Abstract
Ataxia-telangiectasia (A-T) is an autosomal recessive disorder characterized by cerebellar ataxia and oculocutaneous telangiectasias. The gene mutated in this disease, ATM (A-T, mutated), encodes a 370-kDa Ser/Thr protein kinase. ATM not only mediates cellular response to DNA damage but also acts as an activator of Akt in response to insulin. However, despite intensive studies, the mechanism underlying the neuronal degeneration symptoms of human A-T is still poorly understood. We found that the topoisomerase inhibitors etoposide and camptothecin readily induced apoptosis in undifferentiated proliferating SH-SY5Y cells but could not induce apoptosis in neuronally differentiated SH-SY5Y cells. In addition, etoposide induced p53 phosphorylation and H2AX foci formation in proliferating SH-SY5Y cells but failed to do so in differentiated SH-SY5Y cells. Moreover, while inhibition of ATM in undifferentiated SH-SY5Y cells partially protected them from etoposide-induced apoptosis, the same treatment had no effect on cell viability in differentiated SH-SY5Y cells. These results suggest that DNA damage or defective response to DNA damage is not the cause of neuronal cell death in human A-T. In contrast, we discovered that Akt phosphorylation was inhibited when ATM activity was suppressed in differentiated SH-SY5Y cells. Furthermore, inhibition of ATM induced apoptosis following serum starvation in neuronally differentiated SH-SY5Y cells but could not trigger apoptosis under the same conditions in undifferentiated proliferating SH-SY5Y cells. These results demonstrate that ATM mediates the Akt signaling and promotes cell survival in neuron-like human SH-SY5Y cells, suggesting that impaired activation of Akt is the reason for neuronal degeneration in human A-T.
Keywords: ATM, Akt, DNA damage, neuronal degeneration, neuronal differentiation
Ataxia-telangiectasia (A-T) is a rare, inherited autosomal recessive disorder. The hallmark of the A-T disease is cerebellar neuronal degeneration, shown by the death of Purkinje and granular cells in the cerebellar cortex[1]. ATM (A-T, mutated) is the sole gene mutated in this disease and encodes a Ser/Thr protein kinase that belongs to the phosphatidylinositol 3-kinase (PI3K) superfamily. ATM mediates cellular responses to DNA damage by phosphorylating its many downstream targets, thereby activating cell cycle checkpoints and causing cell cycle arrest to facilitate DNA damage repair and DNA recombination. When DNA damage is irreparable, ATM can induce apoptosis by promoting the accumulation of p53[2].
The mechanism by which the loss of ATM leads to neuronal cell death in A-T patients is still controversial. Some evidence suggests that defective nuclear function of ATM following DNA damage is responsible for neuronal degeneration in A-T[1]. However, the major role of nuclear ATM is to induce cell cycle arrest in proliferating cells in response to DNA damage. Human Purkinje cells and other neuronal cells are post-mitotic cells that do not need cell cycle arrest to facilitate DNA damage repair. ATM is reported to be largely cytoplasmic in human Purkinje cells and mouse cerebellar neuronal cells[3]–[5]. A study performed in human SH-SY5Y cells also showed that ATM translocates from the nucleus to the cytoplasm after the cells differentiate into neuron-like cells[6].
Cytoplasmic ATM is known as an insulin-responsive protein[7] that stimulates the phosphorylation of Akt at Ser 473[8],[9]. Defects in insulin signaling were reported to account for neuronal cell death[10]. Furthermore, the activation of Akt is required for differentiation of SH-SY5Y cells into neuron-like cells. Without activated Akt, SH-SY5Y cells have impaired differentiation[11]. In fact, ATM was reported to promote insulin-mediated cell survival, thereby preventing differentiated SH-SY5Y cells from undergoing apoptosis[6]. The transfection of kinase-dead ATM failed to prevent differentiated SH-SY5Y cells from cell death even in the presence of insulin[6]. Since ATM stimulates the phosphorylation of Akt at Ser473 in response to insulin, it is likely that ATM mediates growth factor-induced neuronal cell differentiation and survival by stimulating the phosphorylation of Akt, and the lack of ATM cytoplasmic function in A-T patients may contribute to the neuronal degeneration phenotype[12].
Since ATM may exhibit distinct functionality because of its different localization in proliferating and differentiated SH-SY5Y cells, we compared the response of ATM to DNA damage and growth factor signaling in differentiated and undifferentiated SH-SY5Y cells. Our results show that ATM is mainly responsible for nuclear response to DNA damage in undifferentiated SH-SY5Y cells, whereas ATM mediates the Akt signaling and promotes cell survival in neuronally differentiated SH-SY5Y cells. Our results suggest that while DNA damage or defective DNA damage response is not the cause of neuronal cell death in human A-T, impaired activation of Akt is the reason for neuronal degeneration in human A-T.
Materials and Methods
Materials
All trans-retinoic acid (RA), insulin, insulin-like growth factor-I (IGF-I) and brain-derived neurotrophic factor (BDNF), rapamycin, and LY294002 were purchased from Sigma. KU-55933 and NU7026 were from Calbiochem. The nerve growth factor (NGF) was from PeproTech. Antibodies against PARP, caspase-3, phospho-ATM (Ser1981), and phospho-Akt at Ser473 or Thr308 were from Cell Signaling. Anti-phospho-histone H2AX (Ser139) antibody (clone JBW301) was from Millipore. The antibodies against neural cell adhesion molecule (N-CAM) and β-actin were from Sigma.
Differentiation of SH-SY5Y cells
Human neuroblastoma SH-SY5Y cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) and antibiotics. For differentiation of SH-SY5Y cells, 2 × 105 cells were seeded in a 75-cm2 flask. After overnight culture, 15 µmol/L RA was added into the medium to promote differentiation. Medium and RA were replaced every 5 days. Differentiation usually took 21 days.
SDS-PAGE and cell lysis
Both floating and attached cells were collected and lysed with cold cell lysis buffer (50 mmol/L HEPES, 0.5% NP-40, 1.0% Tween-20, 150 mmol/L NaCl, 1 mmol/L PMSF, 1 mmol/L Na3VO4) supplemented with complete protease inhibitor cocktail for 2 h. The lysates were centrifuged at 14 000 r/min at 4°C for 10 min. The concentration of the collected supernatants was measured by using the Lowry Method (Bio-Rad). Proteins were separated in 4%-12% or 3%-8% NuPage gels (Invitrogen).
Immunoblotting
After SDS-PAGE, proteins were transferred to a PVDF or nitrocellulose membrane using XCell II™ Blot Module (Invitrogen). The membrane was blocked with 5% non-fat dry milk dissolved in TBST buffer (10 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 0.1% Tween-20) for 2 h at room temperature. The membrane was incubated with primary antibodies for 2 h at room temperature or overnight at 4°C. The membrane was washed using TBST buffer, and incubated for 1 h at room temperature with horseradish peroxidase (HRP) -conjugated secondary antibody. After being washed again, the membrane was incubated with Super Signal West Pico Chemiluminescent Substrate (Pierce) for 5 min. The resultant signal was detected using Blue Lite Autorad Film (ISC Bio Express).
Indirect immunofluorescence staining
Undifferentiated and differentiated SH-SY5Y cells were seeded in collagen (type I from rat tail)-coated chambered slides. Differentiated SH-SY5Y cells were cultured in RA-containing medium. After drug treatment, cells were washed with PBS and then fixed in 4% buffered paraformaldehyde for 20 min. Cells were then permeabilized after incubation in PBS containing 0.2% Triton X-100. After permeabilization, cells were first incubated with phospho-H2AX antibody overnight at 4°C and then incubated with Cy3-conjugated secondary antibody (Jackson ImmunoResearch). Following immunostaining, DAPI was used to stain the nuclei of cells. Images were recorded using Olympus FV1000 confocal laser scanning microscope at 60×.
Results
Etoposide and camptothecin induce apoptosis in undifferentiated but not neuronally differentiated SH-SY5Y cells
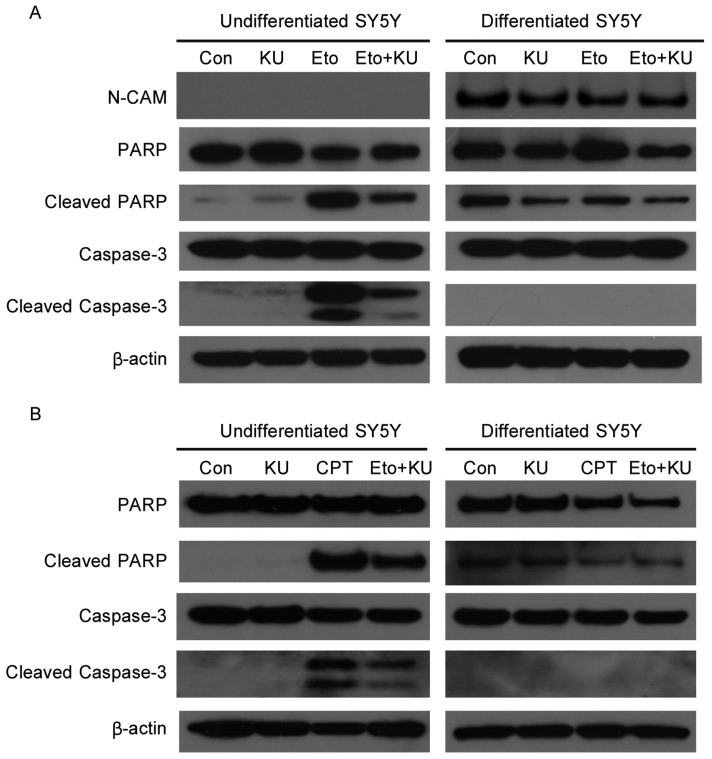
DNA damage responses are largely nuclear events because damaged DNA is in the nuclei and requires nuclear proteins to sense it. To test the functional difference of ATM in mediating DNA damage responses in undifferentiated versus neuronally differentiated SH-SY5Y cells, levels of apoptosis were compared after cells were treated with etoposide, a DNA-damaging agent that mimics ionizing radiation and induces DNA double strand breaks. In undifferentiated cells, apoptosis was triggered by etoposide, as indicated by increased cleavage of PARP and caspase-3. The inhibition of ATM by KU-55933, a specific inhibitor of ATM[13], partially prevented the cleavage of PARP and caspase-3 (Figure 1A), suggesting that the apoptosis induced by etoposide is dependent on ATM. In contrast, in differentiated SH-SY5Y cells, etoposide failed to induce apoptosis, shown by similar levels of cleaved PARP and cleaved caspase-3 between control and etoposide-treated samples.
Figure 1. Etoposide and camptothecin induce apoptosis in undifferentiated but not neuronally differentiated SH-SY5Y cells.
A, SH-SY5Y (simplified as SY5Y in the figure) cells were pretreated with KU-55933 (10 µmol/L) for 1 h before the addition of etoposide (Eto, 10 µmol/L) for another 3 h. Cells were lysed, and cell lysates were subjected to SDS-PAGE and Western blotting for the detection of PARP, Caspase-3, N-CAM, and β-actin. N-CAM is a marker of post-mitotic neuron-like cells, and β-actin is a loading control. B, cells were pretreated with KU-55933 (10 µmol/L) for 1 h before the addition of camptothecin (CPT, 10 µmol/L) for another 3 h. Following cell lysis and SDS-PAGE, the same set of proteins were detected by immunoblotting as described above.
To determine whether other DNA damaging agents also induce apoptosis in undifferentiated and differentiated SH-SY5Y cells, we treated cells with camptothecin, which induces the formation of double- as well as single-strand breaks in chromosomal DNA. Similar to etoposide, camptothecin induced apoptosis in undifferentiated but not differentiated SH-SY5Y cells, and KU-55933 partially prevented the apoptosis induced by camptothecin in differentiated SH-SY5Y cells (Figure 1B).
The traditional nuclear substrates of ATM cannot be activated by etoposide in differentiated SH-SY5Y cells
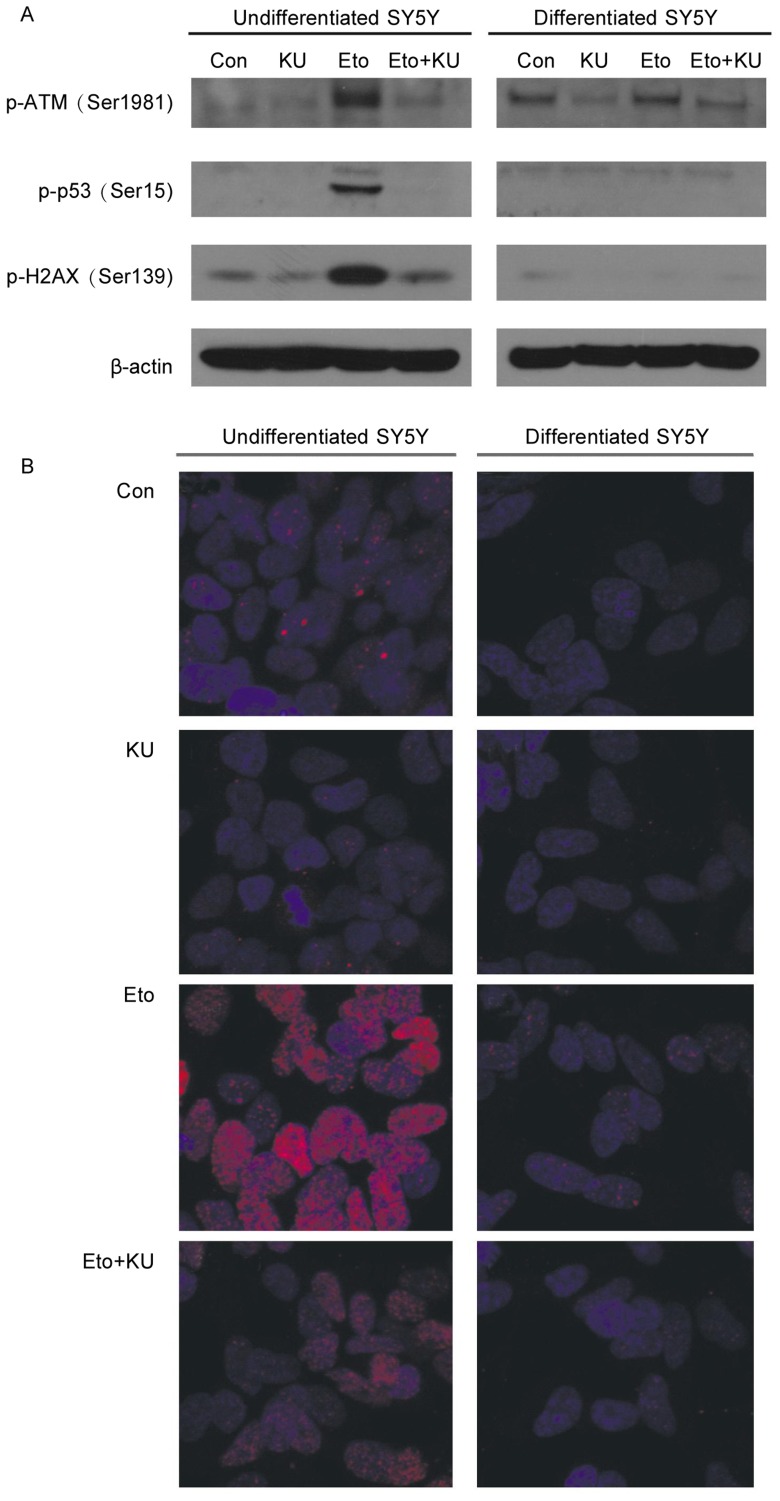
To determine the reason why etoposide could only induce apoptosis in undifferentiated proliferating SH-SY5Y cells, we compared the activation of ATM and its downstream targets in response to etoposide in undifferentiated versus differentiated SH-SY5Y cells. In undifferentiated SH-SY5Y cells, the phosphorylation of ATM at Ser1981, p53 at Ser15, and histone H2AX at Ser139 readily increased in response to etoposide, and KU-55933 blocked these phosphorylation events. Surprisingly, in differentiated SH-SY5Y cells, these phosphorylation events did not occur after etoposide treatment (Figure 2A).
Figure 2. ATM and its nuclear substrates cannot be activated by etoposide in neuronally differentiated SH-SY5Y cells.
A, cells were pretreated with KU-55933 (10 µmol/L) for 1 h before the addition of etoposide (10 µmol/L) for another 3 h. Cells were lysed, and cell lysates were subjected to SDS-PAGE and Western blotting for the detection of phospho-ATM (Serf 981), phospho-p53 (Ser15), phospho-H2AX (Ser139), and β-actin. B, both undifferentiated and differentiated SH-SY5Y cells were immunostained with phospho-H2AX antibody (shown by red foci) as described in Materials and Methods, after being treated with KU-55933 for 1 h followed by etoposide (10 µmol/L) treatment for another 3 h. Nuclei DNA were stained by DAPI (blue).
To further examine the activation status of histone H2AX in differentiated and undifferentiated SH-SY5Y cells, we immunostained these cells with a phospho-H2AX antibody after the cells were treated with KU-55933 and etoposide. In undifferentiated SH-SY5Y cells, etoposide induced the formation of a large amount of H2AX foci, which were not seen in differentiated SH-SY5Y cells (Figure 2B). The foci formation was markedly inhibited by KU-55933, suggesting that the foci formation is dependent on ATM activation.
KU-55933 inhibits Akt phosphorylation in differentiated but not undifferentiated SH-SY5Y cells
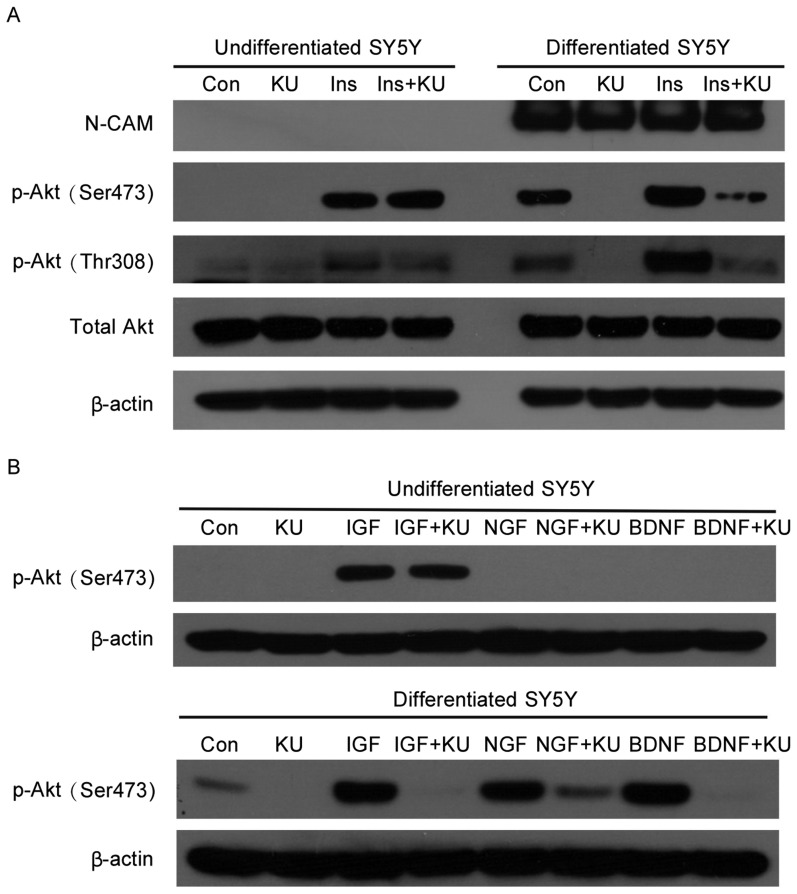
DNA damage signals produced dramatically different responses in differentiated and undifferentiated SH-SY5Y cells. Thus, to further determine whether there is a difference in their responses to growth factor signaling, both types of cells were treated with insulin in the presence or absence of KU-55933. In undifferentiated SH-SY5Y cells, insulin induced Akt phosphorylation at Ser473 and Thr308, and ATM inhibition by KU-55933 did not block these effects, suggesting that Akt phosphorylation may be mediated by kinases other than ATM. After differentiation, the basal level of phospho-Akt increased. More importantly, KU-55933 inhibited the phosphorylation of Akt in neuronally differentiated SH-SY5Y cells (Figure 3A).
Figure 3. KU-55933 blocks growth factor-mediated Akt phosphorylation in differentiated but not undifferentiated SH-SY5Y cells.
A, cells were serum-starved for 6 h and treated with KU-55933 (10 µmol/L) for 1 h before treatment with insulin (100 nmol/L) for 45 min. Cells were lysed, and cell lysates were subjected to SDS-PAGE and Western blotting for the detection of phospho-Akt at Ser473 and Thr308. β-actin was the loading control. B, cells were serum-starved for 6 h and treated with KU-55933 (10 µmol/L) for 1 h before treatment with IGF-I (25 ng/mL), NGF (25 ng/mL) or BDNF (25 ng/mL) for 45 min. Cells were lysed, and cell lysates were subjected to SDS-PAGE and Western blotting for the detection of phospho-Akt at Ser473. β-actin was the loading control.
To further confirm the role of cytoplasmic ATM in mediating growth factor signaling pathways, both types of cells were treated with known neuronal growth factors, including IGF-I and the specific neuronal growth factors NGF and BDNF. Interestingly, in undifferentiated SH-SY5Y cells, IGF-I, like insulin, induced Akt phosphorylation, whereas NGF and BDNF could not, suggesting that undifferentiated SH-SY5Y cells do not exhibit neuron-specific features. Similarly, KU-55933 could not inhibit IGF-I induced Akt phosphorylation in undifferentiated SH-SY5Y cells (Figure 3B). In differentiated neuron-like SH-SY5Y cells, all three growth factors, IGF-I, NGF, and BDNF, stimulated Akt phosphorylation, which was markedly reduced by KU-55933. This result further demonstrates that ATM is an important signal transducer of Akt-mediated growth factor signaling in neuron-like cells.
ATM-mediated Akt activation in response to insulin is possibly through PI3K, not mTOR or DNA-PK, in neuron-like SH-SY5Y cells
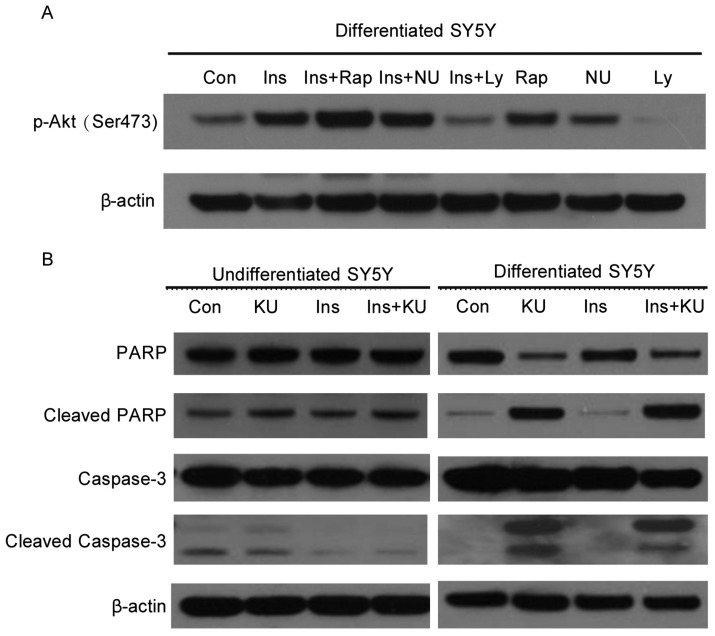
To test whether other PI3K superfamily members, such as mTOR or DNA-dependent protein kinase (DNA-PK), are important for Akt activation in differentiated SH-SY5Y cells, we serum-starved the cells and then treated them with the mTOR inhibitor rapamycin, DNA-PK inhibitor NU7026, or PI3K inhibitor LY294002 before the addition of insulin. Only LY294002 suppressed Akt phosphorylation, similar to the inhibitory effect of KU-55933. The inhibition of mTOR by rapamycin led to enhanced Akt phosphorylation, probably because of rapamycin-induced feedback activation of Akt[14]. Although DNA-PK was considered a potential candidate for kinases that can directly phosphorylate Akt, the inhibition of DNA-PK did not have effect on Akt phosphorylation (Figure 4A). These results suggest that ATM-mediated Akt activation in response to insulin involves PI3K, not mTOR or DNA-PK.
Figure 4. ATM is important for the survival of human neuron-like SH -SY5Y cells following growth factor deprivation.
A, ATM may mediate Akt phosphorylation at Ser473 through PI3K in response to insulin in differentiated SH-SY5Y cells. Cells were serum-starved for 6 h and treated with mTOR inhibitor rapamycin (25 nmol/L), DNA-PK inhibitor NU7026 (10 µmol/L), or PI3K inhibitor LY294002 (16 µmol/L) for 1 h before insulin stimulation of another 45 min. Cells were then collected, and cell lysates were subjected to SDS-PAGE and Western blotting for the detection of phospho-Akt at Ser473 and β-actin. B, ATM inhibition by KU-55933 induces apoptosis in differentiated but not undifferentiated SH-SY5Y cells. Cells were starved for 6 h in serum-free medium. KU-55933 (10 µmol/L) was added 1 h before insulin (100 nmol/L) treatment. After 3 h of insulin stimulation, cells were collected, and cell lysates were subjected to SDS-PAGE and Western blotting for the detection of PARP, caspase-3, and β-actin.
KU-55933 induces apoptosis in differentiated but not undifferentiated SH-SY5Y cells
Akt is important for neuronal cell survival[11]. Since ATM can stimulate Akt activity in differentiated SH-SY5Y cells, it is conceivable that ATM inhibition may result in reduced cell survival. To test this hypothesis, both undifferentiated and differentiated SH-SY5Y cells were serum-starved and then treated with KU-55933 in the presence or absence of insulin. In undifferentiated cells, KU-55933 treatment did not induce increased apoptosis as compared to control samples. In neuronally differentiated SH-SY5Y cells, KU-55933 induced strong cleavage of PARP and Caspase-3 even in the presence of insulin, indicating the occurrence of apoptosis (Figure 4B). This result suggests that ATM is required for the survival of human neuron-like SH-SY5Y cells.
Discussion
While ATM is traditionally considered a nuclear protein that functions in controlling cell cycle progression after DNA damage, recent results have shown that cytoplasmic ATM also plays an important role in stimulating growth factor signaling[12]. However, despite intensive studies aimed at characterizing the mechanism underlying neuronal degeneration of human A-T, there is no direct comparison of ATM's responses to DNA damage or to growth factor stimulation in post-mitotic, neuron-like cells. Our study is the first to investigate and distinguish the functions of different pools of ATM, which is primarily located in either the nucleus or the cytoplasm, depending on differentiation status of SH-SY5Y cells[6]. Our results clearly demonstrate that ATM switches function from a sensor of DNA damage in proliferating cells to a mediator of growth factor signaling and a promoter of cell survivor in post-mitotic neuron-like cells.
In proliferating cells, ATM mainly localizes in the nucleus. Our results show that the activation of ATM in these cells by etoposide causes cells to undergo apoptosis. Inhibition of ATM actually protects cells from apoptosis. After differentiation, the same concentration of etoposide failed to induce apoptosis. This is probably because ATM translocates to the cytoplasm in differentiated SH-SY5Y cells[6], thereby making ATM deficient in sensing DNA damage. Our results were further confirmed when cells were treated with camptothecin, another DNA-damaging agent. These findings are also consistent with those from a previous report indicating that differentiated SH-SY5Y cells are more resistant to DNA damage-induced cell death than undifferentiated SH-SY5Y cells[15].
Since differentiated SH-SY5Y cells exhibit postmitotic, neuron-like properties and mimic human neuronal cells, this result implies that neuronal degeneration is not likely caused by DNA damage-induced neuronal cell death. Importantly, the differentiation method used in our study induces cytoplasmic translocation of ATM[6], which mimics the cytoplasmic localization of ATM in human Purkinje cells and mouse cerebellar neuronal cells[3]–[5]. It should be noted that our results are contradictory with those from another report[16], which suggested that neuronal degeneration in A-T may result from defective DNA damage responses. However, in that study, SH-SY5Y cells were only differentiated for 7 days and ATM translocation might not occur in such a short differentiation time. Also, results from that report show that ATM was not required for neuronal differentiation[16]. This conclusion is in contrast to the findings of Fernandes et al.[17], who demonstrated that ATM is required for differentiation of human neuroblastoma cells.
The failure of ATM to respond to DNA damage in differentiated SH-SY5Y cells is also shown by diminished activation of multiple nuclear substrates of ATM. Although DNA damage can readily induce phosphorylation of ATM, p53, and H2AX, including the formation of H2AX foci in proliferating SH-SY5Y cells, these events do not occur in neuron-like SH-SY5Y cells. In addition, we also incubated differentiated SH-SY5Y cells with etoposide for a prolonged period of time (24 h), and we did not observe enhanced cell apoptosis as compared to control cells (data not shown). These results further suggest that DNA damage is not likely the cause of neuronal cell death in human A-T.
Furthermore, we also observed differential effects of ATM on Akt phosphorylation in response to insulin and other growth factors in differentiated and undifferentiated SH-SY5Y cells. The inhibition of ATM in undifferentiated SH-SY5Y cells did not block the phosphorylation of Akt, suggesting that ATM in these cells is not responsible for Akt phosphorylation. This is probably because in undifferentiated cells, ATM mainly localizes in the nucleus, and the phosphorylation of Akt is mediated by other kinases. Surprisingly, Akt phosphorylation at Ser473 could only be inhibited by KU-55933 in differentiated SH-SY5Y cells, suggesting that Akt phosphorylation becomes ATM-dependent only after ATM translocates to the cytoplasm[6]. One recent article reported that in mouse ocular and cerebellar tissue sections, ATM localizes in both cytoplasm and nucleus; however, ATM is phosphorylated under normal growth conditions only in the cytoplasm[18]. Purportedly, cytoplasmic ATM is active, but nuclear ATM is not active in post-mitotic cells. The functional analysis of ATM in our study agrees with this model. Thus, it is possible that although a portion of ATM still remains in the nucleus, only cytoplasmic ATM is active in neuronal cells of the cerebellum.
Similar to insulin, IGF-I is a neuroprotective growth factor that contributes to neuronal differentiation. ATM was reported to positively regulate the expression of the IGF-I receptor[19]. Our results show that both insulin and IGF-I signaling are mediated by cytoplasmic ATM in differentiated SH-SY5Y cells because insulin- and IGF-I-mediated Akt phosphorylation could be inhibited by a specific ATM inhibitor in these cells. In addition, the activation of Akt was reported to promote the survival of differentiated neuron-like SH-SY5Y cells in response to neuronal-specific growth factors BDNF and NGF[11],[20]. Consistent with these results, we also found that ATM participates in NGF- and BDNF-mediated Akt phosphorylation in differentiated neuron-like SH-SY5Y cells.
Under normal growth conditions, differentiated SH-SY5Y cells are more susceptible to ATM inhibition-induced cell death than undifferentiated SH-SY5Y cells. This suggests that ATM is required for the survival of neuronal cells and protects cells from serum starvation-induced apoptosis after ATM translocates to the cytoplasm. Even in the presence of insulin, differentiated SH-SY5Y cells underwent apoptosis when treated with KU-55933. This is consistent with the reports that ATM mediates insulin signaling[7] and that insulin promotes neurite outgrowth and neuronal survival in the brain, including the cerebellum[21].
The protective role of ATM is probably through mediating the activation of Akt, which is activated in response to growth factors in neuronal cells. Activated Akt can phosphorylate many substrates, such as GSK-3β, FOXO1, FOXO3a, and the Bcl-2-associated protein Bad. Phosphorylation of these proteins results in the inhibition of their pro-apoptotic functions. In differentiated SH-SY5Y cells, FOXO3a is especially important for cell apoptosis[22]. Akt activation can also lead to the activation of the NF-kB pathway, which transcriptionally induces the expression of anti-apoptotic proteins XIAP and Bcl-2. Upon activation of Akt and phosphorylation of its substrates, the release of cytochrome c from mitochondria and the cleavage of Caspases can be prevented. This is in accordance with one of many reports showing that Akt pathway is up-regulated upon differentiation to protect neuronal cells against neurotoxins[23].
Although our study has shown that ATM protein is a mediator of growth factor signaling and a promoter of cell survival in differentiated human neuron-like cells, further investigations are needed to elucidate the downstream pathways regulated by ATM in response to growth factor signaling in neuronal cells. It would also be ideal to further evaluate the functional link between ATM and Akt in human neuronal cells that are either differentiated from human stem cells or isolated from human tissue samples. In undifferentiated proliferating SH-SY5Y cells, KU-55933 only partially rescues the cells from etoposide-induced apoptosis. It will be interesting to further test whether the addition of an inhibitor of ATR, a homolog of the ATM protein, can completely rescue proliferating SH-SY5Y cells from programmed cell death.
Conclusions
Previous studies suggest that ATM has many functions other than initiating DNA damage responses, including mediating signaling cascades in response to insulin and other growth factors. In this report, our results clearly demonstrate that nuclear ATM in proliferating cells mediates DNA damage responses, whereas cytoplasmic ATM in post-mitotic neuron-like cells mediates growth factor signaling pathways. It appears that DNA damage is not the reason for neuronal cell death in A-T. Since inhibition of ATM induces cell apoptosis and abolishes insulin-mediated cell survival in human neuron-like SH-SY5Y cells, the deficiency of cytoplasmic Akt signaling may be the cause of neuronal cell death in human A-T. The elucidation of separate functions executed by nuclear and cytoplasmic ATM highlights the importance of cytoplasmic ATM in stimulating growth factor-mediated Akt signaling pathways and promoting cell survival, thereby providing novel insights into the mechanism underlying the neuronal degeneration observed in patients with A-T.
Acknowledgments
This project was supported by a research grant from A-T Children's Project (ATCP) received by Da-Qing Yang.
References
- 1.Shiloh Y, Kastan MB. ATM: genome stability, neuronal development, and cancer cross paths. Adv Cancer Res. 2001;83:209–254. doi: 10.1016/s0065-230x(01)83007-4. [DOI] [PubMed] [Google Scholar]
- 2.Abraham RT. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001;15:2177–2196. doi: 10.1101/gad.914401. [DOI] [PubMed] [Google Scholar]
- 3.Oka A, Takashima S. Expression of the ataxia-telangiectasia gene (ATM) product in human cerebellar neurons during development. Neurosci Lett. 1998;252:195–198. doi: 10.1016/s0304-3940(98)00576-x. [DOI] [PubMed] [Google Scholar]
- 4.Barlow C, Ribaut-Barassin C, Zwingman TA, et al. et al. ATM is a cytoplasmic protein in mouse brain required to prevent lysosomal accumulation. Proc Natl Acad Sci U S A. 2000;97:871–876. doi: 10.1073/pnas.97.2.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J, Han YR, Plummer MR, et al. et al. Cytoplasmic ATM in neurons modulates synaptic function. Curr Biol. 2009;19:2091–2096. doi: 10.1016/j.cub.2009.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boehrs JK, He J, Halaby M J, et al. et al. Constitutive expression and cytoplasmic compartmentalization of ATM protein in differentiated human neuron-like SH-SY5Y cells. J Neurochem. 2007;100:337–345. doi: 10.1111/j.1471-4159.2006.04254.x. [DOI] [PubMed] [Google Scholar]
- 7.Yang DQ, Kastan MB. Participation of ATM in insulin signalling through phosphorylation of eIF-4E-binding protein 1. Nat Cell Biol. 2000;2:893–898. doi: 10.1038/35046542. [DOI] [PubMed] [Google Scholar]
- 8.Viniegra JG, Martinez N, Modirassari P, et al. et al. Full activation of PKB/Akt in response to insulin or ionizing radiation is mediated through ATM. J Biol Chem. 2005;280:4029–4036. doi: 10.1074/jbc.M410344200. [DOI] [PubMed] [Google Scholar]
- 9.Halaby MJ, Hibma JC, He J, et al. et al. ATM protein kinase mediates full activation of Akt and regulates glucose transporter 4 translocation by insulin in muscle cells. Cell Signal. 2008;20:1555–1563. doi: 10.1016/j.cellsig.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Rivera EJ, Goldin A, Fulmer N, et al. et al. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer's disease: link to brain reductions in acetylcholine. J Alzheimers Dis. 2005;8:247–268. doi: 10.3233/jad-2005-8304. [DOI] [PubMed] [Google Scholar]
- 11.Lopez-Carballo G, Moreno L, Masia S, et al. et al. Activation of the phosphatidylinositol 3-kinase/Akt signaling pathway by retinoic acid is required for neural differentiation of SH-SY5Y human neuroblastoma cells. J Biol Chem. 2002;277:25297–25304. doi: 10.1074/jbc.M201869200. [DOI] [PubMed] [Google Scholar]
- 12.Yang DQ, Halaby MJ, Li Y, et al. et al. Cytoplasmic ATM protein kinase: an emerging therapeutic target for diabetes, cancer and neuronal degeneration. Drug Discov Today. 2011;16:332–338. doi: 10.1016/j.drudis.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 13.Hickson I, Zhao Y, Richardson CJ, et al. et al. Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res. 2004;64:9152–9159. doi: 10.1158/0008-5472.CAN-04-2727. [DOI] [PubMed] [Google Scholar]
- 14.Sun SY, Rosenberg LM, Wang X, et al. et al. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 15.Lasorella A, Iavarone A, Israel MA. Differentiation of neuroblastoma enhances Bcl-2 expression and induces alterations of apoptosis and drug resistance. Cancer Res. 1995;55:4711–4716. [PubMed] [Google Scholar]
- 16.Biton S, Dar I, Mittelman L, et al. et al. Nuclear ataxia-telangiectasia mutated (ATM) mediates the cellular response to DNA double strand breaks in human neuron-like cells. J Biol Chem. 2006;281:17482–17491. doi: 10.1074/jbc.M601895200. [DOI] [PubMed] [Google Scholar]
- 17.Fernandes ND, Sun Y, Price BD. Activation of the kinase activity of ATM by retinoic acid is required for CREB-dependent differentiation of neuroblastoma cells. J Biol Chem. 2007;282:16577–16584. doi: 10.1074/jbc.M609628200. [DOI] [PubMed] [Google Scholar]
- 18.Leemput J, Masson C, Bigot K, et al. et al. ATM localization and gene expression in the adult mouse eye. Mol Vis. 2009;15:393–416. [PMC free article] [PubMed] [Google Scholar]
- 19.Peretz S, Jensen R, Baserga R, et al. et al. ATM-dependent expression of the insulin-like growth factor-I receptor in a pathway regulating radiation response. Proc Natl Acad Sci U S A. 2001;98:1676–1681. doi: 10.1073/pnas.041416598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Encinas M, Iglesias M, Liu Y, et al. et al. Sequential treatment of SH-SY5Y cells with retinoic acid and brain-derived neurotrophic factor gives rise to fully differentiated, neurotrophic factor-dependent, human neuron-like cells. J Neurochem. 2000;75:991–1003. doi: 10.1046/j.1471-4159.2000.0750991.x. [DOI] [PubMed] [Google Scholar]
- 21.van der Heide LP, Ramakers GM, Smidt MP. Insulin signaling in the central nervous system: learning to survive. Prog Neurobiol. 2006;79:205–221. doi: 10.1016/j.pneurobio.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Zhu W, Bijur GN, Styles NA, et al. et al. Regulation of FOXO3a by brain-derived neurotrophic factor in differentiated human SH-SY5Y neuroblastoma cells. Brain Res Mol Brain Res. 2004;126:45–56. doi: 10.1016/j.molbrainres.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Cheung YT, Lau WK, Yu MS, et al. et al. Effects of all-trans-retinoic acid on human SH-SY5Y neuroblastoma as in vitro model in neurotoxicity research. Neurotoxicology. 2009;30:127–135. doi: 10.1016/j.neuro.2008.11.001. [DOI] [PubMed] [Google Scholar]