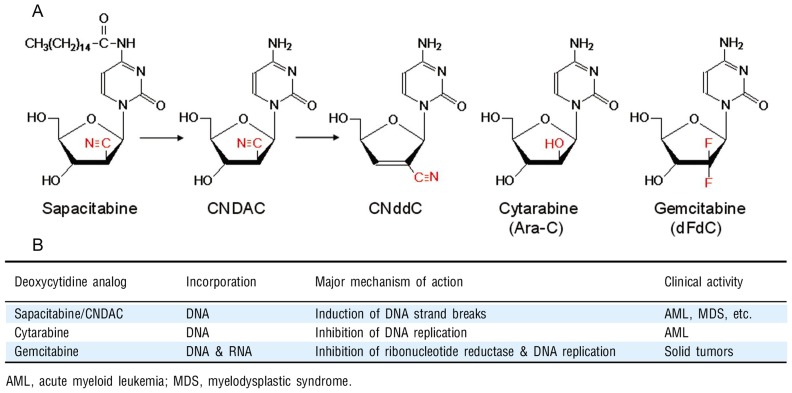
The growth inhibitory actions and cytotoxic activities of many nucleoside analogs require the incorporation of the triphosphate into DNA. Therefore, the fraction of cell population in S phase at the time of drug exposure is specifically impacted. Figure 1 compares sapacitabine/CNDAC with cytarabine and gemcitabine in respect to their structures, major mechanisms of action, and clinical disease targets. All analogs depend on the same deoxycytidine kinase to phosphorylate them into monophosphates. Upon incorporation into DNA, both cytarabine and gemcitabine inhibit chain extension, stall replication forks, and arrest cells in S phase [22]. In addition to inhibition of DNA replication, gemcitabine inhibits ribonucleotide reductase and has a mode of self-potentiation[23]. Gemcitabine has also been reported to have epigenetic function in which it specifically inhibits Gadd45a-mediated reporter gene activation and DNA demethylation[24].
Figure 1. Comparison of deoxycytidine analogs. A, structures; B, mechanisms and clinical activities.
CNDAC, designed as a mechanism-based DNA self-strand breaking nucleoside[17], has a unique mechanism of action distinct from the above two cytosine analogs. Incorporation of CNDAC into DNA does not stall the replication fork. However, addition of other deoxynucleotides at the 3′-terminus of the DNA chain by DNA polymerase leads to structural instability and a subsequent rearrangement by β-elimination, due to the electron-drawing nature of the cyano group in the sugar moiety of CNDAC[25]. This process results in the formation of 2′-C-cyano-2′, 3′-didehydro-2′, 3′-dideoxycytidine (CNddC, Figure 1A), which lacks a 3′-hydroxyl group and therefore is a de facto chain terminator. This signature nucleoside at the 3′-terminus is unique and linked to formation of an SSB that could not be repaired by ligation. CNDAC-induced SSBs can be repaired to some extent by the TC-NER pathway, whereas cytarabine-induced damage repair is independent of this pathway[18]. Unrepaired CNDAC-induced SSBs are converted to one-ended DSBs when cells enter the next cell cycle. Evidence indicates that HR, not the non-homologous end-joining (NHEJ) pathway, is the major mechanism for this type of DSB repair[19]. This is supported by the fact that CNDAC greatly sensitizes cells lacking key components in this pathway, such as ATM, Rad51D(a Rad51 paralog), and two Rad51-interacting proteins, XRCC3 and BRCA2. This finding is of particular interest for two reasons. First, it could serve as a resistance mechanism for cancers treated by sapacitabine/CNDAC-containing regimens. Second and more importantly, it raises the rationale for targeting cancer subtypes with a deficiency in HR function[1].
Dependence of sapacitabine/CNDAC and independence of cytarabine and gemcitabine on HR-mediated repair of DNA damage
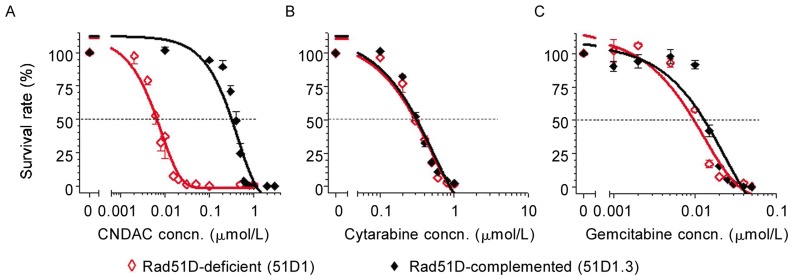
To maximize the clinical potential of sapacitabine, we sought to focus on the HR pathway and ask if HR is also required in cellular response to cytarabine or gemcitabine. CHO cells deficient in Rad51D and complemented with Rad51D were used to compare clonogenic sensitivity to CNDAC, cytarabine, and gemcitabine. The Rad51D-null cell line was approximately 50-fold more sensitive to CNDAC (IC50 = 0.006 µmol/L) compared to 51D1.3, the Rad51D-repleted line (IC50 = 0.32 µmol/L) (Figure 2A). By contrast, the deficient cells were not sensitized to either cytarabine (IC50, ∼0.3 µmol/L) or gemcitabine (IC50, ∼0.01 µmol/L) (Figures 2B and 2C).
Figure 2. Lack of Rad51D sensitizes cells to sapacitabine/CNDAC, but not to cytarabine or gemcitabine.
Clonogenic assays were performed in Rad51D-deficient 51D1 (◊) and Rad51D-complemented 51D1.3 (♦) CHO cells and representative data are shown. Cells were exposed to a range of concentrations of nucleoside analogs (A, CNDAC; B, cytarabine; C, gemcitabine) 24 h after plating and incubated for 24 h with drugs. Then, cells were incubated for an additional 4 days after washing out drugs to allow colonies to grow. Each point on the survival curves is the mean ± SD of triplicates.
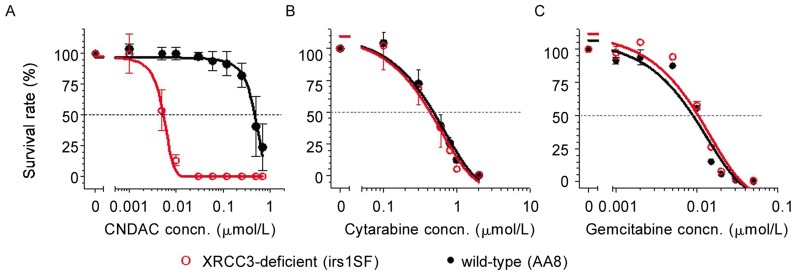
To further address the dependency of cells on HR for survival after CNDAC treatment, we compared cells deficient in XRCC3, which is essential for the function of the key recombinase Rad51 in HR, with wild-type cells. The sensitization due to the lack of XRCC3 was even greater than that of Rad51D. AA8 cells (wild-type, IC50 = 0.48 µmol/L) were almost 90-fold more resistant to CNDAC than cells lacking XRCC3 (IC50 = 0.0053 µmol/L) (Figure 3A). This result is consistent with our previous studies using these cell lines[19]. Similar to Rad51D-null cells, XRCC3-deficient cells were equally sensitive to cytarabine (IC50 = 0.4–0.5 µmol/L) and gemcitabine (IC50 = 0.009–0.010 µmol/L) relative to proficient cells (Figures 3B and 3C). Fold sensitization of cells lacking Rad51D or XRCC3 to CNDAC and to the other two deoxycytidine analogs is summarized in Table 2, which demonstrates that HR is essential for DNA damage repair after treatment with sapacitabine/CNDAC, whereas this pathway is dispensable for repair after treatment with cytarabine or gemcitabine.
Figure 3. Lack of XRCC3 sensitizes cells to sapacitabine/CNDAC, but not to cytarabine or gemcitabine.
Clonogenic assays were performed in wild-type AA8 (○) and XRCC3-deficient irs1SF (•) CHO cell lines, and representative data are shown. Cells were exposed to a range of concentrations of nucleoside analogs (A, CNDAC; B, cytarabine; C, gemcitabine) 24 h after plating and incubated for 24 h with drugs. Then cells were incubated for an additional 4 days (AA8) or 6 days (irs1SF) after washing out drugs to allow colonies to grow. Each point on the survival curves is the mean ± SD of triplicates.
Table 2. Deficiency in homologous recombination (HR) confers sensitivity to CNDAC, but not to cytarabine or gemcitabine.
| Agent | Fold sensitization in HR-deficient cells |
|
| Rad51D | XRCC3 | |
| CNDAC | 50** | 89** |
| Cytarabine | 1.3 | 1.1 |
| Gemcitabine | 1.2 | 0.9 |
| MMC | 53* | 168** |
*P < 0.01; **P < 0.001.
Validati on of cell lines deficient in key HR components
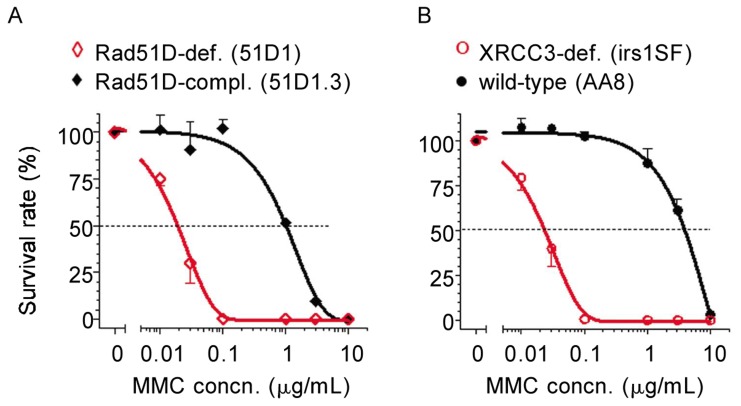
To validate the two pairs of CHO cell lines used in this study, we tested their clonogenic sensitivities to mitomycin C (MMC), an alkylating and inter-strand crosslinking agent that causes DNA DSBs. It is well documented that cells lacking HR function are greatly sensitized to a panel of DNA damaging agents, including MMC[26]. Complementation of Rad51D in deficient cells conferred about 50-fold more resistance to MMC (Figure 4A and Table 2), whereas XRCC3-deficient cells were sensitized more than 160-fold to MMC compared to wild-type cells (Figure 4B and Table 2). The IC50 values in both Rad51D- and XRCC3-deficient cells were close to 0.02 µg/mL (equivalent to 0.06 µmol/L), consistent with the original studies with these cell lines[20],[21]. These results clearly demonstrated that the cell lines were valid for evaluating the functional role of HR proteins in DNA damage repair after nucleoside analogs.
Figure 4. Validation of CHO cell lines. Clonogenic assays were performed in paired CHO lines.
A, Rad51D-deficient 51D1 (◊) and Rad51D-complemented 51D1.3 (♦); B, wild-type AA8 (•) and XRCC3-deficient irs1SF (○). After 24 h of plating, cells were exposed to a range of concentrations of mitomycin C (MMC) for 24 h. Then, cells were incubated for an additional 4 days (51D1, 51D1.3, and AA8) or 6 days (irs1SF) after washing out MMC to allow colonies to grow. Each point on the survival curves is the mean ± SD of triplicates.
Discussion
In this study, we used isogenically paired cell lines deficient in selected proteins in the HR pathway for DSB repair to compare the role the pathway plays in the cytotoxic action of three cytosine nucleoside analogs. The results demonstrate that the viability of cells treated with CNDAC is substantially compromised by the lack of this repair pathway, whereas no sensitization was evident for either cytarabine or gemcitabine.
NER functions in concert with HR, and HR plays a predominant role; however, base excision repair (BER), mismatch repair (MMR), and NHEJ pathways are all dispensable. Our study on the repair mechanism for CNDAC may help determine subtypes of tumor (i.e. those dysfunctional in HR) that may be greatly sensitized to sapacitabine. In this sense, personalized treatment strategies will be enabled in patient subpopulations whose tumors have been identified to have deficiency in HR proteins, such as ATM and BRCA2. Our findings also suggest that sapacitabine may overcome resistance to current front-line therapies and identify synergistic interactions of sapacitabine with known anticancer drugs. Overall, such investigations may provide the rationale for the design of sapacitabine- based clinical trials[1].
Because of its unique mechanism of action, ease of administration, favorable tolerability, and defined dose-limiting toxicity of neutropenia in solid tumors, sapacitabine has attracted interest for investigations in hematologic malignancies, especially AML and myelodysplatic syndrome (MDS). Related phase I and II trials have indicated the benefit of sapacitabine therapy for AML in elderly patients, who have poor tolerance to intensive chemotherapy[16]. Trials of sapacitabine in combination with established agents, such as decitabine, have been initiated[27] and phase I/II trials are ongoing[8]. It is noteworthy that sapacitabine is currently being studied in combination with Cytoxan and rituximab (SCR regimen) for chronic lymphocytic leukemia (CLL) patients with del(11q22-23) in an attempt to substitute fludarabine with sapacitabine to overcome resistance to the front-line fludarabine- cytoxan-rituximab (FCR) regimen[6]. The rationale for this trial is based on the repair mechanism for CNDAC-induced DNA damage.
So far, the repair mechanisms for the DNA damage caused by cytarabine and gemcitabine are largely unclear. Fordham et al.[28] found that a B-lymphoblastoid cell line with biallelic mutations in MSH6 was sensitized to cytarabine, suggesting that MMR is involved in repair of cytarabine-initiated damage. Crul et al.[29] reported that none of the other four DNA repair pathways (BER, NER, HR, and NHEJ) is likely capable of modulating the cytotoxicity of gemcitabine. Consistently, our studies indicate that neither NER nor HR is responsible for removal of these two nucleoside analogs from DNA. The mechanism by which sapacitabine acts differs remarkably from the mechanism by which these two analogs act. The distinct dependency of sapacitabine on NER and more significantly on the HR pathway is uncommon among nucleoside analogs, providing a novel alternative to nucleoside analog-based therapeutic regimens. Moreover, it strongly suggests that sapacitabine could be a potential candidate for overcoming resistance to cytarabine or gemcitabine in cancer chemotherapy.
Conclusions
Sapacitabine, the orally bioavailable prodrug of CNDAC, has shown encouraging anticancer activities in clinical trials. CNDAC has a unique action mechanism of inducing DNA SSBs and DSBs after incorporation and cell cycle progression into a second S phase, respectively. The HR pathway is indispensable for repair of CNDAC-induced DSBs, whereas it is not required for repair of damage by cytarabine or gemcitabine. Together, this study indicates that sapacitabine/CNDAC may have clinical applications different from and/or complementary to the latter two deoxycytidine analogs.
Acknowledgments
This study was supported by grants from the National Cancer Institute, Department of Health and Human Services, USA (CA28596, CA81534 and CA100632).
References
- 1.Liu X, Kantarjian H, Plunkett W. Sapacitabine for cancer. Expert Opin Investig Drugs. 2012;21:541–555. doi: 10.1517/13543784.2012.660249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghaddar HM, Plunkett W, Kantarjian HM, et al. et al. Long-term results following treatment of newly-diagnosed acute myelogenous leukemia with continuous-infusion high-dose cytosine arabinoside. Leukemia. 1994;8:1269–1274. [PubMed] [Google Scholar]
- 3.Hertel LW, Boder GB, Kroin JS, et al. et al. Evaluation of the antitumor activity of gemcitabine (2′,2′-difluoro-2′-deoxy-cytidine) Cancer Res. 1990;50:4417–4422. [PubMed] [Google Scholar]
- 4.Kaye SB. Gemcitabine: current status of phase I and II trials. J Clin Oncol. 1994;12:1527–1531. doi: 10.1200/JCO.1994.12.8.1527. [DOI] [PubMed] [Google Scholar]
- 5.Hanaoka K, Suzuki M, Kobayashi T, et al. et al. Antitumor activity and novel DNA-self-strand-breaking mechanism of CNDAC (1-(2-C-cyano-2-deoxy-beta-D-arabino-pentofuranosyl)cytosine) and its N4-palmitoyl derivative (CS-682) Int J Cancer. 1999;82:226–236. doi: 10.1002/(sici)1097-0215(19990719)82:2<226::aid-ijc13>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 6.Sapacitabine, cyclophosphamide, rituximab for relapsed chronic lymphocytic leukemia, small lymphocytic lymphoma (CLL/SLL) with deletion (11q22-23) ClinicalTrials.gov, Identifier: NCT01253460.
- 7.A study of oral sapacitabine in elderly patients with newly diagnosed acute myeloid leukemia (SEAMLESS) ClinicalTrials.gov, Identifier: NCT01303796. [DOI] [PMC free article] [PubMed]
- 8.Study of sapacitabine in acute myeloid leukemia (AML) ClinicalTrials.gov, Identifier: NCT01211457.
- 9.A study of oral sapacitabine and oral seliciclib in patients with advanced solid tumors. ClinicalTrials.gov, Identifier: NCT00999401.
- 10.Efficacy study of oral sapacitabine to treat acute myeloid leukemia in elderly patients. ClinicalTrials.gov, Identifier: NCT00590187.
- 11.A study of oral sapacitabine in patients with previously treated non-small cell lung cancer. ClinicalTrials.gov, Identifier: NCT00885963.
- 12.Safety and pharmacology study of sapacitabine to treat advanced leukemias or myelodysplastic syndromes. ClinicalTrials.gov, Identifier: NCT00380653.
- 13.A randomized phase II study of oral sapacitabine in patients with advanced cutaneous T-cell lymphoma. ClinicalTrials.gov, Identifier: NCT00476554.
- 14.Delaunoit T, Burch PA, Reid JM, et al. et al. A phase IL clinical and pharmacokinetic study of CS-682 administered orally in advanced malignant solid tumors. Invest New Drugs. 2006;24:327–333. doi: 10.1007/s10637-006-5392-0. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert J, Carducci MA, Baker SD, et al. et al. A phase I study of the oral antimetabolite, CS-682, administered once daily 5 days per week in patients with refractory solid tumor malignancies. Invest New Drugs. 2006;24:499–508. doi: 10.1007/s10637-006-8219-0. [DOI] [PubMed] [Google Scholar]
- 16.Kantarjian H, Garcia-Manero G, O'Brien S, et al. et al. Phase I clinical and pharmacokinetic study of oral sapacitabine in patients with acute leukemia and myelodysplastic syndrome. J Clin Oncol. 2010;28:285–291. doi: 10.1200/JCO.2009.25.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuda A, Nakajima Y, Azuma A, et al. et al. Nucleosides and nucleotides. 100. 2′-C-cyano-2′-deoxy-1-beta-D-arabinofuranosyl-cytosine (CNDAC): design of a potential mechanism-based DNA-strand-breaking antineoplastic nucleoside. J Med Chem. 1991;34:2917–2919. doi: 10.1021/jm00113a034. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Liu X, Matsuda A, et al. et al. Repair of 2′-C-cyano-2′-deoxy-1 -beta-D-arabino-pentofuranosylcytosine-induced DNA single-strand breaks by transcription-coupled nucleotide excision repair. Cancer Res. 2008;68:3881–3889. doi: 10.1158/0008-5472.CAN-07-6885. [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Wang Y, Benaissa S, et al. et al. Homologous recombination as a resistance mechanism to replication-induced double-strand breaks caused by the antileukemia agent CNDAC. Blood. 2010;116:1737–1746. doi: 10.1182/blood-2009-05-220376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tebbs RS, Zhao Y, Tucker JD, et al. et al. Correction of chromosomal instability and sensitivity to diverse mutagens by a cloned cDNA of the XRCC3 DNA repair gene. Proc Natl Acad Sci U S A. 1995;92:6354–6358. doi: 10.1073/pnas.92.14.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hinz JM, Tebbs RS, Wilson PF, et al. et al. Repression of mutagenesis by Rad51D-mediated homologous recombination. Nucleic Acids Res. 2006;34:1358–1368. doi: 10.1093/nar/gkl020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi Z, Azuma A, Sampath D, et al. et al. S-phase arrest by nucleoside analogues and abrogation of survival without cell cycle progression by 7-hydroxystaurosporine. Cancer Res. 2001;61:1065–1072. [PubMed] [Google Scholar]
- 23.Plunkett W, Huang P, Xu YZ, et al. et al. Gemcitabine: metabolism, mechanisms of action, and self-potentiation. Semin Oncol. 1995;22:3–10. [PubMed] [Google Scholar]
- 24.Schafer A, Schomacher L, Barreto G, et al. et al. Gemcitabine functions epigenetically by inhibiting repair mediated DNA demethylation. PLoS One. 2010;5:e14060. doi: 10.1371/journal.pone.0014060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azuma A, Huang P, Matsuda A, et al. et al. 2′-C-cyano-2′-deoxy-1-beta-D-arabino-pentofuranosylcytosine: a novel anticancer nucleoside analog that causes both DNA strand breaks and G2 arrest. Mol Pharmacol. 2001;59:725–731. doi: 10.1124/mol.59.4.725. [DOI] [PubMed] [Google Scholar]
- 26.Al-Minawi AZ, Lee YF, Hakansson D, et al. et al. The ERCC1/XPF endonuclease is required for completion of homologous recombination at DNA replication forks stalled by inter-strand cross-links. Nucleic Acids Res. 2009;37:6400–6413. doi: 10.1093/nar/gkp705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ravandi F, Faderl S, Cortes JE, et al. et al. Phase I/II study of sapacitabine and decitabine administered sequentially in elderly patients with newly diagnosed acute myeloid leukemia. ASCO Meeting Abstracts. 2011;29:6587. [Google Scholar]
- 28.Fordham SE, Matheson EC, Scott K, et al. et al. DNA mismatch repair status affects cellular response to Ara-C and other anti-leukemic nucleoside analogs. Leukemia. 2011;25:1046–1049. doi: 10.1038/leu.2011.38. [DOI] [PubMed] [Google Scholar]
- 29.Crul M, van Waardenburg RC, Bocxe S, et al. et al. DNA repair mechanisms involved in gemcitabine cytotoxicity and in the interaction between gemcitabine and cisplatin. Biochem Pharmacol. 2003;65:275–282. doi: 10.1016/s0006-2952(02)01508-3. [DOI] [PubMed] [Google Scholar]