Abstract
The nodal stage of colorectal cancer is based on the number of positive nodes. It is inevitably affected by the number of removed lymph nodes, but lymph node ratio can be unaffected. We investigated the value of lymph node ratio in stage III colorectal cancer in this study. The clinicopathologic factors and follow-up data of 145 cases of stage III colorectal cancer between January 1998 and December 2008 were analyzed retrospectively. The Pearson and Spearman correlation analyses were used to determine the correlation coefficient, the Kaplan-Meier method was used to analyze survival, and the Cox proportional hazard regression model was used for multivariate analysis in forward stepwise regression. We found that lymph node ratio was not correlated with the number of removed lymph nodes (r = -0.154, P = 0.065), but it was positively correlated with the number of positive lymph nodes (r = 0.739, P < 0.001) and N stage (r = 0.695, P < 0.001). Kaplan-Meier survival analysis revealed that tumor configuration, intestinal obstruction, serum carcinoembryonic antigen (CEA) concentration, T stage, N stage, and lymph node ratio were associated with disease-free survival of patients with stage III colorectal cancer (P < 0.05). Multivariate analysis showed that serum CEA concentration, T stage, and lymph node ratio were prognostic factors for disease-free survival (P < 0.05), whereas N stage failed to achieve significance (P = 0.664). We confirmed that lymph node ratio was a prognostic factor in stage III colorectal cancer and had a better prognostic value than did N stage.
Keywords: Colorectal neoplasm, lymph node ratio, prognosis
Lymph node metastasis is the predominant prognostic indicator in nonmetastatic colorectal cancer and remains the principal determinant of adjuvant treatment following potentially curative resection. Furthermore, the number of lymph nodes removed surgically and evaluated pathologically has been demonstrated to affect both staging accuracy and oncologic outcomes in both node-negative and node-positive patients. The N staging of the American Joint Committee on Cancer (AJCC) staging system for colorectal cancer is based on the number of positive nodes: as the number of removed lymph nodes increases, the number of positive lymph nodes tends to increase as well, which corresponds to a higher N stage. However, the number of removed lymph nodes is affected by the patient, surgeon, pathologist, and others[1]. Therefore, the AJCC staging system for colorectal cancer has some limitations. The ratio of positive lymph nodes to the total number of removed lymph nodes (lymph node ratio, LNR) was originally investigated in esophagogastric tumors. The question of whether LNR is a prognostic factor in colorectal cancer has received increasing attention[2],[3]. If LNR can be considered a prognostic factor, it may also have the advantage of being less dependent on the number of removed lymph nodes than do N staging. Hence, we sought to determine if LNR could provide reliable and clinically relevant prognostic information and if this information was independent of N stage.
Materials and Methods
Patient information
Between January 1998 and December 2008, 157 patients with stage III colorectal cancer underwent radical surgery in the Guangzhou Red Cross Hospital. After excluding cases with familial adenomatous polyposis, multiple primary colorectal cancer, other malignancies at the same time or previously, or N1c stage disease, cases in which the patient underwent preoperative radio-chemotherapy or experienced recurrence or death within 3 months, and cases that were lost or had incomplete information, a total of 145 cases were included in this study. All cases were confirmed by pathology. In the group, 67 were men and 78 were women, ranging in age from 24 to 87 years (median age, 66 years). There were 88 cases of colon cancer and 57 cases of rectal cancer; 9 cases were at stage IIIA, 97 at stage IIIB, and 39 at stage IIIC. In total, 130 patients (89.66%) underwent adjuvant chemotherapy based on the 5-fluorouracil (5-FU) regimen, including 5-FU plus calcium folinate (CF) or oxaliplatin plus 5-FU and CF.
Methods
N stage was divided into N1 and N2 according to the number of regional positive lymph nodes: N1 = 1 to 3 positive lymph nodes and N2 = more than 3 positive lymph nodes. There were 101 cases of N1 stage disease and 44 cases of N2. LNR was defined as the ratio of positive lymph nodes to the total number of removed lymph nodes, and LNR was divided into four groups according to quartile: LNR1 (LNR ≤ 0.111), LNR2 (0.111 < LNR ≤ 0.200), LNR3 (0.200 < LNR ≤ 0.429), and LNR4 (LNR > 0.429). There were 36 cases for LNR1, 37 cases for LNR2, 37 cases for LNR3, and 35 cases for LNR4.
Follow-up
After operation, patients were followed up every 3 months for 2 years after operation, every 6 months for 3 years, and every year thereafter. The follow-up content included detailed history, physical examination, serology [routine blood test, biochemistry, carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9)], imaging (X-ray, B-ultrasound, CT, MRI, and so on), and colonoscopy. The follow-up deadline was June 2011. The median follow-up time was 35.4 months (6.6 to 138.9 months). No patients were lost in this study.
Statistical methods
SPSS18.0 software was applied for statistical analysis. The chi-square (χ2) test or Student's t test was applied to compare the clinical and pathologic factors. Pearson and Spearman correlation analyses were used to determine the correlation coefficient. The survival was analyzed using the Kaplan-Meier method. Multivariate analysis was performed using Cox proportional hazard regression model in forward stepwise regression. P < 0.05 was considered significant.
Results
LNR and clinicopathologic factors
A total of 1917 lymph nodes were removed from all 145 patients, with the average number of removed lymph nodes per patient being 13.22. Overall, 546 positive lymph nodes were detected. The average number of positive lymph nodes per patient was 3.77, and the average LNR was 0.279. A positive correlation was found between the number of positive lymph nodes and the number of removed lymph nodes (r = 0.421, P < 0.001). LNR was not correlated with the number of removed lymph nodes (r = -0.154, P = 0.065), but was positively correlated with the number of positive lymph nodes (r = 0.739, P < 0.001) and N stage (r = 0.695, P < 0.001), as shown in Table 1.
Table 1. The relationship between lymph node ratio and clinicopathologic factors of stage III colorectal cancer.
| Factor | LNR1 | LNR2 | LNR3 | LNR4 | P |
| Gender | 0.565 | ||||
| Male | 13 | 18 | 18 | 18 | |
| Female | 23 | 19 | 19 | 17 | |
| Age (years) | 0.752 | ||||
| <70 | 20 | 21 | 20 | 23 | |
| ≥70 | 16 | 16 | 17 | 12 | |
| BMI | 21.47±2.86 | 22.11 ±2.63 | 22.74±2.45 | 22.79±2.69 | 0.126 |
| Blood type | 0.079 | ||||
| O | 13 | 16 | 9 | 13 | |
| A | 12 | 12 | 12 | 8 | |
| B | 11 | 8 | 8 | 10 | |
| AB | 0 | 1 | 8 | 4 | |
| Anemia | 0.937 | ||||
| No | 28 | 29 | 27 | 26 | |
| Yes | 8 | 8 | 10 | 9 | |
| Blood transfusion | 0.794 | ||||
| No | 10 | 7 | 9 | 7 | |
| Yes | 26 | 30 | 28 | 28 | |
| Serum CEA concentration | 0.351 | ||||
| Normal | 17 | 12 | 16 | 18 | |
| Abnormal | 19 | 25 | 21 | 16 | |
| Unknown | 0 | 0 | 0 | 1 | |
| Tumor location | 0.092 | ||||
| Colon | 28 | 20 | 19 | 21 | |
| Rectum | 8 | 17 | 18 | 14 | |
| Tumor size (cm) | 6.08±2.51 | 5.29±1.87 | 5.66±2.03 | 5.37±1.70 | 0.351 |
| Intestinal obstruction | 0.344 | ||||
| No | 26 | 28 | 24 | 20 | |
| Yes | 10 | 9 | 13 | 15 | |
| Tumor configuration | 0.033 | ||||
| Exophytic | 18 | 8 | 10 | 6 | |
| Ulcerative | 10 | 23 | 19 | 21 | |
| Infiltrative | 8 | 6 | 8 | 8 | |
| Differentiation | 0.507 | ||||
| Well | 2 | 3 | 0 | 2 | |
| Morderate | 22 | 26 | 22 | 21 | |
| Poor | 12 | 8 | 15 | 12 | |
| Average number of removed lymph nodes | 15.25±6.20 | 12.14±3.74 | 13.49±5.65 | 12.00±7.18 | 0.065 |
| Average number of positive lymph nodes | 1.22±0.59 | 2.00±0.71 | 4.03±2.13 | 7.97±4.84 | <0.001 |
| N stage | <0.001 | ||||
| N1 | 35 | 37 | 25 | 4 | |
| N2 | 1 | 0 | 12 | 31 |
Univariate analysis
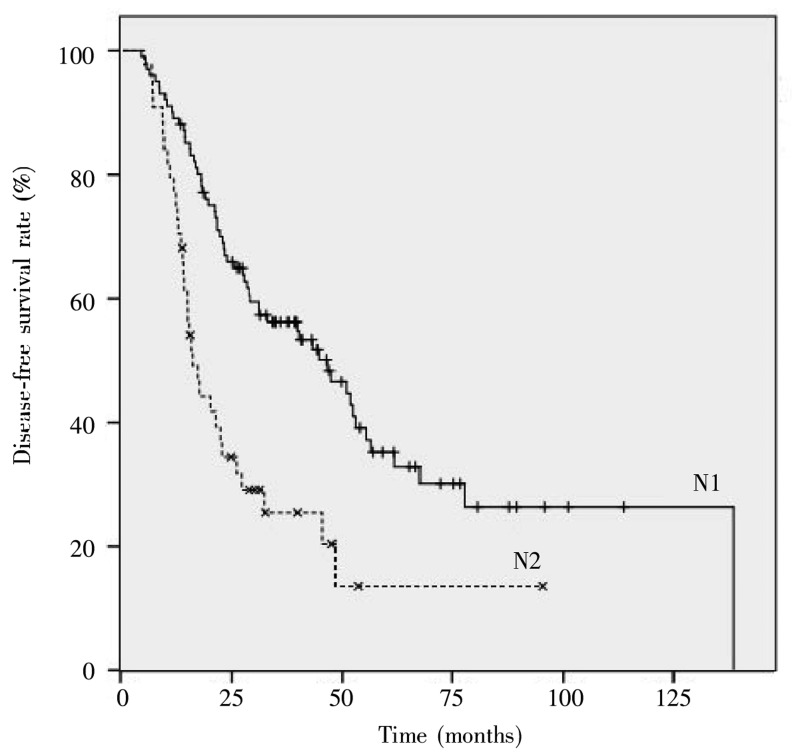
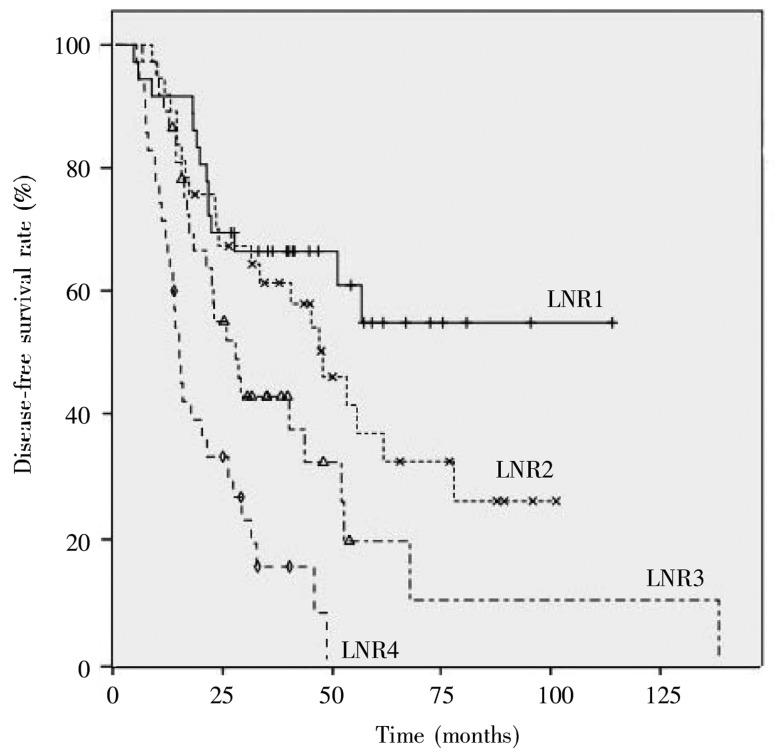
Univariate analysis showed that tumor configuration, intestinal obstruction, serum CEA concentration, T stage, N stage, and LNR were associated with disease-free survival of patients with stage III colorectal cancer, whereas gender, age, blood type, BMI, tumor location, blood transfusion, tumor size, differentiation, and the number of removed lymph nodes were not. The 5-year disease-free survival rates for patients with N1 and N2 diseases were 35.2% and 13.6%, respectively (P < 0.001), as shown in Figure 1. The 5-year disease-free survival rates for the LNR groups were 54.8% for LNR1, 36.8% for LNR2, 19.2% for LNR3, and 0% for LNR4, and the difference was significant (P < 0.001), as shown in Figure 2.
Figure 1. Disease-free survival curves of stage III colorectal cancer patients at different N stages.
Figure 2. Disease-free survival curves of stage III colorectal cancer patients with different lymph node ratios.
Multivariate analysis
Multivariate analysis showed that serum CEA concentration, T stage, and LNR were independent prognostic factors for disease-free survival of patients with stage III colorectal cancer (P < 0.05), whereas N stage failed to achieve significance (P = 0.664), as shown in Table 2.
Table 2. Multivariate analysis for prognosis in stage III colorectal cancer.
| Variate | B | SE | Wald | df | Sig. | Exp(B) | 95% CI for Exp(B) |
| Tumor configuration | 0.277 | 0.155 | 3.199 | 1 | 0.074 | 1.319 | 0.974–1.786 |
| Intestinal obstruction | 0.388 | 0.228 | 2.883 | 1 | 0.090 | 1.473 | 0.942–2.305 |
| Serum CEA concentration | 0.668 | 0.226 | 8.757 | 1 | 0.003 | 1.950 | 1.253–3.035 |
| T stage | 0.721 | 0.211 | 11.717 | 1 | 0.001 | 2.057 | 1.361–3.108 |
| N stage | -0.147 | 0.339 | 0.189 | 1 | 0.664 | 0.863 | 0.444–1.677 |
| LNR | 2.464 | 0.663 | 13.789 | 1 | 0.000 | 11.748 | 3.200–43.122 |
Discussion
In this study, we found that LNR was not correlated with the number of removed lymph nodes, but it was positively correlated with the number of positive lymph nodes and N stage. Kaplan-Meier survival analysis revealed that tumor configuration, intestinal obstruction, serum CEA concentration, T stage, N stage, and LNR were associated with disease-free survival of patients with stage III colorectal cancer. Multivariate analysis showed that serum CEA concentration, T stage, and LNR were prognostic factors for disease-free survival, whereas N stage failed to achieve significance. Hence, we confirmed that LNR was a prognostic factor in stage III colorectal cancer and found that its prognostic value was better than that of N stage.
The presence of metastatic spread to locoregional lymph nodes has long been established as an important prognostic factor in most solid cancers. The classical Halstedian paradigm views lymphatic barrier as the first line of defense against stepwise systemic spread. Assuming this model reflects the reality, timely and adequate lymphadenectomy would prevent systemic spread and improve outcome. On the contrary, Punglia et al.[4] contends that systemic spread starts early in the course of the tumor-host relationship and is independent from lymph node metastasis, and therefore, the natural history of the disease will hardly be influenced by extensive locoregional surgery. A similar debate surrounds the role of lymphadenectomy in colorectal cancer. This debate has been fueled by the following paradox: on one hand, clinical trials examining extensive (extramesenteric) lymphadenectomy in colorectal cancer failed to show any survival benefit[5], whereas on the other hand, data from large retrospective studies have consistently shown a positive association between survival and the number of lymph nodes examined in the surgical specimen[6]. It is commonly assumed that this observation is not explained by a therapeutic effect of removing more lymph nodes, but rather reflects stage migration[7].
According to the 7th edition of the AJCC staging system for colorectal cancer, N staging is based on the number of positive nodes. Hence, this staging system is inevitably affected by the number of removed lymph nodes. Indeed, as the number of removed lymph nodes increases, the number of positive nodes will likely increase, which will result in a higher N stage. The National Comprehensive Cancer Network (NCCN) recommends the removal and pathologic examination of at least 12 nodes for resectable primary colorectal cancer. The number 12 does not hold any particular biological significance; it likely results from a statistical probability distribution indicating that once more than 12 nodes have been assessed, the likelihood of missing any positive mesenteric nodes becomes very small[8]. In fact, the optimal number of lymph nodes removed is still controversi. A recent analysis of the pathologic staging of 131 953 patients from the Surveillance, Epidemiology and End Results (SEER) database with a beta-binomial model suggested that the minimum number of nodes required for adequate N staging depended on the T stage: to achieve correct staging with a probability of 90%, 1 node needed to be examined for T1 disease, 4 nodes for T2 disease, 13 nodes for T3 disease, and 21 nodes for T4 disease[9]. Some investigators even believed that the stage would improve as the number of removed lymph nodes increases[10]. However, several possible factors may influence the number of removed lymph nodes, including the operating surgeon, the examining pathologist, the patient, and the biology of the disease itself. For example, more nodes are removed from young, thin, female patients with cancer of the right colon[1]. Furthermore, more node-positive patients are typically reported by high-volume surgical centers[11]. It is generally known that as the number of removed lymph nodes increases, so too does the likelihood that their pathologic stage will change. However, LNR can be unaffected by the number of removed lymph nodes. Indeed, in our study, a positive correlation was found between the number of positive lymph nodes and the number of removed lymph nodes, but LNR was not correlated with the total number of removed lymph nodes. Intuitively, it seems evident, for example, that the prognostic significance of 3 positive nodes on a total of 3 nodes examined is completely different when a total of 35 nodes were removed. Moug et al.[12] demonstrated a noteworthy result: the 5-year overall survival for pN1 patients with an LNR greater than 40% was much worse (60%) than that for N2 patients with an LNR less than 20% (73%). These findings suggest that it is difficult to use simple N stage to accurately determine the prognosis in colorectal cancer and indicate the necessity of LNR for determining the prognosis in stage III colorectal cancer and for guiding treatment[2],[3].
Moug et al.[12] are the first to report that LNR had a high prognostic impact on colon cancer. Afterward, several studies showed that LNR was a more accurate prognostic parameter for tumor staging than established prognostic factors or the pN stage[13]-[16]. According to multivariate analyses in these studies, LNR was the only prognostic indicator, whereas T stage and lymph node metastasis, described as prognostic factors in the 7th edition of the AJCC TNM staging system, were not[13]. In a recent report, 514 cases of colorectal cancer were categorized into four groups on the basis of quartiles for LNR[14]. More specifically, for LNR1, LNR2, LNR3, and LNR4, the 5-year overall survival rates were 79%, 72%, 62%, and 55%, respectively (P < 0.001) and the 5-year disease-free survival rates were 73%, 67%, 54%, and 42%, respectively (P < 0.001). LNR was an independent prognostic factor for both overall survival and disease-free survival in multivariate analysis, and the study suggested that it could be a good staging complement for patients with stage III colorectal cancer when less than 12 lymph nodes are removed. In another report, 36 712 patients with node-positive nonmetastatic colon cancer diagnosed between 1992 and 2004 were identified from the SEER database and stratified according to LNR and the number of nodes examined, and LNR was significantly associated with survival[15]. Importantly, the hazard ratios across all LNR strata (HR = 1.1235–5.116) exceeded those of AJCC N stage (HR = 1.126–1.221), suggesting that LNR may better estimate overall survival than does N stage. Ceelen et al.[16] retrospectively analyzed 16 studies on LNR, which included 33 984 cases of colorectal cancer, conducted between 1975 and 2009. All identified studies showed LNR to be an independent predictor of overall survival, disease-free survival, or cancer-specific survival by multivariate analysis (15 studies) or univariate analysis (1 study). Importantly, in 7 studies (44% ), the total number of positive nodes (N stage) was no longer an independent prognostic factor when LNR was included in the regression model. Four studies (25%) reported that the number of positive nodes was a significant predictor of outcome but with a lower significance compared to the LNR. In 4 studies (25%), the prognostic significance of the number of positive nodes was not available. Our study showed that LNR was one of the prognostic indicators in stage III colorectal cancer by multivariate analysis, but N stage failed to achieve significance because its statistical weight was obscured by the LNR. These results were similar to those described by Hong et al.[13] and Vaccaro et al.[17], who reported that the prognostic value of LNR was better than that of N stage in stage III colorectal cancer.
Here, we have confirmed that LNR is a prognostic factor in stage III colorectal cancer and that its prognostic value is better than that of N stage. Nevertheless, this study was retrospective and included a small sample size of colon cancer and rectal cancer cases. Furthermore, the adjuvant chemotherapy regimen was not the same for all patients. Thus, large scale and prospective studies are required to further confirm our results.
Although the value of LNR is widely accepted, appropriate stratification for LNR remains unclear. The cutoff value is not universal, and methods used to choose the cutoff also vary[18],[19], with different parameters such as quartiles, median values, and arbitrary values being used in each study. In this study, LNR was divided into four groups by quartiles, and survival curves of the four groups were completely separate, suggesting that quartile was a good cutoff point. Nevertheless, further large scale studies are still necessary to determine a specific valid cutoff point for LNR to achieve prognostic stratification.
References
- 1.Rosenberg R, Friederichs J, Schuster T, et al. et al. Prognosis of patients with colorectal cancer is associated with lymph node ratio: a single-center analysis of 3026 patients over a 25-year time period. Ann Surg. 2008;248:968–978. doi: 10.1097/SLA.0b013e318190eddc. [DOI] [PubMed] [Google Scholar]
- 2.Dekker JW, Peeters KC, Putter H, et al. et al. Metastatic lymph node ratio in stage III rectal cancer, prognostic significance in addition to the 7th edition of the TNM classification. Eur J Surg Oncol. 2010;36:1080–1086. doi: 10.1016/j.ejso.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Berger AC, Sigurdson ER, LeVoyer T, et al. et al. Colon cancer survival is associated with decreasing ratio of metastatic to examined lymph nodes. J Clin Oncol. 2005;23:8706–8712. doi: 10.1200/JCO.2005.02.8852. [DOI] [PubMed] [Google Scholar]
- 4.Punglia RS, Morrow M, Winer EP, et al. et al. Local therapy and survival in breast cancer. N Engl J Med. 2007;356:2399–2405. doi: 10.1056/NEJMra065241. [DOI] [PubMed] [Google Scholar]
- 5.Titu LV, Tweedle E, Rooney PS. High tie of the inferior mesenteric artery in curative surgery for left colonic and rectal cancers: a systematic review. Dig Surg. 2008;25:148–157. doi: 10.1159/000128172. [DOI] [PubMed] [Google Scholar]
- 6.Chang GJ, Rodriguez-Bigas MA, Skibber JM, et al. et al. Lymph node evaluation and survival after curative resection of colon cancer:systematic review. J Natl Cancer Inst. 2007;99:433–441. doi: 10.1093/jnci/djk092. [DOI] [PubMed] [Google Scholar]
- 7.Sargent D, Sobrero A, Grothey A, et al. et al. Evidence for cure by adjuvant therapy in colon cancer: observations based on individual patient data from 20898 patients on 18 randomized trials. J Clin Oncol. 2009;27:872–877. doi: 10.1200/JCO.2008.19.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner J, Vollmer RT. Lymph nodes in colorectal carcinoma-the Poisson probability paradigm. Am J Clin Pathol. 2006;125:866–872. doi: 10.1309/35AE-PKTA-AGUT-HQKQ. [DOI] [PubMed] [Google Scholar]
- 9.Gonen M, Schrag D, Weiser MR. Nodal staging score: a tool to assess adequate staging of node-negative colon cancer. J Clin Oncol. 2009;27:6166–6171. doi: 10.1200/JCO.2009.23.7958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein NS. Lymph node recoveries from 2427 pT3 colorectal resection specimens spanning 45 years-recommendations for a minimum number of recovered lymph nodes based on predictive probabilities. Am J Surg Pathol. 2002;26:179–189. doi: 10.1097/00000478-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Galizia G, Orditura M, Ferraraccio F, et al. et al. The lymph node ratio is a powerful prognostic factor of node-positive colon cancers undergoing potentially curative surgery. World J Surg. 2009;33:2704–2713. doi: 10.1007/s00268-009-0207-z. [DOI] [PubMed] [Google Scholar]
- 12.Moug SJ, Saldanha JD, McGregor JR, et al. et al. Positive lymph node retrieval ratio optimises patient staging in colorectal cancer. Br J Cancer. 2009;100:1530–1533. doi: 10.1038/sj.bjc.6605049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong KD, Lee SI, Moon HY, et al. et al. Lymph node ratio as determined by the 7th edition of the American Joint Committee on Cancer staging system predicts survival in stage III colon cancer. J Surg Oncol. 2011;103:406–410. doi: 10.1002/jso.21830. [DOI] [PubMed] [Google Scholar]
- 14.Huh JW, Kim YJ, Kim HR, et al. Ratio of metastatic to resected lymph nodes as a prognostic factor in node-positive colorectal cancer. Ann Surg Oncol. 2010;17:2640–2646. doi: 10.1245/s10434-010-1015-2. [DOI] [PubMed] [Google Scholar]
- 15.Chen SL, Steele SR, Eberhardt J, et al. et al. Lymph node ratio as a quality and prognostic indicator in stage III colon cancer. Ann Surg. 2011;253:82–87. doi: 10.1097/SLA.0b013e3181ffa780. [DOI] [PubMed] [Google Scholar]
- 16.Ceelen W, Van Nieuwenhove Y. Prognostic value of the lymph node ratio in stage III colorectal cancer: a systematic review. Ann Surg Oncol. 2010;17:2847–2855. doi: 10.1245/s10434-010-1158-1. [DOI] [PubMed] [Google Scholar]
- 17.Vaccaro CA, Im V, Rossi GL, et al. et al. Lymph node ratio as prognosis factor for colon cancer treated by colorectal surgeons. Dis Colon Rectum. 2009;52:1244–1250. doi: 10.1007/DCR.0b013e3181a65f0b. [DOI] [PubMed] [Google Scholar]
- 18.Peng J, Xu Y, Guan Z, et al. et al. Prognostic significance of the metastatic lymph node ratio in node-positive rectal cancer. Ann Surg Oncol. 2008;15:3118–3123. doi: 10.1245/s10434-008-0123-8. [DOI] [PubMed] [Google Scholar]
- 19.Schumacher P, Dineen S, Barnett C, Jr, et al. et al. The metastatic lymph node ratio predicts survival in colon cancer. Am J Surg. 2007;194:827–831. doi: 10.1016/j.amjsurg.2007.08.030. [DOI] [PubMed] [Google Scholar]