Abstract
Background
Strategies for patient selection for intra-arterial therapy (IAT) in acute ischemic stroke (AIS) are highly variable. The degree of protocol adoption and treatment rates associated with implementation of a service-wide patient selection IAT protocol were assessed.
Methods
All patients with AIS prospectively recorded in our stroke database from January 2007 to June 2009 were reviewed. The IAT patient selection protocol was implemented in March 2008. Patients were defined as likely to benefit (LTB) from IAT if they had brain imaging completed within 6 h from last known well time, NIH Stroke Scale score ≥8, infarct volume ≤100 ml and evidence of proximal artery occlusion.
Results
Of 1348 subjects identified, 118 (8.7%) met the criteria for LTB and 62 (52%) underwent IAT. There was a significant increase in rates of IAT among LTB patients after protocol implementation (61% vs 40%, p<0.02). In LTB patients, factors associated with IAT were stroke duration (OR 0.78, 95% CI 0.6 to 0.9 per hour), arrival within later calendar months during study period (OR 1.1, 95% CI 1.02 to 1.2 per month), intravenous tissue plasminogen activator (OR 0.6, 95% CI 0.4 to 0.9) and age (OR 0.98, 95% CI 0.95 to 1.02 per year). After multivariable adjustment, only stroke duration (OR 0.65, 95% CI 0.5 to 0.8 per hour) remained an independent predictor of IAT.
Conclusions
Most patients with AIS did not meet our criteria for LTB and only 52% of those defined as LTB received IAT. Protocol adoption increased the use of IAT over time; however, further exploration of factors associated with the reasons for non-treatment and the impact of IAT on outcomes is necessary.
INTRODUCTION
Intra-arterial brinolysis and/or endovascular mechanical recanalization are frequently offered as an open-label or off-label treatment option to patients with acute ischemic stroke (AIS) who present with persistent symptoms outside the 3 h window for intravenous (IV) thrombolysis.1 Despite its increasing utilization,2 there are no uniform criteria for patient selection for intra-arterial therapy (IAT)3,4 and the evidence supporting current approaches is limited.5–7
Patients who present with intracranial proximal artery occlusion (PAO), small infarct volume and large territory of cerebral tissue deemed ‘at-risk’ have been proposed to be ‘most likely to benefit’ from intra-arterial intervention.8–10 We sought to determine the characteristics, degree of protocol adoption and rates of IAT after implementation of a service-wide consensus-driven patient selection protocol for IAT.
METHODS
Protocol for patient selection for IAT in patients with AIS
In July 2007 a year-long process was initiated in our institution to develop a service-wide patient selection protocol for open-label or off-label IAT based on expert guidelines, past site experience and local opinion leader consensus. Two separate protocols were developed based on the available evidence for anterior versus posterior circulation PAO. This process was intended to reduce variation in patient selection and provide a more homogeneous group of patients selected for IAT across various combinations of stroke neurology and endovascular providers. All members of the acute stroke team were invited to participate, including the staff and fellows of our Acute Stroke Service, emergency department, neuroradiology division and the multispecialty endovascular division. The evidence reviewed included published clinical trial and registry data (PROACT-II, MERCI and Multi-MERCI inclusion criteria)5–7,11 as well as the prospectively collected data from the our service-wide IAT experience. A review of our local institutional data suggested that (1) earlier time to IATwas associated with better outcomes (p<0.05); (2) patients with AIS with low NIH Stroke Scale (NIHSS) scores (0–3) had very good outcomes (64% discharged directly to home) without IAT; and (3) outcomes were similar for patients with terminal internal carotid artery versus proximal middle cerebral artery (MCA) occlusions, while distal MCA M2 or anterior cerebral artery clots were felt to be more difficult to reach and to manipulate. These variables and those identified from the published clinical trial and registry data as independently associated with good clinical outcome were included as selection criteria for the IAT protocol.
All patients with AIS were categorized as those who were ‘likely to benefit’ (LTB), ‘uncertain to benefit’ (UCTB) or ‘unlikely to benefit’ (ULTB) from IAT. Patients were defined as LTB if they had brain imaging completed within 6 h from last known well (LKW) time, initial NIHSS score ≥8 (or evidence of moderate to severe aphasia), baseline infarct volume ≤100 ml and evidence of PAO (internal carotid artery or MCA M1 or proximal M2 segments) on angiography (table 1).
Table 1.
The Acute Stroke Service proposed consensus criteria for patient selection for intra-arterial therapy in anterior circulation stroke in the absence of proven benefit among currently available treatments
| Likely to benefit | Uncertain to benefit | Unlikely to benefit | Levels/source of evidence |
|---|---|---|---|
| Imaging completed <6 h since LKW* | Imaging completed within 6–8 h since LKW | Imaging completed >8 h since LKWy | MERCI/Penumbra time window, our data (unlikely to begin therapy within time window of MERCI) |
| NCCT hypodensity or DWI hyperintensity <100 ml (or <1/3 of MCA territory)‡ | NCCT hypodensity or DWI hyperintensity <100 ml (or <1/3 of MCA territory)‡ | NCCT hypodensity or DWI hyperintensity ≥100 ml (or ≥1/3 of MCA territory)‡ | PROACT-II inclusion criteria (no benefit in post hoc analysis, risk of hemorrhagic complications) |
| NIHSS ≥8§ or moderate to severe aphasia | NIHSS 4–7 | NIHSS <4§ or without significant aphasia | PROACT-II, our data (stroke mortality exceeds sICH with NIHSS ≥8 but complete recovery is likely with NIHSS ≤4) |
| Acute vascular occlusion of the cervical or intracranial ICA, MCA M1 or proximal M2 segments | Acute vascular occlusion present in ACA-A1-3 segments or distal M2 but not in the ICA or MCA M1/proximal M2 | Acute vascular occlusion present in ACA-A1-3 segments or distal M2 but not in the ICA or MCA M1/proximal M2 | PROACT-II, MERCI, Multi-MERCI, our data |
6 h from onset and additional 1.5 h to access clot; treatment expected to start by 7.5 h in all cases.
8 h exceeds all currently FDA approved therapies.
Calculated by abc/2 rule.
Use NIHSS value closest to imaging; if fluctuating, use highest NIHSS.
ACA, anterior cerebral artery; DWI, diffusion weighted imaging; ICA, internal carotid artery; LKW, last known well time; MCA, middle cerebral artery; NCCT, non-contrast computerized tomography; NIHSS, National Institutes of Health Stroke Scale; sICH, symptomatic intracranial hemorrhage.
Patient selection for IAT was based on the following algorithm: patients in whom all favorable criteria were present were defined as LTB, those in whom any unfavorable criteria were present were defined as ULTB and those remaining were assigned to UCTB (figure 1). Patients who were UCTB were to be offered clinical trial enrollment preferentially but could be offered IAT on a compassionate basis. All LTB patients were considered for IAT, including using mechanical thrombectomy for up to 8 h from the LKW time.6
Figure 1.
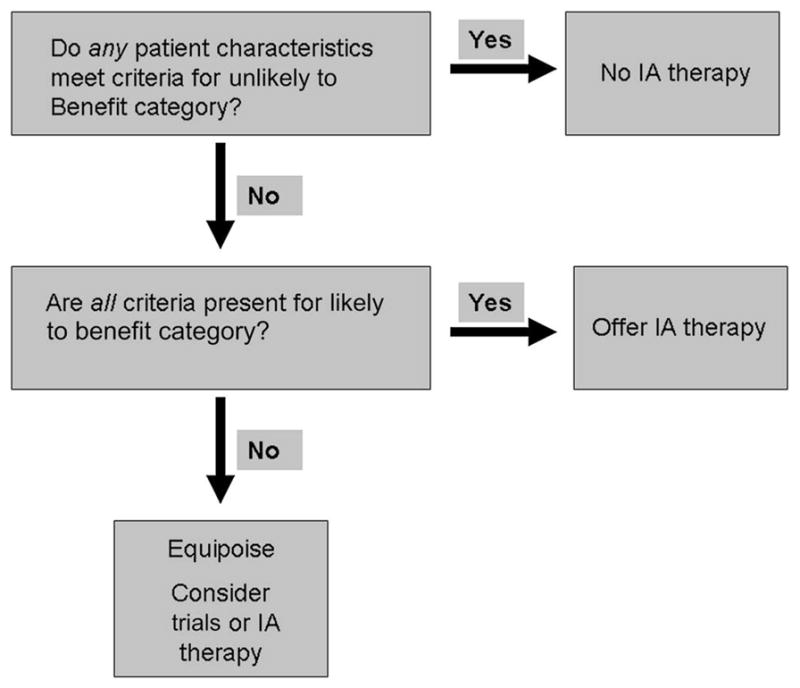
The Acute Stroke Service protocol for patient selection for intra-arterial (IA) therapy in anterior circulation acute ischemic stroke (implemented March 2008).
The protocol was reviewed by the community of acute stroke care providers and underwent several iterations until a version acceptable to all groups was crafted. The final version was accepted for adoption and implementation began in March 2008. While the protocol remained stable, all constituents agreed that it could be modified as future data might dictate.
This current analysis includes a review of all AIS cases that presented within the equal chronological time intervals before and after the protocol implementation in March 2008.
Patient characteristics
We conducted a retrospective review of 1348 patients prospectively enrolled into our Get With the Guidelines Stroke (GWTG-S) database (1 January 2007 to 30 June 2009).
Patient characteristics, clinical presentation, acute treatments including intravenous tissue plasminogen activator (tPA) and IAT as well as contraindications or warnings documented as the reason for withholding acute treatments were abstracted from medical records by a trained abstracter in accordance with the GWTG-S. All patients underwent clinical evaluation by a neurologist, diagnostic neuroimaging and laboratory testing on arrival at the emergency department. All patients who presented within 9 h from the LKW time were considered in the analysis.
For each subject considered LTB based on the IAT protocol criteria, additional medical record review was completed to identify treating acute stroke staff member. For the purpose of this analysis, a level of experience based on the number of years in practice was assigned to each acute stroke staff member as follows: level 1 (<4 years of experience), level 2 (4–8 years of experience) or level 3 (>8 years of experience). Characteristics of PAO and infarct volume were abstracted by a single investigator from the neuroimaging data considered in decision-making for IAT in each case. IAT treatment status (treated vs untreated) for LTB subjects was the primary outcome of interest.
Statistical methods
Continuous variables were analyzed using the t test or Wilcoxon rank sum test as appropriate. The Fisher exact test was used to analyze proportions. Univariate and multivariate logistic regression analyses were performed to evaluate predictors of IAT in LTB patients. The significance level was set at p<0.05 for all analyses.
RESULTS
Among the 1348 patients with AIS who presented to our hospital within the study period, 118 (8.7%) met the criteria for LTB (mean age 66±14.8 years, 40% women, 93% Caucasian). Of these, 62 (52%) underwent IAT but only 13 of the 62 IAT subjects (21%) received an intervention based on the current practice guidelines as ‘an option for treatment of selected patients who have major stroke of <6 h duration due to occlusion of the MCA and who are not otherwise candidates for IV thrombolysis’.3 The clinical characteristics of the LTB cohort are presented in table 2.
Table 2.
Clinical characteristics of patients considered likely to benefit from intra-arterial treatment based on the Acute Stroke Service protocol (n=118)
| Characteristics | |
|---|---|
| Age, years (±SD) | 72.4 (±14.8) |
| Gender (male,%) | 44.1 |
| Ethnicity (white, %) | 94.5 |
| NIHSS score, median (IQR) | 15.5 (12–19) |
| IV tPA (%) | 62.7 |
| Stroke duration, h (±SD) | 3.1 (±1.8) |
| Hypertension (%) | 67.8 |
| DM (%) | 19.5 |
| CAD (%) | 34.75 |
| Hyperlipidemia (%) | 44.9 |
| Tobacco use (%) | 37.1 |
| Prior stroke/TIA (%) | 4.8 |
| Antiplatelet agent use (%) | 32.5 |
| Warfarin use (%) | 13.7 |
| Antihypertensive agent use (%) | 62.7 |
| Statin use (%) | 41.5 |
| Hypoglycemic agent use (%) | 18.8 |
| WBC count, 103/mm3 (± SD) | 10.2 (±3.8) |
| Hemoglobin, g/dl (± SD) | 13.2 (±1.9) |
| Platelet count, 103/mm3 (± SD) | 263 |
| Blood glucose, mg/dl (± SD) | 139.5 (±52.8) |
| INR, mean (± SD) | 1.2 (±0.3) |
AF, atrial fibrillation; CAD, coronary artery disease; DM, diabetes mellitus; INR, international normalized ratio; IV tPA, intravenous tissue plasminogen activator; NIHSS, National Institutes of Health Stroke Scale; PMH, past medical history; TIA, transient ischemic attack; WBC, white blood cell.
In univariate analysis, longer stroke duration measured as increasing LKW to arrival time (OR 0.78, 95% CI 0.6 to 0.9 per hour) and age (OR 0.9, 95% CI 0.9 to 1.01 per year) decreased the odds of IAT among the LTB subjects, whereas IV tPA use (OR 1.8, 95% CI 0.8 to 3.9) and arrival for stroke treatment in the later months after the start of the study period (OR 1.1, 95% CI 1.02 to 1.2 per calendar month) increased the odds of IAT (table 3). However, following multivariable adjustment, only stroke duration (time from LKW to arrival) independently reduced the odds of IAT among the LTB subjects (OR 0.65, 95% CI 0.5 to 0.8 per hour; table 3).
Table 3.
Univariate and multivariate predictors of intra-arterial therapy among patients likely to benefit (n=118)
| Univariate analysis OR (95% CI) | Multivariate analysis OR (95% CI) | |
|---|---|---|
| Demographics | ||
| Age, years | 0.98 (0.95 to 1.02)* | 0.98 (0.95 to 1.16) |
| Gender, female | 0.9 (0.95 to 1.01) | 1.12 (0.4 to 3.0) |
| AIS-related factors | ||
| Stroke duration, h | 0.78 (0.6 to 0.9)† | 0.65 (0.5 to 0.8) † |
| NIHSS, points | 1.04 (0.9 to 1.13) | 1.1 (0.9 to 1.2) |
| IV tPA | 1.8 (0.8 to 3.9)* | 0.7 (0.2 to 2.1) |
| Date of arrival, month | 1.1 (1.02 to 1.2) † | 1.1 (0.9 to 1.1) |
| Attending level | 0.9 (0.5 to 1.5) | 0.8 (0.1 to 1.5) |
| Vascular risk factors | ||
| Hypertension | 1.16 (0.5 to 2.5) | 1.7 (0.4 to 6.3) |
| Diabetes mellitus | 1.2 (0.5 to 3.05) | 2.0 (0.4 to 9.5) |
| Hyperlipidemia | 1.02 (0.5 to 2.1) | 1.2 (0.4 to 3.8) |
| CAD | 0.9 (0.4 to 1.9) | 1.7 (0.5 to 5.8) |
| Tobacco use | 0.8 (0.4 to 1.7) | 0.8 (0.3 to 1.7) |
| Admission medication use | ||
| Antiplatelet agent | 1.4 (0.6 to 3.1) | 0.8 (0.2 to 2.7) |
| Warfarin | 0.5 (0.2 to 1.5) | 1.1 (0.1 to 11.8) |
| Antihypertensive agent | 0.9 (0.4 to 1.8) | 0.4 (0.1 to 1.8) |
| Statin | 0.8 (0.3 to 1.6) | 0.7 (0.2 to 2.4) |
| Hypoglycemic agent | 1.35 (0.5 to 3.5) | 1.4 (0.4 to 3.6) |
| Admission laboratory values | ||
| WBC count, per ml | 0.9 (0.8 to 1.1) | 1.1 (0.9 to 1.2) |
| Hemoglobin, mg/dl | 1.004 (0.8 to 1.2) | 0.8 (0.6 to 1.3) |
| Platelet count, per ml | 1.0 (0.9 to 1.004) | 0.9 (0.9 to 1.01) |
| Blood glucose, mg/dl | 0.9 (0.9 to 1.01) | 0.9 (0.9 to 1.01) |
| INR | 0.5 (0.2 to 1.6) | 0.4 (0.05 to 4.1) |
Univariate variables with p value <0.2.
Statistically significant variables (p<0.05).
AIS, acute ischemic stroke; CAD, coronary artery disease; DM, diabetes mellitus; INR, international normalized ratio; IV tPA, intravenous tissue plasminogen activator; NIHSS, National Institutes of Health Stroke Scale; WBC, white blood cell count.
There was a significant increase in the rate of IAT after protocol implementation. Of the 48 patients classified as LTB between January 2007 and February 2008, 19 (40%) underwent IAT compared with 43/70 (61%) between March 2008 and June 2009 (p<0.02). There was no association between use of IATand either the individual stroke staff involved or years of experience (p=0.6).
Descriptively, the reasons for not performing IAT in the LTB group were: late presentation in the intervention window or concerns about reliability of the reported LKW time (21.4%), rapid improvement (16.1%), family refusal (14.3%), neuro-imaging features of limited tissue at risk (11%), extensive clot burden identified by endovascular team (7.2%), reason unclear/unrecorded (7.2%), advanced age (5.3%), technical challenge identified by endovascular team (5.3%), carotid dissection (4%), advanced directives (4%), hemorrhagic conversion (1.7%) and trial enrollment (1.7%).
DISCUSSION
Limited evidence, lack of guideline recommendations and differences in reimbursement among treatment options all probably contribute to variability of practice among the advanced centers caring for patients with acute cerebral ischemia in the USA.1 IAT is frequently used as part of an off-label (endovascular brinolysis) or an on-label unproven treatment (mechanical recanalization) in patients with PAO who present with persistent symptoms of AIS, both within and outside the 3 h window for IV thrombolysis.3 Lack of a uniform approach to patient selection for open-label IAT and a uniform IAT treatment algorithm in acute stroke also contributes to unease about randomization against placebo in current clinical trials studying the benefits of IAT,12 and may ultimately result in failure of current trials to demonstrate the benefit due to selection bias or incomplete enrollment.13,14 In the absence of randomized clinical trial data, prospective data collection in a uniform cohort of patients may be useful to evaluate the effect of quality-based interventions.
Our findings indicate that a process for consensus development of an evidence-based patient selection protocol led to rapid adoption and a significant increase over time in the use of IAT among patients defined as LTB. This effect was not limited to specific stroke staff members or influenced by the time in which they trained. We suggest that increased IAT rates may be due to fewer disagreements over patient eligibility between the stroke neurologist and the endovascular specialist on call. The fact that none of the multiple variables traditionally considered in decision-making for IAT other than stroke duration were associated with IAT selection suggests strong adherence to the protocol.
The larger number of patients who were classified as LTB but not treated prior to protocol implementation suggests that there was inconsistent application of the decision-making process which was amenable to a quality improvement intervention. Given that a significant proportion of patients who would otherwise be considered LTB from IAT did not receive endo-vascular treatment prior to the protocol implementation, this increase may indicate a successful service-wide quality improvement intervention that, in the future, may be expected to contribute to better outcome metrics in patients with acute cerebral ischemia.
Despite increasing the rate of IATafter implementation of the protocol, the overall rate of IAT for those patients defined as LTB was only 52%. Considering that only stroke duration independently predicted whether a subject otherwise deemed LTB from IAT actually underwent the procedure, the role of other factors in decision-making regarding IAT needs further investigation. Previous reports have indicated that age, stroke duration beyond 6 h and mild or rapidly improving symptoms precluded patients from receiving IAT.15 Interestingly, our data indicate that treatment with IV tPA prior to consideration for IAT decreased the odds of undergoing IAT, probably due to clinical improvement from early reperfusion. On the other hand, those patients with persistent PAO and severe disability who were unlikely to improve significantly from IV tPA alone were still eligible for IAT within this subgroup.16
Based on our definition of LTB patients, they constitute only a small proportion of all patients with AIS. Statistical modeling of the IAT data collected prospectively at our institution and the published IAT trial data produced the variables defining LTB criteria including (1) neuroimaging definition of stroke severity (volume of cerebral infarct on diffusion-weighted imaging or head CT if MRI is contraindicated); (2) presence of PAO on CTor MR angiography; (3) evidence of clinical-radiographic mismatch (stroke severity measured as NIHSS score immediately before and/or after neuroimaging); (4) imaging available within an acceptable time limit to allow for timely IAT initiation; and (5) stroke severity. These criteria alone or in various combinations have been used by previous investigators to select patients who were likely to have a good outcome with IAT5–7,17; however, with advancing knowledge, these criteria continue to evolve and will probably undergo further revision over time based on emerging data.8,18 Moreover, prospective validation of the LTB criteria and the role of protocol-based patient selection for IAT in improvement of clinical stroke outcomes will be required. Clinical outcomes among patients at varying levels of anticipated IAT benefit (LTB, UCTB and ULTB) who are considered for various acute reperfusion strategies are currently underway.
CONCLUSION
These data provide evidence that a uniform approach to patient selection for open-label or compassionate use of IAT may improve the rates of patient inclusion for intervention as well as the homogeneity of the patient cohort. Further exploration of factors associated with the reasons for non-treatment and the impact of IAT on outcomes is warranted.
Footnotes
Contributors Study design: NSR, LHS. Data acquisition: NSR, EES, KMF, RGN, AJY, JAH, LHS. Data analysis: NSR, LHS. Study management: LHS. Manuscript preparation: NSR. Manuscript review: NSR, EES, KMF, RGN, AJY, JAH, LHS.
Competing interests None.
Ethics approval Ethics approval was provided by the IRB.
Provenance and peer review Not commissioned; externally peer reviewed.
Disclosures NSR, EES and KMF have nothing to disclose. RGN is a member of the Physician Advisory Board for Concentric Medical, ev3 Neurovascular, Coaxia, Rapid Medical, and Neurointervention. He is also the PI for the TREVO-2 Trial but does not receive any consulting fees. AJY has received research funding from Penumbra Inc (core imaging laboratory for the START trial). JAH has received consulting fees from Intra-tech, a development stage stroke device company. LHS serves as paid stroke systems consultant to the Massachusetts Department of Public Health and the Chair of the GWTG National Steering Committee (unpaid).
References
- 1.Saver JL, Albers GW, Dunn B, et al. for the SVIC. Stroke therapy academic industry roundtable (stair) recommendations for extended window acute stroke therapy trials. Stroke. 2009;40:2594–600. doi: 10.1161/STROKEAHA.109.552554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirsch JA, Yoo AJ, Nogueira RG, et al. Case volumes of intra-arterial and intravenous treatment of ischemic stroke in the USA. J Neurointerv Surg. 2009;1:27–31. doi: 10.1136/jnis.2009.000166. [DOI] [PubMed] [Google Scholar]
- 3.Adams HP, Jr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655–711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 4.Abou-Chebl A. Endovascular treatment of acute ischemic stroke may be safely performed with no time window limit in appropriately selected patients. Stroke. 2010;41:1996–2000. doi: 10.1161/STROKEAHA.110.578997. [DOI] [PubMed] [Google Scholar]
- 5.Furlan A, Higashida R, Wechsler L, et al. for the PI. Intra-arterial prourokinase for acute ischemic stroke: the PROACT II study: a randomized controlled trial. JAMA. 1999;282:2003–11. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
- 6.Smith WS, Sung G, Starkman S, et al. MERCI Trial Investigators. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke. 2005;36:1432–8. doi: 10.1161/01.STR.0000171066.25248.1d. [DOI] [PubMed] [Google Scholar]
- 7.Smith WS, Sung G, Saver J, et al. Multi MERCI Investigators. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke. 2008;39:1205–12. doi: 10.1161/STROKEAHA.107.497115. [DOI] [PubMed] [Google Scholar]
- 8.Yoo AJ, Verduzco LA, Schaefer PW, et al. MRI-based selection for intra-arterial stroke therapy: value of pretreatment diffusion-weighted imaging lesion volume in selecting patients with acute stroke who will benefit from early recanalization. Stroke. 2009;40:2046–54. doi: 10.1161/STROKEAHA.108.541656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lev MH, Segal AZ, Farkas J, et al. Utility of perfusion-weighted CT imaging in acute middle cerebral artery stroke treated with intra-arterial thrombolysis: prediction of final infarct volume and clinical outcome editorial comment: prediction of final infarct volume and clinical outcome. Stroke. 2001;32:2021–8. doi: 10.1161/hs0901.095680. [DOI] [PubMed] [Google Scholar]
- 10.Gasparotti R, Grassi M, Mardighian D, et al. Perfusion CT in patients with acute ischemic stroke treated with intra-arterial thrombolysis: predictive value of infarct core size on clinical outcome. AJNR Am J Neuroradiol. 2009;30:722–7. doi: 10.3174/ajnr.A1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bose A, Henkes H, Alfke K, et al. Penumbra Phase 1 Stroke Trial Investigators. The Penumbra system: a mechanical device for the treatment of acute stroke due to thromboembolism. AJNR Am J Neuroradiol. 2008;29:1409–13. doi: 10.3174/ajnr.A1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El Khoury R, Fisher M, Savitz SI. Current practice versus willingness to enroll in clinical trials: paradox among vascular neurologists about treatment for acute ischemic stroke. Stroke. 2010;41:2038–43. doi: 10.1161/STROKEAHA.110.586511. [DOI] [PubMed] [Google Scholar]
- 13.Higashida RT, Furlan AJ, Roberts H, et al. Technology Assessment Committee of the American Society of Interventional and Therapeutic Neuroradiology; Technology Assessment Committee of the Society of Interventional Radiology. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. 2003;34:e109–37. doi: 10.1161/01.STR.0000082721.62796.09. [DOI] [PubMed] [Google Scholar]
- 14.Lee M, Hong KS, Saver JL. Efficacy of intra-arterial fibrinolysis for acute ischemic stroke: meta-analysis of randomized controlled trials. Stroke. 2010;41:932–7. doi: 10.1161/STROKEAHA.109.574335. [DOI] [PubMed] [Google Scholar]
- 15.Isenegger J, Nedeltchev K, Arnold M, et al. Reasons to withhold intra-arterial thrombolysis in clinical practice. J Neurol. 2006;253:1552–6. doi: 10.1007/s00415-006-0220-1. [DOI] [PubMed] [Google Scholar]
- 16.Mattle HP, Arnold M, Georgiadis D, et al. Comparison of intra-arterial and intravenous thrombolysis for ischemic stroke with hyperdense middle cerebral artery sign. Stroke. 2008;39:379–83. doi: 10.1161/STROKEAHA.107.492348. [DOI] [PubMed] [Google Scholar]
- 17.Schellinger PD, Thomalla G, Fiehler J, et al. MRI-based and CT-based thrombolytic therapy in acute stroke within and beyond established time windows: an analysis of 1210 patients. Stroke. 2007;38:2640–5. doi: 10.1161/STROKEAHA.107.483255. [DOI] [PubMed] [Google Scholar]
- 18.Yoo AJ, Barak ER, Copen WA, et al. Combining acute diffusion-weighted imaging and mean transmit time lesion volumes with National Institutes of Health Stroke Scale score improves the prediction of acute stroke outcome. Stroke. 2010;41:1728–35. doi: 10.1161/STROKEAHA.110.582874. [DOI] [PubMed] [Google Scholar]


