Abstract
Secreted protein, acidic and rich in cysteine (SPARC) is expressed in numerous types of tumors and is suggested to have prognostic value. Moreover, because of its strong affinity for albumin, and hence albumin-bound drugs, SPARC has increasingly become a focus for research. In this study, we aimed to determine SPARC expression in patients with non-small cell lung cancer (NSCLC) and investigate the association of SPARC with disease prognosis. Tissue microarrays were constructed with specimens from 105 patients with NSCLC treated at Sun Yat-sen University Cancer Center, and immunohistochemical analysis was performed on these tissue microarrays to assess SPARC expression. Our results showed that SPARC expression status did not significantly relate with age, gender, and tumor stage. However, SPARC was expressed more frequently in squamous cell carcinoma than in adenocarcinoma (75% vs. 43.5%, P = 0.004). Patients with smoking history had higher SPARC expression than non-smokers (68.2% vs. 33.3%, P = 0.002). In both univariate and multivariate analyses, SPARC was a prognostic factor of overall survival (HR = 0.32; 95% CI: 0.16–0.65) but not disease-free survival. Our study indicates that SPARC expression is higher in squamous cell carcinoma than in adenocarcinoma in NSCLC. Most notably, SPARC can be used as a prognostic factor for NSCLC.
Keywords: Non-small cell lung cancer, prognosis, SPARC
Secreted protein, acidic and rich in cysteine (SPARC, also called osteonectin or BM-40), a glycosylated, 43-kDa, matricellular protein, is highly conserved and multifunctional, regulating matrix remodeling and turnover. As a non-structural component of extracellular matrices, SPARC can bind to extracellular matrix components such as collagens, laminin, fibronectin, and vitronectin[1] and mediate cell-matrix interactions. It also has counter-adhesive properties, inducing cell rounding, inhibiting cell spreading[2], and mediating focal adhesion disassembly and reorganization of actin stress fibers[3]. SPARC also delays cell cycle in the G1 phase in many types of cells[4]. Thus, this protein regulates multiple biological processes, including cell proliferation, survival, apoptosis, adhesion, and migration[5],[6].
In normal tissues, SPARC expression is limited to the epithelia of the bone or gut or to tissues undergoing development, remodeling, and repair[7]. However, because of the extensive matrix remodeling process in tumorigenesis, differential patterns of SPARC expression are also detectable in cancer-associated stroma and some malignant cells, affecting tumor development, invasion, metastases, and angiogenesis[8]. Up-regulated SPARC expression was reported in breast cancer, prostate cancer, melanoma, and glioblastoma[9]. In clear contrast, down-regulation of SPARC expression has been demonstrated in ovarian cancer[10], colorectal cancer[11], pancreatic cancer[12], and acute myelogenous leukemia[13].
There is considerable controversy regarding the prognostic value of SPARC. SPARC up-regulation was reported as an independent prognostic factor for disease progression and poor overall survival (OS) in several studies on tumor types that include head and neck cancer[14], pancreatic adenocarcinoma[15], gastric cancer[16], breast cancer[17]–[20], osteosarcoma[21], glioblastoma[22], oesophageal carcinoma[23], bladder cancer[24], meningioma[25], tongue carcinoma[26], and cutaneous malignant melanoma[27]. However, other studies have provided evidence to validate that low expression of SPARC is predictive of poor outcome[1]. SPARC-induced changes can either suppress or promote neoplastic progression, depending on the requirements for cell-matrix and tumor-stroma interactions. Modulating tumor microenvironment is a clinically relevant therapeutic strategy, and as a natural regulator of this microenvironment, SPARC represents a highly promising therapeutic modality. Nevertheless, the implications of SPARC expression on prognosis are still unclear. Because SPARC facilitates the accumulation of albumin and, thus, albumin-bound drugs in tumors[28],[29], this protein has recently generated considerable interest as a predictor for the efficacy of albumin-bound drugs.
In this study, we determined SPARC expression in 105 patients with non-small cell lung cancer (NSCLC) using immunohistochemistry to analyze tissue microarrays. We also assessed the relationship between SPARC expression and disease prognosis.
Materials and Methods
Tumor specimens and tissue microarrays
Paraffin-embedded, formalin-fixed specimens were obtained from 105 patients histologically diagnosed with NSCLC between August 2000 and June 2004 at Sun Yat-sen University Cancer Center (Guangzhou, China). To confirm the original diagnosis of NSCLC, all pathologic reports were reviewed by a single pathologist. All patients were staged according to the 1997 TNM staging system based on initial radiologic imaging evaluation. The date of latest follow-up is August 20, 2012. The protocol was approved by the Ethics Committee of Sun Yat-sen Cancer Center, and written informed consent was obtained from each patient.
Tissue microarrays were constructed using a Tissue Microarrayer[30] (Beecher Instruments®, Silver Spring, MD, USA). Two 1.0-mm diameter cylinders were punched from each of the 105 specimens and arrayed in three tissue microarray blocks. According to the histological image of hematoxylin-eosin (HE) stained slides, core biopsies on each tissue microarray block were randomly selected from two different parts of each specimen, but areas of necrosis were avoided. Sections of 4-µm thick were cut from each block.
Immunohistochemical staining and scoring
SPARC expression in NSCLC was examined by immunohistochemical staining. Briefly, paraffin-embedded sections were heated at 60°C for 30 min before being deparaffinized by incubation in Citrisolv (Fisher Scientific, Pittsburgh, PA) 3 times for 10 min, rinsed in absolute ethanol, and then treated with methanol containing 0.5% hydrogen peroxide for 30 min. The slides were then washed in water, incubated in PBS-Tween (0.1% Tween-20 in PBS) for 10 min, and blocked in Dulbecco's Modified Eagle's Medium containing 10% FBS for 1 h. The slides were next incubated with 10 µg/mL of polyclonal anti-human SPARC antibody (R&D Systems, Minneapolis, MN) in PBS-Tween for 1 h, washed twice in PBS-Tween for 5 min, and incubated in a 1:100 dilution of anti-rabbit alkaline phosphatase-conjugated secondary antibody (Pierce, Rockford, IL) for 1 h. Sections were washed in PBS-Tween and incubated in Vector Red Alkaline Phosphatase Substrate (Victor Laboratories, Burlingame, CA) for 20 min. Then, they were counterstained with Mayer's hematoxylin (Electron Microscopy Sciences, Hatfield, PA) for 30 s and mounted with Crystal Mount (BioMeda, Corp, Foster City, CA). The stained sections were scanned on a GenePix 4000B microarray scanner (Molecular Device, Sunnyvale, CA) using GenePix Pro 6.0 software. The staining was scored using F532 total intensities. Positive SPARC expression was defined as staining ≥ median staining, and negative SPARC expression was defined as staining < median staining.
Statistical analysis
Disease-free survival (DFS) was defined as the duration from the date of NSCLC diagnosis to the date of local recurrence or metastasis or the date of last follow-up. OS was calculated from the date of the histological diagnosis to the date of death for any cause or the date of last follow-up. The chi-square test was performed to evaluate the relationship between clinicopathologic variables and SPARC expression, and t test was used to evaluate the relationship between age and SPARC expression. DFS and OS were estimated using the Kaplan-Meier method. The log-rank test was used to determine the difference in survival among patients with different clinicopathologic characteristics. Independent prognostic factors for DFS and OS were determined by multivariate Cox proportional hazards regression analysis. All statistical analyses were performed using SPSS version 16.0 software. P values were two-sided and were deemed significant when < 0.05.
Results
Patient characteristics
Detailed patient characteristics are summarized in Table 1. Eighty-five patients were men, and 20 were women, with a median age of 60 years (range, 34–78 years). At the time of NSCLC diagnosis, the proportions of patients with stage I, II, and III disease were 47.6%, 19.0%, and 33.3% respectively. No patients had metastatic disease. All patients underwent radical therapy.
Table 1. Characteristics of 105 patients with non-small cell lung cancer.
| Characteristic | No. of patients | Percentage (%) |
| Gender | ||
| Male | 85 | 81.0 |
| Female | 20 | 19.0 |
| Age (years) | ||
| Median | 60 | |
| Range | 34–78 | |
| Stage | ||
| IA | 11 | 10.5 |
| IB | 39 | 37.1 |
| IIA | 2 | 1.9 |
| IIB | 18 | 17.1 |
| IIIA | 31 | 29.5 |
| IIIB | 4 | 3.8 |
| Histology | ||
| Adenocarcinoma | 69 | 65.7 |
| Non-adenocarcinoma | 36 | 34.3 |
| Smoking history | ||
| Yes | 63 | 60.0 |
| No | 39 | 37.1 |
| Unknown | 3 | 2.9 |
SPARC expression and association with clinicopathologic features
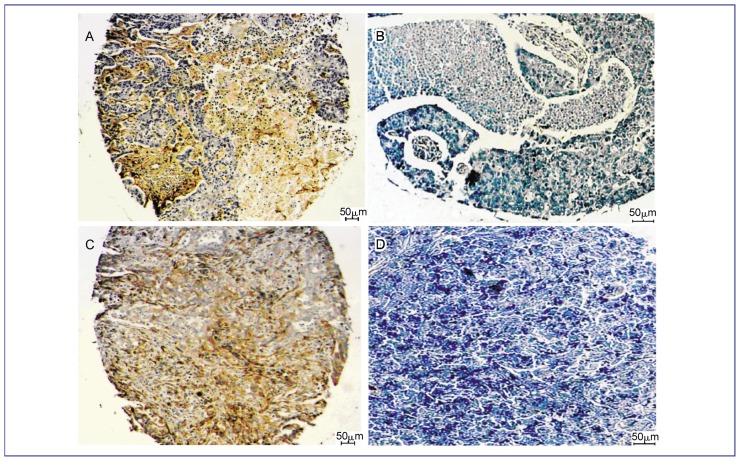
Of the 105 cases of NSCLC, 57 (54.3%) showed positive SPARC staining, whereas 48 (45.7%) showed no SPARC staining. Representative immunohistochemical staining results showed positive cytoplasmic staining in the tumor and a positive stromal reaction (Figure 1).
Figure 1. SPARC protein expression in non-small cell lung cancer (NSCLC).
Paraffin-embedded sections were stained with SPARC antibody and counterstained with Mayer's hematoxylin as described in Materials and Methods. SPARC positive expression was defined as staining ≥ median staining, and negative expression was defined as staining < median staining. SPARC staining in the cytoplasm and stroma was brown. A, SPARC-positive squamous cell carcinoma; B, SPARC-negative squamous cell carcinoma; C, SPARC-positive adenocarcinoma; D, SPARC-negative adenocarcinoma.
Patient characteristics in SPARC-negative and -positive arms are shown in Table 2. SPARC expression was not correlated with age, gender, and disease stage. Patients with squamous cell carcinoma expressed SPARC more frequently than patients with adenocarcinoma (P = 0.004). Likewise, patients with smoking history also showed a higher SPARC-positive rate than non-smokers (P = 0.002). Furthermore, of all smokers with squamous cell carcinoma, 21 patients showed positive SPARC expression (21/28, 75.0%).
Table 2. Relationship between patient characteristics and SPARC expression in 105 patients with non-small cell lung cancer.
| Characteristic | SPARC expressiona |
P | |
| Positive | Negative | ||
| Gender | 0.213 | ||
| Male | 49 | 36 | |
| Female | 8 | 12 | |
| Age (years) | 0.297 | ||
| Median | 60 | 60.5 | |
| Stage | 0.564 | ||
| I | 26 | 24 | |
| II | 13 | 7 | |
| III | 18 | 17 | |
| Histology | 0.004 | ||
| Adenocarcinoma | 30 | 39 | |
| Squamous cell carcinoma | 27 | 9 | |
| Smoking history | 0.002 | ||
| Yes | 43 | 20 | |
| No | 13 | 26 | |
| Unknown | 1 | 2 | |
a Except for age, the values are presented as patient number.
SPARC expression and association with prognosis
The median OS for the entire cohort and for patients with stage I and II disease has not yet been reached, whereas the median OS for patients with stage III disease was 79.3 months (95% CI: 50.8–107.9 months). The median DFS was 25.3 months for all patients, 49.5 months (95% CI: 14.0–85.0 months) for patients with stage II disease, and 14.7 months (95% CI: 9.9–19.5 months) for patients with stage III disease. For patients with stage I disease, the median DFS has not yet been reached.
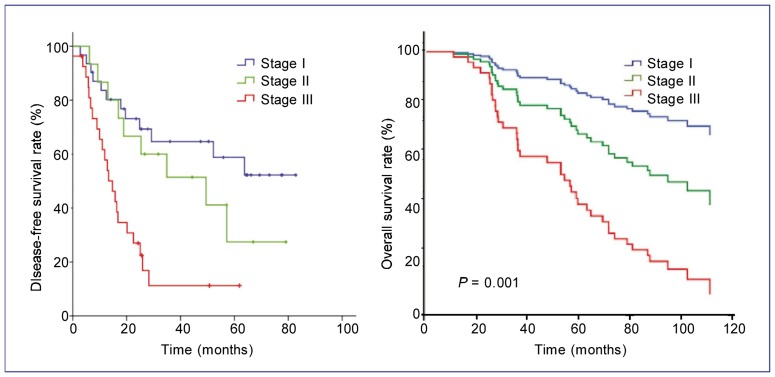
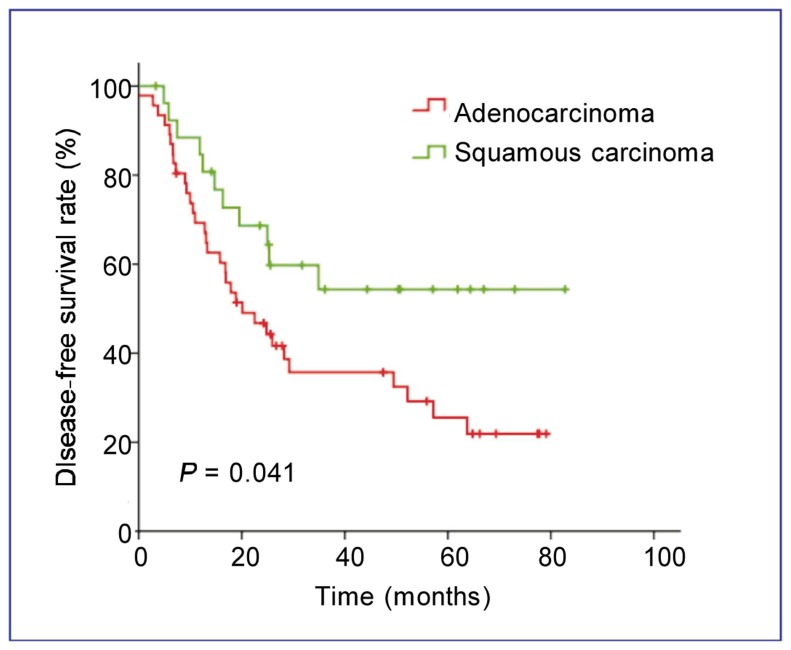
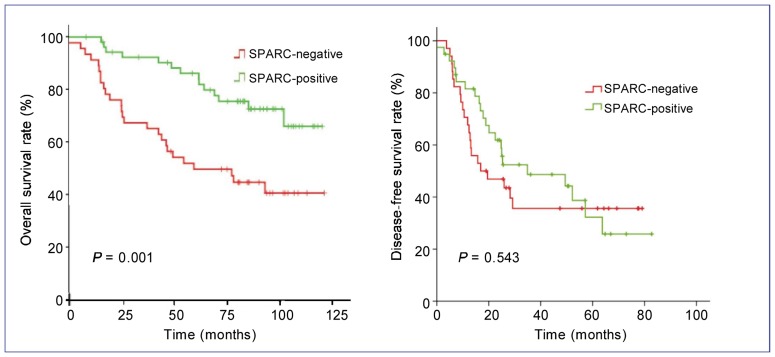
In univariate analysis, disease stage was an independent prognostic factor for DFS (P < 0.001) and OS (P = 0.001) (Figure 2). Patients with squamous cell carcinoma showed a longer DFS than those with adenocarcinoma (P = 0.041) (Figure 3). The absence of SPARC expression was an adverse prognostic factor for OS (P = 0.001) but not for DFS (P = 0.543) (Figure 4). The median OS of patients in the SPARC-negative arm was 86.9 months (95% CI: 63.2–110.7 months), but has not been reached for patients in the SPARC-positive arm.
Figure 2. Kaplan-Meier estimates of disease-free survival (DFS) and overall survival (OS) for patients with NSCLC at different stages.
All 105 patients with NSCLC were staged according to the 1997 TNM staging system based on initial radiologic imaging evaluation. The proportions of patients with stage I, II, and III disease were 47.6%, 19.0%, and 33.3%, respectively. Disease stage is an independent prognostic factor for DFS (A) and OS (B). The median DFS was 25.3 months for all patients, 49.5 months (95% CI: 14.0–85.0 months) for patients with stage II disease, and 14.7 months (95% CI: 9.9–19.5 months) for patients with stage III disease. For patients with stage I disease, the median DFS has not yet been reached. The median OS for all patients was 68.2 months, and the median OS for patients with stage I, II, and III disease was 74.0 months (95% CI: 69.0–79.1 months), 71.7 months (95% CI: 64.0–79.3 months), and 57.8 months (95% CI: 50.2–65.4 months), respectively.
Figure 3. Kaplan-Meier estimates of DFS for patients with NSCLC of different histological types.
Patients with squamous carcinoma show a longer DFS than those with adenocarcinoma (P = 0.041).
Figure 4. Kaplan-Meier estimates of OS (A) and DFS (B) for patients with different SPARC expression statuses.
Negative expression of SPARC was an adverse prognostic factor for OS (P = 0.001) but not DFS (P = 0.543). The median OS of the SPARC-negative arm was 86.9 months (95% CI: 63.2–110.7 months), but has not yet been reached for the SPARC-positive arm.
In multivariate Cox regression analyses, disease stage and histological type were identified as independent prognostic factors for DFS, with hazard ratios (HR) of 2.11 (95% CI: 1.44–3.08) and 0.69 (95% CI: 0.50–0.96), respectively (Table 3). Independent prognostic factors for the OS of NSCLC patients were disease stage (HR = 2.05, 95% CI: 1.40–3.00) and SPARC expression status (HR = 0.32, 95% CI: 0.16–0.65) (Table 3).
Table 3. Multivariate Cox regression analysis for disease-free survival and overall survival in 105 patients with non-small cell lung cancer.
| Variable | HR | 95% CI for HR | P |
| Disease-free survival | |||
| Stage | 2.11 | 1.44–3.08 | <0.001 |
| Histological type | 0.69 | 0.50–0.96 | 0.029 |
| Overall survival | |||
| Stage | 2.05 | 1.40–3.00 | <0.001 |
| SPARC expression status | 0.32 | 0.16–0.65 | 0.002 |
HR, hazard ratio; CI, confidence interval.
Discussion
This study aimed to determine if SPARC protein expression is a clinically useful prognostic factor for NSCLC. We performed a systematic evaluation of SPARC by immunohistochemical staining of tissue microarrays containing 1 05 tumor specimens obtained from patients with primary NSCLC. The data presented here strongly indicate that positive SPARC expression was associated with better survival (HR = 0.32). Our data was in disagreement with a previous study suggesting that SPARC overexpression was associated with poor prognosis in NSCLC[31]. However, our findings corroborated data from recent two studies on breast cancer that confirmed the positive effect of SPARC overexpression on prognosis[32],[33]. Other studies on colorectal and ovarian cancer also verified the hypothesis that SPARC functions as a tumor suppressor[22],[23],[34]. Indeed, SPARC has been reported to inhibit angiogenesis[24] and increase apoptosis[25], which further supports our results.
With its high binding affinity for albumin, tumoral SPARC prompts the accumulation of albumin in tumors and increases the effectiveness of albumin-bound drugs. Preliminary evidence suggests that tumor accumulation of nab-paclitaxel, a solvent-free, albumin-bound formulation of paclitaxel[28],[29], may be facilitated by binding to SPARC[26]. In head and neck cancer, response to nab-paclitaxel was higher in SPARC-positive patients than in SPARC-negative patients (83% vs. 25%)[27]. These findings suggest that SPARC is a candidate biomarker for predicting response to albumin-bound drugs.
In the current study, high SPARC expression was observed more frequently in patients with squamous cell carcinoma than in patients with adenocarcinoma (75% vs. 43.5%, P = 0.004). This finding is accordant with results from a phase II clinical trial of nab-paclitaxel for advanced NSCLC. Like in our study, the response rate was higher for patients with squamous cell carcinoma (5/29, 17.2%) than for patients with adenocarcinoma (1/11, 9.1%)[28]. These observations suggest that nab-paclitaxel accumulates in tumors through interacting with SPARC, making squamous cell carcinomas, which have a higher rate of SPARC expression, more responsive to treatment than adenocarcinomas.
Recent results from a phase III trial provide additional evidence in favor of our conclusion[29]. In this trial, 1053 patients with NSCLC were randomized to receive carbo/nab-paclitaxel and carbo/paclitaxel as first-line chemotherapy. Nab-paclitaxel garnered a significantly higher overall response than paclitaxel. More interestingly, this difference was far more pronounced between the squamous cell carcinoma subset in nab-paclitaxel arm and that in paclitaxel arm (41% vs. 25%, P < 0.001), but was not significant between the non-squamous cell carcinoma subset of those arms (26% vs. 25%). Given that SPARC overexpression associates with higher response to nab-paclitaxel, our results partially explain why patients with squamous cell carcinoma benefit more from albumin-bound drug treatment. However, the predictive value of SPARC needs further investigation.
In the present study, SPARC expression was observed more frequently in smokers than in non-smokers. However, to date, there is no evidence that SPARC expression, smoking history, and effectiveness of albumin-bound drugs are related. Additionally, the positive rate of SPARC expression in squamous cell carcinoma was 75%, whereas smokers with squamous cell carcinoma show a comparable positive rate. Thus, we attributed the difference of SPARC expression between smokers and non-smokers to a larger population of smokers among patients with squamous cell carcinoma.
Previously, Gail et al.[35] suggested that recurrence rate was much lower in squamous carcinoma than in nonsquamous/mixed type for stage I NSCLC. Several studies also indicated longer survival for patients with squamous carcinoma compared with other types, though this difference was not significant. Similarly, in this study, we provide evidence that recurrence is less likely in patients with squamous carcinoma than in those with adenocarcinoma.
In conclusion, we report here the results from our study of SPARC expression in NSCLC. SPARC was expressed more frequently in squamous cell carcinoma than in adenocarcinoma. High SPARC expression in smokers may relate with a larger population of smokers among patients with squamous cell carcinoma. Furthermore, SPARC is an independent prognostic factor for OS in NSCLC.
Acknowledgments
We thank all patients and their families for their participation in this study. This work was supported by State Key Laboratory Fund of China (No. 010829).
References
- 1.Podhajcer OL, Benedetti LG, Girotti MR, et al. The role of the matricellular protein SPARC in the dynamic interaction between the tumor and the host. Cancer Metastasis Rev. 2008;27:691–705. doi: 10.1007/s10555-008-9146-7. [DOI] [PubMed] [Google Scholar]
- 2.Brekken RA, Sage EH. SPARC, a matricellular protein: at the crossroads of cell-matrix communication. Matrix Biol. 2001;19:816–827. doi: 10.1016/s0945-053x(00)00133-5. [DOI] [PubMed] [Google Scholar]
- 3.Arnold SA, Brekken RA. SPARC: a matricellular regulator of tumorigenesis. J Cell Commun Signal. 2009;3:255–723. doi: 10.1007/s12079-009-0072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradshaw AD, Sage EH. SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J Clin Invest. 2001;107:1049–1054. doi: 10.1172/JCI12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chlenski A, Cohn SL. Modulation of matrix remodeling by SPARC in neoplastic progression. Semin Cell Dev Biol. 2010;21:55–65. doi: 10.1016/j.semcdb.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 6.Tai IT, Tang MJ. SPARC in cancer biology: its role in cancer progression and potential for therapy. Drug Resist Updat. 2008;11:231–246. doi: 10.1016/j.drup.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Chin D, Boyle GM, Williams RM, et al. Novel markers for poor prognosis in head and neck cancer. Int J Cancer. 2005;113:789–797. doi: 10.1002/ijc.20608. [DOI] [PubMed] [Google Scholar]
- 8.Miyoshi K, Sato N, Ohuchida K, et al. SPARC mRNA expression as a prognostic marker for pancreatic adenocarcinoma patients. Anticancer Res. 2010;30:867–871. [PubMed] [Google Scholar]
- 9.Zhao ZS, Wang YY, Chu YQ, et al. SPARC is associated with gastric cancer progression and poor survival of patients. Clin Cancer Res. 2010;16:260–268. doi: 10.1158/1078-0432.CCR-09-1247. [DOI] [PubMed] [Google Scholar]
- 10.Said N, Socha MJ, Olearczyk JJ, et al. Normalization of the ovarian cancer microenvironment by SPARC. Mol Cancer Res. 2007;5:1015–1030. doi: 10.1158/1541-7786.MCR-07-0001. [DOI] [PubMed] [Google Scholar]
- 11.Taghizadeh F, Tang MJ, Tai IT. Synergism between vitamin D and secreted protein acidic and rich in cysteine-induced apoptosis and growth inhibition results in increased susceptibility of therapy-resistant colorectal cancer cells to chemotherapy. Mol Cancer Ther. 2007;6:309–317. doi: 10.1158/1535-7163.MCT-06-0517. [DOI] [PubMed] [Google Scholar]
- 12.Infante JR, Matsubayashi H, Sato N, et al. Peritumoral fibroblast SPARC expression and patient outcome with resectable pancreatic adenocarcinoma. J Clin Oncol. 2007;25:319–325. doi: 10.1200/JCO.2006.07.8824. [DOI] [PubMed] [Google Scholar]
- 13.DiMartino JF, Lacayo NJ, Varadi M, et al. Low or absent SPARC expression in acute myeloid leukemia with MLL rearrangements is associated with sensitivity to growth inhibition by exogenous SPARC protein. Leukemia. 2006;20:426–432. doi: 10.1038/sj.leu.2404102. [DOI] [PubMed] [Google Scholar]
- 14.Watkins G, Douglas-Jones A, Bryce R, et al. Increased levels of SPARC (osteonectin) in human breast cancer tissues and its association with clinical outcomes. Prostaglandins Leukot Essent Fatty Acids. 2005;72:267–272. doi: 10.1016/j.plefa.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Gradishar WJ. Albumin-bound paclitaxel: a next-generation taxane. Expert Opin Pharmacother. 2006;7:1041–1053. doi: 10.1517/14656566.7.8.1041. [DOI] [PubMed] [Google Scholar]
- 16.Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23:7794–7803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 17.Yao X, Qian CN, Zhang ZF, et al. Two distinct types of blood vessels in clear cell renal cell carcinoma have contrasting prognostic implications. Clin Cancer Res. 2007;13:161–169. doi: 10.1158/1078-0432.CCR-06-0774. [DOI] [PubMed] [Google Scholar]
- 18.Koukourakis MI, Giatromanolaki A, Brekken RA, et al. Enhanced expression of SPARC/osteonectin in the tumor-associated stroma of non-small cell lung cancer is correlated with markers of hypoxia/acidity and with poor prognosis of patients. Cancer Res. 2003;63:5376–5380. [PubMed] [Google Scholar]
- 19.Beck AH, Espinosa I, Gilks CB, et al. The fibromatosis signature defines a robust stromal response in breast carcinoma. Lab Invest. 2008;88:591–601. doi: 10.1038/labinvest.2008.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagai MA, Gerhard R, Fregnani JH, et al. Prognostic value of NDRG1 and SPARC protein expression in breast cancer patients. Breast Cancer Res Treat. 2010;126:1–14. doi: 10.1007/s10549-010-0867-2. [DOI] [PubMed] [Google Scholar]
- 21.Tai IT, Dai M, Owen DA, et al. Genome-wide expression analysis of therapy-resistant tumors reveals SPARC as a novel target for cancer therapy. J Clin Invest. 2005;115:1492–1502. doi: 10.1172/JCI23002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheetham S, Tang MJ, Mesak F, et al. SPARC promoter hypermethylation in colorectal cancers can be reversed by 5-Aza-2′deoxycytidine to increase SPARC expression and improve therapy response. Br J Cancer. 2008;98:1810–1819. doi: 10.1038/sj.bjc.6604377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Socha MJ, Said N, Dai Y, et al. Aberrant promoter methylation of SPARC in ovarian cancer. Neoplasia. 2009;11:126–135. doi: 10.1593/neo.81146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chlenski A, Liu S, Guerrero LJ, et al. SPARC expression is associated with impaired tumor growth, inhibited angiogenesis and changes in the extracellular matrix. Int J Cancer. 2006;118:310–316. doi: 10.1002/ijc.21357. [DOI] [PubMed] [Google Scholar]
- 25.Yiu GK, Chan WY, Ng SW, et al. SPARC (secreted protein acidic and rich in cysteine) induces apoptosis in ovarian cancer cells. Am J Pathol. 2001;159:609–622. doi: 10.1016/S0002-9440(10)61732-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schiemann BJ, Neil JR, Schiemann WP. SPARC inhibits epithelial cell proliferation in part through stimulation of the transforming growth factor-beta-signaling system. Mol Biol Cell. 2003;14:3977–3988. doi: 10.1091/mbc.E03-01-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desai N, Trieu V, Damascelli B, et al. SPARC expression correlates with tumor response to albumin-bound paclitaxel in head and neck cancer patients. Transl Oncol. 2009;2:59–64. doi: 10.1593/tlo.09109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Green MR, Manikhas GM, Orlov S, et al. Abraxane, a novel Cremophor-free, albumin-bound particle form of paclitaxel for the treatment of advanced non-small-cell lung cancer. Ann Oncol. 2006;17:1263–1268. doi: 10.1093/annonc/mdl104. [DOI] [PubMed] [Google Scholar]
- 29.Socinski MA, Bondarenko IN, Karaseva NA, et al. Results of a randomized, phase III trial of nab-paclitaxel (nab-P) and carboplatin (C) compared with cremophor-based paclitaxel (P) and carboplatin as first-line therapy in advanced non-small cell lung cancer (NSCLC) J Clin Oncol. 2010;28:18s (suppl; abstr LBA7511). [Google Scholar]
- 30.Yao X, Qian CN, Zhang ZF, et al. Two distinct types of blood vessels in clear cell renal cell carcinoma have contrasting prognostic implications. Clin Cancer Res. 2007;13:161–169. doi: 10.1158/1078-0432.CCR-06-0774. [DOI] [PubMed] [Google Scholar]
- 31.Koukourakis MI, Giatromanolaki A, Brekken RA, et al. Enhanced expression of SPARC/osteonectin in the tumor-associated stroma of non-small cell lung cancer is correlated with markers of hypoxia/acidity and with poor prognosis of patients. Cancer Res. 2003;63:5376–5380. [PubMed] [Google Scholar]
- 32.Beck AH, Espinosa I, Gilks CB, et al. The fibromatosis signature defines a robust stromal response in breast carcinoma. Lab Invest. 2008;88:591–601. doi: 10.1038/labinvest.2008.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagai MA, Gerhard R, Fregnani JH, et al. Prognostic value of NDRG1 and SPARC protein expression in breast cancer patients. Breast Cancer Res Treat. 2010;126:1–14. doi: 10.1007/s10549-010-0867-2. [DOI] [PubMed] [Google Scholar]
- 34.Tai IT, Dai M, Owen DA, et al. Genome-wide expression analysis of therapy-resistant tumors reveals SPARC as a novel target for cancer therapy. J Clin Invest. 2005;115:1492–1502. doi: 10.1172/JCI23002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gail MH, Eagan RT, Feld R, et al. Prognostic factors in patients with resected stage I non-small cell lung cancer. A report from the Lung Cancer Study Group. Cancer. 1984;54:1802–1813. doi: 10.1002/1097-0142(19841101)54:9<1802::aid-cncr2820540908>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]